Electrochemotherapy and Other Clinical Applications of Electroporation for the Targeted Therapy of Metastatic Melanoma
Abstract
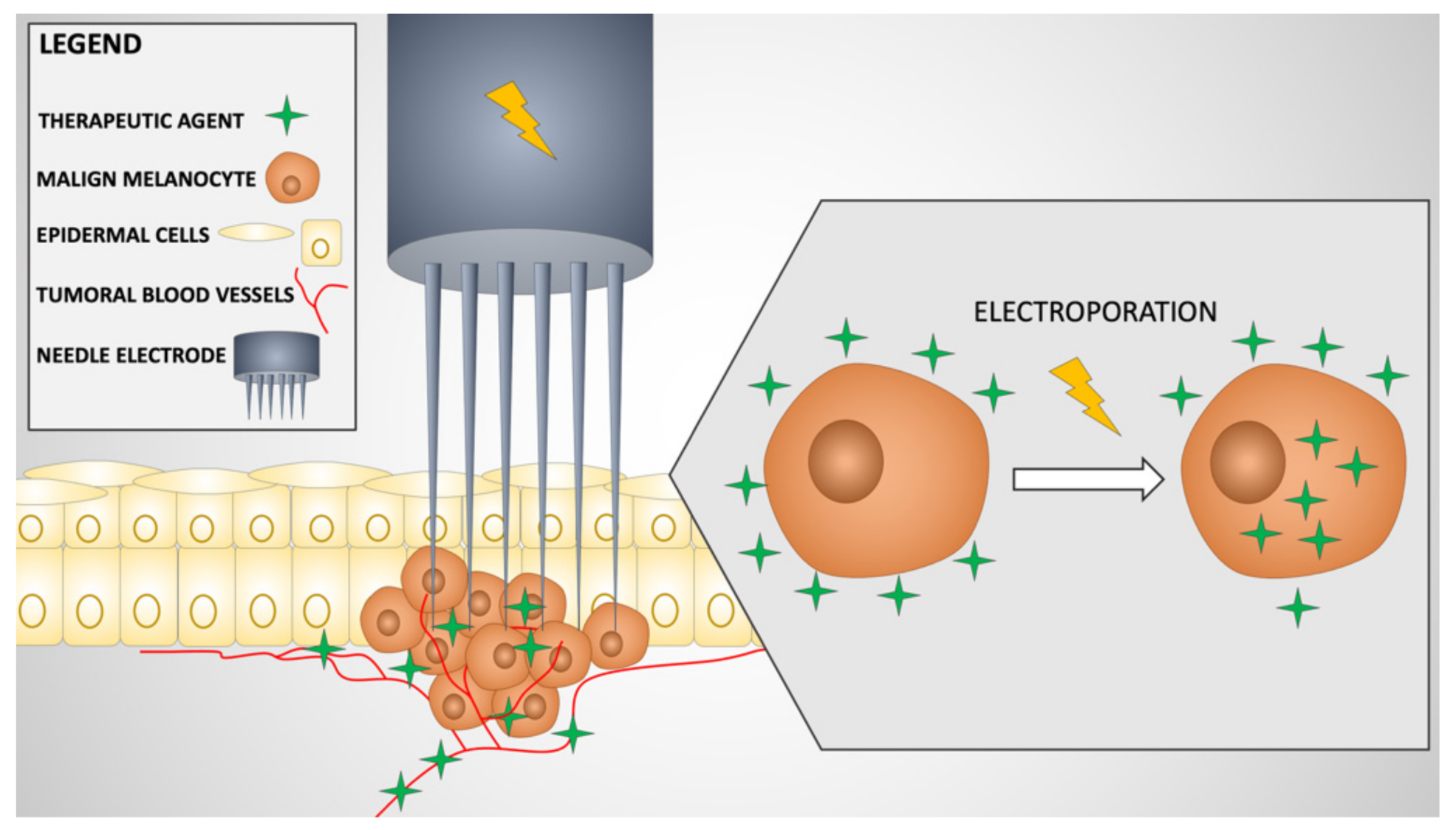
:1. Introduction
2. Mechanism of Action of Electrochemotherapy
3. Electrochemotherapy Treatment Regimen
4. Advantages, Contraindications, Side Effects and Limitations of ECT
5. Clinical Applications of ECT in Melanoma
| No | Author | Clinical Trial Number | Therapeutic Agent | Reference |
|---|---|---|---|---|
| 1 | DeConti et al. | NCT00006035 | Intratumoral bleomycin | [82] |
| 2 | Ricotti et al. | - | Intravenous bleomycin | [83] |
| 3 | Kunte et al. | - | Intratumoral or intravenous bleomycin | [84] |
| 4 | Ferrucci et al. | NCT03448666 | Pembrolizumab and intravenous bleomycin ECT | [85] |
| 5 | Kis et al. | NCT03628417 | Intratumoral calcium electroporation vs. intratumoral bleomycin ECT | [87,88] |
| 6 | Falk et al. | Intratumoral calcium electroporation vs. intratumoral bleomycin ECT | [59] | |
| 7 | Simioni et al. | ISRCTN.11667954 | Intravenous bleomycin with variable electrode-geometry ECT (VEG-ECT) | [88] |
6. Other Clinical Applications of Electroporation in Melanoma
6.1. Intralesional Gene Transfer by Electroporation of Interleukin Plasmids
6.2. Gene Transfer of Human Telomerase Reverse Transcriptase (hTERT) DNA Plasmid
6.3. Gene Transfer of Tyrosinase DNA Plasmid
6.4. SCIB 1—A Human Immunoglobulin G1 Antibody DNA Vaccine
6.5. Gene Transfer of Antiangiogenic Metargidin Peptide Plasmid
6.6. mRNA Electroporated Autologous Dendritic Cells
| No | Author | Clinical Trial Number | Therapeutic Agent | Reference |
|---|---|---|---|---|
| Intralesional gene transfer by electroporation of interleukin plasmids | ||||
| 1 | Daud et al. | NCT00323206 | IL-12p DNA | [91,92] |
| 2 | Algazi et al. | NCT01502293 | Tavokinogene telseplasmid (IL-12p) | [93,94,95] |
| 3 | Tsay et al. | NCT02493361 | IL-12p and pembrolizumab | [96] |
| 4 | Malloy et al. | NCT03132675 | Tavokinogene telseplasmid (IL-12p) and pembrolizumab | [97] |
| 5 | Kharkevitch et al. | NCT00223899 | VCL-IM01 (IL-2 encoding plasmid) | [98] |
| Gene transfer of human telomerase reverse transcriptase (hTERT) DNA plasmid | ||||
| 6 | Aurisicchio et al. | NCT00753415 | V934-EP/V935 vaccine | [99,100] |
| Gene transfer of tyrosinase DNA plasmid | ||||
| 7 | Wolchok et al. | NCT00471133 | Intramuscular tyrosinase DNA plasmid vaccine | [101] |
| SCIB 1—a human immunoglobulin G1 antibody DNA vaccine | ||||
| 8 | Lorigan et al. | NCT01138410 | Intramuscular SCIB1 | [103,104,105] |
| 9 | Patel et al. | NCT04079166 | Intramuscular SCIB1 and pembrolizumab | [106] |
| Gene transfer of antiangiogenic metargidin peptide plasmid | ||||
| 10 | Vasseur et al. | NCT01764009 | Intramuscular AMEP | [108] |
| 11 | Pierre et al. | NCT01045915 | Intratumoral AMEP | [109] |
| 12 | Spanggaard et al. | - | Intratumoral AMEP | [107,110] |
| mRNA electroporated autologous dendritic cells | ||||
| 13 | Young et al. | NCT01456104 | Subcutaneous autologous Langerhans-type dendritic cells electroporated with mRNA encoding a melanoma-associated antigen | [111,112] |
| 14 | Neyns et al. | NCT01676779 | Intravenous and intradermal mRNA electroporated autologous dendritic cells | [113] |
| 15 | Punt et al. | NCT01530698 | Intranodal trimix dendritic cells electroporated with mRNA encoding melanoma-associated antigens | [114] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snoj, M.; Matthiessen, L.W. Electrochemotherapy of Cutaneous Metastases. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–14. [Google Scholar]
- Neophytou, C.M.; Kyriakou, T.C.; Papageorgis, P. Mechanisms of Metastatic Tumor Dormancy and Implications for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 6158. [Google Scholar] [CrossRef] [Green Version]
- Campana, L.G.; Miklavčič, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumors—Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Goggins, C.A.; Khachemoune, A. The use of electrochemotherapy in combination with immunotherapy in the treatment of metastatic melanoma: A focused review. Int. J. Dermatol. 2019, 58, 865–870. [Google Scholar] [CrossRef]
- Stampfli, R.; Willi, M. Membrane potential of a Ranvier node measured after electrical destruction of its membrane. Experientia 1957, 13, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Neu, W.K.; Neu, J.C. Theory of Electroporation BT—Cardiac Bioelectric Therapy: Mechanisms and Practical Implications; Efimov, I.R., Kroll, M.W., Tchou, P.J., Eds.; Springer US: Boston, MA, USA, 2009; pp. 133–161. [Google Scholar]
- Zygogianni, A.; Kyrgias, G.; Scarlatos, J.; Koukourakis, M.; Souliotis, K.; Kouvaris, J.; Kelekis, N.; Kouloulias, V. Potential Role of Electrochemotherapy as Anticancer Treatment for Cutaneous and Subcutaneous Lesions. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 3753–3757. [Google Scholar] [PubMed]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rai, B. Electroporation of Skin Stratum Corneum Lipid Bilayer and Molecular Mechanism of Drug Transport: A Molecular Dynamics Study. Langmuir 2018, 34, 5860–5870. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, L. Changes in living cell morphology induced by electroporation. Bioint. Res. App. Chem. 2015, 5, 975–977. [Google Scholar]
- Sharma, A.K.; Debarshi Ghosh, D.; Saluja, N.K.; Singh, T.G. A Mathematical Model to Expedite Electroporation Based Vaccine Development for COVID-19. Bioint. Res. App. Chem. 2021, 12, 1951–1961. [Google Scholar] [CrossRef]
- Menegazzo, I.; Mammi, S.; Sieni, E.; Sgarbossa, P.; Bartolozzi, A.; Mozzon, M.; Bertani, R.; Forzan, M.; Sundararajan, R. Single-sided Time Domain-Nuclear Magnetic Resonance to Study the Effect of Cell Membrane Electroporation on the Water Mobility in Vegetal Tissues. Bioint. Res. App. Chem. 2021, 11, 14127–14141. [Google Scholar] [CrossRef]
- Rems, L.; Miklavčič, D. Tutorial: Electroporation of cells in complex materials and tissue. J. Appl. Phys. 2016, 119, 201101. [Google Scholar] [CrossRef]
- Sersa, G.; Krzic, M.; Sentjurc, M.; Ivanusa, T.; Beravs, K.; Kotnik, V.; Coer, A.; Swartz, H.M.; Cemazar, M. Reduced blood flow and oxygenation in SA-1 tumours after electrochemotherapy with cisplatin. Br. J. Cancer 2002, 87, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, R.; Jaroszeski, M.J.; Reintgen, D.S.; Puleo, C.A.; DeConti, R.C.; Gilbert, R.A.; Glass, L.F. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–157. [Google Scholar] [CrossRef]
- Sersa, G.; Stabuc, B.; Cemazar, M.; Miklavcic, D.; Rudolf, Z. Electrochemotherapy with cisplatin: The systemic antitumour effectiveness of cisplatin can be potentiated locally by the application of electric pulses in the treatment of malignant melanoma skin metastases. Melanoma Res. 2000, 10, 381–385. [Google Scholar] [CrossRef]
- Giri, P.; Mittal, L.; Ignacio, G.; Camarillo, I.G.; Sundararajan, R. Analysis of Pathways in Triple-Negative Breast Cancer Cells Treated with the Combination of Electrochemotherapy and Cisplatin. Bioint. Res. App. Chem. 2021, 11, 13453–13464. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gordon Spratt, E.A.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef] [Green Version]
- Mali, B.; Jarm, T.; Snoj, M.; Sersa, G.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 4–16. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehld, J.; Sersae, G.; Collins, C.G.; Garbaya, J.R.; Billarda, V.; Geertsend, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Martya, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Pizzichetta, M.A. Cutaneous metastatic melanoma. In Color Atlas of Melanocytic Lesions of the Skin; Springer: Berlin/Heidelberg, Germany, 2007; pp. 260–264. [Google Scholar]
- Savoia, P.; Fava, P.; Nardò, T.; Osella-Abate, S.; Quaglino, P.; Bernengo, M.G. Skin metastases of malignant melanoma: A clinical and prognostic survey. Melanoma Res. 2009, 19, 321–326. [Google Scholar] [CrossRef]
- Rubegni, P.; Lamberti, A.; Mandato, F.; Perotti, R.; Fimiani, M. Dermoscopic patterns of cutaneous melanoma metastases. Int. J. Dermatol. 2014, 53, 404–412. [Google Scholar] [CrossRef]
- Pereira, W.L.; Toledo de Oliveira, T.; Kanashiro, M.M.; Filardi, M.A.; Marcelo Rocha da Costa, M.; Marciano da Costa, L. Anticarcinogenic potential of the Morin bioflavonoid against SK-MEL-5 human melanoma cells. Bioint. Res. App. Chem. 2017, 7, 2098–2102. [Google Scholar]
- Kelidari, H.R.; Alipanah, H.; Roozitalab, G.; Ebrahimi, M.; Osanloo, M. Anticancer Effect of Solid-Lipid Nanoparticles Containing Mentha longifolia and Mentha pulegium Essential Oils: In Vitro Study on Human Melanoma and Breast Cancer Cell Lines. Bioint. Res. App. Chem. 2021, 12, 2128–2137. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Möller, M.G.; Salwa, S.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy as an adjunct or alternative to other treatments for unresectable or in-transit melanoma. Expert Rev. Anticancer Ther. 2009, 9, 1611–1630. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Hronik-Tupaj, M.; Kaplan, D.L. A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng. Part B Rev. 2012, 18, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Miklavcic, D. Analytical description of transmembrane voltage induced by electric fields on spheroidal cells. Biophys. J. 2000, 79, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Pucihar, G.; Miklavcic, D. Induced transmembrane voltage and its correlation with electroporation-mediated molecular transport. J. Membr. Biol. 2010, 236, 3–13. [Google Scholar] [CrossRef]
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A Review of Current Status, Alternative IGP Approaches, and Future Perspectives. J. Healthc. Eng. 2019, 2019, 2784516. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Banoun, H.; Paoletti, C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: Direct access to the cytosol. Exp. Cell Res. 1988, 175, 15–25. [Google Scholar] [CrossRef]
- Wichtowski, M.; Murawa, D. Electrochemotherapy in the treatment of melanoma. Contemp. Oncol. 2018, 22, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.O.; Collins, C.G.; Aarons, S.; Tangney, M.; Whelan, M.; O’Reily, S.; Breathnach, O.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy: Aspects of preclinical development and early clinical experience. Ann. Surg. 2007, 245, 469–479. [Google Scholar] [CrossRef]
- Bigi, L.; Galdo, G.; Cesinaro, A.M.; Vaschieri, C.; Marconi, A.; Pincelli, C.; Fantini, F. Electrochemotherapy induces apoptotic death in melanoma metastases: A histologic and immunohistochemical investigation. Clin. Cosmet. Investig. Dermatol. 2016, 9, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Miklavcic, D.; Corovic, S.; Pucihar, G.; Pavselj, N. Importance of tumour coverage by sufficiently high local electric field for effective electrochemotherapy. Eur. J. Cancer Suppl. 2006, 4, 45–51. [Google Scholar] [CrossRef]
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajsek, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br. J. Cancer 2008, 98, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. CII 2015, 64, 1315–1327. [Google Scholar] [CrossRef]
- Heller, L.; Pottinger, C.; Jaroszeski, M.J.; Gilbert, R.; Heller, R. In vivo electroporation of plasmids encoding GM-CSF or interleukin-2 into existing B16 melanomas combined with electrochemotherapy induces long-term antitumour immunity. Melanoma Res. 2000, 10, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Insp-ECT-Purposes and History. Available online: http://www.insp-ect.org/ (accessed on 10 September 2020).
- Overview|Electrochemotherapy for Metastases in the Skin from Tumours of Non-Skin Origin and Melanoma|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ipg446 (accessed on 10 September 2020).
- Deore, A.B.; Sapakal, V.D.; Shikalgar, T.S.; Jagtap, M.J.; Bhinde, C.J. Elecrochemotherapy: An enhancement of cytotoxicity of anticancer drugs. Innov. Pharm. Pharmacother. 2017, 5, 147–153. [Google Scholar]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.H.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 1147–1154. [Google Scholar] [CrossRef]
- Curatolo, P.; Quaglino, P.; Marenco, F.; Mancini, M.; Nardò, T.; Mortera, C.; Rotunno, R.; Calvieri, S.; Bernengo, M.G. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann. Surg. Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef]
- Landström, F.; Kristiansson, S.; Appelros, P. Neurological Complications After Electrochemotherapy Treatment in the Head and Neck Area. Anticancer Res. 2021, 41, 3519–3522. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Jabbary Lak, F.; Gazdhar, A.; Shafighi, M.; Borradori, L.; Hunger, R.E. Application of electrochemotherapy in the management of primary and metastatic cutaneous malignant tumours: A systematic review and meta-analysis. Eur. J. Dermatol. EJD 2018, 28, 287–313. [Google Scholar] [CrossRef] [PubMed]
- Cabuy, E. Electrochemotherapy in Cancer Treatment. Reliab. Cancer Ther. 2012, 4, 1–29. [Google Scholar]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, E.; Oláh, J.; Ócsai, H.; Baltas, E.; Gyulai, R.; Kemény, L.; Horvath, A.R. Electrochemotherapy of cutaneous metastases of melanoma--a case series study and systematic review of the evidence. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2011, 37, 816–824. [Google Scholar] [CrossRef]
- Mali, B.; Jarm, T.; Corovic, S.; Paulin-Kosir, M.S.; Cemazar, M.; Sersa, G.; Miklavcic, D. The effect of electroporation pulses on functioning of the heart. Med. Biol. Eng. Comput. 2008, 46, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Cadossi, R.; Ronchetti, M.; Cadossi, M. Locally enhanced chemotherapy by electroporation: Clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014, 10, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Sersa, G.; Cemazar, M.; Miklavcic, D.; Chaplin, D.J. Tumor blood flow modifying effect of electrochemotherapy with bleomycin. Anticancer Res. 1999, 19, 4017–4022. [Google Scholar]
- Quaglino, P.; Mortera, C.; Osella-Abate, S.; Barberis, M.; Illengo, M.; Rissone, M.; Savoia, P.; Bernengo, M.G. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann. Surg. Oncol. 2008, 15, 2215–2222. [Google Scholar] [CrossRef]
- Kristiansson, S.; Reizenstein, J.; von Beckerath, M.; Landström, F. Long-term follow-up in patients treated with electrochemotherapy for non-melanoma skin cancer in the head and neck area. Acta Otolaryngol. 2019, 139, 195–200. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Perri, F.; Pavone, E.; Aversa, C.; Maglione, M.G.; Guida, A.; Montano, M.; Villano, S.; Daponte, A.; Caponigro, F.; et al. Electrochemotherapy as palliative treatment in patients with advanced head and neck tumours: Outcome analysis in 93 patients treated in a single institution. Oral Oncol. 2019, 92, 77–84. [Google Scholar] [CrossRef]
- Wichtowski, M.; Potocki, P.; Kufel-Grabowska, J.; Streb, J.; Murawa, D. Electrochemotherapy in the Treatment of Massive, Multisite Breast Cancer Metastasis to the Skin and Subcutaneous Tissue: A Case Report. Breast Care 2016, 11, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Campana, L.G.; Marconato, R.; Valpione, S.; Galuppo, S.; Alaibac, M.; Rossi, C.R.; Mocellin, S. Basal cell carcinoma: 10-year experience with electrochemotherapy. J. Transl. Med. 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, E.G.; Baltás, E.; Ócsai, H.; Vass, A.; Németh, I.B.; Varga, E.; Oláh, J.; Kemény, L.; Tóth-Molnár, E. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci. Rep. 2019, 9, 4285. [Google Scholar] [CrossRef]
- Kreuter, A.; van Eijk, T.; Lehmann, P.; Fischer, M.; Horn, T.; Assaf, C.; Schley, G.; Herbst, R.; Kellner, I.; Weisbrich, C.; et al. Electrochemotherapy in advanced skin tumors and cutaneous metastases—A retrospective multicenter analysis. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2015, 13, 308–315. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Baltás, E.; Kis, E.; Nagy, N.; Sohár, N.; Varga, E.; Széll, M.; Kemény, L.; Oláh, J. Electrochemotherapy for Non-melanoma Skin Cancer in a Child with Xeroderma Pigmentosum. Acta Derm. Venereol. 2017, 97, 962–964. [Google Scholar] [CrossRef] [Green Version]
- Di Chiacchio, N.G.; Di Chiacchio, N.; Criado, P.R.; Brunner, C.H.M.; Suaréz, M.V.R.; Belda Junior, W. Ungual warts: Comparison of treatment with intralesional bleomycin and electroporation in terms of efficacy and safety. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 2349–2354. [Google Scholar] [CrossRef]
- Starita, N.; Di Monta, G.; Cerasuolo, A.; Marone, U.; Anniciello, A.M.; Botti, G.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Effect of electrochemotherapy on human herpesvirus 8 kinetics in classic Kaposi sarcoma. Infect. Agent Cancer 2017, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scelsi, D.; Mevio, N.; Bertino, G.; Occhini, A.; Brazzelli, V.; Morbini, P.; Benazzo, M. Electrochemotherapy as a new therapeutic strategy in advanced Merkel cell carcinoma of head and neck region. Radiol. Oncol. 2013, 47, 366–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichtowski, M.; Murawa, D.; Czarnecki, R.; Piechocki, J.; Nowecki, Z.; Witkiewicz, W. Electrochemotherapy in the Treatment of Breast Cancer Metastasis to the Skin and Subcutaneous Tissue—Multicenter Experience. Oncol. Res. Treat. 2019, 42, 47–51. [Google Scholar] [CrossRef]
- Campana, L.G.; Bianchi, G.; Mocellin, S.; Valpione, S.; Campanacci, L.; Brunello, A.; Donati, D.; Sieni, E.; Rossi, C.R. Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: Results of a non-comparative phase II study. World J. Surg. 2014, 38, 813–822. [Google Scholar] [CrossRef]
- Di Meo, N.; Conforti, C.; Gatti, A.; Nan, K.; Degrassi, F.; Cova, M.A.; Stacul, F.; Zalaudek, I. Ultrasound-guided electrochemotherapy for the treatment of skin metastases of breast cancer: A winning combination of techniques. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, e432–e434. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Stinco, G.; Trevisini, S.; di Meo, N.; Signoretto, D.; Leonardo, E.; Bonin, S.; Trevisan, G. Electrochemotherapy as a novel treatment for primary cutaneous marginal zone B-cell lymphomas. Dermatol. Ther. 2014, 27, 244–247. [Google Scholar] [CrossRef]
- Guida, M.; Ruggieri, E.; Fucci, L.; Ressa, M.; D’Aluisio, L.; Fanelli, G.; Strippoli, S. Image Gallery: A case of cutaneous giant angiosarcoma treated successfully with electrochemotherapy. Br. J. Dermatol. 2017, 177, e27. [Google Scholar] [CrossRef]
- Guida, M.; Campana, L.G.; Curatolo, P.; Strippoli, S.; Bonadies, A.; Grilz, G.; Cabula, C.; Rotunno, R.; Bucher, S.; Solari, N.; et al. Local treatment with electrochemotherapy of superficial angiosarcomas: Efficacy and safety results from a multi-institutional retrospective study. J. Surg. Oncol. 2016, 114, 246–253. [Google Scholar] [CrossRef]
- Gehl, J.; Geertsen, P.F. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res. 2000, 10, 585–589. [Google Scholar] [CrossRef]
- Morley, J.; Grocott, P.; Purssell, E.; Murrells, T. Electrochemotherapy for the palliative management of cutaneous metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 2257–2267. [Google Scholar] [CrossRef]
- Gehl, J.; Geertsen, P.F. Palliation of haemorrhaging and ulcerated cutaneous tumours using electrochemotherapy. Eur. J. Cancer Suppl. 2006, 4, 35–37. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S.; Belehradek, J., Jr.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Mir, L.M.; Belehradek, M.; Domenge, C.; Orlowski, S.; Poddevin, B.; Belehradek, J., Jr.; Schwaab, G.; Luboinski, B.; Paoletti, C. Electrochemotherapy, a new antitumor treatment: First clinical trial. C. R. Acad. Sci. III 1991, 313, 613–618. [Google Scholar] [PubMed]
- Bleomycin with or without Electroporation Therapy in Treating Patients with Stage III or Stage IV Melanoma, NCT00006035. Available online: https://clinicaltrials.gov/ct2/show/NCT00006035 (accessed on 30 May 2021).
- Ricotti, F.; Giuliodori, K.; Cataldi, I.; Campanati, A.; Ganzetti, G.; Ricotti, G.; Offidani, A. Electrochemotherapy: An effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol. Ther. 2014, 27, 148–152. [Google Scholar] [CrossRef]
- Kunte, C.; Letulé, V.; Gehl, J.; Dahlstroem, K.; Curatolo, P.; Rotunno, R.; Muir, T.; Occhini, A.; Bertino, G.; Powell, B.; et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br. J. Dermatol. 2017, 176, 1475–1485. [Google Scholar] [CrossRef]
- ECT-Pembrolizumab in Patients with Unresectable Melanoma with Superficial or Superficial and Visceral Metastases, NCT03448666. Available online: https://clinicaltrials.gov/ct2/show/NCT03448666 (accessed on 30 May 2021).
- Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial, NCT03628417. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03628417 (accessed on 30 May 2021).
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, É.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simioni, A.; Valpione, S.; Granziera, E.; Rossi, C.R.; Cavallin, F.; Spina, R.; Sieni, E.; Aliberti, C.; Stramare, R.; Campana, L.G. Ablation of soft tissue tumours by long needle variable electrode-geometry electrochemotherapy: Final report from a single-arm, single-centre phase-2 study. Sci. Rep. 2020, 10, 2291. [Google Scholar] [CrossRef] [Green Version]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Morgan, R.A.; Beane, J.D.; Zheng, Z.; Dudley, M.E.; Kassim, S.H.; Nahvi, A.V.; Ngo, L.T.; Sherry, R.M.; Phan, G.Q.; et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015, 21, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Phase I Trial of Intratumoral pIL-12 Electroporation in Malignant Melanoma, NCT00323206. Available online: https://clinicaltrials.gov/ct2/show/NCT00323206 (accessed on 30 May 2021).
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trial of pIL-12 Electroporation Malignant Melanoma (IL-12MEL), NCT01502293. Available online: https://clinicaltrials.gov/ct2/show/NCT01502293 (accessed on 30 May 2021).
- A Multicenter Phase II Trial of Intratumoral pIL-12 Electroporation in Advanced Stage Cutaneous and in Transit Malignant Melanoma, NCT01502293. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01502293 (accessed on 30 May 2021).
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef] [Green Version]
- A Multicenter Phase II Trial of Intratumoral pIL-12 Electroporation in Advanced Stage Cutaneous and in Transit Malignant Melanoma, NCT02493361. Available online: https://clinicaltrials.gov/ct2/show/NCT02493361 (accessed on 30 May 2021).
- Tavo and Pembrolizumab in Patients with Stage III/IV Melanoma Progressing on Pembrolizumab or Nivolumab Treatment (Keynote695), NCT03132675. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03132675 (accessed on 30 May 2021).
- A Trial to Evaluate the Safety of Intratumoral VCL-IM01 Followed by Electroporation in Metastatic Melanoma, NCT00223899. Available online: https://clinicaltrials.gov/ct2/show/NCT00223899 (accessed on 30 May 2021).
- A Study of V934/V935 Vaccine in Cancer Participants with Selected Solid Tumors (V934-002), NCT00753415. Available online: https://clinicaltrials.gov/ct2/show/NCT00753415 (accessed on 30 May 2021).
- Aurisicchio, L.; Fridman, A.; Mauro, D.; Sheloditna, R.; Chiappori, A.; Bagchi, A.; Ciliberto, G. Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: A phase I study. J. Transl. Med. 2020, 18, 39. [Google Scholar] [CrossRef] [Green Version]
- Safety and Immunogenicity of a Melanoma DNA Vaccine Delivered by Electroporation, NCT00471133. Available online: https://clinicaltrials.gov/ct2/show/NCT00471133 (accessed on 30 May 2021).
- Yuan, J.; Ku, G.Y.; Adamow, M.; Mu, Z.; Tandon, S.; Hannaman, D.; Chapman, P.; Schwartz, G.; Carvajal, R.; Panageas, K.S.; et al. Immunologic responses to xenogeneic tyrosinase DNA vaccine administered by electroporation in patients with malignant melanoma. J. Immunother. Cancer 2013, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Study of a DNA Immunotherapy to Treat Melanoma, NCT01138410. Available online: https://clinicaltrials.gov/ct2/show/NCT01138410 (accessed on 30 May 2021).
- Xue, W.; Brentville, V.A.; Symonds, P.; Cook, K.W.; Yagita, H.; Metheringham, R.L.; Durrant, L.G. SCIB1, a huIgG1 antibody DNA vaccination, combined with PD-1 blockade induced efficient therapy of poorly immunogenic tumors. Oncotarget 2016, 7, 83088–83100. [Google Scholar] [CrossRef]
- Patel, P.M.; Ottensmeier, C.H.; Mulatero, C.; Lorigan, P.; Plummer, R.; Pandha, H.; Elsheikh, S.; Hadjimichael, E.; Villasanti, N.; Adams, S.E.; et al. Targeting gp100 and TRP-2 with a DNA vaccine: Incorporating T cell epitopes with a human IgG1 antibody induces potent T cell responses that are associated with favourable clinical outcome in a phase I/II trial. Oncoimmunology 2018, 7, e1433516. [Google Scholar] [CrossRef] [Green Version]
- SCIB1 in Melanoma Patients Receiving Pembrolizumab SCIB1-002, NCT04079166. Available online: https://clinicaltrials.gov/ct2/show/NCT04079166 (accessed on 30 May 2021).
- Spanggaard, I.; Snoj, M.; Cavalcanti, A.; Bouquet, C.; Sersa, G.; Robert, C.; Cemazar, M.; Dam, E.; Vasseur, B.; Attali, P.; et al. Gene electrotransfer of plasmid antiangiogenic metargidin peptide (AMEP) in disseminated melanoma: Safety and efficacy results of a phase I first-in-man study. Hum. Gene Ther. Clin. Dev. 2013, 24, 99–107. [Google Scholar] [CrossRef]
- Safety and Efficacy of Intramuscular Electrotransfer of Plasmid AMEP in Patients Suffering from Advanced or Metastatic Melanoma: An Open-Label Phase I/II Clinical Trial—The AIMM Study (AMEP in Metastatic Melanoma), NCT01764009. Available online: https://clinicaltrials.gov/ct2/show/NCT01764009 (accessed on 30 May 2021).
- Safety and Efficacy of Intratumoural Electrotransfer of Plasmid AMEP in Patients Suffering from Advanced or Metastatic Melanoma: An Open Phase 1 Trial, NCT01045915. Available online: https://clinicaltrials.gov/ct2/show/NCT01045915 (accessed on 30 May 2021).
- Spanggaard, I.; Gehl, J. Antiangiogenic Metargidin Peptide (AMEP) Gene Therapy in Disseminated Melanoma. Methods Mol. Biol. 2015, 1317, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Immune Responses to Autologous Langerhans-type Dendritic Cells Electroporated with mRNA Encoding a Tu-Mor-Associated Antigen in Patients with Malignancy: A Single-Arm Phase I Trial in Melanoma, NCT01456104. Available online: https://clinicaltrials.gov/ct2/show/NCT01456104 (accessed on 30 May 2021).
- Chung, D.J.; Carvajal, R.D.; Postow, M.A.; Sharma, S.; Pronschinske, K.B.; Shyer, J.A.; Singh-Kandah, S.; Dickson, M.A.; D’Angelo, S.P.; Wolchok, J.D.; et al. Langerhans-type dendritic cells electroporated with TRP-2 mRNA stimulate cellular immunity against melanoma: Results of a phase I vaccine trial. Oncoimmunology 2017, 7, e1372081. [Google Scholar] [CrossRef] [PubMed]
- mRNA Electroporated Autologous Dendritic Cells for Stage III/IV Melanoma (DC-MEL), NCT01676779. Available online: https://clinicaltrials.gov/ct2/show/NCT01676779 (accessed on 30 May 2021).
- Single-step Antigen Loading and TLR Activation of Dendritic Cells by mRNA Electroporation for Vaccination in Stage III and IV Melanoma Patients, NCT01530698. Available online: https://clinicaltrials.gov/ct2/show/NCT01530698 (accessed on 30 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucu, C.I.; Giurcăneanu, C.; Popa, L.G.; Orzan, O.A.; Beiu, C.; Holban, A.M.; Grumezescu, A.M.; Matei, B.M.; Popescu, M.N.; Căruntu, C.; et al. Electrochemotherapy and Other Clinical Applications of Electroporation for the Targeted Therapy of Metastatic Melanoma. Materials 2021, 14, 3985. https://doi.org/10.3390/ma14143985
Cucu CI, Giurcăneanu C, Popa LG, Orzan OA, Beiu C, Holban AM, Grumezescu AM, Matei BM, Popescu MN, Căruntu C, et al. Electrochemotherapy and Other Clinical Applications of Electroporation for the Targeted Therapy of Metastatic Melanoma. Materials. 2021; 14(14):3985. https://doi.org/10.3390/ma14143985
Chicago/Turabian StyleCucu, Corina Ioana, Călin Giurcăneanu, Liliana Gabriela Popa, Olguța Anca Orzan, Cristina Beiu, Alina Maria Holban, Alexandru Mihai Grumezescu, Bogdan Mircea Matei, Marius Nicolae Popescu, Constantin Căruntu, and et al. 2021. "Electrochemotherapy and Other Clinical Applications of Electroporation for the Targeted Therapy of Metastatic Melanoma" Materials 14, no. 14: 3985. https://doi.org/10.3390/ma14143985














