Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer
Abstract
1. Introduction
1.1. General Considerations
1.2. Gold Nanoparticles for Biomedical Applications
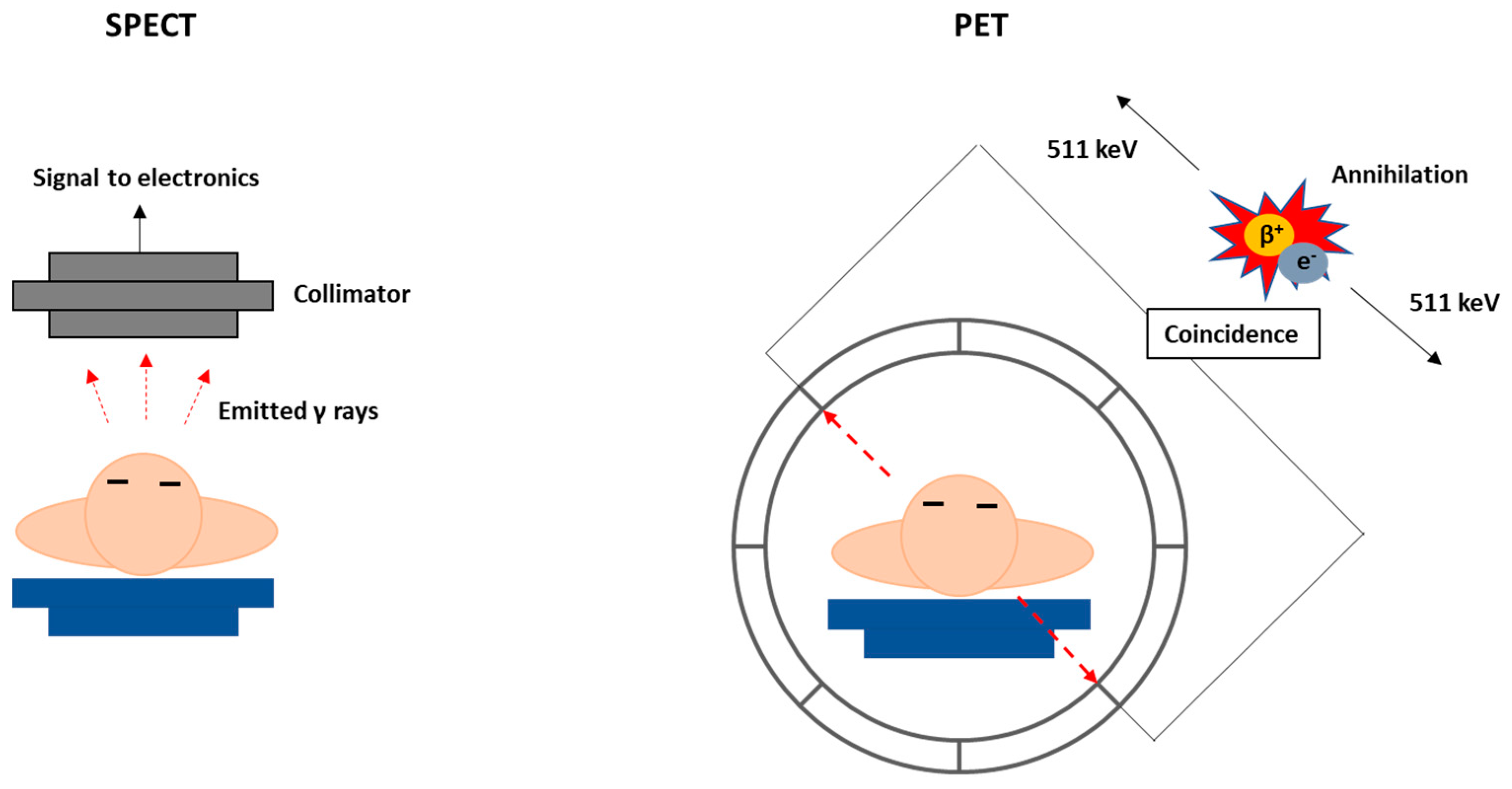
1.3. Nuclear Medicine Modalities and Medical Radionuclides
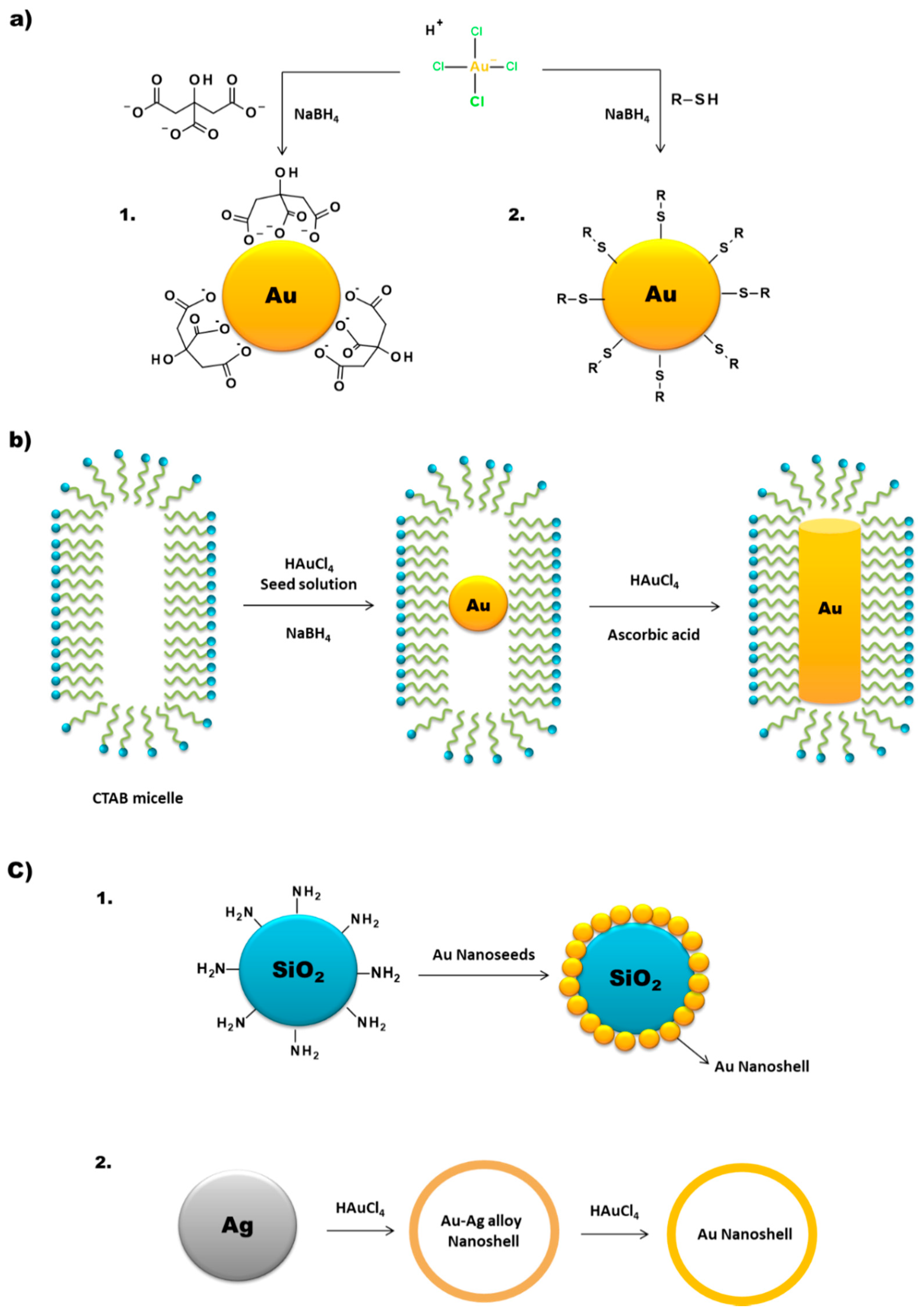
2. Synthesis of Gold Nanoparticles
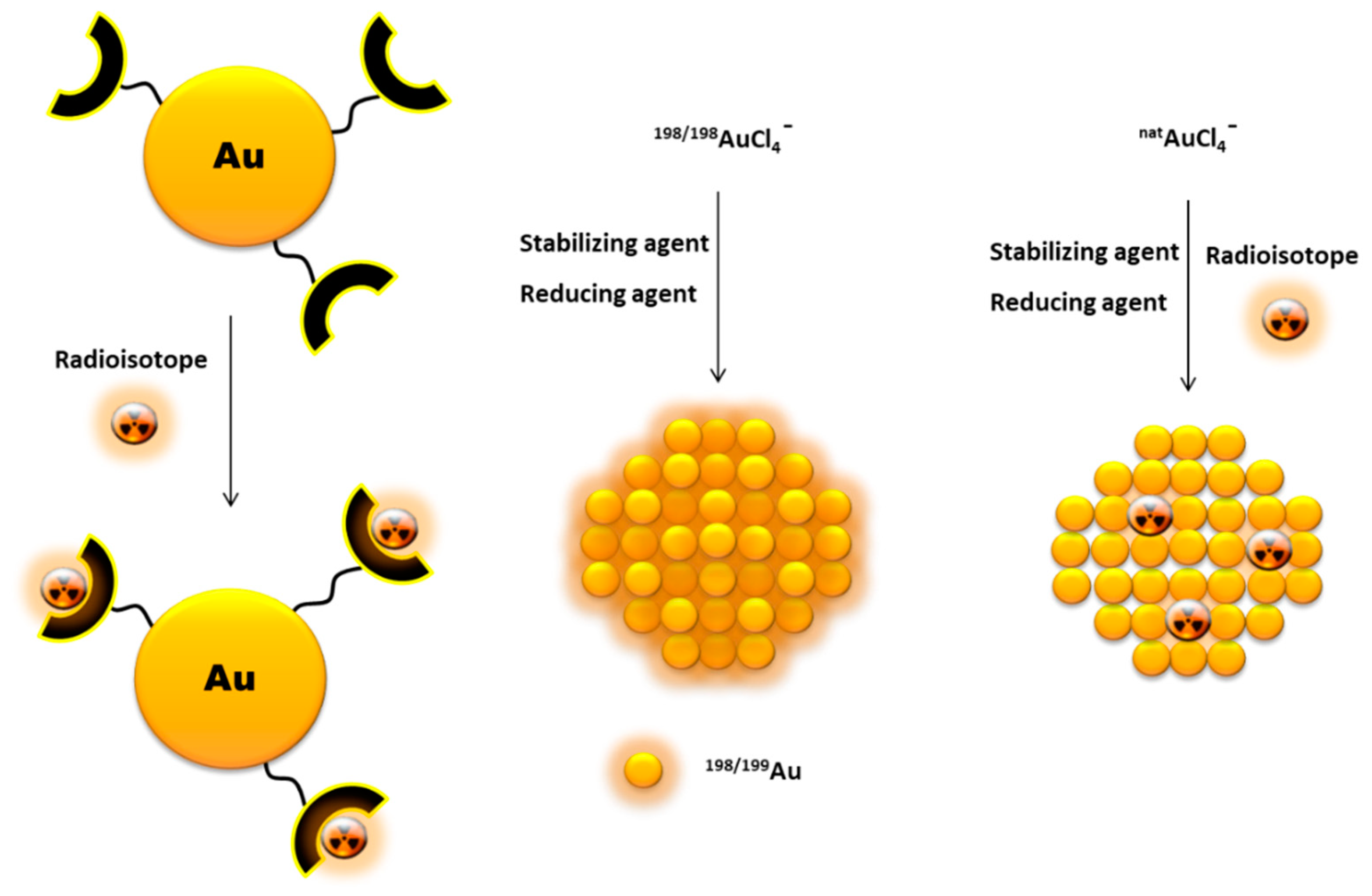
3. Radiolabelling of Gold Nanoparticles
4. Examples of Radiolabeled AuNPs for Nuclear Imaging Applications
4.1. Radiohalogens
4.1.1. Fluorine-18 (18F)
4.1.2. Iodine-124 (124I)
4.1.3. Iodine-125 (125I)
4.2. Radiometals
4.2.1. Copper-64
4.2.2. Gallium-67/Gallium-68
4.2.3. Technetium-99m
4.2.4. Indium-111
4.2.5. Gold-198/199
5. Examples of Radiolabeled AuNPs for Therapeutic Applications
5.1. Beta-Emitting Isotopes
5.1.1. Yttrium-90
5.1.2. Iodine-131
5.1.3. Lutetium-177
5.2. Alpha-Emitting Isotopes
5.2.1. Astatine-211
5.2.2. Actinium-225
5.3. Boron Neutron Capture Therapy (BNCT)
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α | Alpha |
| α-MSH | α-Melanocyte-stimulating hormone |
| AuNCs | Gold nanocages |
| AuNP | Gold nanoparticle |
| AuNR | Gold nanorod |
| AuNS | Gold nanoshell |
| APTES | 3-Aminopropyltriethoxysilane |
| β | Beta |
| BBB | Blood brain barrier |
| BBN | Bombesin |
| BNCT | Boron neutron capture therapy |
| CLI | Cerenkov luminescence imaging |
| CT | Computed tomography |
| CTAB | Cetyltrimethylammonium bromide |
| CTX | Chlorotoxin |
| DAPTA | D-Ala1-peptide T-amide |
| DC | Dendritic cell |
| DLS | Dynamic light scattering |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOX | Doxorubicin |
| DPLNs | Draining popliteal lymph nodes |
| DTDTPA | Dithiolated DTPA |
| DTPA | Diethylene triamine pentaacetic acid |
| EC | Electron capture |
| ECIS. | European Cancer Information System |
| EGFr | Epidermal growth factor receptor |
| EPR | Enhanced permeability and retention |
| GRPr | Gastrin releasing peptide receptor |
| HNSCC | Head and neck squamous cell carcinoma |
| HPAO | 3-(4-Hydroxyphenyl)propionic acid-OSu |
| HPMA | N-(2-Hydroxypropyl) methacrylamide |
| HER | Human epidermal growth factor receptor |
| IT | Isomeric transition |
| LET | Linear energy transfer |
| LPFFD | Cys-Leu-Pro-Phe-Phe-Asp |
| MC1R | Melanocortin 1 receptor |
| MRI | Magnetic resonance imaging |
| NAA | Neutron activation analysis |
| NET | Neuroendocrine tumours |
| NIR | Near-infrared |
| NMR | Nuclear magnetic resonance |
| NODAGA | 1,4,7-Triazacyclononane-1-glutaric acid-4,7-acetic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| NP | Nanoparticle |
| NPD | Nanoparticle depots |
| NTA | Nanoparticle tracking analyses |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| p.i | Post-injection |
| PRRT | Peptide receptor radionuclide therapy |
| PSI | Permanent seed implantation |
| PSMA | Prostate-specific membrane antigen |
| RES | Reticuloendothelial system |
| RGD | Cyclic arginine-glycine-aspartic acid |
| RIe-AuNP | Radionuclide-embedded gold nanoparticles |
| ROI | Region of interest |
| SEM | Scanning electron microscopy |
| SFB | N-Succinimidyl-4-fluorobenzoate |
| SPECT | Single-photon emission cumputed tomography |
| SPR | Surface plasmon resonance |
| SUV | Standardized uptake value |
| TATE | Octreotate |
| TEM | Transmission electron microscopy |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumour necrosis factor alpha |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Source: ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu (accessed on 10 October 2020).
- Jeon, J. Review of Therapeutic Applications of Radiolabeled Functional Nanomaterials. Int. J. Mol. Sci. 2019, 20, 2323. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Holland, J.P. Advanced Methods for Radiolabeling Multimodality Nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018, 59, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Koziorowski, J.; Stanciu, A.E.; Gomez-Vallejo, V.; Llop, J. Radiolabeled Nanoparticles for Cancer Diagnosis and Therapy. Anti-Cancer Agents Med. Chem. 2017, 17, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Saboktakin, M. The biological and biomedical naparticles—Synthesis and applications. Adv. Mater. Sci. 2017. [Google Scholar] [CrossRef][Green Version]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.B.; Ashrafizadeh, M.; Zare, E.N.; Agarwal, T.; Padil, V.V.T.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117–139. [Google Scholar] [CrossRef]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci 2016, 4, 1600279. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, K. Multimodal Molecular Imaging Strategies using Functionalized Nano Probes. J. Nanotechnol. Res. 2019, 1, 119. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lina, L.; Shuhe, K.; Chao, S.; Chufeng, S.; Zhong, G.; Jia, L.; Taofeng, Z.; Xingping, L.; Bin, L. Multifunctional Nanoparticles in Precise Cancer Treatment: Considerations in Design and Functionalization of Nanocarriers. Curr. Top. Med. Chem. 2020, 20, 2427–2441. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2018, 14, 93–126. [Google Scholar] [CrossRef]
- Brown, T.D.; Habibi, N.; Wu, D.; Lahann, J.; Mitragotri, S. Effect of Nanoparticle Composition, Size, Shape, and Stiffness on Penetration Across the Blood–Brain Barrier. ACS Biomater. Sci. Eng. 2020, 6, 4916–4928. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; Baptista, J.G.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, S.; Dong, D.; Wei, J.W.; Fang, C.; Zhou, X.Z.; Sun, K.; Li, L.F.; Li, B.; Wang, M.Y.; et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, J. Multimodal Molecular Imaging: Current Status and Future Directions. Contrast Media Mol. Imaging 2018, 2018, 1382183. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.; Hofman, M. Is there still a role for SPECt CT in oncology in the PEt CT era? Nat. Rev. Clin. Oncol. 2012, 9, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; England, C.G.; Chen, F.; Cai, W.B. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. Ejnmmi Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Gharatape, A.; Salehi, R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- FDA.gov. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-digestive-tract-cancers (accessed on 21 December 2020).
- Aghanejad, A.; Omidi, Y. Chapter 25—Radiolabeled Theranostics: Magnetic and Gold Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 535–547. [Google Scholar] [CrossRef]
- Turkavich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Chen, S.W. 4-hydroxythiophenol-protected gold nanoclusters in aqueous media. Langmuir 1999, 15, 7551–7557. [Google Scholar] [CrossRef]
- Chen, S.W.; Murray, R.W. Arenethiolate monolayer-protected gold clusters. Langmuir 1999, 15, 682–689. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Kumar, D.; Mohandoss, D.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine—Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathy, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkami, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanopartictes. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- An, L.; Wang, Y.Y.; Tian, Q.W.; Yang, S.P. Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy. Materials 2017, 10, 1372. [Google Scholar] [CrossRef]
- Ahmed, W.; Bhatti, A.S.; van Ruitenbeek, J.M. Efficient seed-mediated method for the large-scale synthesis of Au nanorods. J. Nanoparticle Res. 2017, 19, 115. [Google Scholar] [CrossRef]
- Ji, C.X.; Searson, P.C. Synthesis and characterization of nanoporous gold nanowires. J. Phys. Chem. B 2003, 107, 4494–4499. [Google Scholar] [CrossRef]
- Moon, J.M.; Wei, A. Uniform gold nanorod arrays from polyethylenimine-coated alumina templates. J. Phys. Chem. B 2005, 109, 23336–23341. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chang, S.S.; Lee, C.L.; Wang, C.R.C. Gold nanorods: Electrochemical synthesis and optical properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Wang, Y.C.; Rheaume, E.; Lesage, F.; Kakkar, A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Xia, Y.N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Silva, F.; Zambre, A.; Campello, M.P.C.; Gano, L.; Santos, I.; Ferraria, A.M.; Ferreira, M.J.; Singh, A.; Upendran, A.; Paulo, A.; et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016, 27, 1153–1164. [Google Scholar] [CrossRef]
- Alric, C.; Taleb, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Mendoza-Sanchez, A.N.; Ferro-Flores, G.; Ocampo-Garcia, B.E.; Morales-Avila, E.; Ramirez, F.D.; De Leon-Rodriguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderon, E.L.; Camacho-Lopez, M.A. Lys(3)-Bombesin Conjugated to Tc-99m-Labelled Gold Nanoparticles for In Vivo Gastrin Releasing Peptide-Receptor Imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef]
- Reznickova, A.; Slavikova, N.; Kolska, Z.; Kolarova, K.; Belinova, T.; Kalbacova, M.H.; Cieslar, M.; Svorcik, V. PEGylated gold nanoparticles: Stability, cytotoxicity and antibacterial activity. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 560, 26–34. [Google Scholar] [CrossRef]
- Kluenker, M.; Mondeshki, M.; Tahir, M.N.; Tremel, W. Monitoring Thiol-Ligand Exchange on Au Nanoparticle Surfaces. Langmuir 2018, 34, 1700–1710. [Google Scholar] [CrossRef]
- Zhang, J.J.; Mou, L.; Jiang, X.Y. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936. [Google Scholar] [CrossRef]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z.M. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugao, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-(198)AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Guleria, A.; Shukla, R.; Kumar, C.; Nair, K.V.V.; Sarma, H.D.; Tyagi, A.K.; Dash, A. Facile One-Pot Synthesis of Intrinsically Radiolabeled and Cyclic RGD Conjugated Au-199 Nanoparticles for Potential Use in Nanoscale Brachytherapy. Ind. Eng. Chem. Res. 2018, 57, 14337–14346. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Sadeghi, M.; Aboudzadeh, M.R.; Mohseni, M. Production and modeling of radioactive gold nanoparticles in Tehran research reactor. Appl. Radiat. Isot. 2016, 118, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, Y.X.; Yin, L.L.; Xia, X.T.; Hu, F.; Liu, Q.Y.; Qin, C.X.; Lan, X.L. Synthesis and Bioevaluation of Iodine-131 Directly Labeled Cyclic RGD-PEGylated Gold Nanorods for Tumor-Targeted Imaging. Contrast Media Mol. Imaging 2017. [Google Scholar] [CrossRef]
- Frellsen, A.F.; Hansen, A.E.; Jolck, R.I.; Kempen, P.J.; Severin, G.W.; Rasmussen, P.H.; Kjaer, A.; Jensen, A.T.I.; Andresen, T.L. Mouse Positron Emission Tomography Study of the Biodistribution of Gold Nanoparticles with Different Surface Coatings Using Embedded Copper-64. ACS Nano 2016, 10, 9887–9898. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Sultan, D.; Detering, L.; Cho, S.H.; Sun, G.R.; Pierce, R.; Wooley, K.L.; Liu, Y.J. Copper-64-Alloyed Gold Nanoparticles for Cancer Imaging: Improved Radiolabel Stability and Diagnostic Accuracy. Angew. Chem. Int. Ed. 2014, 53, 156–159. [Google Scholar] [CrossRef]
- Sun, X.L.; Huang, X.L.; Yan, X.F.; Wang, Y.; Guo, J.X.; Jacobson, O.; Liu, D.B.; Szajek, L.P.; Zhu, W.L.; Niu, G.; et al. Chelator-Free Cu-64-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano 2014, 8, 8438–8446. [Google Scholar] [CrossRef]
- Guerrero, S.; Herance, J.R.; Rojas, S.; Mena, J.F.; Gispert, J.D.; Acosta, G.A.; Albericio, F.; Kogan, M.J. Synthesis and In Vivo Evaluation of the Biodistribution of a F-18-Labeled Conjugate Gold-Nanoparticle-Peptide with Potential Biomedical Application. Bioconjugate Chem. 2012, 23, 399–408. [Google Scholar] [CrossRef]
- Zhu, J.; Chin, J.; Wangler, C.; Wangler, B.; Lennox, R.B.; Schirrmacher, R. Rapid F-18-Labeling and Loading of PEGylated Gold Nanoparticles for In Vivo Applications. Bioconjugate Chem. 2014, 25, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. Part A 2019, 107, 251–285. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.A.; Diagaradjane, P.; Deorukhkar, A.A.; Goins, B.; Bao, A.; Phillips, W.T.; Wang, Z.; Schwartz, J.; Krishnan, S. Integrin alpha(v)beta(3)-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int. J. Nanomed. 2011, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.L.; Melancon, M.P.; Abdelsalam, M.; Figueira, T.A.; Dixon, K.; McWatters, A.; Zhou, M.; Huang, Q.; Mawlawi, O.; Dunner, K.; et al. Imaging Intratumoral Nanoparticle Uptake After Combining Nanoembolization with Various Ablative Therapies in Hepatic VX2 Rabbit Tumors. J. Biomed. Nanotechnol. 2016, 12, 296–307. [Google Scholar] [CrossRef]
- Tian, M.; Lu, W.; Zhang, R.; Xiong, C.Y.; Ensor, J.; Nazario, J.; Jackson, J.; Shaw, C.; Dixon, K.A.; Miller, J.; et al. Tumor Uptake of Hollow Gold Nanospheres After Intravenous and Intra-arterial Injection: PET/CT Study in a Rabbit VX2 Liver Cancer Model. Mol. Imaging Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Pang, B.; Detering, L.; Luehmann, H.; Yang, M.X.; Black, K.; Sultan, D.; Xia, Y.N.; Liu, Y.J. Melanocortin I Receptor Targeted Imaging of Melanoma With Gold Nanocages and Positron Emission Tomography. Mol. Imaging 2018, 17. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wangler, C.; Schibli, R.; Wangler, B. Targeted Cu-64-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Zhao, Y.; Detering, L.; Sultan, D.; Cooper, M.L.; You, M.; Cho, S.; Meier, S.L.; Luehmann, H.; Sun, G.; Rettig, M.; et al. Gold Nanoclusters Doped with (64)Cu for CXCR4 Positron Emission Tomography Imaging of Breast Cancer and Metastasis. ACS Nano 2016, 10, 5959–5970. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, Y.; Luehmann, H.; Yang, X.; Detering, L.; You, M.; Zhang, C.; Zhang, L.; Li, Z.-Y.; Ren, Q.; et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121–3131. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Luehmann, H.; Xia, X.; Brown, P.; Jarreau, C.; Welch, M.; Xia, Y. Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano 2012, 6, 5880–5888. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.J.; Bao, A.; Goins, B.; Phillips, W.T. In Vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int. J. Pharm. 2010, 395, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Jurado, A.S.; et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials 2020, 13, 513. [Google Scholar] [CrossRef]
- Chilug, L.E.; Leonte, R.A.; Barbinta Patrascu, M.E.; Ion, A.C.; Tuta, C.S.; Raicu, A.; Manda, G.; Niculae, D. In Vitro binding kinetics study of gold nanoparticles functionalized with Ga-68-DOTA conjugated peptides. J. Radioanal. Nucl. Chem. 2017, 311, 1485–1493. [Google Scholar] [CrossRef]
- Pretze, M.; Hien, A.; Rädle, M.; Schirrmacher, R.; Wängler, C.; Wängler, B. Gastrin-Releasing Peptide Receptor- and Prostate-Specific Membrane Antigen-Specific Ultrasmall Gold Nanoparticles for Characterization and Diagnosis of Prostate Carcinoma via Fluorescence Imaging. Bioconjugate Chem. 2018, 29, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, Z.G.; Xu, X.Y.; Luo, Y.; Peng, C.; Shen, M.W.; Shi, X.Y. Tc-99m-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 19883–19891. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.H.; Zhao, L.Z.; Zhao, Q.H.; Li, D.; Liu, C.C.; Yu, Z.B.; Shen, M.W.; Majoral, J.P.; Mignani, S.; Zhao, J.H.; et al. A promising dual mode SPECT/CT imaging platform based on Tc-99m-labeled multifunctional dendrimer-entrapped gold nanoparticles. J. Mater. Chem. B 2017, 5, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhao, L.Z.; Xu, X.Y.; Sun, N.; Qiao, W.L.; Xing, Y.; Shen, M.W.; Zhu, M.L.; Shi, X.Y.; Zhao, J.H. Design of Tc-99(m)-Labeled Low Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted Single Photon Emission Computed Tomography/Computed Tomography Imaging of Gliomas. J. Biomed. Nanotechnol. 2019, 15, 1201–1212. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, J.Y.; Zhao, L.Z.; Xiong, Z.J.; Li, Y.J.; Wu, S.; Chand, G.; Shi, X.Y.; Zhao, J.H. SPECT/CT imaging of chemotherapy-induced tumor apoptosis using Tc-99m-labeled dendrimer-entrapped gold nanoparticles. Drug Deliv. 2018, 25, 1384–1393. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, L.Z.; Li, X.; Wang, P.; Zhao, J.H.; Shi, X.Y.; Shen, M.W. Targeted tumor SPECT/CT dual mode imaging using multifunctional RGD-modified low generation dendrimer-entrapped gold nanoparticles. Biomater. Sci. 2017, 5, 2393–2397. [Google Scholar] [CrossRef]
- Peiris, P.M.; Deb, P.; Doolittle, E.; Doron, G.; Goldberg, A.; Govender, P.; Shah, S.; Rao, S.; Carbone, S.; Cotey, T.; et al. Vascular Targeting of a Gold Nanoparticle to Breast Cancer Metastasis. J. Pharm. Sci. 2015, 104, 2600–2610. [Google Scholar] [CrossRef]
- Kamal, R.; Chadha, V.D.; Dhawan, D.K. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles (Tc-99m-Res-AuNP) in colon cancer tissue. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Zhao, L.Z.; Yang, J.X.; Chen, L.; Shi, J.H.; Zhao, J.H.; Shi, X.Y. Tc-99m-Labeled Polyethylenimine-Entrapped Gold Nanoparticles with pH-Responsive Charge Conversion Property for Enhanced Dual Mode SPECT/CT Imaging of Cancer Cells. Langmuir 2019, 35, 13405–13412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Tan, H.; Cheng, L.L.; Liu, G.B.; Yang, Y.; Zhao, Y.Z.; Zhang, Y.Q.; Li, Y.L.; Zhan, C.F.; et al. Gold nanoparticles-based SPECT/CT imaging probe targeting for vulnerable atherosclerosis plaques. Biomaterials 2016, 108, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sakr, T.M.; Morsy, S.A.G.; Mahmoud, N.A.; Rashed, H.M.; El-Rehim, H.A.A.; Khoobchandani, M.; Katti, K.K.; Katti, K.V. Preparation, Characterization, Cytotoxicity and Biological Evaluation of 99mTc-Doxorubicin-Epigallocatechingallate Functionalized Gold Nanoparticles as a New Genaration of Theranostic Radiopharmaceutical. Preprints 2018, 2018080331. [Google Scholar] [CrossRef]
- Sakr, T.M.; El-Hashash, M.A.; El-Mohty, A.A.; Essa, B.M. 99mTc-gallic-gold nanoparticles as a new imaging platform for tumor targeting. Appl. Radiat. Isot. 2020, 164. [Google Scholar] [CrossRef]
- El-Ghareb, W.I.; Swidan, M.M.; Ibrahim, I.T.; El-Bary, A.A.; Tadros, M.I.; Sakr, T.M. 99mTc-doxorubicin-loaded gallic acid-gold nanoparticles (99mTc-DOX-loaded GA-Au NPs) as a multifucntional theranostic agent. Int. J. Pharm. 2020, 586, 119514. [Google Scholar] [CrossRef]
- Black, K.C.L.; Akers, W.J.; Sudlow, G.; Xu, B.G.; Laforest, R.; Achilefu, S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale 2015, 7, 440–444. [Google Scholar] [CrossRef]
- Song, L.; Falzone, N.; Vallis, K.A. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR-positive cancer. Int. J. Radiat. Biol. 2016, 92, 716–723. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Fonge, H.; Cai, Z.L.; Scollard, D.; Lechtman, E.; Done, S.J.; Pignol, J.P.; Reilly, R.M. Role of Antibody-Mediated Tumor Targeting and Route of Administration in Nanoparticle Tumor Accumulation In Vivo. Mol. Pharm. 2012, 9, 2168–2179. [Google Scholar] [CrossRef]
- Cai, Z.L.; Chattopadhyay, N.; Yang, K.Y.; Kwon, Y.L.; Yook, S.; Pignol, J.P.; Reilly, R.M. In-111-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl. Med. Biol. 2016, 43, 818–826. [Google Scholar] [CrossRef]
- Lee, S.B.; Ahn, S.B.; Lee, S.W.; Jeong, S.Y.; Ghilsuk, Y.; Ahn, B.C.; Kim, E.M.; Jeong, H.J.; Lee, J.; Lim, D.K.; et al. Radionuclide-embedded gold nanoparticles for enhanced dendritic cell-based cancer immunotherapy, sensitive and quantitative tracking of dendritic cells with PET and Cerenkov luminescence. NPG Asia Mater. 2016, 8, e281. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, H.W.; Singh, T.D.; Li, Y.; Kim, S.K.; Cho, S.J.; Lee, S.-W.; Jeong, S.Y.; Ahn, B.-C.; Choi, S.; et al. Visualization of Macrophage Recruitment to Inflammation Lesions using Highly Sensitive and Stable Radionuclide-Embedded Gold Nanoparticles as a Nuclear Bio-Imaging Platform. Theranostics 2017, 7, 926–934. [Google Scholar] [CrossRef][Green Version]
- Lee, S.B.; Lee, Y.J.; Cho, S.J.; Kim, S.K.; Lee, S.W.; Lee, J.; Lim, D.K.; Jeon, Y.H. Antigen-Free Radionuclide-Embedded Gold Nanoparticles for Dendritic Cell Maturation, Tracking, and Strong Antitumor Immunity. Adv. Healthc. Mater. 2018, 7, e1701369. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kumar, D.; Li, Y.; Lee, I.-K.; Cho, S.J.; Kim, S.K.; Lee, S.-W.; Jeong, S.Y.; Lee, J.; Jeon, Y.H. PEGylated crushed gold shell-radiolabeled core nanoballs for in vivo tumor imaging with dual positron emission tomography and Cerenkov luminescent imaging. J. Nanobiotechnol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.E.; Cho, S.J.; Chin, J.; Kim, S.K.; Lee, I.K.; Lee, S.W.; Lee, J.; Jeon, Y.H. Crushed Gold Shell Nanoparticles Labeled with Radioactive Iodine as a Theranostic Nanoplatform for Macrophage-Mediated Photothermal Therapy. Nano-Micro Lett. 2019, 11, 36. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.L.; Wang, H.L.; Li, Q.; Li, X.; Zhou, C.Q.; Xu, J.; Chai, Y.M.; Liang, X.W.; Xiong, L.Q.; et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics 2019, 9, 1893–1908. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Pang, B.; Luehmann, H.; Detering, L.; Yang, X.; Sultan, D.; Harpstrite, S.; Sharma, V.; Cutler, C.S.; Xia, Y.N.; et al. Gold Nanoparticles Doped with Au-199 Atoms and Their Use for Targeted Cancer Imaging by SPECT. Adv. Healthc. Mater. 2016, 5, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Hazra, D.K. Conjugation of Antibodies with Radiogold Nanoparticles, as an Effector Targeting Agents in Radiobioconjugate Cancer Therapy: Optimized Labeling and Biodistribution Results. Indian J. Nucl. Med. 2017, 32, 296–303. [Google Scholar] [CrossRef]
- Al-Yasiri, A.Y.; White, N.E.; Katti, K.V.; Loyalka, S.K. Estimation of tumor and local tissue dose in gold nanoparticles radiotherapy for prostate cancer. Rep. Pract. Oncol. Radiother. 2019, 24, 288–293. [Google Scholar] [CrossRef]
- Huang, Y.-Y. An Overview of PET Radiopharmaceuticals in Clinical Use: Regulatory, Quality and Pharmacopeia Monographs of the United States and Europe. In Nuclear Medicine Physics; Shahzad, A., Bashir, S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Chuang, Y.C.; Chen, N.T.; Lo, L.W. Seeing Better and Going Deeper in Cancer Nanotheranostics. Int. J. Mol. Sci. 2019, 20, 3490. [Google Scholar] [CrossRef]
- Koehler, L.; Gagnon, K.; McQuarrie, S.; Wuest, F. Iodine-124: A promising positron emitter for organic PET chemistry. Molecules 2010, 15, 2686–2718. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, S.W.; Jeong, S.Y.; Yoon, G.; Cho, S.J.; Kim, S.K.; Lee, I.K.; Ahn, B.C.; Lee, J.; Jeon, Y.H. Engineering of Radioiodine-Labeled Gold Core Shell Nanoparticles As Efficient Nuclear Medicine Imaging Agents for Trafficking of Dendritic Cells. ACS Appl. Mater. Interfaces 2017, 9, 8480–8489. [Google Scholar] [CrossRef]
- Lee, S.B.; Yoon, G.; Lee, S.W.; Jeong, S.Y.; Ahn, B.C.; Lim, D.K.; Lee, J.; Jeon, Y.H. Combined Positron Emission Tomography and Cerenkov Luminescence Imaging of Sentinel Lymph Nodes Using PEGylated Radionuclide-Embedded Gold Nanoparticles. Small 2016, 12, 4894–4901. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Teunissen, A.J.P.; Kluza, E.; Mulder, W.J.M.; van der Meel, R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Ge, J.X.; Zhang, Q.Y.; Zeng, J.F.; Gu, Z.; Gao, M.Y. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2020, 228, 119553. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445–1495. [Google Scholar] [CrossRef]
- Boswell, C.A.; Sun, X.K.; Niu, W.J.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.T.; Persson, M.; Madsen, J.; Kjaer, A. High tumor uptake of Cu-64: Implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol. 2013, 40, 345–350. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Osso, J., Jr. The Current Status and Future of Theranostic Copper-64 Radiopharmaceuticals; Research Center for Nuclear Medicine, Tehran University of Medical Sciences: Tehran, Iran, 2017; Volume 25, pp. 1–10. [Google Scholar]
- Kubicek, V.; Bohmova, Z.; Sevcikova, R.; Vanek, J.; Lubal, P.; Polakova, Z.; Michalicova, R.; Kotek, J.; Hermann, P. NOTA Complexes with Copper(II) and Divalent Metal Ions: Kinetic and Thermodynamic Studies. Inorg. Chem. 2018, 57, 3061–3072. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircso, G.; Rosch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zambre, A.; Silva, F.; Upendran, A.; Afrasiabi, Z.; Xin, Y.; Paulo, A.; Kannan, R. Synthesis and characterization of functional multicomponent nanosized gallium chelated gold crystals. Chem. Commun. 2014, 50, 3281–3284. [Google Scholar] [CrossRef] [PubMed]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Debouttiere, P.J.; Roux, S.; Vocanson, F.; Billotey, C.; Beuf, O.; Favre-Reguillon, A.; Lin, Y.; Pellet-Rostaing, S.; Lamartine, R.; Perriat, P.; et al. Design of gold nanoparticles for magnetic resonance imaging. Adv. Funct. Mater. 2006, 16, 2330–2339. [Google Scholar] [CrossRef]
- Alric, C.; Miladi, I.; Kryza, D.; Taleb, J.; Lux, F.; Bazzi, R.; Billotey, C.; Janier, M.; Perriat, P.; Roux, S.; et al. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale 2013, 5, 5930–5939. [Google Scholar] [CrossRef]
- Silva, F.; Gano, L.; Campello, M.P.C.; Marques, R.; Prudencio, I.; Zambre, A.; Upendran, A.; Paulo, A.; Kannan, R. In vitro/in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable Tc-99m-label. Dalton Trans. 2017, 46, 14572–14583. [Google Scholar] [CrossRef]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Molecular Nanomedicine Towards Cancer: In-111-Labeled Nanoparticles. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]
- Reilly, R.M.; Kiarash, R.; Cameron, R.G.; Porlier, N.; Sandhu, J.; Hill, R.P.; Vallis, K.; Hendler, A.; Gariépy, J. 111In-labeled EGF Is Selectively Radiotoxic to Human Breast Cancer Cells Overexpressing EGFR. J. Nucl. Med. 2000, 41, 429. [Google Scholar]
- Chakravarty, R.; Chakraborty, S.; Guleria, A.; Kumar, C.; Kunwar, A.; Nair, K.V.V.; Sarma, H.D.; Dash, A. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized Au-198 nanoparticles for targeted cancer therapy. Nucl. Med. Biol. 2019, 72–73, 1–10. [Google Scholar] [CrossRef]
- Lai, P.; Cai, Z.L.; Pignol, J.P.; Lechtman, E.; Mashouf, S.; Lu, Y.J.; Winnik, M.A.; Jaffray, D.A.; Reilly, R.M. Monte Carlo simulation of radiation transport and dose deposition from locally released gold nanoparticles labeled with In-111, Lu-177 or Y-90 incorporated into tissue implantable depots. Phys. Med. Biol. 2017, 62, 8581–8599. [Google Scholar] [CrossRef]
- Buckway, B.; Frazier, N.; Gormley, A.J.; Ray, A.; Ghandehari, H. Gold nanorod-mediated hyperthermia enhances the efficacy of HPMA copolymer-Y-90 conjugates in treatment of prostate tumors. Nucl. Med. Biol. 2014, 41, 282–289. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Li, Y.J.; Zhu, J.Y.; Sun, N.; Song, N.N.; Xing, Y.; Huang, H.; Zhao, J.H. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramirez, F.D.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorin-Vega, E.; Morales-Avila, E.; Isaac-Olive, K. Lu-177-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomater. 2016. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramirez, F.D.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Azorin-Vega, E.; Jimenez-Mancilla, N.; Luna-Gutierrez, M.; Isaac-Olive, K. Fluorescent, Plasmonic, and Radiotherapeutic Properties of the Lu-177-Dendrimer-AuNP-Folate-Bombesin Nanoprobe Located Inside Cancer Cells. Mol. Imaging 2017, 16. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.L.; Lu, Y.J.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Intratumorally Injected Lu-177-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Lu, Y.J.; Jeong, J.J.; Cai, Z.L.; Tong, L.; Alwarda, R.; Pignol, J.P.; Winnik, M.A.; Reilly, R.M. Stability and Biodistribution of Thiol-Functionalized and Lu-177-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef]
- Dziawer, L.; Majkowska-Pilip, A.; Gawel, D.; Godlewska, M.; Pruszynski, M.; Jastrzebski, J.; Was, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with At-211 as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef]
- Lai, P.; Lechtman, E.; Mashouf, S.; Pignol, J.-P.; Reilly, R.M. Depot system for controlled release of gold nanoparticles with precise intratumoral placement by permanent brachytherapy seed implantation (PSI) techniques. Int. J. Pharm. 2016, 515, 729–739. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossío, U.; Llop, J. Gold Nanoparticles as Boron Carriers for Boron Neutron Capture Therapy: Synthesis, Radiolabelling and In Vivo Evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef]
- Feiner, I.V.J.; Pulagam, K.R.; Gómez-Vallejo, V.; Zamacola, K.; Baz, Z.; Caffarel, M.M.; Lawrie, C.H.; Ruiz-de-Angulo, A.; Carril, M.; Llop, J. Therapeutic Pretargeting with Gold Nanoparticles as Drug Candidates for Boron Neutron Capture Therapy. Part. Part. Syst. Charact. 2020. [Google Scholar] [CrossRef]


| Radionuclide | Half-Life | Mode of Decay (%) | Application |
|---|---|---|---|
| 11C | 20.3 min | β+ (100) | PET |
| 18F | 109.8 min | β+ (97) EC a (3) | PET |
| 61Cu | 3.3 h | β+ (100) | PET |
| 62Cu | 9.7 min | β+ (100) | PET |
| 64Cu | 12.7 h | β− (40) β+ (19) EC (41) | PET/Therapy |
| 67Ga | 3.27 d | EC (100) | SPECT |
| 68Ga | 67.8 min | β+ (90) EC (10) | PET |
| 86Y | 14.7 h | β+ (33) EC (66) | PET |
| 89Zr | 78.4 h | β+ (100) | PET |
| 99mTc | 6.0 h | IT b (100) | SPECT |
| 111In | 2.83 d | EC (100) | SPECT |
| 123I | 13.2 h | EC (100) | SPECT |
| 124I | 100.8 h | β+ (100) | PET |
| Radionuclide | Half-Life (h) | Mode of Decay (%) |
|---|---|---|
| 67Cu | 61.8 | β− (100) |
| 90Y | 64.1 | β− (100) |
| 131I | 192.0 | β− (100) |
| 153Sm | 46.3 | β− (100) |
| 166Ho | 26.8 | β− (100) |
| 177Lu | 161.0 | β− (100) |
| 186Re | 89.2 | β− (92) EC a (8) |
| 188Re | 17.0 | β− (100) |
| 198Au | 64.7 | β− (100) |
| 199Au | 75.3 | β− (100) |
| 211At | 7.2 | α (100) |
| 223Ra | 274.5 | α (100) |
| 225Ac | 238.1 | α (100) |
| Radioisotope | Type of AuNPs/Size/Coating (Radiolabeling Approach) | Imaging Application/Study | References |
|---|---|---|---|
| 18F | Spherical AuNPs/12 nm/LPFFD (18F-fluorobenzoate conjugation) | Biodistribution studies and in vivo PET imaging in healthy mice. | [63,64,65] |
| 64Cu | AuNSs/120 nm/cyclic-RGD (Chelator-based) | PET imaging and thermoablation treatment in HCT116 human colorectal cancer xenograft mice. | [66] |
| Spherical AuNPs/9.4 nm/PEG (64Cu/Au alloying) | Biodistribution and in vivo PET imaging in rats bearing EMT-6 breast cancer. | [61] | |
| AuNSs/44.7 nm/doxorubicin, lipiodol (chelator-based) | Biodistribution and chemotherapeutic drug delivery studies, laser induced thermal treatment and in vivo PET imaging in hepatic VX2 tumours in mice. | [67,68] | |
| AuNCs/35 nm/PEG, α-MSH (chelator-based) | Biodistribution studies and PET/CT imaging in vivo in B16/F10 melanoma mouse model. | [69] | |
| Spherical AuNPs/3 nm/PEG, bombesin, LUG, NIR dye SIDAG (chelator-based) | In vitro radiotoxicity studies in PC3 and LNCaP cell lines. Biodistribution studies and PET/CT imaging in healthy mice. | [70] | |
| Nanoclusters/4.2 nm/AMD3100 (chelator-based) | Biodistribution studies and PET/CT imaging in 4T1 mouse orthotopic breast cancer mouse model with lung metastases. | [71] | |
| Spherical, hexapodal and rod shaped AuNPs/10, 30, 80 nm/PEG, cyclic RGD (64Cu epitaxial growth on NP surface) | Biodistribution studies and in vivo PET imaging in U87MG glioblastoma xenograft mice. | [62] | |
| Tripod shaped AuNPs/25, 35 nm/DAPTA (64Cu-doped NPs) | In vivo PET imaging and image-guided photothermal treatment in 4T1-TNBC xenograft mice. | [72] | |
| AuNCs/30.4, 54.4 nm/PEG (chelator-based) | Biodistribution and in vivo PET imaging in EMT-6 murine breast cancer mouse model. | [73] | |
| AuNSs/120 nm/PEG (chelator-based) | In vivo PET imaging of 64Cu-NS-RGDfKS pharmacodynamics in nude rats xenografted with head and neck squamous cell carcinoma (HNSCC) | [74] | |
| 67Ga | Spherical AuNPs/4 nm/bombesin, DOTA (chelator-based) | In vitro radiotoxicity studies in PC3 cells. Biodistribution studies in PC3 xenograft mice. | [49,75] |
| 68Ga | Spherical AuNPs/42.5 nm/NOC, TOC (chelator-based) | In vitro binding kinetics studies in human colon cancer cell line (HT-29) and AR42J cell line of acinar pancreatic rat. | [76] |
| Spherical AuNPs/3 nm/PEG, bombesin, LUG, NIR dye SIDAG (chelator-based) | Ex vivo biodistribution studies and in vivo fluorescence imaging in LNCaP tumour bearing mice. | [77] | |
| 99mTc | Dendrimer-entraped spherical AuNPs/1.6 nm (Au core), 291.2 nm (dendrimer)/PAMAM (chelator-based) | Biodistribution studies in xenograft mice tumours with HeLa cells. | [78] |
| Dendrimer-entraped spherical AuNPs/2–6 nm (Au core), 127–139 nm (dendrimer)/PEG, cyclic RGD (chelator-based) | Ex vivo biodistribution studies in albino mice. In vivo Micro-SPECT/CT imaging, in albino mice and nude mice bearing C6 xenografted tumours. Therapeutic efficacy studies in C6 xenografted mice. | [79,80,81,82] | |
| Spherical AuNPs/5 nm/cyclic RGD (chelator-based) | Scintigraphy imaging in xenografted mice harboring 4T1 metastasis breast cancer. | [83] | |
| Spherical AuNPs/16.7 nm/Resveratrol (chelator-based) | In vivo biodistribution studies in HT 29 tumour bearing rats. | [84] | |
| PEI-entraped spherical AuNPs/3.3 nm (Au core)/PEG, fluorescein isothiocyanate, alkoxyphenyl acylsulfonamide (chelator-based) | In vitro CT and SPECT imaging of fribrosarcoma HT1080 cells. | [85] | |
| Spherical AuNPs/30.2 nm/Annexin V (chelator-based) | SPECT/CT imaging of mice with high fat diet-induced atherosclerosis. | [86] | |
| Spherical AuNPs/58.9 nm/doxorubicin, EGCG (chelator-based) | In vitro cytotoxicity studies in breast carcinoma MCF-7 and hepatocellular carcinoma HepG-2 cell lines. Biodistribution studies in Ehrlich ascites carcinoma tumour bearing albino mice. | [87] | |
| Spherical AuNPs/10 nm/gallic, doxorubicin (chelator-based) | In vitro anti-proliferative activity studies in MCF7 cell lines. Biodistribution studies in Ehrlich ascites carcinoma tumour bearing albino mice. | [88,89] | |
| 111In | Spherical AuNPs/10 nm/MMP9 (chelator-based) | In vivo SPECT/CT imaging in nude mice bearing bilateral tumours (A431 with high MMP9 expression and 4T1Luc with low MMP9 expression). | [90] |
| Spherical AuNPs/14 nm/EGF (chelator-based) | Internalization and radiotoxicity studies in MDA-MB-468 and MCF-7 cells. | [91] | |
| Spherical AuNPs/30 nm/trastuzumab (chelator-based) | Micro-SPECT/CT imaging in MDA-MB-361 human breast cancer xenograft mice. | [92,93] | |
| 124I | Spherical AuNPs/5, 10, 20 nm/oligotyrosine (124I-embeded NPs) | Dendritic cell and macrophages labeling in vivo for PET imaging detection of Sentinel Lymph Nodes. | [94,95,96] |
| Crushed Au Shell-covered spherical AuNPs/0.25 nm/ poly(N-vinyl-2-pyrrolidone (chloramine T oxidation combined with 124I-embeded NPs) | PET/CT imaging in 4T1 and CT26 tumour bearing mice and photothermal therapy in CT26 tumour bearing mice. | [97,98] | |
| 125I | Spherical and rod shaped AuNPs/56 nm/cyclic RGD (NP adsorption) | Biodistribution studies and SPECT/CT imaging in H1299 tumour bearing mice. | [99] |
| Spherical AuNPs/10 nm/MMP9 (chelator-based) | In vivo SPECT/CT imaging in nude mice bearing bilateral tumours (A431 with high MMP9 expression and 4T1Luc with low MMP9 expression). | [90] | |
| Spherical AuNPs/5, 10, 20 nm/ ogotyrosine (chloramine T oxidation combined with 125I-embeded NPs) | Dendritic cell and macrophages labeling in vivo for SPECT/PET imaging detection of Sentinel Lymph Nodes. | [94,95,96] | |
| 199Au | Spherical AuNPs/5, 18 nm/DAPTA (199Au-doped NPs) | Biodistribution studies and SPECt/CT imaging in 4T1 tumour bearing mice. | [100] |
| Amorphous/25–85 nm/PEG, folic acid, human immunoglobulin, Bharglob, M3-monoclonal antibody (199Au NP synthesis) | In vivo biodsitribution studies in healthy mice. | [101] | |
| Spherical AuNPs/not applicable (199AuNPs) | Assessment of dose distribution in human prostate cancer using Monte-Carlo simulations. | [102] |
| Radioisotope | Type of AuNPs/Size/Coating (Radiolabeling Approach) | Application/Study | Refs. |
|---|---|---|---|
| 90Y | Spherical AuNPs-loaded nanoparticle depots/15 nm/PEG, polyglutamide (chelator-based) | Monte Carlo simulations of permanent seed implantation brachytherapy. | [125] |
| AuNRs/40 nm/PEG (chelator-based) | Biodistribution studies, combined radiotherapy and hyperthermia treatment in prostate DU145 xenograft mice. | [126] | |
| 131I | PEI-entraped spherical AuNPs/4.4 nm (AuNP core), 151 nm (PEI)/HPAO, PEG, CTX (chloramine T oxidation) | Targeted SPECT/CT imaging and radionuclide therapy in subcutaneous glioma tumour model in vivo. | [127] |
| AuNRs/93 nm/PEG, cyclic RGD (NP adsorption) | SPECT/CT imaging and biodistribution analyses in B16F10 and MCF7 tumour bearing mice | [59] | |
| 177Lu | Dendrimer-entraped spherical AuNPs/2.5 nm (AuNP core), 5.6 nm (dendrimer)/folate, bombesin (chelator-based) | Radiocytotocixity studies in T47D cells. Biodistribution studies and optical imaging in T47D xenograft mice. | [128,129] |
| Spherical AuNPs/30 nm/orthopyridyl disulfide, PEG, panitumumab(chelator-based) | Biodistribution/radiotoxicity studies and small-animal SPECT/CT imaging in MDA-MB-468 xenograft mice. | [130,131] | |
| AuNRs/15 nm/PEG, polyglutamide (chelator-based) | Monte Carlo simulations of permanent seed implantation brachytherapy. | [125] | |
| 198Au | Spherical AuNPs/12.5 nm/cyclic RGD (198Au NP synthesis) | Biodistribution and tumour regression studies in melanoma C57BL/6 tumour bearing mice. | [124] |
| Spherical AuNPs/35 nm/mangiferin (198Au NP synthesis) | Biodistribution and therapeutic efficacy studies in prostate PC3 xenograft mice. | [56] | |
| 211At | Spherical AuNPs/5 nm/PEG, trastuzumab (NP adsorption) | In vitro radiotoxicity studies in human ovarian cancer cell line SKOV-3 | [132] |
| 225Ac | Spherical AuNPs/2–3 nm/DOTAGA (chelator-based) | Biodistribution and therapeutic efficacy studies in glioblastoma multiform cell line U87 xenograft mice. | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials 2021, 14, 4. https://doi.org/10.3390/ma14010004
Silva F, Cabral Campello MP, Paulo A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials. 2021; 14(1):4. https://doi.org/10.3390/ma14010004
Chicago/Turabian StyleSilva, Francisco, Maria Paula Cabral Campello, and António Paulo. 2021. "Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer" Materials 14, no. 1: 4. https://doi.org/10.3390/ma14010004
APA StyleSilva, F., Cabral Campello, M. P., & Paulo, A. (2021). Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials, 14(1), 4. https://doi.org/10.3390/ma14010004






