Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement
Abstract
:1. Introduction
2. Materials and Methods
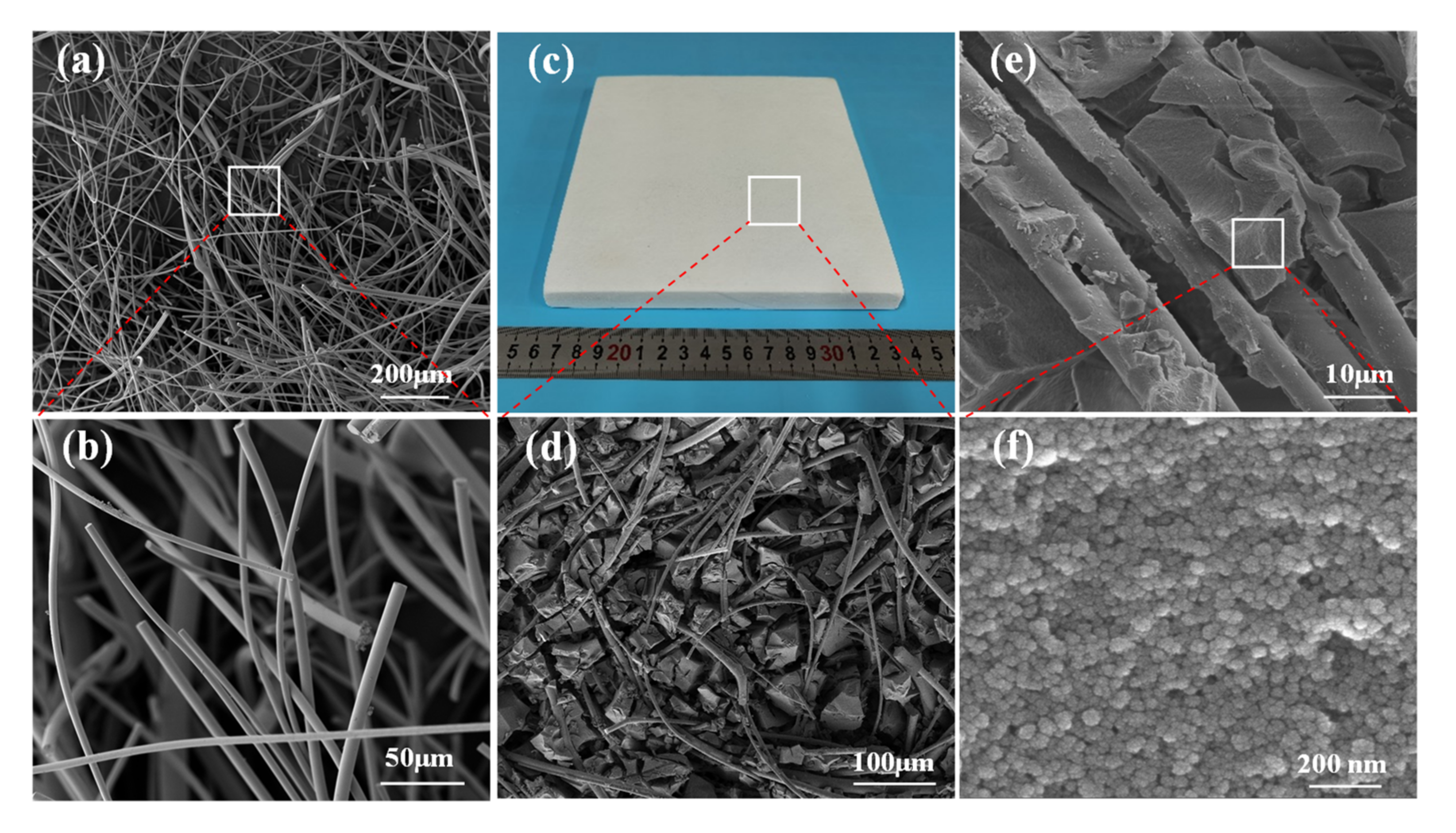
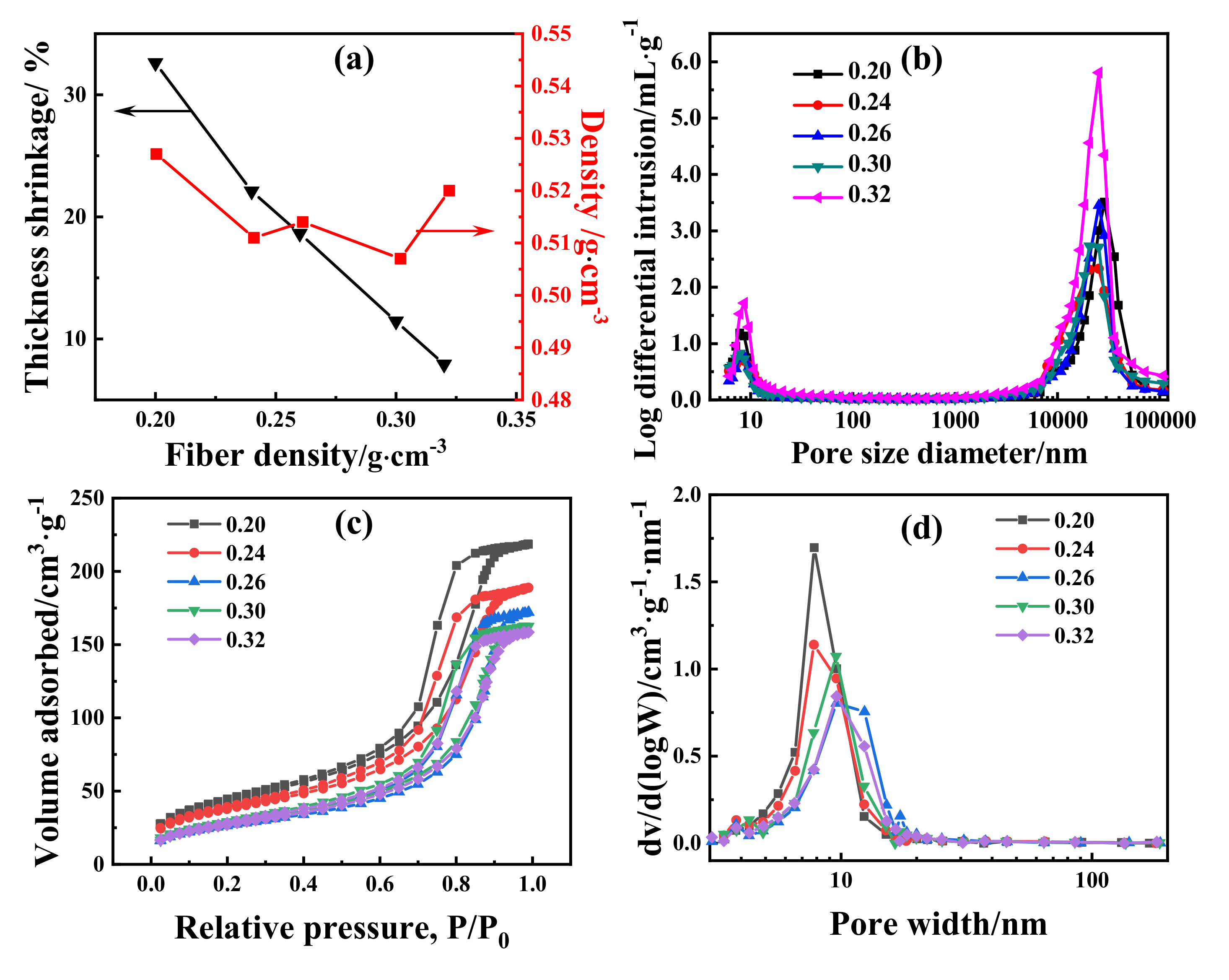
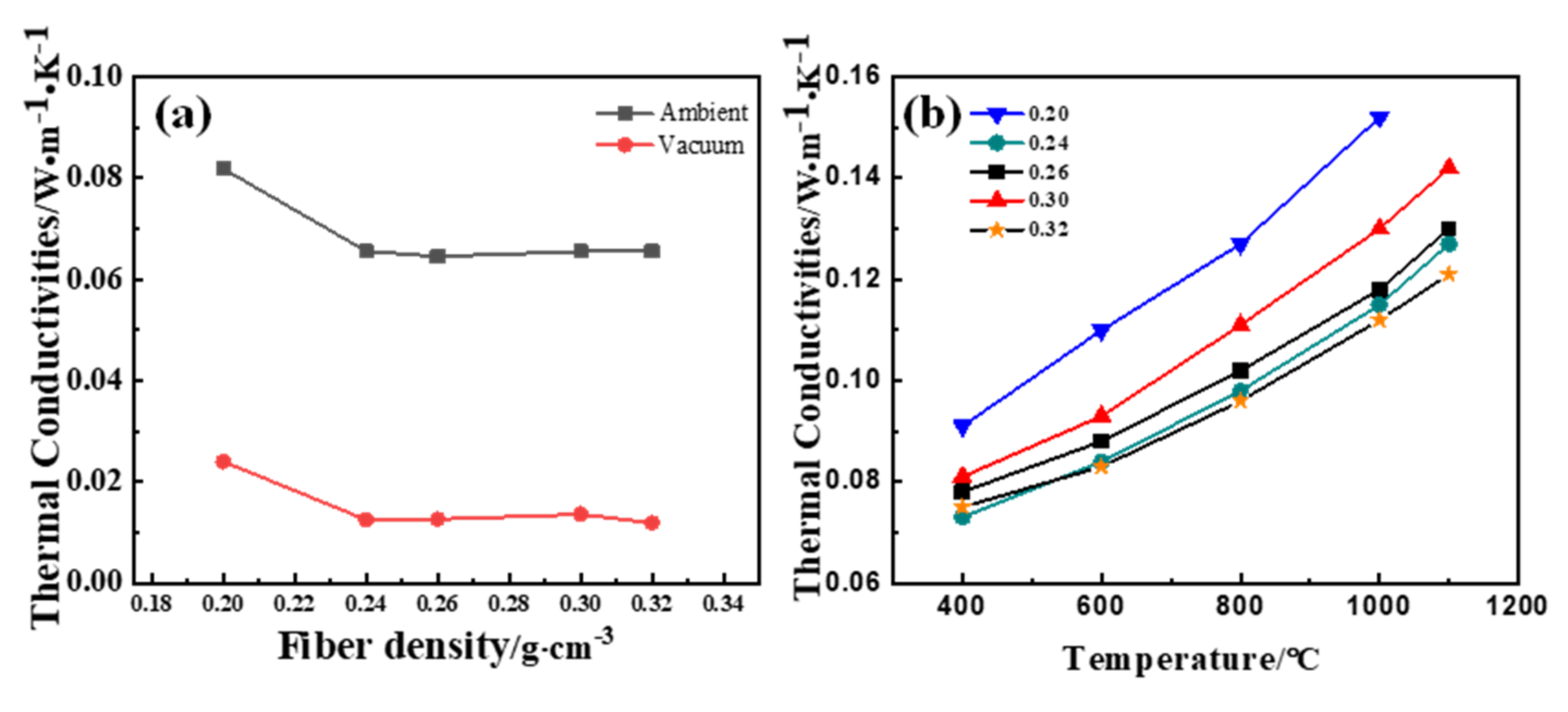
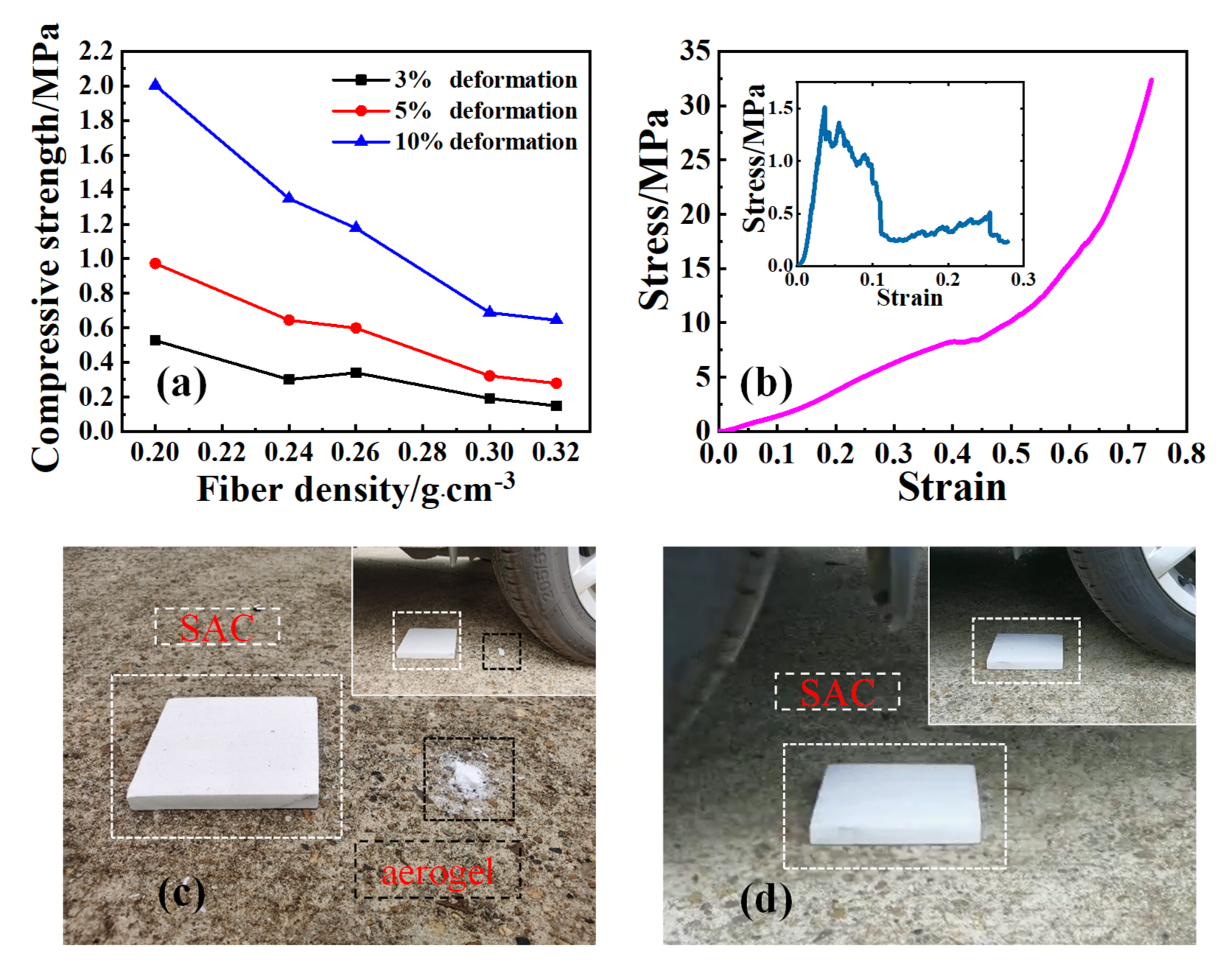
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Li, L.; Feng, J.; Feng, J. Preparation of monodispersed silica sol with small particle size, narrow size distribution, and high conversion. J. Sol-Gel Sci. Technol. 2019, 91, 44–53. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Feng, J.; Zhang, S.; Peng, F.; Xiao, Y.; Li, L.; Feng, J. Preparation of silica aerogels with high temperature resistance and low thermal conductivity by monodispersed silica sol. Mater. Des. 2020, 191, 108640. [Google Scholar] [CrossRef]
- Niu, Z.; He, X.; Huang, T.; Tang, B.; Cheng, X.; Zhang, Y.; Shao, Z. A facile preparation of transparent methyltriethoxysilane based silica xerogel monoliths at ambient pressure drying. Microporous Mesoporous Mater. 2019, 286, 98–104. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Li, K.; Zhao, S.; Fei, Z.; Zhang, Z. A flexible silica aerogel with good thermal and acoustic insulation prepared via water solvent system. J. Sol-Gel Sci. Technol. 2019, 92, 652–661. [Google Scholar] [CrossRef]
- Guo, T.; Yun, S.; Li, Y.; Chen, Z.; Cao, C.; Gao, Y. Facile synthesis of highly flexible polymethylsilsesquioxane aerogel monoliths with low density, low thermal conductivity and superhydrophobicity. Vacuum 2021, 183, 109825. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chang, T.F.; Lu, S.Y.; Chang, Y.C. Preparation of monolithic silica aerogel of low thermal conductivity by ambient pressure drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Oh, C.S.; Kim, Y.H.; Ahn, Y.S.; Yeo, J.G. Methyltrimethoxysilane based monolithic silica aerogels via ambient pressure drying. Microporous Mesoporous Mater. 2007, 100, 350–355. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zhang, W.; Yan, H.; Wang, Y.; Li, M.; Liu, Q. Rapid synthesis and characterization of ambient pressure dried monolithic silica aerogels in ethanol/water co-solvent system. J. Non-Cryst. Solids 2019, 503–504, 214–223. [Google Scholar] [CrossRef]
- Cheng, X.; Li, C.; Shi, X.; Li, Z.; Gong, L.; Zhang, H. Rapid synthesis of ambient pressure dried monolithic silica aerogels using water as the only solvent. Mater. Lett. 2017, 204, 157–160. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- Xu, B.; Cai, J.Y.; Finn, N.; Cai, Z. An improved method for preparing monolithic aerogels based on methyltrimethoxysilane at ambient pressure Part I: Process development and macrostructures of the aerogels. Microporous Mesoporous Mater. 2012, 148, 145–151. [Google Scholar] [CrossRef]
- Yun, S.; Guo, T.; Zhang, J.; He, L.; Li, Y.; Li, H.; Zhu, X.; Gao, Y. Facile synthesis of large-sized monolithic methyltrimethoxysilane-based silica aerogel via ambient pressure drying. J. Sol-Gel Sci. Technol. 2017, 83, 53–63. [Google Scholar] [CrossRef]
- Cheng, E.J.; Sakamoto, J.; Salvador, J.; Wang, H.; Maloney, R.; Thompson, T. Cast-in-place, ambiently-dried, silica-based, high-temperature insulation. Acta Mater. 2017, 127, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Jiang, Y.; Feng, J.; Feng, J.; Yue, C. Infrared-opacified Al2O3–SiO2 aerogel composites reinforced by SiC-coated mullite fibers for thermal insulations. Ceram. Int. 2015, 41, 437–442. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Chen, Q.; Zhang, S.; Li, L.; Feng, J.; Feng, J. Sintering behavior of SiO2 aerogel composites reinforced by mullite fibers via in-situ rapid heating TEM observations. J. Eur. Ceram. Soc. 2020, 40, 127–135. [Google Scholar] [CrossRef]
- Du, D.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Facile synthesis of silica aerogel composites via ambient-pressure drying without surface modification or solvent exchange. Vacuum 2020, 173, 109117. [Google Scholar] [CrossRef]
- Xu, Q.; Ren, H.; Zhu, J.; Bi, Y.; Xu, Y.; Zhang, L. Facile fabrication of graphite-doped silica aerogels with ultralow thermal conductivity by precise control. J. Non-Cryst. Solids 2017, 469, 14–18. [Google Scholar] [CrossRef]
- Tsakiroglou, C.D.; Burganos, V.N.; Jacobsen, J. Pore-Structure Analysis by Using Nitrogen Sorption and Mercury Intrusion Data. Aiche J. 2010, 50, 489–510. [Google Scholar] [CrossRef]
- Rigby, S.P.; Fletcher, R.S.; Riley, S.N. Characterisation of porous solids using integrated nitrogen sorption and mercury porosimetry. Chem. Eng. Sci. 2004, 59, 41–51. [Google Scholar] [CrossRef]
- Hansen, W.; Almudaiheem, J. Pore Structure of Hydrated Portland Cement Measured by Nitrogen Sorption and Mercury Intrusion Porosimetry. MRS Online Proc. Libr. OPL 1986, 85, 105. [Google Scholar] [CrossRef]
- Abdel-Jawad, Y.; Hansen, W. Pore Structure of Hydrated Cement Determined By Mercury Porosimetry and Nitrogen Sorption Techniques. MRS Online Proc. Libr. OPL 1988, 137, 105. [Google Scholar] [CrossRef]
- Lee, S.-C.; Cunnington, G.R. Conduction and Radiation Heat Transfer in High-Porosity Fiber Thermal Insulation. J. Thermophys. Heat Transf. 2000, 14, 121–136. [Google Scholar] [CrossRef]
- Swimm, K.; Vidi, S.; Reichenauer, G.; Ebert, H.P. Coupling of gaseous and solid thermal conduction in porous solids. J. Non-Cryst. Solids 2017, 456, 114–124. [Google Scholar] [CrossRef]
- Ng, T.Y.; Yeo, J.J.; Liu, Z.S. A molecular dynamics study of the thermal conductivity of nanoporous silica aerogel, obtained through negative pressure rupturing. J. Non-Cryst. Solids 2012, 358, 1350–1355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, D.; Liu, F.; Jiang, Y.; Feng, J.; Li, L.; Feng, J. Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement. Materials 2021, 14, 3983. https://doi.org/10.3390/ma14143983
Du D, Liu F, Jiang Y, Feng J, Li L, Feng J. Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement. Materials. 2021; 14(14):3983. https://doi.org/10.3390/ma14143983
Chicago/Turabian StyleDu, Dongxuan, Fengqi Liu, Yonggang Jiang, Junzong Feng, Liangjun Li, and Jian Feng. 2021. "Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement" Materials 14, no. 14: 3983. https://doi.org/10.3390/ma14143983
APA StyleDu, D., Liu, F., Jiang, Y., Feng, J., Li, L., & Feng, J. (2021). Facile Preparation of High Strength Silica Aerogel Composites via a Water Solvent System and Ambient Pressure Drying without Surface Modification or Solvent Replacement. Materials, 14(14), 3983. https://doi.org/10.3390/ma14143983





