Settable Polymeric Autograft Extenders in a Rabbit Radius Model of Bone Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyester Triol and Thioketal Diol Synthesis
2.3. AG Extender Fabrication
2.4. AG Extenders in a Rabbit Radius Defect
2.5. µCT Analysis
2.6. Histological Evaluation
2.7. Statistical Analysis
3. Results
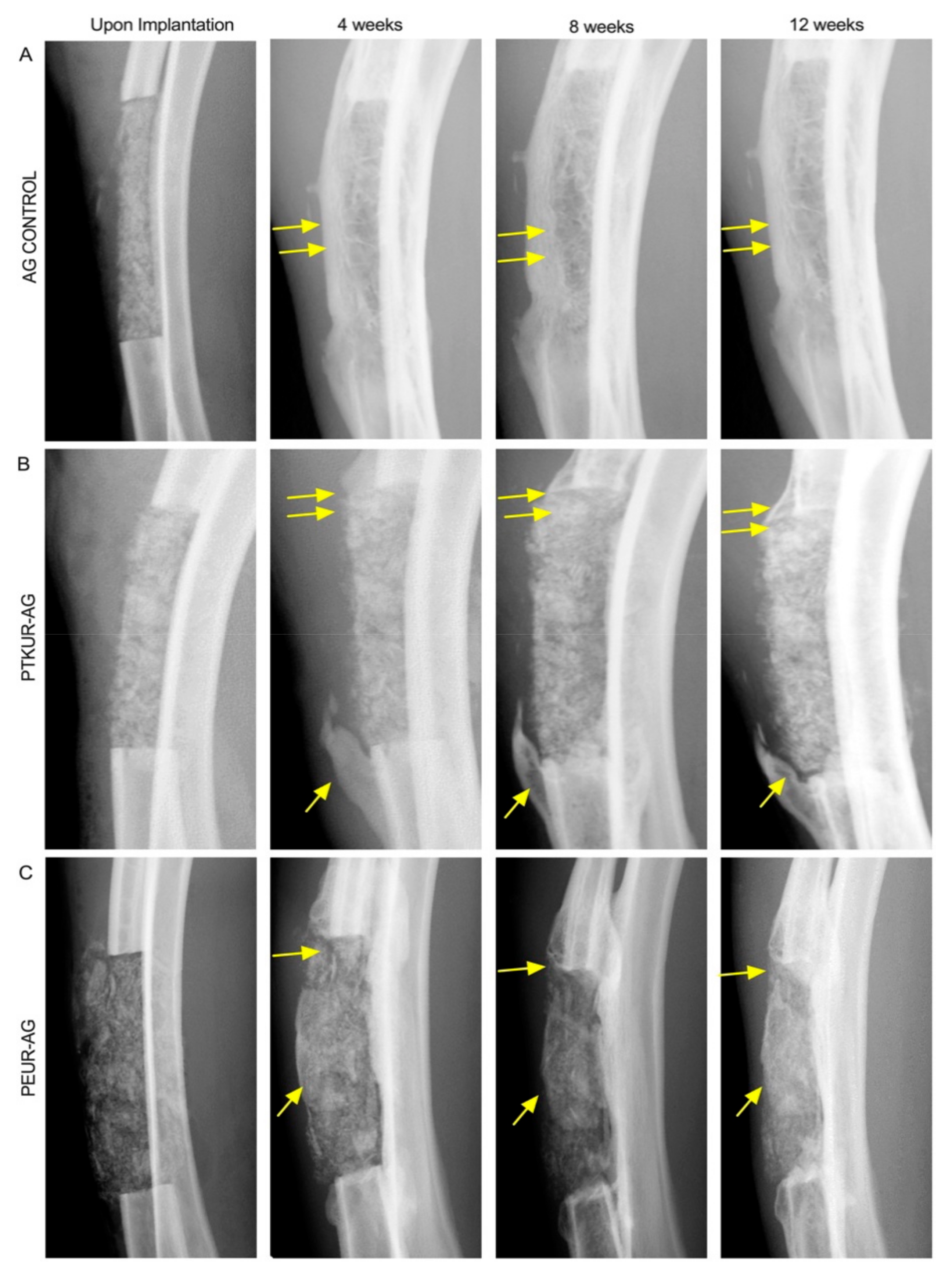
3.1. Surgical Outcomes
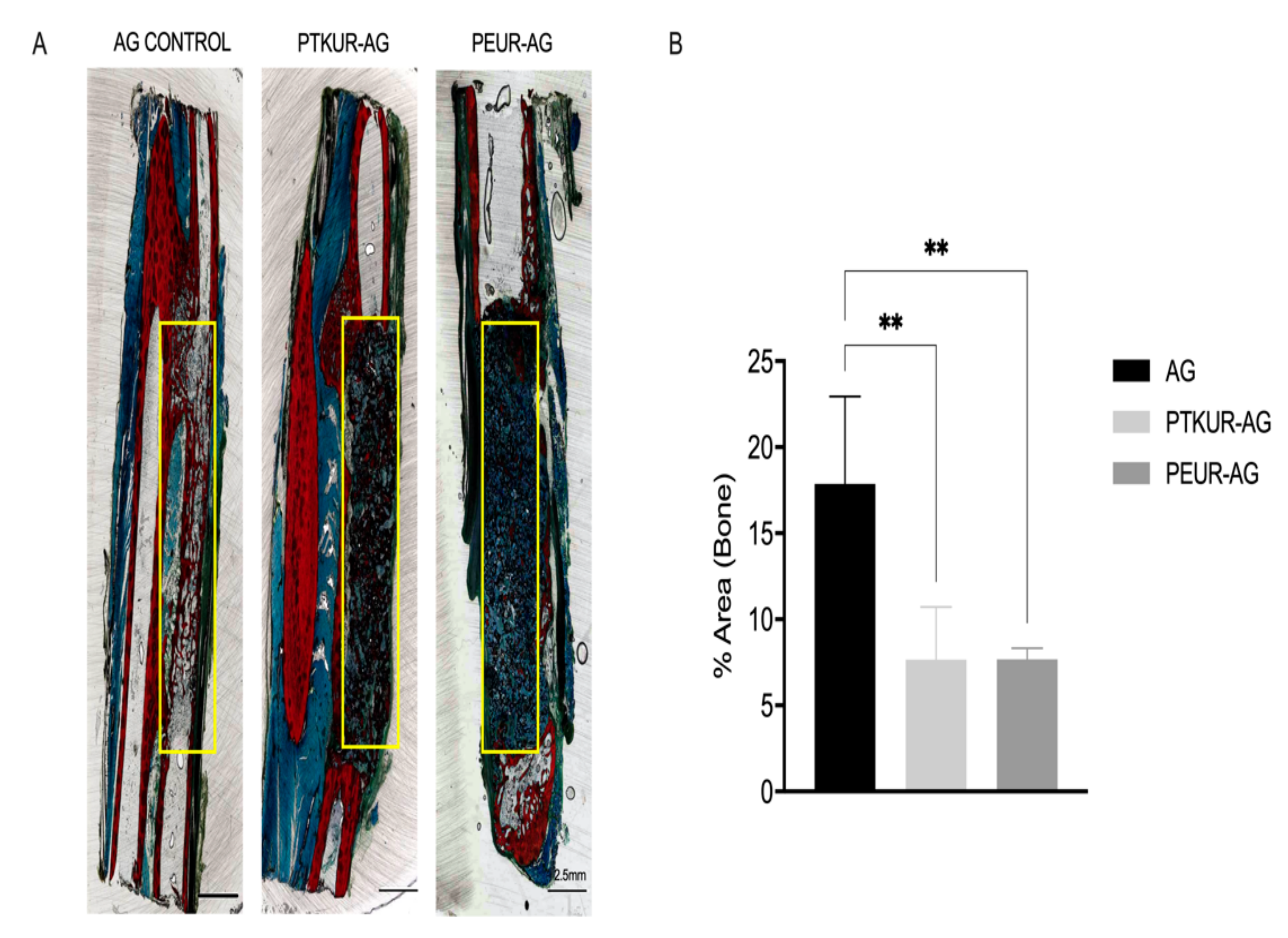
3.2. In Vivo Bone Analysis
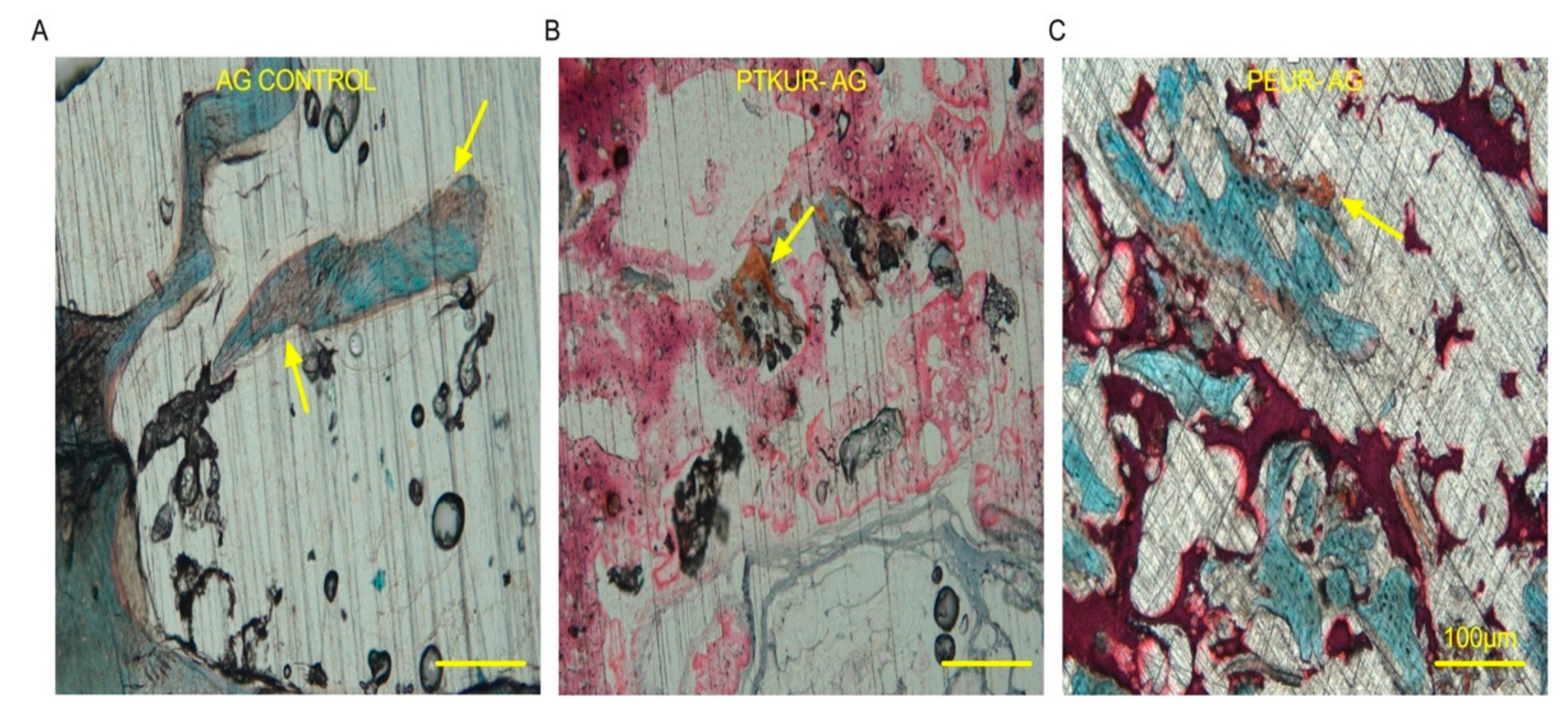
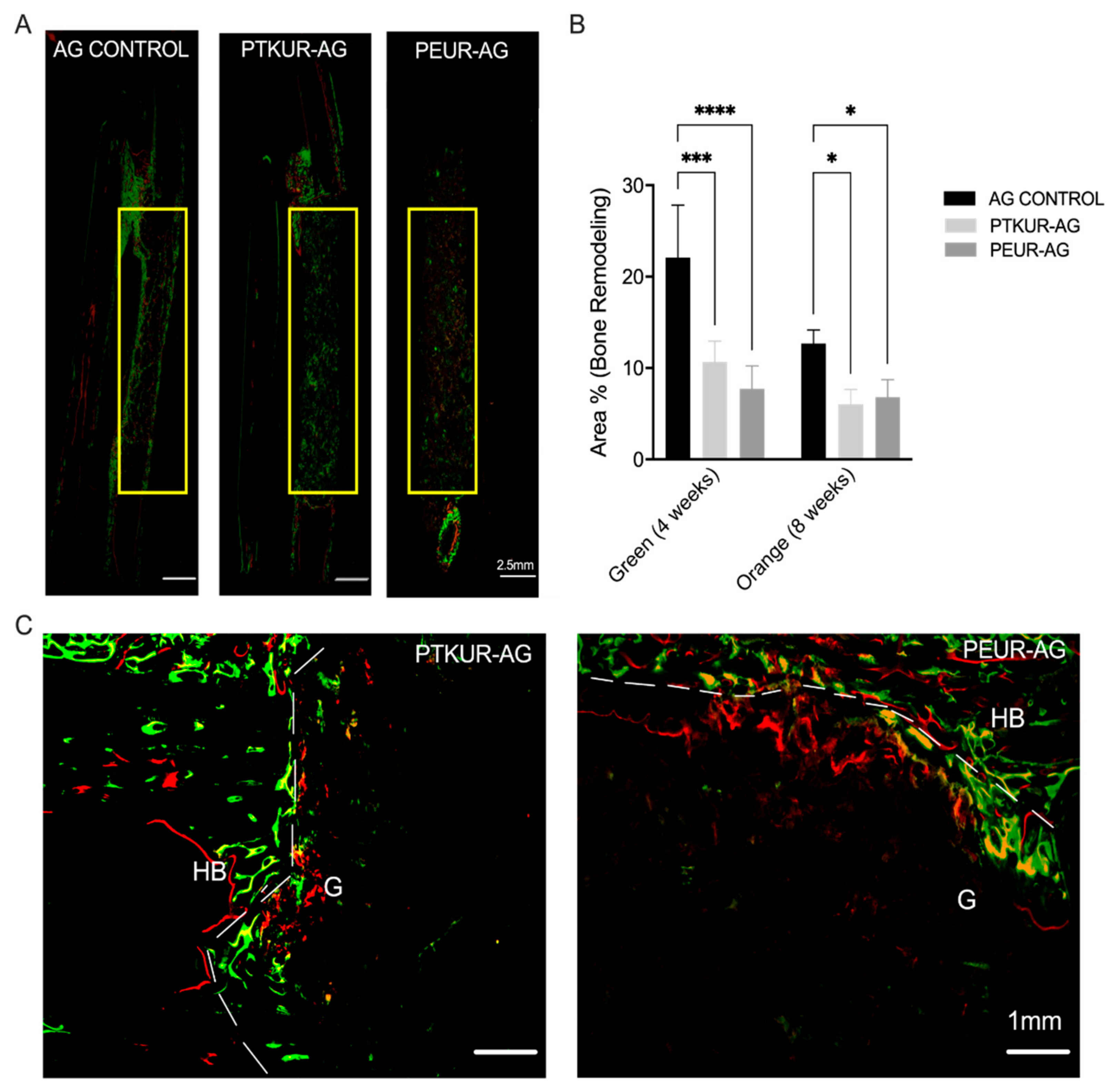
3.3. PTKUR and PEUR Graft Remodeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Shafiei, Z.; Bigham, A.S.; Dehghani, S.N.; Torabi Nezhad, S. Fresh Cortical Autograft versus Fresh Cortical Allograft Effects on Experimental Bone Healing in Rabbits: Radiological, Histopathological and Biomechanical Evaluation. Cell Tissue Bank. 2009, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Myeroff, C.; Archdeacon, M. Autogenous Bone Graft: Donor Sites and Techniques. J. Bone Jt. Surg. Ser. A 2011, 7, 2227–2236. [Google Scholar] [CrossRef]
- Rogers, G.F.; Greene, A.K. Autogenous Bone Graft: Basic Science and Clinical Implications. J. Craniofacial Surg. 2012, 3, 323–327. [Google Scholar] [CrossRef]
- Carlisle, E.R.; Fischgrund, J.S. Bone Graft and Fusion Enhancement. In Surgical Management of Spinal Deformities; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781416033721. [Google Scholar]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of Iliac Crest Bone Graft Harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone Grafts, Bone Substitutes and Orthobiologics the Bridge between Basic Science and Clinical Advancements in Fracture Healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Waters, P.M. Free Vascularized Fibula Grafting: Principles, Techniques, and Applications in Pediatric Orthopaedics. Orthop. J. Harv. Med. Sch. 2006, 8, 86–89. [Google Scholar]
- Lumetti, S.; Galli, C.; Manfredi, E.; Consolo, U.; Marchetti, C.; Ghiacci, G.; Toffoli, A.; Bonanini, M.; Salgarelli, A.; Macaluso, G.M. Correlation between Density and Resorption of Fresh-Frozen and Autogenous Bone Grafts. BioMed Res. Int. 2014, 2014, 508328. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.A.; Ayerza, M.A.; Muscolo, D.L.; Farfalli, G.L. What Are the Risk Factors and Management Options for Infection After Reconstruction With Massive Bone Allografts? Clin. Orthop. Relat. Res. 2016. [Google Scholar] [CrossRef]
- Urban, R.M.; Turner, T.M.; Hall, D.J.; Inoue, N.; Gitelis, S. Increased Bone Formation Using Calcium Sulfate-Calcium Phosphate Composite Graft. Clin. Orthop. Related Res. 2007, 459, 110–117. [Google Scholar] [CrossRef]
- Parikh, S.N. Bone Graft Substitutes in Modern Orthopedics. Orthopedics 2002, 25, 1301–1309. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A Comprehensive Clinical Review of Recombinant Human Bone Morphogenetic Protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Smucker, J.D.; Rhee, J.M.; Singh, K.; Yoon, S.T.; Heller, J.G. Increased Swelling Complications Associated with Off-Label Usage of RhBMP-2 in the Anterior Cervical Spine. Spine 2006, 31, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Stiel, N.; Hissnauer, T.N.; Rupprecht, M.; Babin, K.; Schlickewei, C.W.; Rueger, J.M.; Stuecker, R.; Spiro, A.S. Evaluation of Complications Associated with Off-Label Use of Recombinant Human Bone Morphogenetic Protein-2 (RhBMP-2) in Pediatric Orthopaedics. J. Mater. Sci. Mater. Med. 2016, 27, 184. [Google Scholar] [CrossRef]
- Barrack, R.L. Bone Graft Extenders, Substitutes, and Osteogenic Proteins. J. Arthroplas. 2005, 20, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Girardi, F.P.; Cammisa, F.P. The Effect of Bone Graft Extenders to Enhance the Performance of Iliac Crest Bone Grafts in Instrumented Lumbar Spine Fusion. Orthopedics 2003, 26. [Google Scholar] [CrossRef]
- Cammisa, F.P.; Lowery, G.; Garfin, S.R.; Geisler, F.H.; Klara, P.M.; McGuire, R.A.; Sassard, W.R.; Stubbs, H.; Block, J.E. Two-Year Fusion Rate Equivalency between Grafton® DBM Gel and Autograft in Posterolateral Spine Fusion: A Prospective Controlled Trial Employing a Side-by-Side Comparison in the Same Patient. Spine 2004, 29, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Smucker, J.D.; Petersen, E.B.; Nepola, J.V.; Fredericks, D.C. Assessment of Mastergraft(®) Strip with Bone Marrow Aspirate as a Graft Extender in a Rabbit Posterolateral Fusion Model. Iowa Orthop. J. 2012, 32, 61–68. [Google Scholar]
- Smucker, J.D.; Petersen, E.B.; Fredericks, D.C. Assessment of MASTERGRAFT PUTTY as a Graft Extender in a Rabbit Posterolateral Fusion Model. Spine 2012, 37, 1017–1021. [Google Scholar] [CrossRef]
- Dai, L.Y.; Jiang, L.S. Single-Level Instrumented Posterolateral Fusion of Lumbar Spine with β-Tricalcium Phosphate versus Autograft: A Prospective, Randomized Study with 3-Year Follow-Up. Spine 2008, 33, 1299–1304. [Google Scholar] [CrossRef]
- Lerner, T.; Bullmann, V.; Schulte, T.L.; Schneider, M.; Liljenqvist, U. A Level-1 Pilot Study to Evaluate of Ultraporous β-Tricalcium Phosphate as a Graft Extender in the Posterior Correction of Adolescent Idiopathic Scoliosis. Eur. Spine J. 2009, 18, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hile, D.D.; Kandziora, F.; Lewandrowski, K.U.; Doherty, S.A.; Kowaleski, M.P.; Trantolo, D.J. A Poly(Propylene Glycol-Co-Fumaric Acid) Based Bone Graft Extender for Lumbar Spinal Fusion: In Vivo Assessment in a Rabbit Model. Eur. Spine J. 2006, 15, 936–943. [Google Scholar] [CrossRef]
- Chedid, M.K.; Tundo, K.M.; Block, J.E.; Muir, J.M. Hybrid Biosynthetic Autograft Extender for Use in Posterior Lumbar Interbody Fusion: Safety and Clinical Effectiveness. Open Orthop. J. 2015, 9, 218–225. [Google Scholar] [CrossRef][Green Version]
- Lewandrowski, K.U.; Bondre, S.; Gresser, J.D.; Silva, A.E.; Wise, D.L.; Trantolo, D.J. Augmentation of Osteoinduction with a Biodegradable Poly(Propylene Glycol-Co-Fumaric Acid) Bone Graft Extender. A Histologic and Histomorphometric Study in Rats. Bio-Med Mater. Eng. 1999, 9, 325–334. [Google Scholar]
- Walsh, W.R.; Oliver, R.A.; Gage, G.; Yu, Y.; Bell, D.; Bellemore, J.; Adkisson, H.D. Application of Resorbable Poly(Lactide-Co-Glycolide) with Entangled Hyaluronic Acid as an Autograft Extender for Posterolateral Intertransverse Lumbar Fusion in Rabbits. Tissue Eng. Part A 2011, 17, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Keränen, P.; Itälä, A.; Koort, J.; Kohonen, I.; Dalstra, M.; Kommonen, B.; Aro, H.T. Bioactive Glass Granules as Extender of Autogenous Bone Grafting in Cementless Intercalary Implant of the Canine Femur. Scand. J. Surg. 2007, 96, 243–251. [Google Scholar] [CrossRef]
- Fernando, S.; McEnery, M.; Guelcher, S.A. Polyurethanes for Bone Tissue Engineering. Adv. Polyurethane Biomater. 2016, 481–501. [Google Scholar] [CrossRef]
- Guelcher, S.A. Biodegradable Polyurethanes: Synthesis and Applications in Regenerative Medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef]
- McEnery, M.A.P.; Lu, S.; Gupta, M.K.; Zienkiewicz, K.J.; Wenke, J.C.; Kalpakci, K.N.; Shimko, D.A.; Duvall, C.L.; Guelcher, S.A. Oxidatively Degradable Poly(Thioketal Urethane)/Ceramic Composite Bone Cements with Bone-like Strength. RSC Adv. 2016, 6, 109414–109424. [Google Scholar] [CrossRef]
- McGough, M.A.P.; Boller, L.A.; Groff, D.M.; Schoenecker, J.G.; Nyman, J.S.; Wenke, J.C.; Rhodes, C.; Shimko, D.; Duvall, C.L.; Guelcher, S.A. Nanocrystalline Hydroxyapatite-Poly(Thioketal Urethane) Nanocomposites Stimulate a Combined Intramembranous and Endochondral Ossification Response in Rabbits. ACS Biomater. Sci. Eng. 2020, 6, 564–574. [Google Scholar] [CrossRef]
- Talley, A.D.; Boller, L.A.; Kalpakci, K.N.; Shimko, D.A.; Guelcher, C.S.A. Injectable, Compression-Resistant Polymer / Ceramic Composite Bone Grafts Promote Lateral Ridge Augmentation without Protective Mesh in a Canine Model. Clin. Oral Implant. Res. 2018, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Lu, S.; McGough, M.A.P.; Shiels, S.M.; Zienkiewicz, K.J.; Merkel, A.R.; Vanderburgh, J.P.; Nyman, J.S.; Sterling, J.A.; Tennent, D.J.; Wenke, J.C.; et al. Settable Polymer/Ceramic Composite Bone Grafts Stabilize Weight-Bearing Tibial Plateau Slot Defects and Integrate with Host Bone in an Ovine Model. Biomaterials 2018, 179, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Talley, A.D.; McEnery, M.A.; Kalpakci, K.N.; Zienkiewicz, K.J.; Shimko, D.A.; Guelcher, S.A. Remodeling of Injectable, Low-Viscosity Polymer/Ceramic Bone Grafts in a Sheep Femoral Defect Model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Gupta, M.K.; Page, J.M.; Yu, F.; Davidson, J.M.; Guelcher, S.A.; Duvall, C.L. A Porous Tissue Engineering Scaffold Selectively Degraded by Cell-Generated Reactive Oxygen Species. Biomaterials 2014. [Google Scholar] [CrossRef] [PubMed]
- McGough, M.; Shiels, S.M.; Boller, L.A.; Zienkiewicz, K.J.; Duvall, C.L.; Wenke, J.C.; Guelcher, S.A. Poly(Thioketal Urethane) Autograft Extenders in an Intertransverse Process Model of Bone Formation. Tissue Eng. Part A 2018, 25, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Boller, L.; Jones, A.; Cochran, D.; Guelcher, S. Compression-Resistant Polymer/Ceramic Composite Scaffolds Augmented with RhBMP-2 Promote New Bone Formation in a Nonhuman Primate Mandibular Ridge Augmentation Model. Int. J. Oral. Maxillofac. Implant. 2020, 35, 616–624. [Google Scholar] [CrossRef]
- Dumas, J.E.; Prieto, E.M.; Zienkiewicz, K.J.; Guda, T.; Wenke, J.C.; Bible, J.; Holt, G.E.; Guelcher, S. Balancing the Rates of New Bone Formation and Polymer Degradation Enhances Healing of Weight-Bearing Allograft/Polyurethane Composites in Rabbit Femoral Defects. Tissue Eng. Part A 2014, 20, 115–129. [Google Scholar] [CrossRef]
- Hafeman, A.E.; Zienkiewicz, K.J.; Zachman, A.L.; Sung, H.J.; Nanney, L.B.; Davidson, J.M.; Guelcher, S.A. Characterization of the Degradation Mechanisms of Lysine-Derived Aliphatic Poly(Ester Urethane) Scaffolds. Biomaterials 2011, 32, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Guelcher, S.A.; Srinivasan, A.; Dumas, J.E.; Didier, J.E.; McBride, S.; Hollinger, J.O. Synthesis, Mechanical Properties, Biocompatibility, and Biodegradation of Polyurethane Networks from Lysine Polyisocyanates. Biomaterials 2008, 29, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Bodde, E.W.H.; Spauwen, P.H.M.; Mikos, A.G.; Jansen, J.A. Closing Capacity of Segmental Radius Defects in Rabbits. J. Biomed. Mater. Res. Part A 2008, 85, 206–217. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Stokes, K.E.; Park, H.M.; Hollinger, J.O. Evaluation of Particulate Bioglass in a Rabbit Radius Ostectomy Model. J. Biomed. Mater. Res. 1997, 35, 249–254. [Google Scholar] [CrossRef]
- Talley, A.D.; Kalpakci, K.N.; Shimko, D.A.; Zienkiewicz, K.J.; Cochran, D.L.; Guelcher, S.A. Effects of Recombinant Human Bone Morphogenetic Protein-2 Dose and Ceramic Composition on New Bone Formation and Space Maintenance in a Canine Mandibular Ridge Saddle Defect Model. Tissue Eng. Part A 2016, 22, 469–479. [Google Scholar] [CrossRef]
- Shiels, S.M.; Talley, A.D.; McGough, M.A.P.; Zienkiewicz, K.J.; Kalpakci, K.; Shimko, D.; Guelcher, S.A.; Wenke, J.C. Injectable and Compression-Resistant Low-Viscosity Polymer/Ceramic Composite Carriers for RhBMP-2 in a Rabbit Model of Posterolateral Fusion: A Pilot Study. J. Orthop. Surg. Res. 2017, 12, 107. [Google Scholar] [CrossRef]
- Dumas, J.E.; Zienkiewicz, K.; Tanner, S.A.; Prieto, E.M.; Bhattacharyya, S.; Guelcher, S.A. Synthesis and Characterization of an Injectable Allograft Bone/Polymer Composite Bone Void Filler with Tunable Mechanical Properties. Tissue Eng. Part A 2010, 16, 2505–2518. [Google Scholar] [CrossRef]
- Guda, T.; Walker, J.A.; Singleton, B.M.; Hernandez, J.W.; Son, J.S.; Kim, S.G.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Guided Bone Regeneration in Long-Bone Defects with a Structural Hydroxyapatite Graft and Collagen Membrane. Tissue Eng. Part A 2013, 9, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Guda, T.; Walker, J.A.; Pollot, B.E.; Appleford, M.R.; Oh, S.; Ong, J.L.; Wenke, J.C. In Vivo Performance of Bilayer Hydroxyapatite Scaffolds for Bone Tissue Regeneration in the Rabbit Radius. J. Mater. Sci. Mater. Med. 2011, 22, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Guda, T.; Singleton, B.M.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Effect of Cell-Seeded Hydroxyapatite Scaffolds on Rabbit Radius Bone Regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 1458–1466. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Ehrler, D.M.; Vaccaro, A.R. The Use of Allograft Bone in Lumbar Spine Surgery. Clin. Orthop. Relat. Res. 2000, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S. Onlay Bone Grafts in Head and Neck Reconstruction. Semin. Plast. Surg. 2010, 24, 255–261. [Google Scholar] [CrossRef]
- Antheunis, H.; van der Meer, J.C.; de Geus, M.; Heise, A.; Koning, C.E. Autocatalytic Equation Describing the Change in Molecular Weight during Hydrolytic Degradation of Aliphatic Polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The Biology of Bone Grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A. The physiologic and clinical significance of bone histomorphometric data. In Bone Histomorphometry: Techniques and Interpretations; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Compston, J.; Skingle, L.; Dempster, D.W. Bone Histomorphometry. In Vitamin D, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128099667. [Google Scholar]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, J.; Cao, L.; Qian, X.; Xing, W.; Lu, J.; Liu, C. Segmental Bone Regeneration Using RhBMP-2-Loaded Collagen/Chitosan Microspheres Composite Scaffold in a Rabbit Model. Biomed. Mater. 2012, 7. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boller, L.A.; McGough, M.A.P.; Shiels, S.M.; Duvall, C.L.; Wenke, J.C.; Guelcher, S.A. Settable Polymeric Autograft Extenders in a Rabbit Radius Model of Bone Formation. Materials 2021, 14, 3960. https://doi.org/10.3390/ma14143960
Boller LA, McGough MAP, Shiels SM, Duvall CL, Wenke JC, Guelcher SA. Settable Polymeric Autograft Extenders in a Rabbit Radius Model of Bone Formation. Materials. 2021; 14(14):3960. https://doi.org/10.3390/ma14143960
Chicago/Turabian StyleBoller, Lauren A., Madison A.P. McGough, Stefanie M. Shiels, Craig L. Duvall, Joseph C. Wenke, and Scott A. Guelcher. 2021. "Settable Polymeric Autograft Extenders in a Rabbit Radius Model of Bone Formation" Materials 14, no. 14: 3960. https://doi.org/10.3390/ma14143960
APA StyleBoller, L. A., McGough, M. A. P., Shiels, S. M., Duvall, C. L., Wenke, J. C., & Guelcher, S. A. (2021). Settable Polymeric Autograft Extenders in a Rabbit Radius Model of Bone Formation. Materials, 14(14), 3960. https://doi.org/10.3390/ma14143960





