Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles
Abstract
:1. Introduction
2. Results
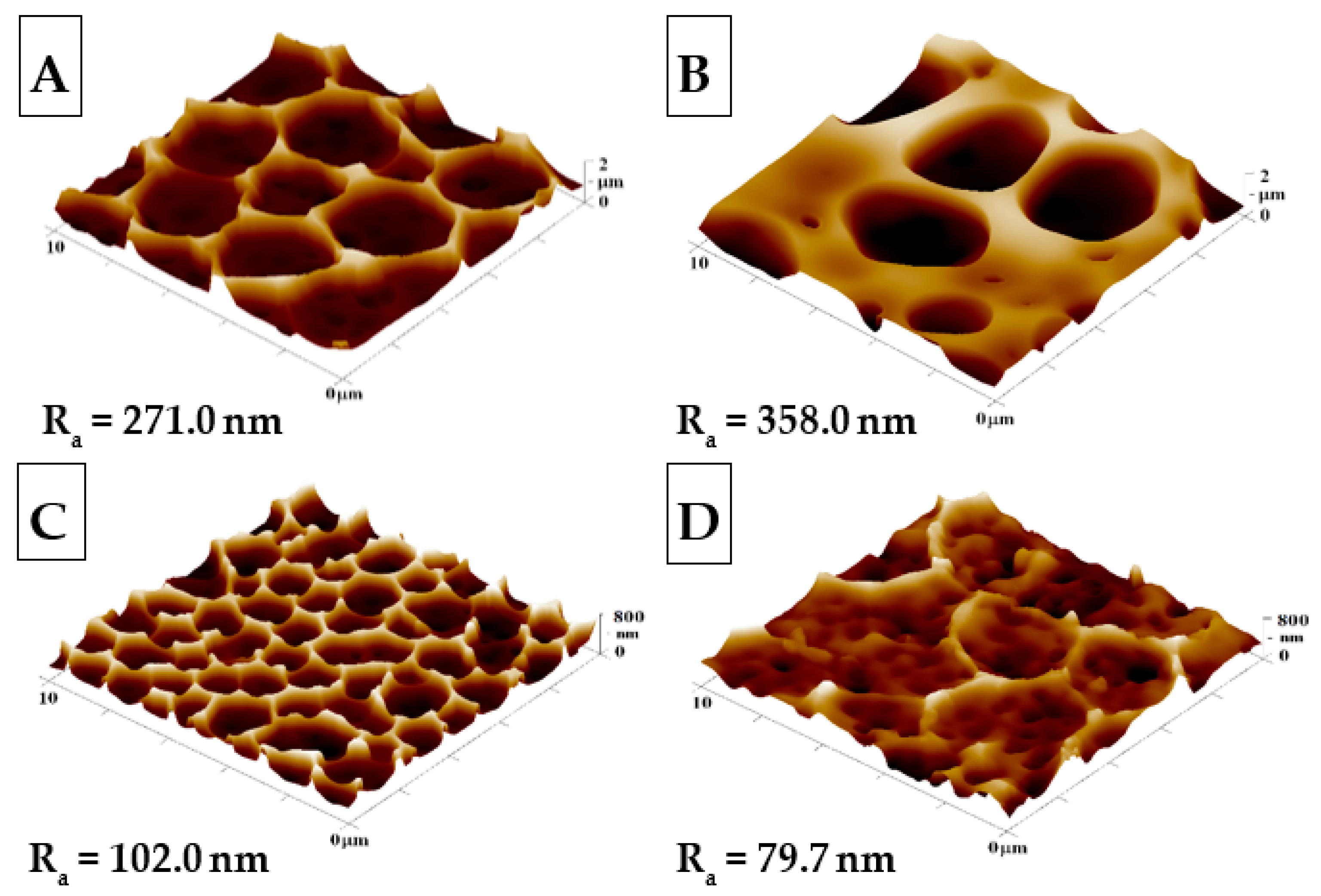
2.1. Surface Morphology and Roughness
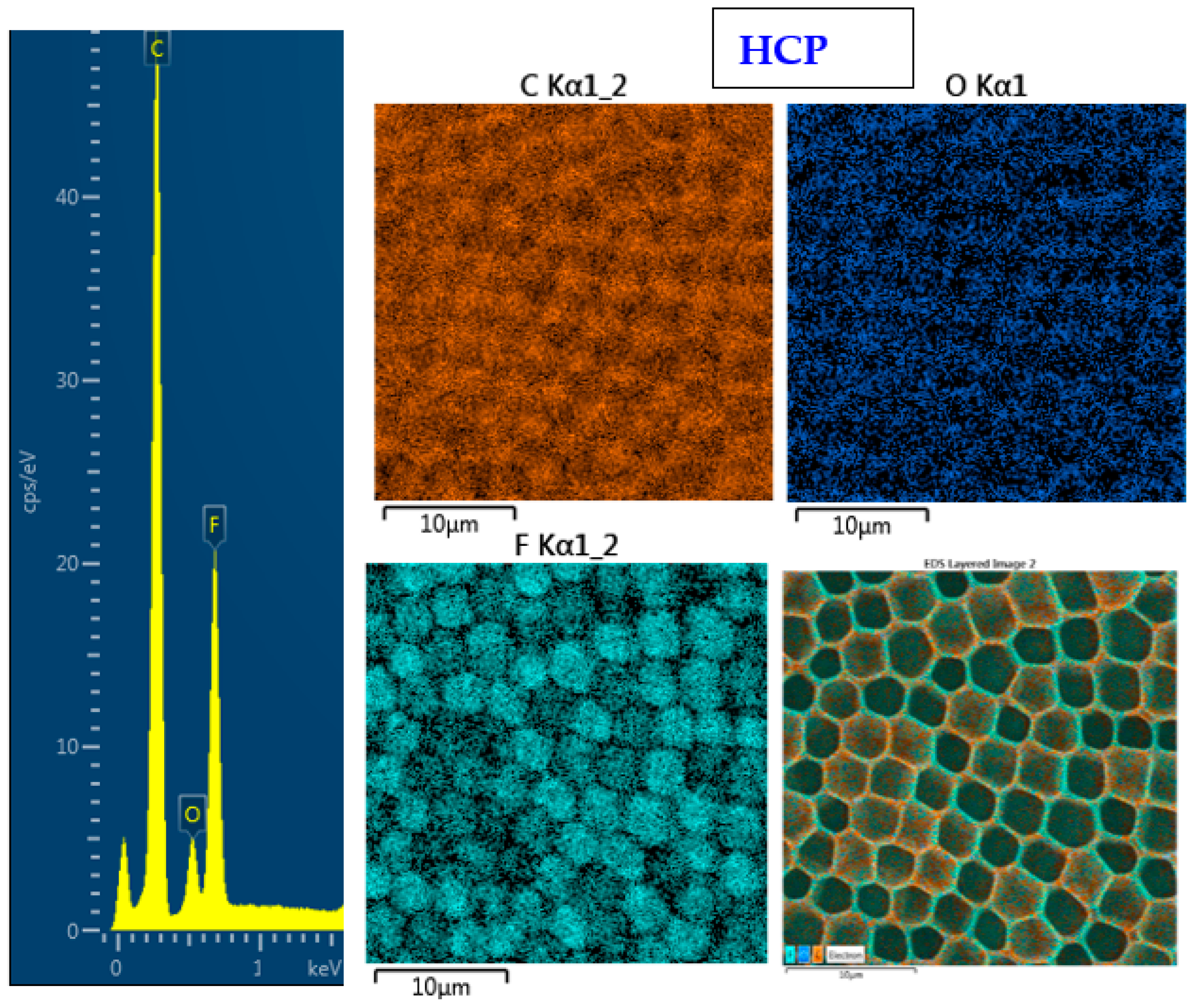
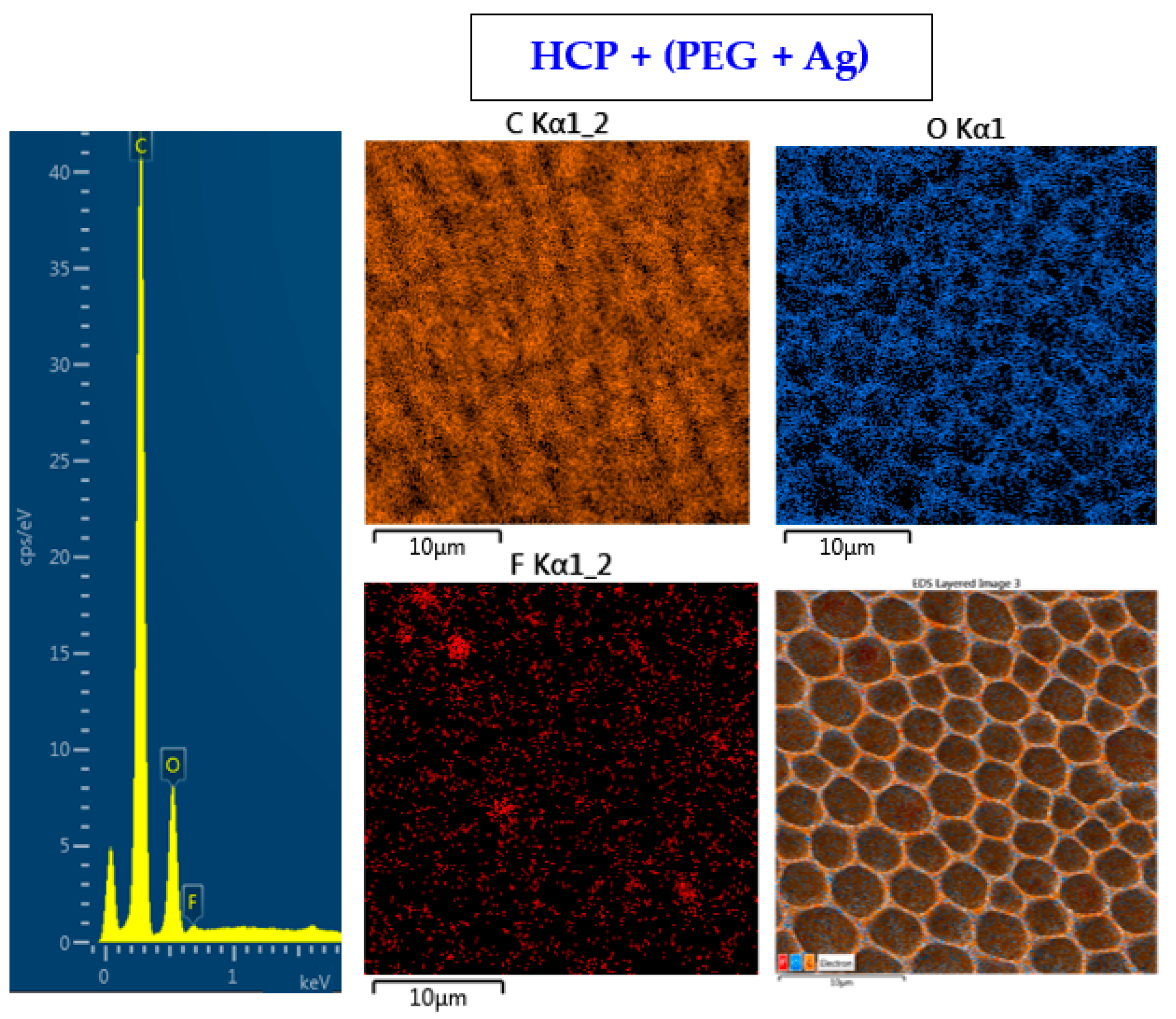
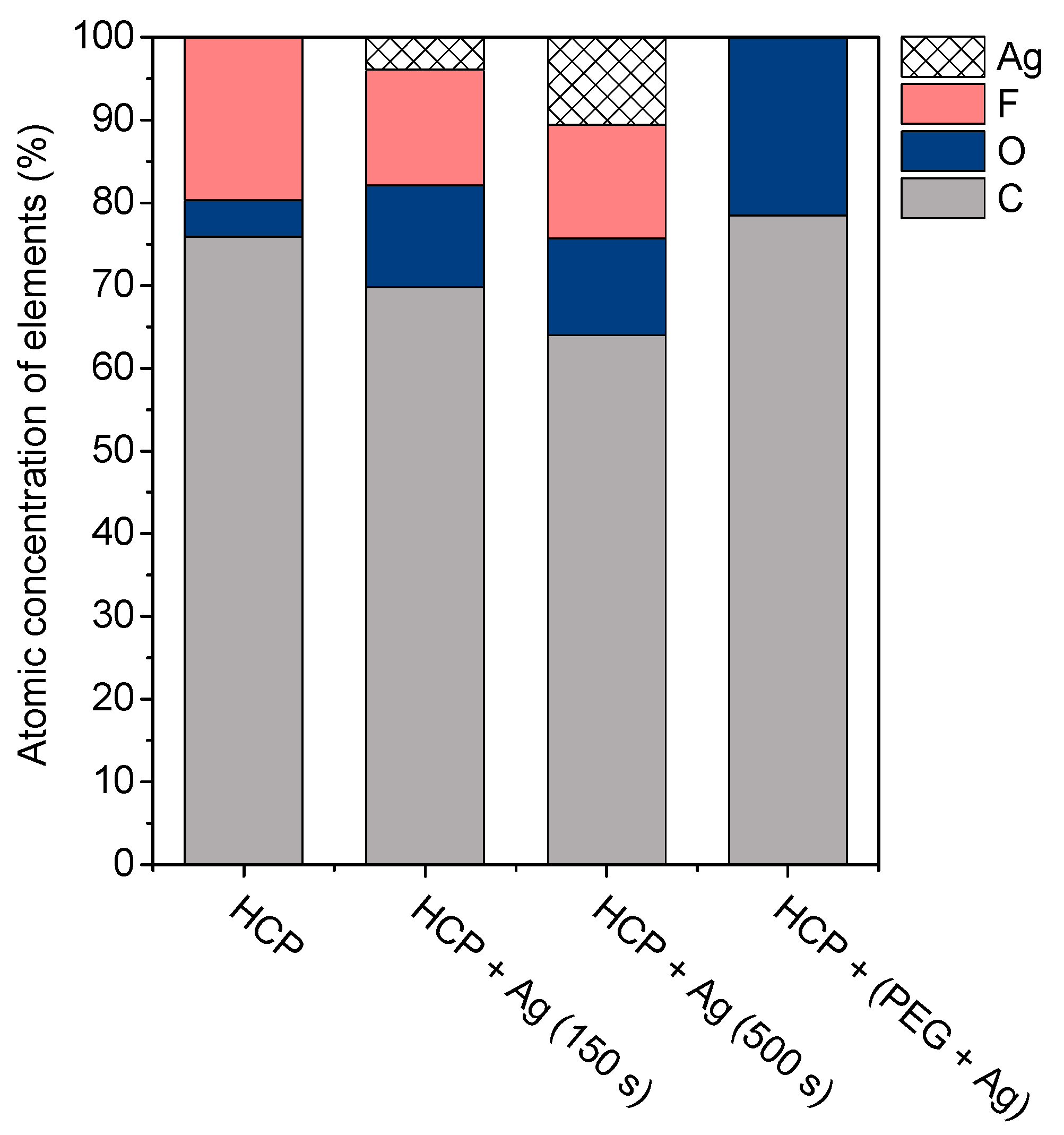
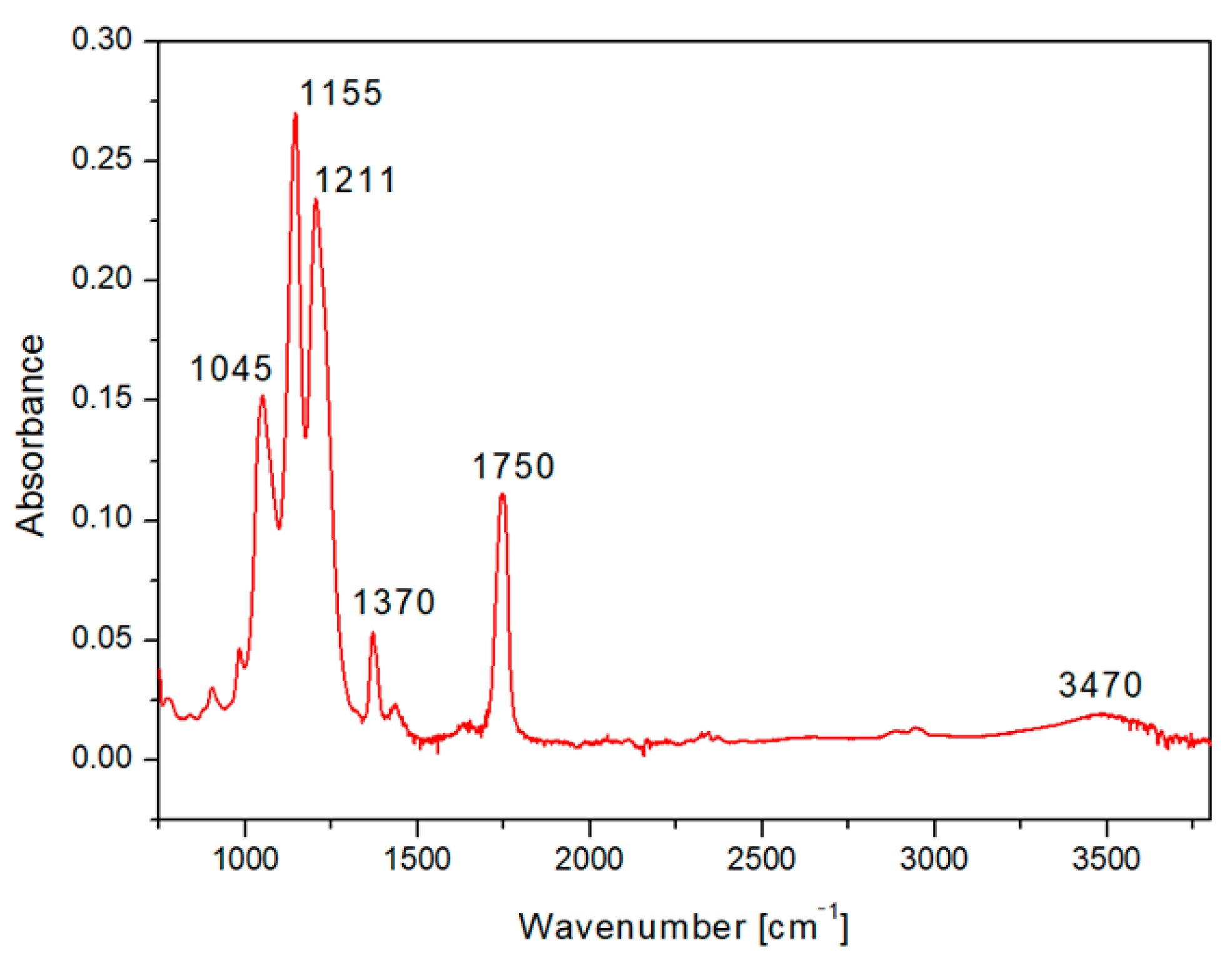
2.2. Surface Morphology Analysis Using SEM and Surface Chemistry Analysis
2.3. Wettability
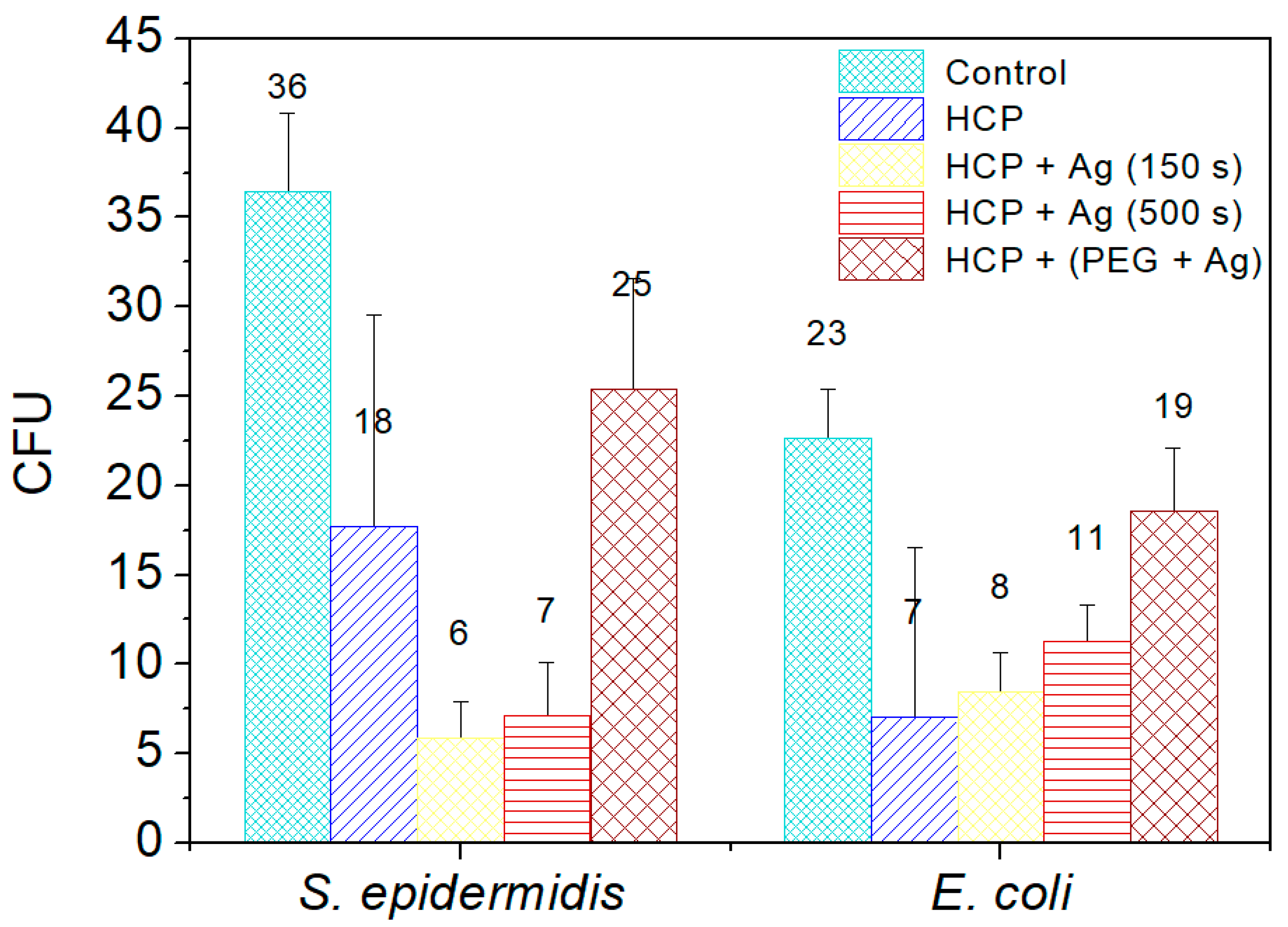
2.4. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.2. Pattern Preparation and Modification
3.3. Silver Nanostructure Preparation
3.4. Analytical Methods
3.5. Antibacterial Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AFM | Atomic force microscopy |
| BF | Breath figure |
| CFU | Colony-forming units |
| ECM | Extracellular matrix |
| EDS | Energy-dispersive X-ray spectroscopy |
| HCP | Honeycomb pattern |
| LB | Luria–Bertani |
| IPS | Improved phase separation |
| PBS | Phosphate-buffered saline |
| PCA | Plate counting agar |
| PEG | Polyethylene glycol |
| PGA | Polyglycolic acid |
| PLA | Poly-L-lactide acid |
| SEM | Scanning electron microscopy |
| TE | Tissue engineering |
References
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.H.; Han, B.; Zhang, X.; Jin, F.; Xu, F.; et al. Bioinspired engineering of honeycomb structure—Using nature to inspire human innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Dong, C.; Hao, J. Honeycomb films with ordered patterns and structures. In Comprehensive Supramolecular Chemistry II; Elsevier: Oxford, UK, 2017; Volume 9, pp. 207–229. [Google Scholar]
- Yin, H.; Zhan, F.; Yu, Y.; Li, Z.; Feng, Y.; Billon, L. Direct formation of hydrophilic honeycomb film by self-assembly in breath figure templating of hydrophobic polylacticacid/ionic surfactant complexes. Soft Mater. 2019, 15, 5052–5059. [Google Scholar] [CrossRef]
- Bencsik, M.; Ramsey, M. We Discovered More about the Honeybee ‘Wake-Up Call’—And It Could Help Save Them, the Conversation. 2018. Available online: https://theconversation.com/we-discovered-more-about-the-honeybee-wake-up-call-and-it-could-help-save-them-105751 (accessed on 15 March 2020).
- Slepička, P.; Neznalová, K.; Fajstavr, D.; Kasálková, N.S.; Švorčík, V. Honeycomb-like pattern formation on perfluoroethylenepropylene enhanced by plasma treatment. Plasma Process Polym. 2019, 16, 1900063. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah, A.-A.A.; Iftikhar, F.J.; Bano, B.; Khan, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common Biocompatible Polymeric Materials for Tissue Engineering and Regenerative Medicine. Mater. Chem. Phys. 2019, 122528. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surfaces B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Ilmarinen, T.; Skottman, H.; Kellomäki, M. Breath figures in tissue engineering and drug delivery: State-of-the-art and future perspectives. Acta Biomater. 2018, 66, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Mahalingam, S.; Edirisinghe, M. Creating “hotels” for cells by electrospinning honeycomb-like polymeric structures. Mater. Sci. Eng. C 2013, 33, 4384–4391. [Google Scholar] [CrossRef] [PubMed]
- Male, U.; Shin, B.K.; Huh, D.S. Coupling of breath figure method with interfacial polymerization: Bottom-surface functionalized honeycomb-patterned porous films. Polymer 2017, 119, 206–211. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M.; Rodríguez-Hernández, J. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 2014, 39, 510–554. [Google Scholar] [CrossRef] [Green Version]
- Bui, V.-T.; Ko, S.H.; Choi, H.-S. Large-Scale Fabrication of Commercially Available, Nonpolar Linear Polymer Film with a Highly Ordered Honeycomb Pattern. ACS Appl. Mater. Interfaces 2015, 7, 10541–10547. [Google Scholar] [CrossRef]
- Dong, R.; Sun, R.; Wang, X.; Chen, Z.; Jin, C. Fabrication of hierarchically structured surfaces with “rose petal” effect by a modified breath figure method. Thin Solid Films 2019, 689, 137503. [Google Scholar] [CrossRef]
- Huang, H.; Dean, D. 3D printed porous cellulose acetate tissue scaffolds for additive manufacturing. Addit. Manuf. 2020, 31, 100927. [Google Scholar] [CrossRef]
- Lukanina, K.I.; Grigoriev, T.E.; Krasheninnikov, S.V.; Mamagulashvilli, V.G.; Kamyshinsky, R.A.; Chvalun, S.N. Multi-hierarchical tissue-engineering ECM-like scaffolds based on cellulose acetate with collagen and chitosan fillers. Carbohydr. Polym. 2018, 191, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.M.; Alavifar, S.S. The role of physicochemical properties in the nanoprecipitation of cellulose acetate. Carbohydr. Polym. 2020, 230, 115628. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Crosslinked pullulan/cellulose acetate fibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2016, 69, 1103–1115. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Kerstin, J.; Thomas, H. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Escudero-Castellanos, A.; Ocampo-García, B.E.; Domínguez-García, M.V.; Flores-Estrada, J.; Flores-Merino, M.V. Hydrogels based on poly(ethylene glycol) as scaffolds for tissue engineering application: Biocompatibility assessment and effect of the sterilization process. J. Mater. Sci. Mater. Med. 2016, 27, 176. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Yabu, H.; Jia, R.; Matsuo, Y.; Ijiro, K.; Yamamoto, S.A.; Nishino, F.; Takaki, T.; Kuwahara, M.; Shimomura, M. Preparation of highly oriented nano-pit arrays by thermal shrinking of honeycomb-patterned polymer films. Adv. Mater. 2008, 20, 4200–4204. [Google Scholar] [CrossRef]
- Tanaka, M.; Takebayashi, M.; Shimomura, M. Fabrication of ordered arrays of biodegradable polymer pincushions using self-organized honeycomb-patterned films. Macromol. Symp. 2009, 279, 175–182. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Sahan, Y.; Gurbuz, O.; Goncagul, G.; Kara, A.; Ozakin, C. Antimicrobial effect of PEG-PLA on food-spoilage microorganisms. Food Sci. Biotechnol. 2017, 26, 1123–1128. [Google Scholar] [CrossRef]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Razza, N.; Breloy, L.; Andaloussi, S.A.; Chiappone, A.; Sangermano, M.; Hélary, C.; Belbekhouche, S.; Coradin, T.; Versace, D. Photoinduced chitosan-PEG hydrogels with long-term antibacterial properties. J. Mater. Chem. 2018, 7, 6526–6538. [Google Scholar] [CrossRef]
- Qing, Y.A.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormena, R.P.L.; Motta, E.V.; Breloy, B.D.F.O.; Chaker, J.A.; Fagg, C.H.W.; Freire, D.O.; Martins, P.M.; da Silva, I.C.R.; Sousa, M.H. Evaluation of the antimicrobial activity of silver nanoparticles obtained by microwave-assisted green synthesis using Handroanthus impetiginosus (Mart. ex DC.) Mattos underbark extract. RSC Adv. 2020, 10, 20676–20681. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Shalumon, K.T.; Chennazhi, K.P.; Nair, S.V. Biomedical Applications of Polymer/Silver Composite Nanofibers. In Biomedical Applications of Polymeric Nanofibers; Jayakumar, R., Nair, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 263–282. [Google Scholar] [CrossRef]
- Vosmanská, V.; Kolářová, K.; Pišlová, M.; Švorčík, V. Chemické a fyzikální modifikace biomateriálů na bázi celulosy. Chem. Listy 2017, 111, 614–621. Available online: http://www.chemicke-listy.cz/docs/full/2017_10_614-621.pdf (accessed on 18 June 2021).
- Slepička, P.; Trostová, S.; Kasálková, N.S.; Kolská, Z.; Sajdl, P.; Švorčík, V. Surface modification of biopolymers by argon plasma and thermal treatment. Plasma Process. Polym. 2011, 9, 197–206. [Google Scholar] [CrossRef]
- Neznalová, K.; Sajdl, P.; Slepička, P.; Švorčík, V. Cellulose acetate honeycomb-like pattern created by improved phase separation. Express Polym. Lett. 2020, 14, 1078–1088. [Google Scholar] [CrossRef]
- Baillot, R.; Deshayes, Y. Tools and analysis methods of encapsulated LEDs. In Reliability Investigation of LED Devices for Public Light Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–206. [Google Scholar]
- Thijssen, W.H.A.; Strange, M.; de Brugh, J.M.J.; van Ruitenbeek, J.M. Formation and properties of metal-oxygen atomic chains. New J. Phys. 2008, 10, 033005. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, K.; Sundberg, J.; Gatenholm, P.; Renneckar, S. Electrospun nanofibrous cellulose scaffolds with controlled microarchitecture. Carbohydr. Polym. 2014, 100, 143–149. [Google Scholar] [CrossRef]
- Gorassinia, A.; Adami, G.; Calvini, P.; Giacomello, A. ATR-FTIR characterization of old pressure sensitive adhesive tapes in historic papers. J. Cult. Herit. 2016, 21, 775–785. [Google Scholar] [CrossRef]
- Li, W. A study of plasma-cleaned Ag-plated Cu leadframe surfaces. J. Electron. Mater. 2010, 39, 295–302. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Ding, Y.; Ge, X.; Leng, Y. Control of bacterial adhesion and growth on honeycomb-like patterned surfaces. Colloids Surfaces B Biointerfaces 2015, 135, 549–555. [Google Scholar] [CrossRef]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of modified biodegradable PHB non-woven fabric. Mater. Sci. Eng. C 2016, 65, 364–368. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Elashnikov, R.; Ulbrich, P.; Staszek, M.; Kolská, Z.; Švorčík, V. Stabilization of sputtered gold and silver nanoparticles in PEG colloid solutions. J. Nanoparticles Res. 2015, 17, 11–26. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtuková, K.; Fajstavrová, K.; Rimpelová, S.; Vokatá, B.; Fajstavr, D.; Kasálková, N.S.; Siegel, J.; Švorčík, V.; Slepička, P. Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles. Materials 2021, 14, 4051. https://doi.org/10.3390/ma14144051
Hurtuková K, Fajstavrová K, Rimpelová S, Vokatá B, Fajstavr D, Kasálková NS, Siegel J, Švorčík V, Slepička P. Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles. Materials. 2021; 14(14):4051. https://doi.org/10.3390/ma14144051
Chicago/Turabian StyleHurtuková, Klaudia, Klára Fajstavrová, Silvie Rimpelová, Barbora Vokatá, Dominik Fajstavr, Nikola Slepičková Kasálková, Jakub Siegel, Václav Švorčík, and Petr Slepička. 2021. "Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles" Materials 14, no. 14: 4051. https://doi.org/10.3390/ma14144051
APA StyleHurtuková, K., Fajstavrová, K., Rimpelová, S., Vokatá, B., Fajstavr, D., Kasálková, N. S., Siegel, J., Švorčík, V., & Slepička, P. (2021). Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles. Materials, 14(14), 4051. https://doi.org/10.3390/ma14144051







