Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys
Abstract
:1. Introduction
2. Materials and Methods
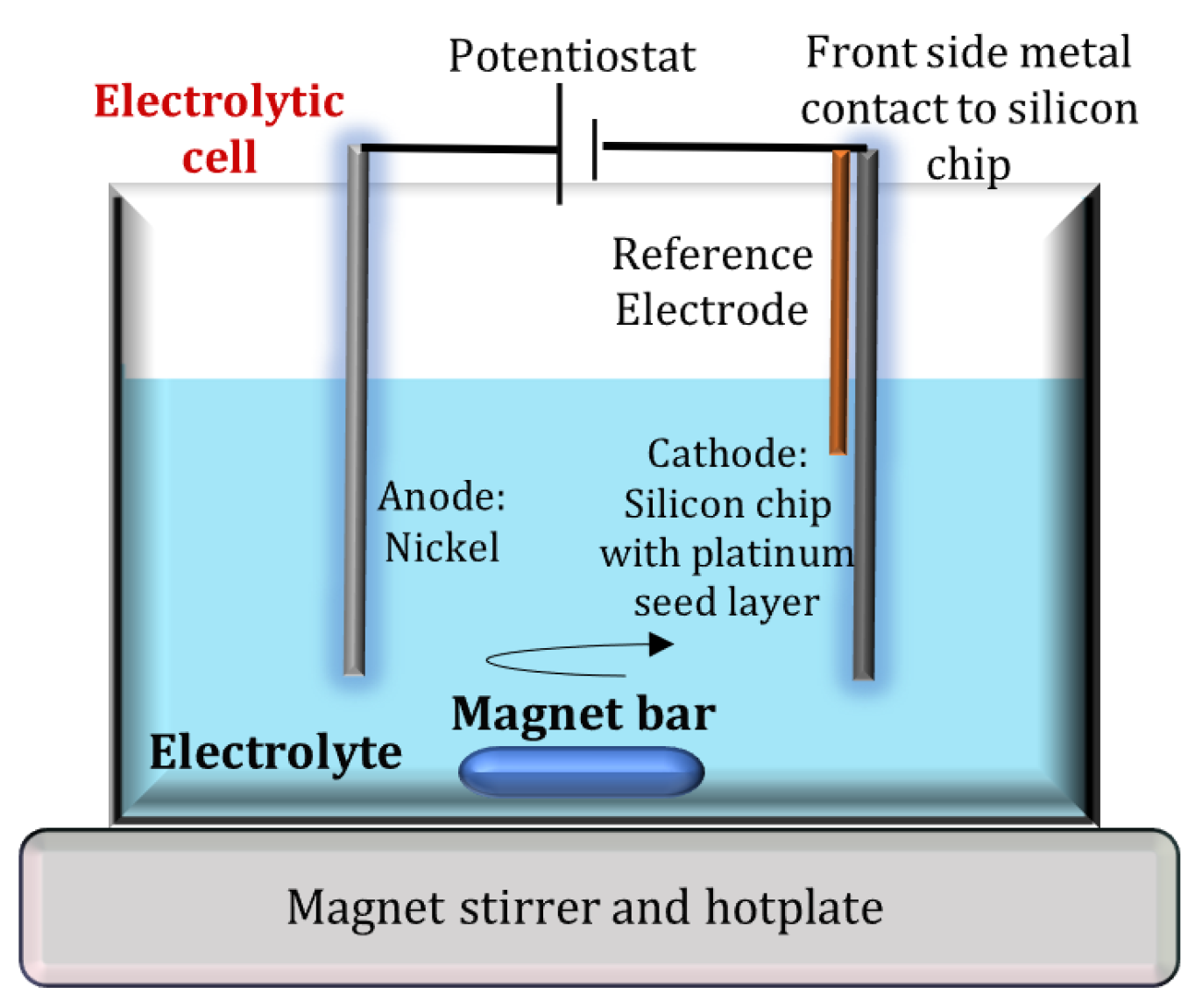
2.1. Electrochemical Deposition Experimental Setup
2.2. Material Characterization
3. Modelling of Electrolyte Vortex Flow Created by Magnetic Stirring and Determination of Optimum Electrode Position within the Vortex
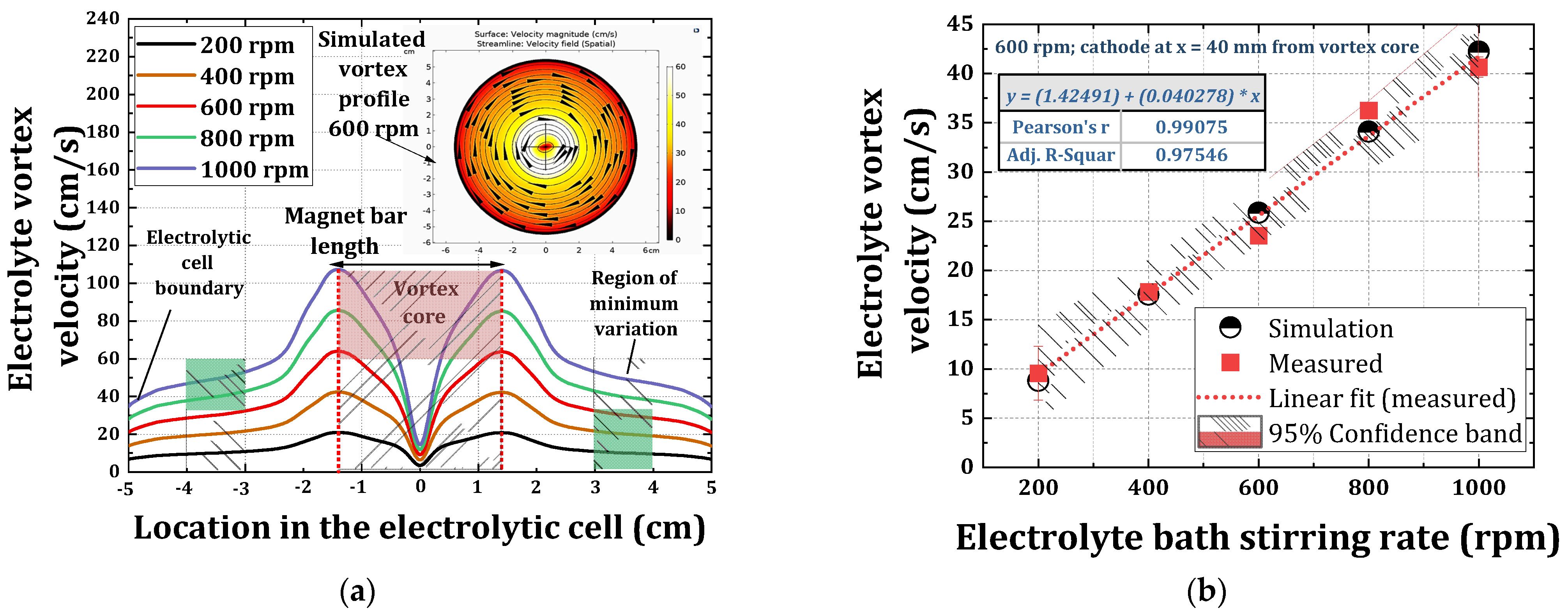
3.1. Simulation of the Electrolyte Vortex Flow Created by Magnetic Stirrer
3.2. Measurement of Electrolyte Vortex Velocity
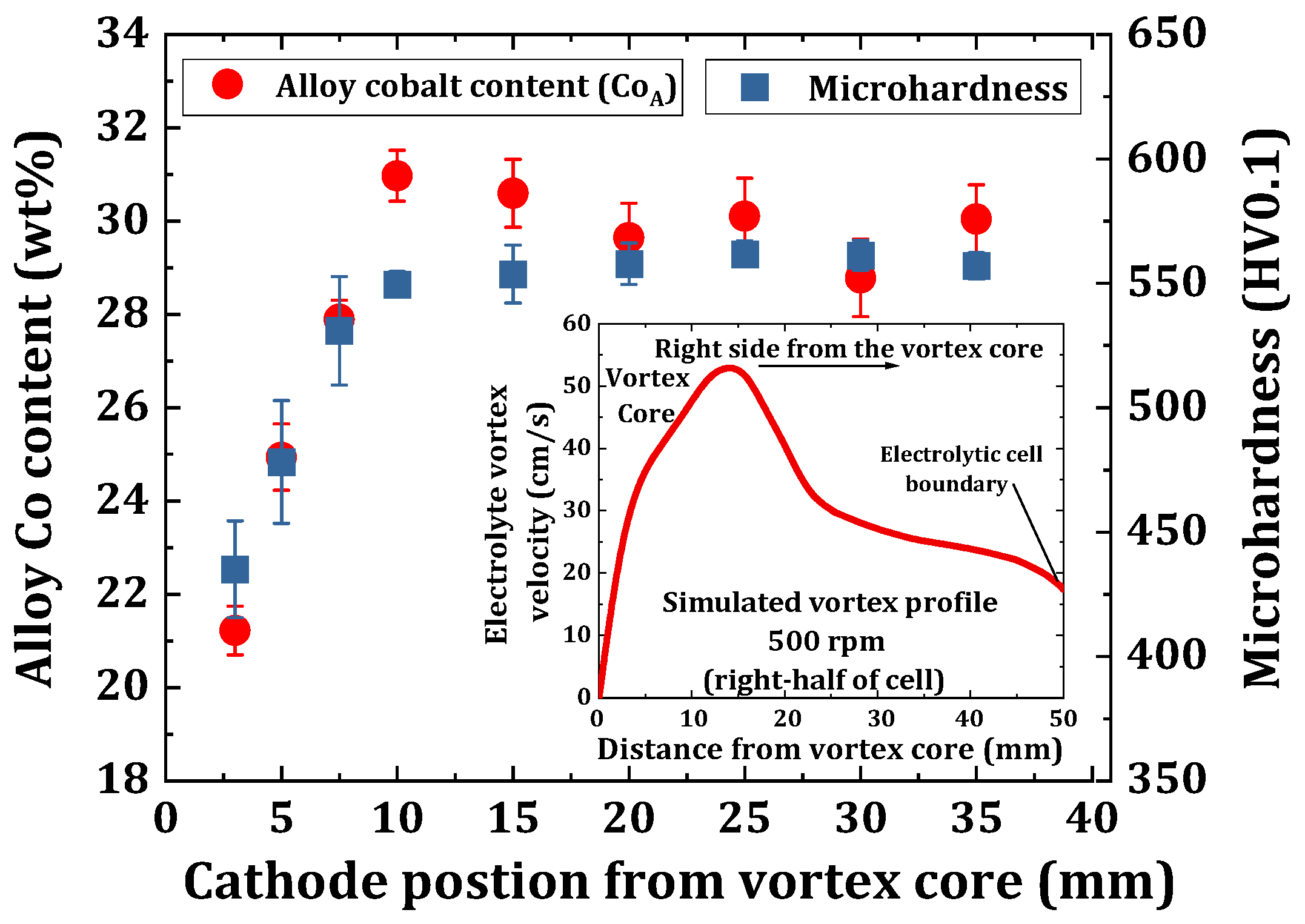
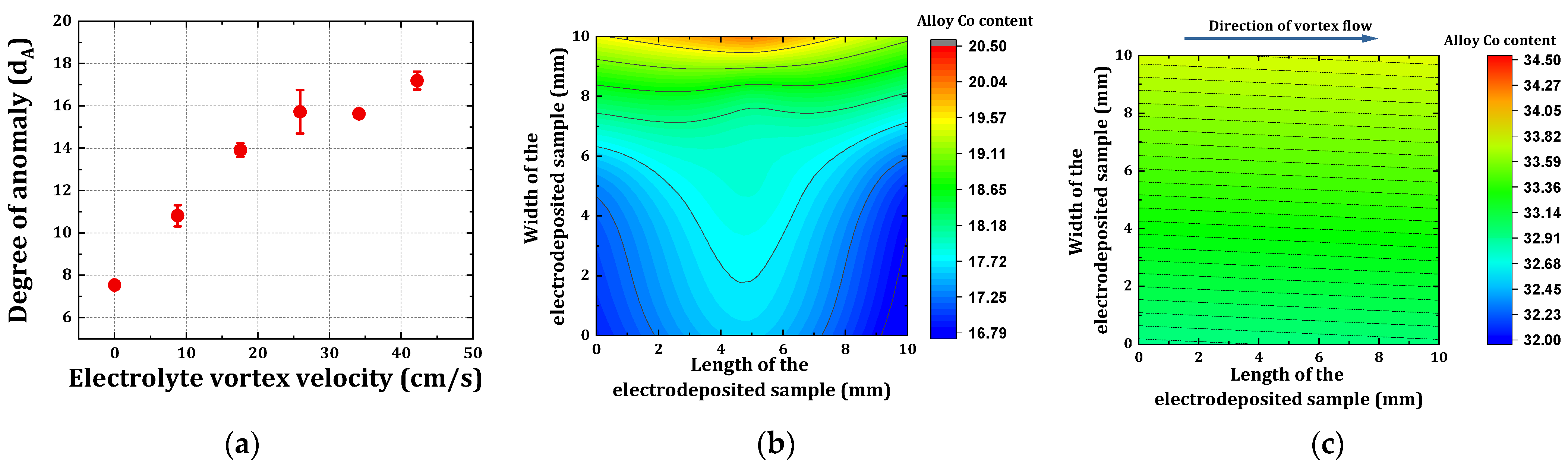
3.3. Determination of an Optimum Electrode Position in the Electrolyte Vortex Flow
4. Results and Discussion
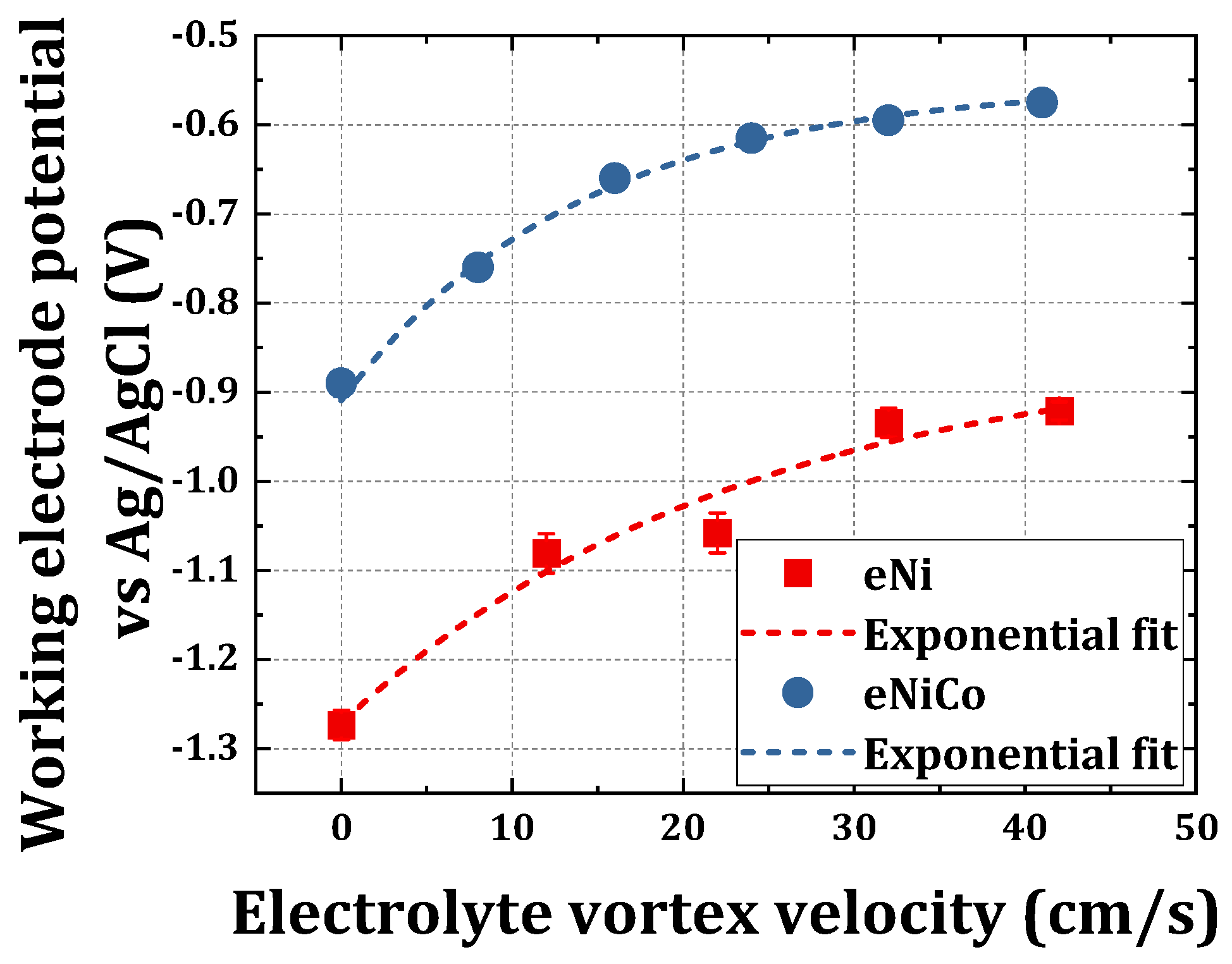
4.1. Chronopotentiometric Electrodeposition
4.2. Degree of Anomaly as a Function of Varying BHD Conditions
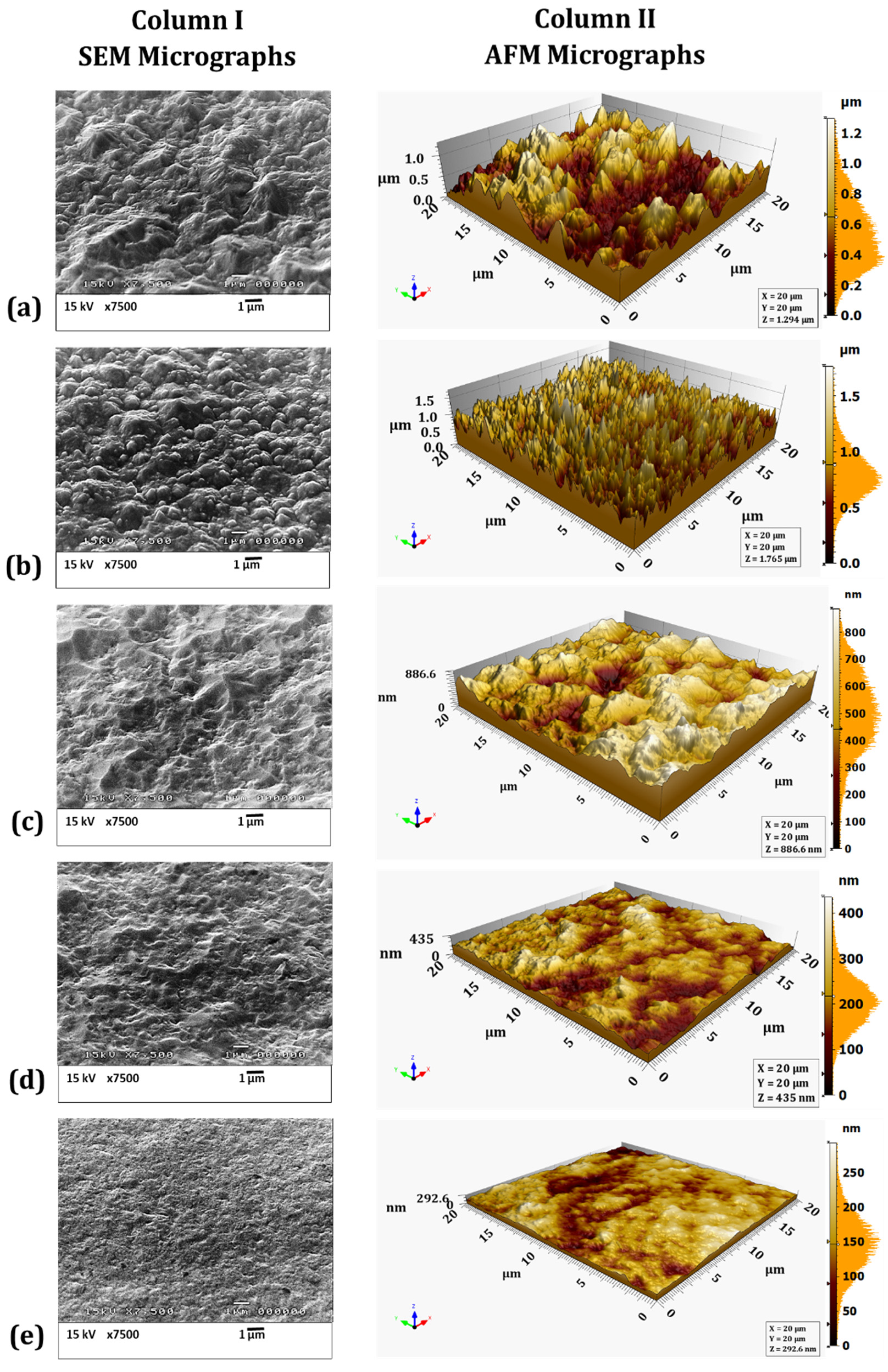
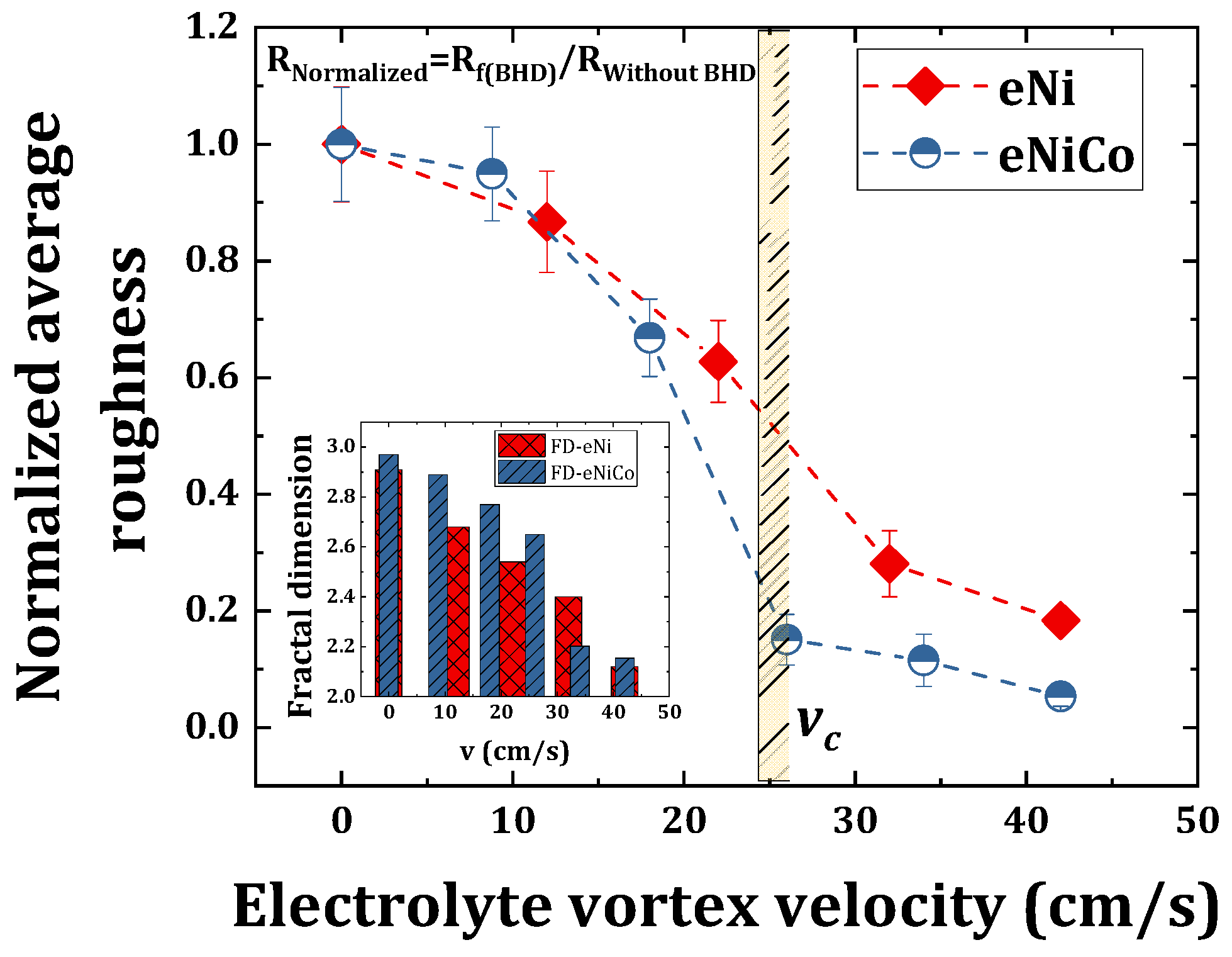
4.3. SEM and AFM Surface Morphology Characterization
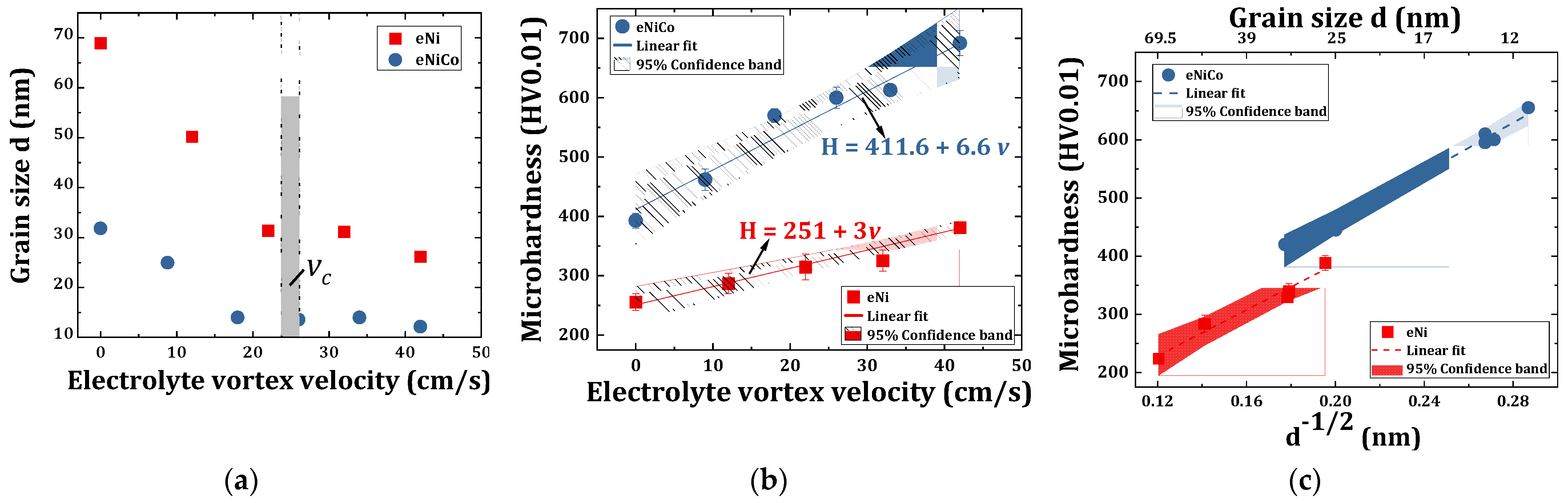
4.4. X-ray Diffractograms, Growth Phase and Grain Size
4.5. Microhardness Characterization of eNi and eNiCo Alloy Samples
5. Model for the Influence of BHD on Nucleation, Growth Kinetics and Micromechanical Properties
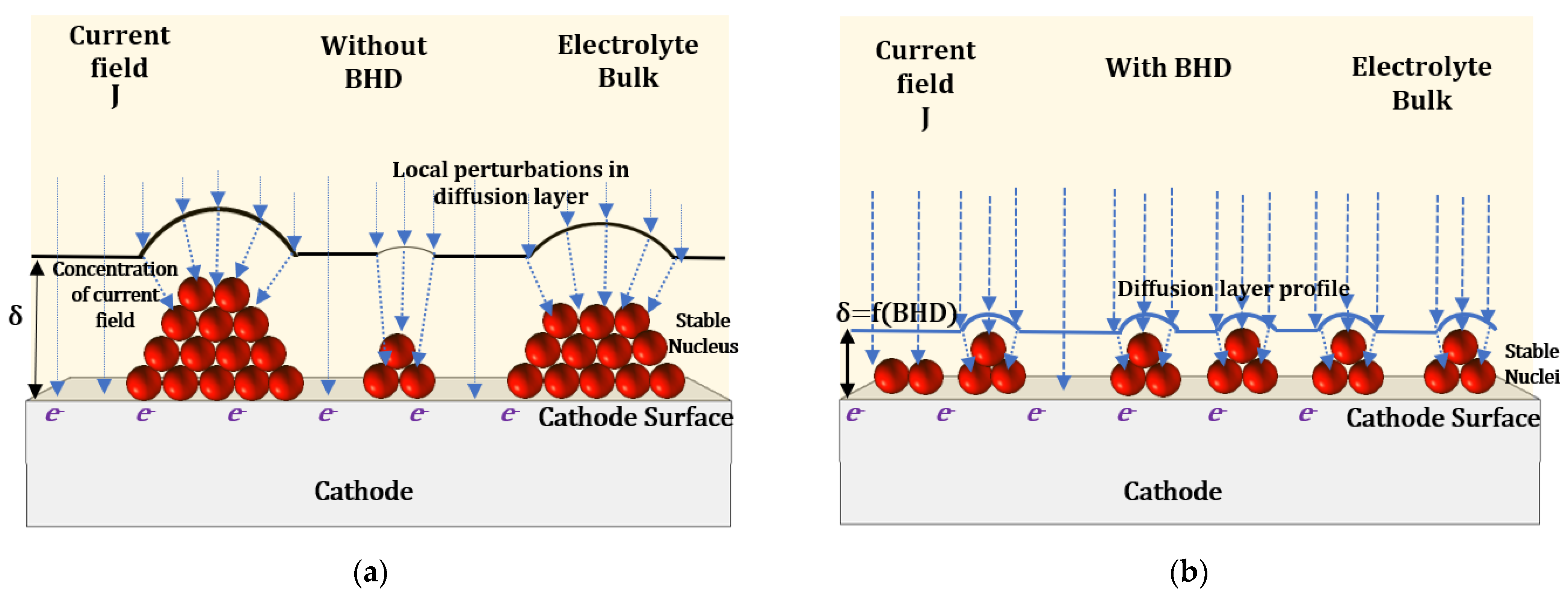
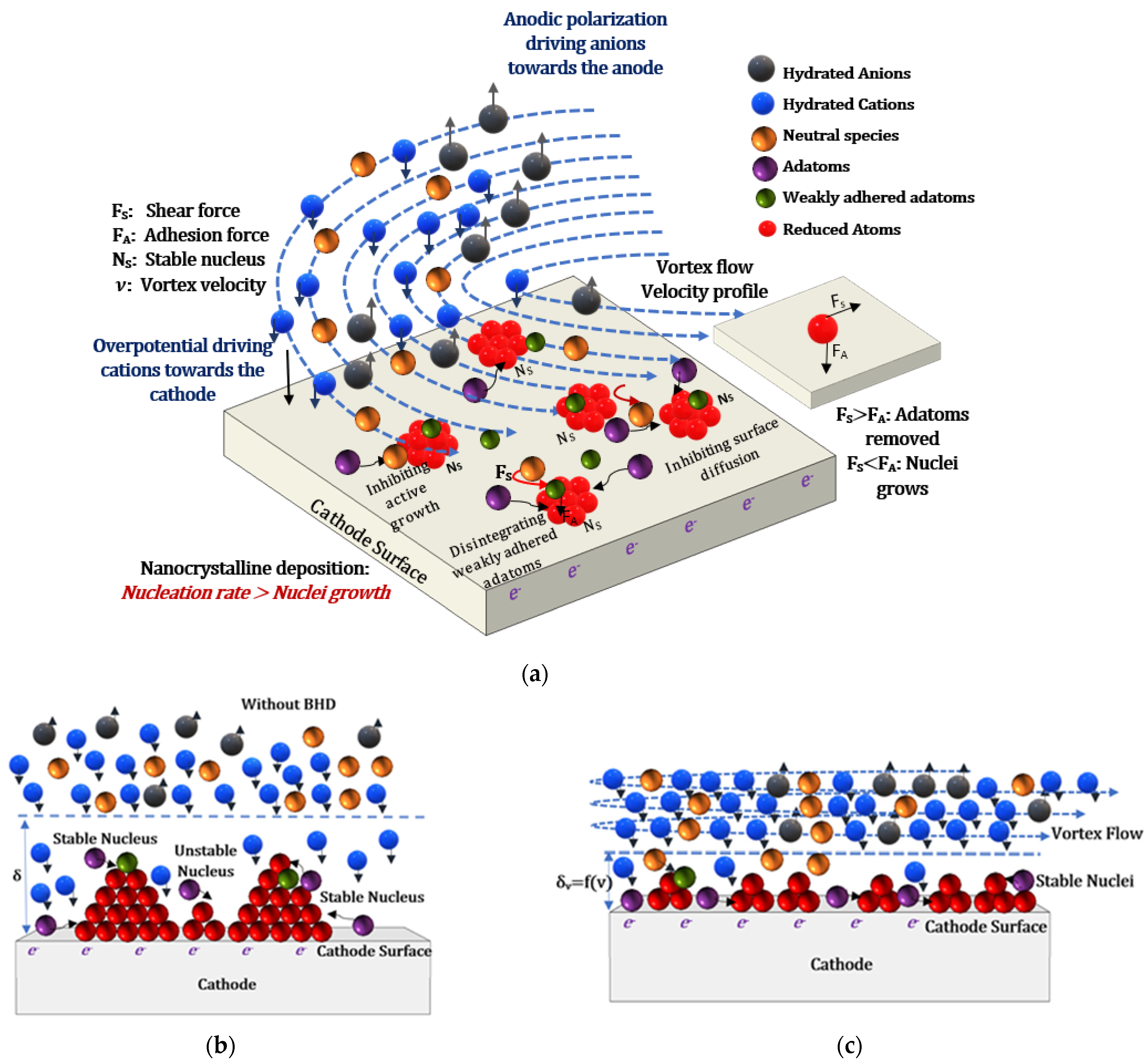
5.1. Electrochemical Effects of BHD on Nucleation and Growth Kinetics during ECD
5.2. Physical Effects of BHD on Nucleation and Growth Kinetics during ECD
6. Conclusions
- The convective velocity profile within the vortex created by magnetic stirrer has an inhomogeneous nature across the electrolytic cell, wherein the electrolytic flow region with a minimum variation occurs near the boundary of the electrolytic cell;
- The degree of anomaly dA, in the case of electrodeposited nickel-cobalt alloys, increased initially strongly as a function of increasing bath hydrodynamic velocity, i.e., from dA = 7.5 to dA = 15.7 for an increment in BHD velocity from 0 to 26 cm/s, and it only gradually increased from dA 15.7 to 17.2 for bath hydrodynamics beyond the critical velocity up to investigated maximum velocity of 42 cm/s. This finding is also a proof that the chosen BHD provides, by magnetic stirring, a suitable range of velocities to investigate the influence of BHD;
- The surface morphology of electrodeposited nickel-cobalt alloy and nickel coating samples changed from granular to more planar as a function of increasing bath hydrodynamic velocity, indicating the electrodeposition of fine grained and compact coatings;
- The AFM micrographs showed that the average surface roughness and the fractal dimension values decreased with increasing bath hydrodynamic velocity, i.e., it decreased from 207 nm (FD = 2.97) to 11 nm (FD = 2.15), and from 181 nm (FD = 2.91) to 33 nm (FD = 2.2) on increasing the velocity from 0 to 42 cm/s for nickel-cobalt and nickel coatings, respectively;
- The X-ray diffraction characterization of electrodeposited nickel-cobalt alloys and nickel coating samples revealed, firstly, the fcc nature of the coatings, and secondly, showed a peak broadening of the diffractograms as a function of increasing bath hydrodynamic velocity;
- The computed grain size using the Debye–Scherrer relation from the diffractograms decreased from 31 nm to 12 nm, and 69 nm to 26 nm as function of increasing bath hydrodynamic velocity (up to 42 cm/s) for nickel-cobalt and nickel coating samples, respectively;
- Consecutively, the microhardness increased by 76% (i.e., from 393 HV0.01 to 692 HV0.01), and by 49% (from 255 HV0.01 to 381 HV0.01) on increasing the convective velocity from 0 to 42 cm/s for nickel-cobalt and nickel coating samples, respectively, which fits well with the Hall–Petch relationship.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saile, V.; Wallrabe, U.; Tabata, O.; Korvink, J.G. Advanced Micro & Nanosystems Volume 7 LIGA and Its Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 3527314946. [Google Scholar]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780470167786. [Google Scholar]
- Ehrfeld, W. Electrochemistry and microsystems. Electrochim. Acta 2003, 48, 2857–2868. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Gilchrist, M.; Fang, F. Advances in precision micro/nano-electroforming: A state-of-the-art review. J. Micromech. Microeng. 2020, 30, 103002. [Google Scholar] [CrossRef]
- Malek, C.K.; Saile, V. Applications of LIGA technology to precision manufacturing of high-aspect-ratio micro-components and -systems: A review. Microelectron. J. 2004, 35, 131–143. [Google Scholar] [CrossRef]
- Genolet, G.; Lorenz, H. UV-LIGA: From Development to Commercialization. Micromachines 2014, 5, 486–495. [Google Scholar] [CrossRef]
- Weißhaar, K.; Weinmann, M.; Jung, A.; Weber, O.; Natter, H. Replication of microstructured tools for electrochemical machining applications. Int. J. Adv. Manuf. Technol. 2016, 82, 197–209. [Google Scholar] [CrossRef]
- Losey, M.W.; Kelly, J.J.; Badgayan, N.D.; Sahu, S.K.; Rama Sreekanth, P.S. Electrodeposition. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128035818. [Google Scholar]
- Karimzadeh, A.; Aliofkhazraei, M.; Walsh, F.C. A review of electrodeposited Ni-Co alloy and composite coatings: Microstructure, properties and applications. Surf. Coat. Technol. 2019, 372, 463–498. [Google Scholar] [CrossRef]
- Schmuki, P.; Virtanen, S. Electrochemistry at the Nanoscale; Springer: New York, NY, USA, 2009; ISBN 978-0-387-73581-8. [Google Scholar]
- Ahn, J.; Hong, S.; Shim, Y.-S.; Park, J. Electroplated Functional Materials with 3D Nanostructures Defined by Advanced Optical Lithography and Their Emerging Applications. Appl. Sci. 2020, 10, 8780. [Google Scholar] [CrossRef]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques and Applications, 2nd ed.; John Wiley & Sons Incorporated: Newark, NJ, USA, 2018; ISBN 9783527341405. [Google Scholar]
- Pan, C.T.; Wu, T.T.; Tsai, H.Y.; Chou, M.C.; Wu, T.C. Fabrication of a micro-punching head using LIGA and stacking processes. J. Micromech. Microeng. 2008, 18, 1–11. [Google Scholar] [CrossRef]
- Khazi, I.; Mescheder, U. Micromechanical properties of anomalously electrodeposited nanocrystalline Nickel-Cobalt alloys: A review. Mater. Res. Express 2019, 6, 82001. [Google Scholar] [CrossRef]
- Mbugua, N.S.; Kang, M.; Zhang, Y.; Ndiithi, N.J.; V Bertrand, G.; Yao, L. Electrochemical Deposition of Ni, NiCo Alloy and NiCo-Ceramic Composite Coatings-A Critical Review. Materials 2020, 13, 3475. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Tanhaei, M.; Ahmadipour, M.F.; Ghaffari Adli, R.; Mahdavi, S.; Walsh, F.C. Electrodeposited Ni-Co alloy-particle composite coatings: A comprehensive review. Surf. Coat. Technol. 2020, 382, 125153. [Google Scholar] [CrossRef]
- Omar, M.A. Electrodeposition of Ni-Co Film: A Review. Int. J. Electrochem. Sci. 2021, 150962. [Google Scholar] [CrossRef]
- Tian, L.; Li, L. A Review on the Strengthening of Nanostructured Materials. Int. J. Curr. Eng. Technol. 2018, 8. [Google Scholar] [CrossRef]
- Kong, J.; Sabatini, M.; Monaco, L.; Tam, J.; McCrea, J.L.; Palumbo, G.; Howe, J.; Erb, U. Characterization of a nanocrystalline NiCo electroformed sheet metal. J. Mater. Sci. 2021, 56, 1749–1767. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.-Y.; Chang, T.-F.M.; Luo, X.; Yamane, D.; Sone, M. Electrodeposition of Ni-Co Alloys and Their Mechanical Properties by Micro-Vickers Hardness Test. Electrochem 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Fundamentals of Materials Science: The Microstructure–Property Relationship Using Metals as Model Systems; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-10499-2. [Google Scholar]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Koch, C.C. Nanostructured Materials: Processing, Properties, and Potential Applications; Noyes Publications/William Andrew Pub: Norwich, NY, USA, 2002; ISBN 0815514514. [Google Scholar]
- Natter, H.; Hempelmann, R. Nanocrystalline Metals Prepared by Electrodeposition. Z. Phys. Chem. 2008, 222, 319–354. [Google Scholar] [CrossRef]
- Erb, U.; Aust, K.T.; Palumbo, G. 6 Electrodeposited Nanocrystalline Metals, Alloys, and Composites. In Nanostructured Materials Processing, Properties, and Applications, 2nd ed.; Noyes Publications/William Andrew Pub: Norwich, NY, USA, 2007; pp. 235–292. ISBN 9780815515340. [Google Scholar]
- Zangari, G. Electrodeposition of Alloys and Compounds in the Era of Microelectronics and Energy Conversion Technology. Coatings 2015, 5, 195–218. [Google Scholar] [CrossRef]
- Qiao, G.; Jing, T.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni–Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar] [CrossRef]
- Qiao, G.-Y.; Xiao, F.-R. Effects of grain size on the properties of bulk nanocrystalline Co–Ni alloys. Mater. Res. Express 2017, 4, 86512. [Google Scholar] [CrossRef]
- Qiao, G.; Jing, T.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. Effect of Current Density on Microstructure and Properties of Bulk Nanocrystalline Ni–Co Alloys Prepared by JED. J. Electrochem. Soc. 2006, 153, C305–C308. [Google Scholar] [CrossRef]
- Gomez, J.E.; Ramirez, E. Valles. Electrodeposition of Co-Ni alloys. J. Appl. Electrochem. 1998, 28, 71–79. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Nanocrystalline Ni–Co alloy coatings: Electrodeposition using horizontal electrodes and corrosion resistance. J. Coat Technol. Res. 2013, 10, 285–295. [Google Scholar] [CrossRef]
- Hu, X.; Qu, N. Improved Corrosion Resistance of Ni-Co Coatings Prepared by Electrodeposition with Large Centrifugal Acceleration. J. Materi. Eng. Perform. 2019, 28, 2104–2114. [Google Scholar] [CrossRef]
- Watanabe, T. Nano-Plating Microstructure Control Theory of Plated Film and Data Base of Plated Film Microstructure; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 0-08-044375-3. [Google Scholar]
- Hyde, M.E.; Compton, R.G. Theoretical and experimental aspects of electrodeposition under hydrodynamic conditions. J. Electroanal. Chem. 2005, 581, 224–230. [Google Scholar] [CrossRef]
- Kreysa, G.; Ota, K.; Robert, F. Savinell. Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; ISBN 978-1-4419-6995-8. [Google Scholar]
- Almeida, A.S.; Souza, V.C.B.d. An alternative method for measuring velocities in open-channel flows: Perfomance evaluation of a Pitot tube compared to an acoustic meter. RBRH 2017, 22, 2080. [Google Scholar] [CrossRef]
- Khazi, I.; Mescheder, U. Influence of electrochemical operating conditions on the micromechanical properties of electrodeposited Nickel-Cobalt alloys for fabrication of microtools. In Proceedings of the MikroSystemTechnik 2019 Congress 2019, Berlin, Germany, 28–30 October 2019; pp. 1–4. [Google Scholar]
- Sknar, Y.; Sknar, I.V.; Savchuk, O.O.; Danilov, F.I. Electrodeposition of Ni Co alloy from methansulfonate electrolyte. Role Electrolyte Ph Anomalous Codeposition Nickel Cobalt. Surf. Coat. Technol. 2020, 387, 125542. [Google Scholar] [CrossRef]
- Liu, P.; Chen, D.; Wang, Q.; Xu, P.; Long, M.; Duan, H. Crystal structure and mechanical properties of nickel–cobalt alloys with different compositions: A first-principles study. J. Phys. Chem. Solids 2020, 137, 109194. [Google Scholar] [CrossRef]
- Tebbakh, S.; Mentar, L.; Messaoudi, Y.; Khelladi, M.R.; Belhadj, H.; Azizi, A. Effect of cobalt content on electrodeposition and properties of Co–Ni alloy thin films. Inorg. Nano-Metal Chem. 2020, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xue, Q.; Liu, H.; Xu, T. Microstructure and tribological properties of electrodeposited Ni–Co alloy deposits. Appl. Surf. Sci. 2005, 242, 326–332. [Google Scholar] [CrossRef]
- Wu, B.Y.C.; Ferreira, P.J.; Schuh, C.A. Nanostructured Ni-Co Alloys with Tailorable Grain Size and Twin Density. Metall. Mater. Trans. A 2005, 36, 1927–1936. [Google Scholar] [CrossRef]
- Bigos, A.; Wolowicz, M.; Janusz-Skuza, M.; Starowicz, Z.; Szczerba, M.J.; Bogucki, R.; Beltowska-Lehman, E. Citrate-based baths for electrodeposition of nanocrystalline nickel coatings with enhanced hardness. J. Alloys Compd. 2021, 850, 156857. [Google Scholar] [CrossRef]
- Chung, C.-K.; Chang, W.-T. Electrochemical Deposition and Mechanical Property Enhancement of the Nickel and Nickel-Cobalt Films. In Handbook of Manufacturing Engineering and Technology; Nee, A.Y.C., Ed.; Springer: London, UK, 2015; pp. 2891–2927. ISBN 978-1-4471-4669-8. [Google Scholar]
- Gilman, J.J. Chemistry and Physics of Mechanical Hardness; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-22652-0. [Google Scholar]
- Hussain, M.S. Synthesis of Bulk Nanostructured Materials by High Speed Turbulent Flow—A Method of Electrodepositing Nanocrystalline Nickel. In Nanomaterials in Chromatography; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–88. ISBN 9780128127926. [Google Scholar]
- Hussain, M.S. Direct Ni-Co alloy plating of titanium alloy surfaces by high speed electrodeposition. Trans. IMF. 2012, 90, 15–19. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-108255-9. [Google Scholar]
- Dolati, A.; Sababi, M.; Nouri, E.; Ghorbani, M. A study on the kinetic of the electrodeposited Co–Ni alloy thin films in sulfate solution. Mater. Chem. Phys. 2007, 102, 118–124. [Google Scholar] [CrossRef]
- Phillips, V.R. Specific gravity, viscosity, and solubility for aqueous nickel sulfate solutions. J. Chem. Eng. Data 1972, 17. [Google Scholar] [CrossRef]
- Jeong, M.; Yokoshima, T.; Nara, H.; Momma, T.; Osaka, T. Influence of the diffusion-layer thickness during electrodeposition on the synthesis of nano core/shell Sn–O–C composite as an anode of lithium secondary batteries. RSC Adv. 2014, 4, 26872–26880. [Google Scholar] [CrossRef]
- Winand, R. Electrocrystallization—Theory and applications. Hydrometallurgy 1992, 29, 567–598. [Google Scholar] [CrossRef]
- Harniman, R.L.; Plana, D.; Carter, G.H.; Bradley, K.A.; Miles, M.J.; Fermín, D.J. Real-time tracking of metal nucleation via local perturbation of hydration layers. Nat. Commun. 2017, 8, 971. [Google Scholar] [CrossRef]
- Kanani, N. Electroplating Basic Principles, Processes and Practice; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 1856174514. [Google Scholar]
- Nagy, K.S.; Kazemiabnavi, S.; Thornton, K.; Siegel, D.J. Thermodynamic Overpotentials and Nucleation Rates for Electrodeposition on Metal Anodes. ACS Appl. Mater. Interfaces 2019, 11, 7954–7964. [Google Scholar] [CrossRef]



| Electrolyte Constituents and ECD Operating Conditions | |
|---|---|
| Ni(NH2SO3)2.6H2O | 1.8 (mol/L) |
| Co(NH2SO3)2.6H2O | 0.03 (mol/L) |
| H3BO3 | 0.5 (mol/L) |
| Deionized H2O | 14 (mol/L) |
| Bath pH value | 4 |
| Temperature | 45 ± 2 °C |
| Current Density | 50 mA/cm2 |
| Sample | v (cm/s) | EWE (V) | t (µm) | CoA (wt%) | dA | Ra (nm) | FD | XRD FWHM (2θ°) | d (nm) | H (HV0.01) |
|---|---|---|---|---|---|---|---|---|---|---|
| eNiCo alloy coatings | 0 | −0.89 ± 0.016 | 13.5 ± 0.9 | 15.1 | 7.5 | 207.5 ± 9.8 | 2.97 | 0.29 | 31.83 | 393 ± 12 |
| 8.8 | −0.76 ± 0.004 | 13.6 ± 1.1 | 21.6 | 10.8 | 197 ± 7 | 2.89 | 0.37 | 24.95 | 462 ± 18 | |
| 18 | −0.66 ± 0.005 | 12.4 ± 0.8 | 27.8 | 13.9 | 138.6 ± 8.5 | 2.77 | 0.66 | 13.98 | 570 ± 11.5 | |
| 26 | −0.61 ± 0.013 | 12.8 ± 1.3 | 31.4 | 15.7 | 31.3 ± 6.7 | 2.65 | 0.68 | 13.57 | 600 ± 17.8 | |
| 34 | −0.59 ± 0.010 | 12.6 ± 1.4 | 31.3 | 15.6 | 23.9 ± 3.9 | 2.21 | 0.66 | 13.98 | 613 ± 9.7 | |
| 42 | −0.57 ± 0.006 | 12.8 ± 1.4 | 34.4 | 17.2 | 11.1 ± 1.65 | 2.15 | 0.76 | 12.14 | 692 ± 21 | |
| eNi coatings | 0 | −1.27 ± 0.016 | 44.8 ± 0.9 | - | - | 181.7 ± 8.7 | 2.91 | 0.13 | 68.95 | 255 ± 13.8 |
| 12 | −1.08 ± 0.02 | 42.2 ± 1.1 | - | - | 157.5 ± 7.7 | 2.68 | 0.18 | 50.21 | 287 ± 17 | |
| 22 | −1.05 ± 0.02 | 42.7 ± 1.3 | - | - | 114 ± 6.9 | 2.54 | 0.28 | 31.42 | 315 ± 22 | |
| 32 | −0.93 ± 0.016 | 41.4 ± 0.8 | - | - | 51 ± 7.4 | 2.4 | 0.29 | 31.19 | 325 ± 17.8 | |
| 42 | −0.92 ± 0.01 | 42.6 ± 0.8 | - | - | 33.4 ± 1.9 | 2.2 | 0.34 | 26.19 | 381 ± 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazi, I.; Mescheder, U.; Wilde, J. Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys. Materials 2021, 14, 3898. https://doi.org/10.3390/ma14143898
Khazi I, Mescheder U, Wilde J. Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys. Materials. 2021; 14(14):3898. https://doi.org/10.3390/ma14143898
Chicago/Turabian StyleKhazi, Isman, Ulrich Mescheder, and Jürgen Wilde. 2021. "Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys" Materials 14, no. 14: 3898. https://doi.org/10.3390/ma14143898
APA StyleKhazi, I., Mescheder, U., & Wilde, J. (2021). Influence of Bath Hydrodynamics on the Micromechanical Properties of Electrodeposited Nickel-Cobalt Alloys. Materials, 14(14), 3898. https://doi.org/10.3390/ma14143898







