Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs
Abstract
:1. Introduction
2. Materials and Methods
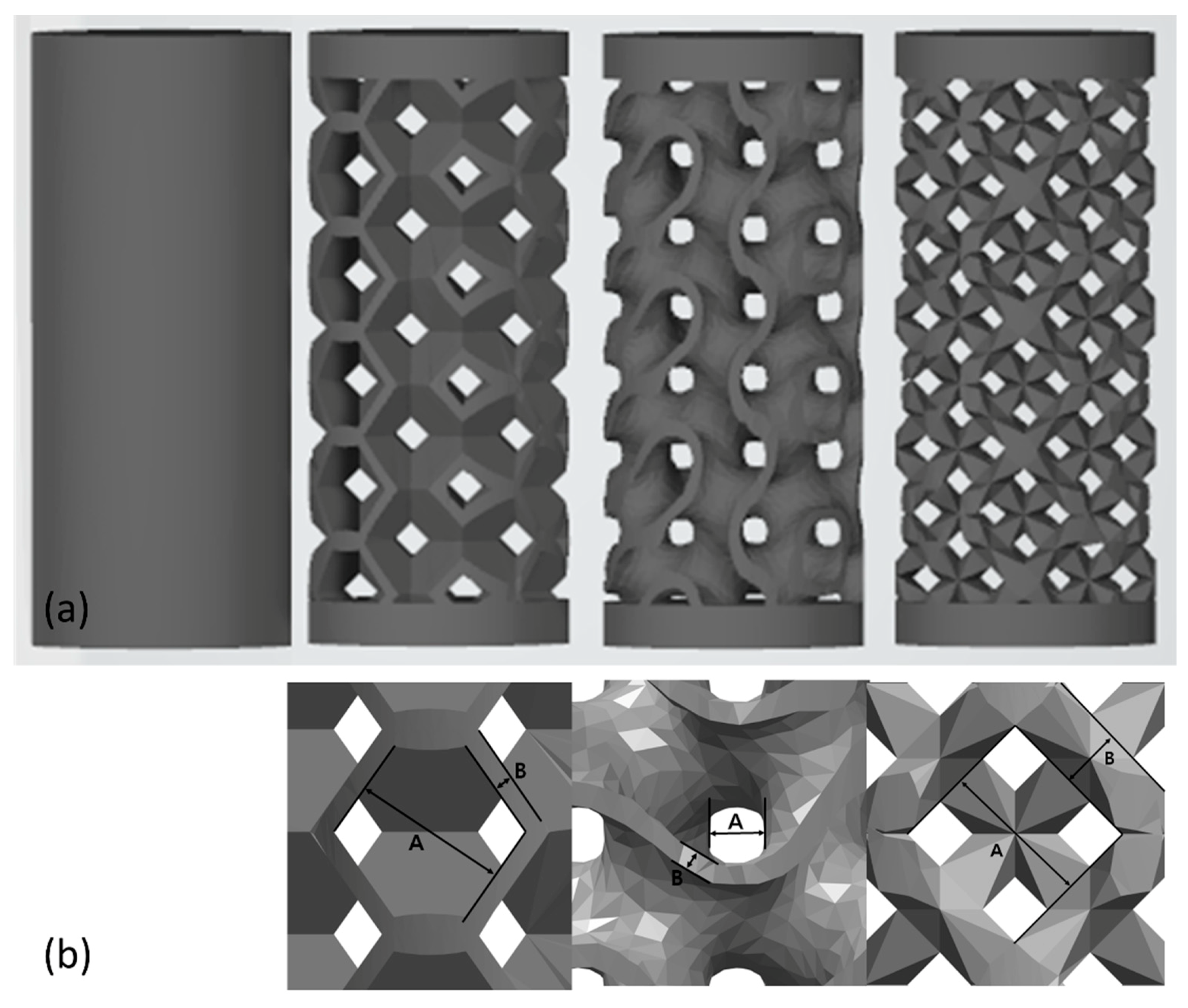
2.1. Selection of the Pore Design
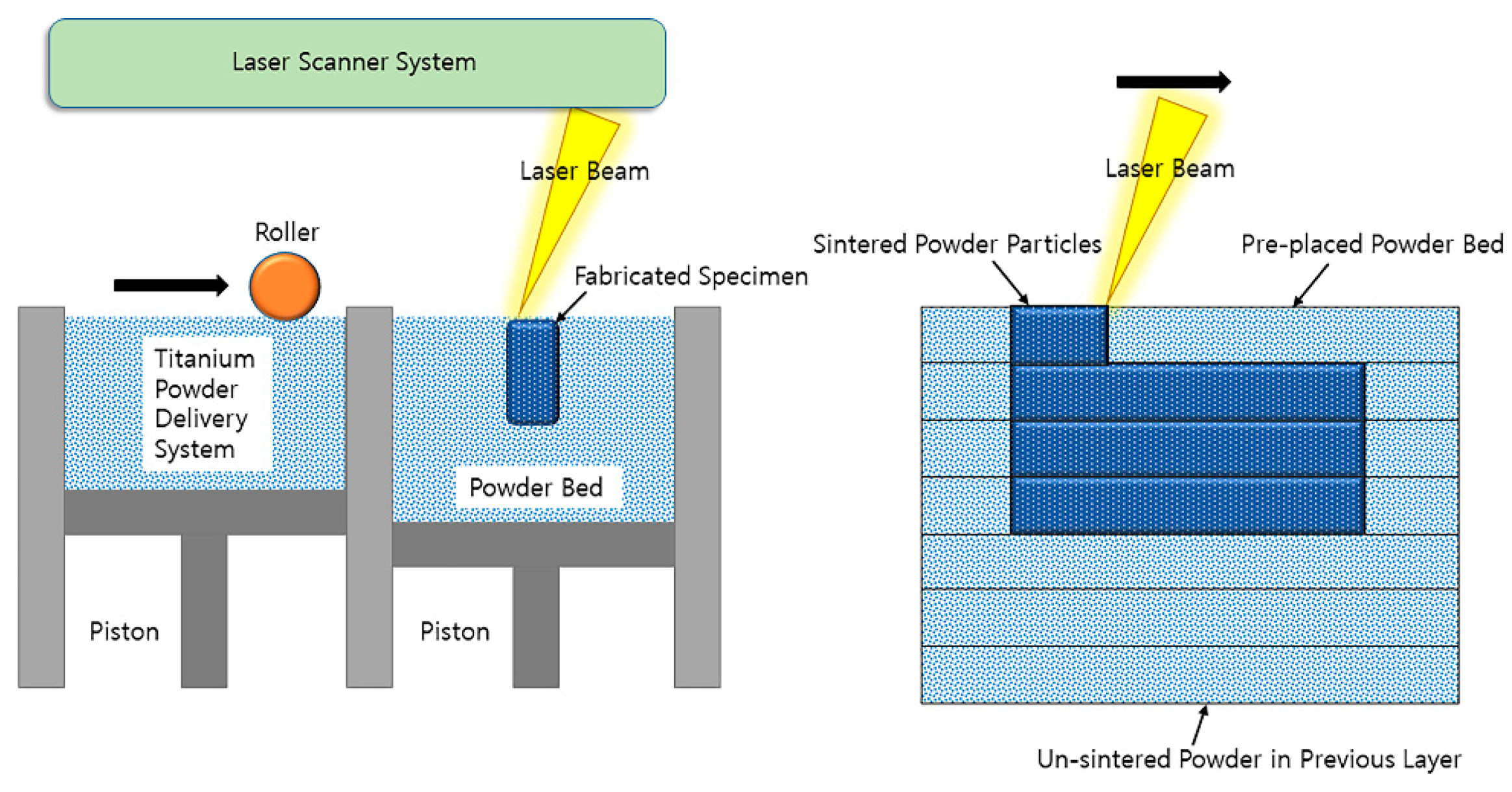
2.2. Manufacture of 3D-Printed Titanium Specimens
2.3. Animal Experiments
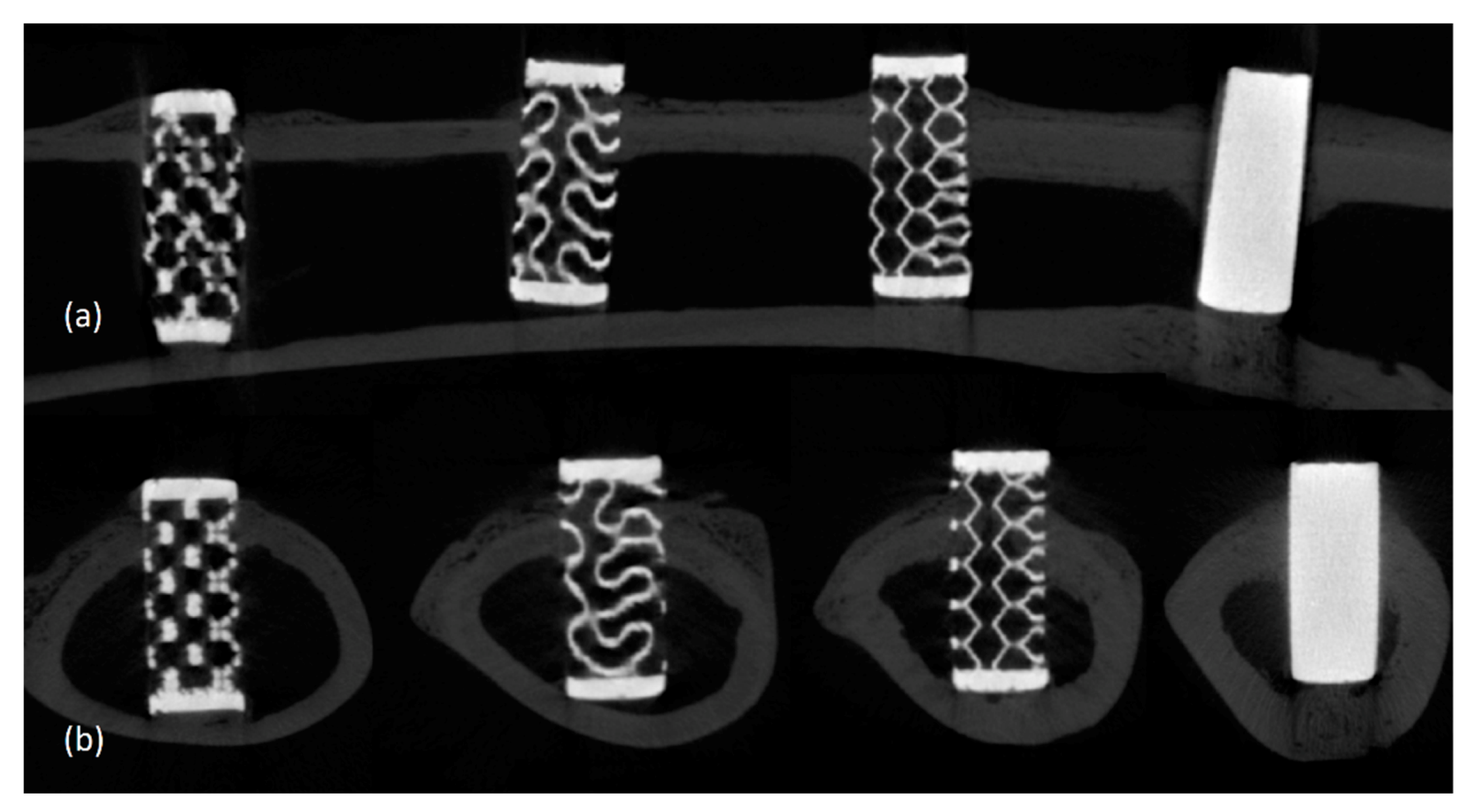
2.4. Micro-CT Imaging and Volumetric Analysis
2.5. Histological Findings
2.6. Statistical Interpretation
3. Results
3.1. Clinical Findings in the Animal Experiment
3.2. Radiological Findings
3.3. Histological Findings
3.4. Statistical Interpretation of Bone Conduction Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rotaru, H.; Schumacher, R.; Kim, S.G.; Dinu, C. Selective laser melted titanium implants: A new technique for the reconstruction of extensive zygomatic complex defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Y.; El-Amin, S.F.; Laurencin, C. In vitro and in vivo evaluation of a novel polymer-ceramic composite scaffold for bone tissue engineering. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 529–530. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murr, L.E. Open-cellular metal implant design and fabrication for biomechanical compatibility with bone using electron beam melting. J. Mech. Behav. Biomed. Mater. 2017, 76, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.W.; Kim, M.Y.; Han, S.J. Cranial bone regeneration according to different particle sizes and densities of demineralized dentin matrix in the rabbit model. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.D.; Yook, S.W.; Jang, T.S.; Li, Y.; Kim, H.E.; Koh, Y.H. Dynamic freeze casting for the production of porous titanium (Ti) scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Lascano, S.; Bris, J.; Pavon, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 148–155. [Google Scholar] [CrossRef]
- Yan, L.; Wu, J.; Zhang, L.; Liu, X.; Zhou, K.; Su, B. Pore structures and mechanical properties of porous titanium scaffolds by bidirectional freeze casting. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar] [CrossRef]
- Kujala, S.; Ryhanen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W. Geometry as a factor for tissue growth: Towards shape optimization of tissue engineering scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rudrich, U.; Lasgorceix, M.; Champion, E.; Pascaud-Mathieu, P.; Damia, C.; Chartier, T.; Brie, J.; Magnaudeix, A. Pre-osteoblast cell colonization of porous silicon substituted hydroxyapatite bioceramics: Influence of microporosity and macropore design. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, L.M.; Leite, D.D.; Nascimento, F.O.; de Vasconcellos, L.G.; Graca, M.L.; Carvalho, Y.R.; Cairo, C.A. Porous titanium for biomedical applications: An experimental study on rabbits. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e407–e412. [Google Scholar] [CrossRef] [PubMed]
- do Prado, R.F.; Esteves, G.C.; Santos, E.L.S.; Bueno, D.A.G.; Cairo, C.A.A.; Vasconcellos, L.G.O.; Sagnori, R.S.; Tessarin, F.B.P.; Oliveira, F.E.; Oliveira, L.D.; et al. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 2018, 13, e0196169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinl, P.; Muller, L.; Korner, C.; Singer, R.F.; Muller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Benzing, J.; Hrabe, N.; Quinn, T.; White, R.; Rentz, R.; Ahlfors, M. Hot isostatic pressing (HIP) to achieve isotropic microstructure and retain as-built strength in an additive manufacturing titanium alloy (Ti-6Al-4V). Mater. Lett. 2019, 257. [Google Scholar] [CrossRef]
- Melli, V.; Juszczyk, M.; Sandrini, E.; Bolelli, G.; Bonferroni, B.; Lusvarghi, L.; Cigada, A.; Manfredini, T.; De Nardo, L. Tribological and mechanical performance evaluation of metal prosthesis components manufactured via metal injection molding. J. Mater. Sci. Mater. Med. 2015, 26, 5332. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Asaoka, K.; Kon, M.; Matsubara, T.; Yoshida, K. Chemical surface modification of high-strength porous Ti compacts by spark plasma sintering. Bio-Med. Mater. Eng. 2006, 16, 83–91. [Google Scholar]
- Prananingrum, W.; Naito, Y.; Galli, S.; Bae, J.; Sekine, K.; Hamada, K.; Tomotake, Y.; Wennerberg, A.; Jimbo, R.; Ichikawa, T. Bone ingrowth of various porous titanium scaffolds produced by a moldless and space holder technique: An in vivo study in rabbits. Biomed. Mater. 2016, 11, 015012. [Google Scholar] [CrossRef]
- Likibi, F.; Assad, M.; Coillard, C.; Chabot, G.; Rivard, C.H. [Bone integration and apposition of porous and non porous metallic orthopaedic biomaterials]. Ann. Chir. 2005, 130, 235–241. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T.; Mabuchi, M. Processing and mechanical properties of autogenous titanium implant materials. J. Mater. Sci. Mater. Med. 2002, 13, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wu, L.; Zhou, C.; Fan, H.; Gou, M.; Li, Z.; Zhang, B.; Lei, H.; Sun, H.; Liang, J.; et al. 3D printed titanium scaffolds with homogeneous diamond-like structures mimicking that of the osteocyte microenvironment and its bone regeneration study. Biofabrication 2020. [Google Scholar] [CrossRef]
- de Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Kruse Gujer, A.; Gratz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C.; et al. Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J. Mater. Chem B 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Hart, C.N.; Halbritter, S.A.; Morton, D.; Buser, D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin. Implant. Dent. Relat Res. 2009, 11, 338–347. [Google Scholar] [CrossRef]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.; Liu, L.; Li, Z.; Liu, J. 3D printed Ti6Al4V bone scaffolds with different pore structure effects on bone ingrowth. J. Biol Eng. 2021, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, Y.; Ao, Y.; Han, C.; Wang, Q.; Li, Y.; Liu, J.; Wei, Q.; Zhang, Z. Effect of pore geometry on the fatigue properties and cell affinity of porous titanium scaffolds fabricated by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 88, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, X.; Yao, Y.; Wei, Y.; Han, C.; Shi, Y.; Wei, Q.; Zhang, Z. Porous niobium coatings fabricated with selective laser melting on titanium substrates: Preparation, characterization, and cell behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 50–59. [Google Scholar] [CrossRef]
- Ishimoto, T.; Kawahara, K.; Matsugaki, A.; Kamioka, H.; Nakano, T. Quantitative Evaluation of Osteocyte Morphology and Bone Anisotropic Extracellular Matrix in Rat Femur. Calcif. Tissue Int. 2021. [Google Scholar] [CrossRef]
- Ishimoto, T.; Yamada, K.; Takahashi, H.; Takahata, M.; Ito, M.; Hanawa, T.; Nakano, T. Trabecular health of vertebrae based on anisotropy in trabecular architecture and collagen/apatite micro-arrangement after implantation of intervertebral fusion cages in the sheep spine. Bone 2018, 108, 25–33. [Google Scholar] [CrossRef]


| Octadense | Gyroid | Dode | p-Value | |
|---|---|---|---|---|
| 2 weeks | 24.888 ± 0.872 | 25.069 ± 1.259 | 24.990 ± 2.715 | 0.957 |
| 4 weeks | 27.874 ± 2.184 | 25.171 ± 1.656 | 26.527 ± 2.311 | 0.491 |
| 6 weeks | 26.835 ± 2.078 | 27.591 ± 1.719 | 27.433 ± 4.143 | 0.733 |
| p-value | 0.252 | 0.193 | 0.670 |
| Octadense | Gyroid | Dode | p-Value | |
|---|---|---|---|---|
| 2 weeks | 8.073 ± 0.170 | 8.100 ± 0.055 | 8.278 ± 0.205 | 0.393 |
| 4 weeks | 8.117 ± 0.399 | 7.727 ± 0.081 | 8.263 ± 0.127 | 0.099 |
| 6 weeks | 7.765 ± 0.181 | 7.746 ± 0.341 | 7.989 ± 0.084 | 0.587 |
| p-value | 0.193 | 0.301 | 0.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-K.; Ryu, M.; Woo, S.-H.; Song, I.-S.; Choi, Y.-J.; Lee, U.-L. Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs. Materials 2021, 14, 3892. https://doi.org/10.3390/ma14143892
Lim H-K, Ryu M, Woo S-H, Song I-S, Choi Y-J, Lee U-L. Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs. Materials. 2021; 14(14):3892. https://doi.org/10.3390/ma14143892
Chicago/Turabian StyleLim, Ho-Kyung, Miyoung Ryu, Su-Heon Woo, In-Seok Song, Young-Jun Choi, and Ui-Lyong Lee. 2021. "Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs" Materials 14, no. 14: 3892. https://doi.org/10.3390/ma14143892
APA StyleLim, H.-K., Ryu, M., Woo, S.-H., Song, I.-S., Choi, Y.-J., & Lee, U.-L. (2021). Bone Conduction Capacity of Highly Porous 3D-Printed Titanium Scaffolds Based on Different Pore Designs. Materials, 14(14), 3892. https://doi.org/10.3390/ma14143892







