Characteristics of Graphite Felt Electrodes Treated by Atmospheric Pressure Plasma Jets for an All-Vanadium Redox Flow Battery
Abstract
:1. Introduction
2. Materials and Methods
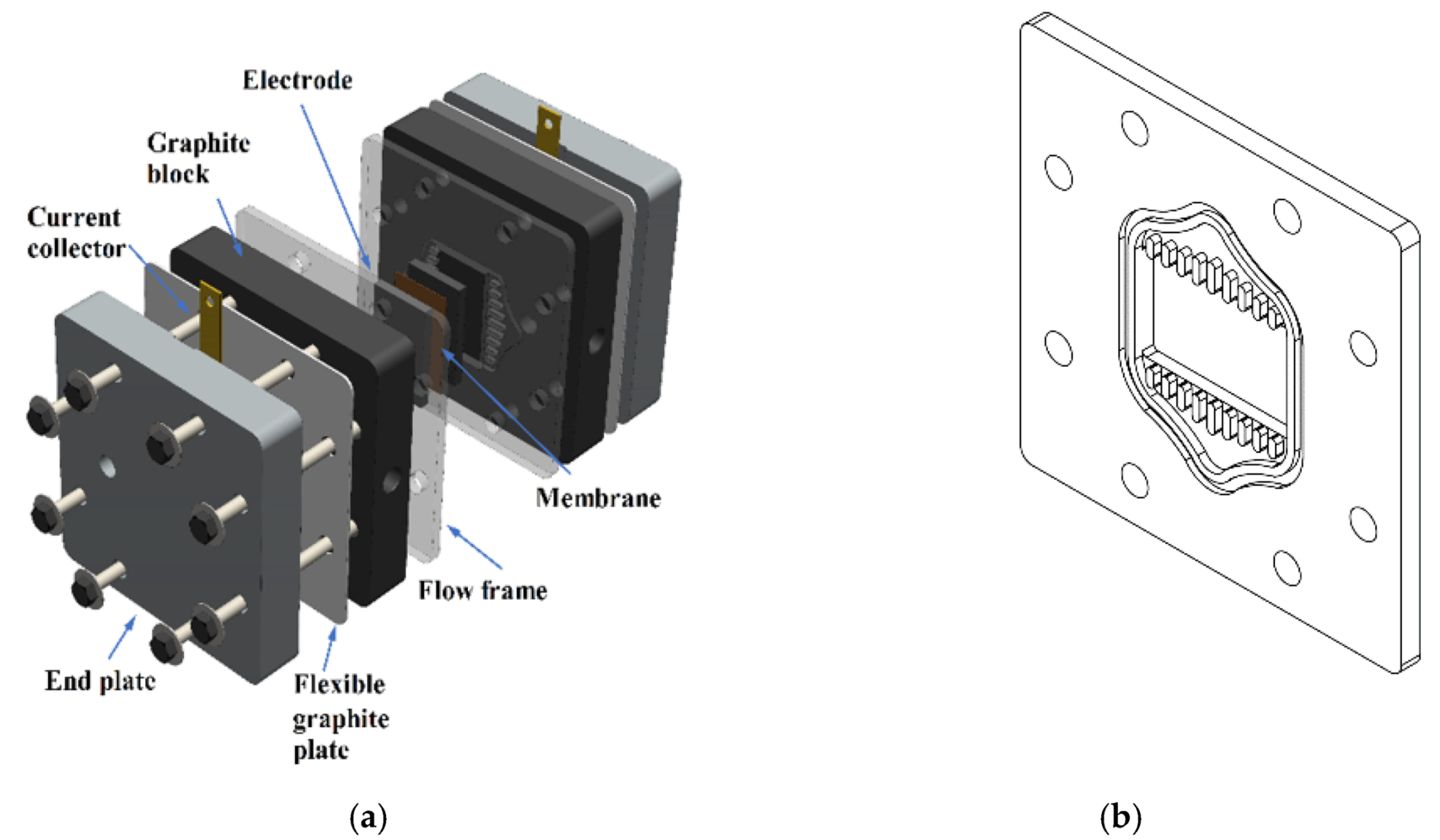
2.1. Preparation of a VRFB
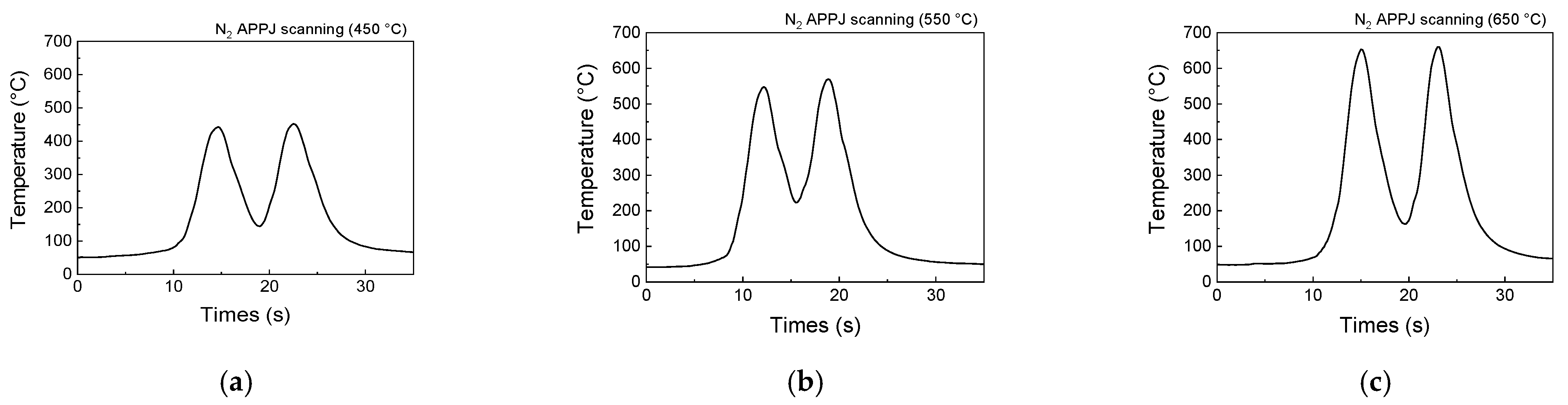
2.2. Experimental Procedure
3. Results and Discussion
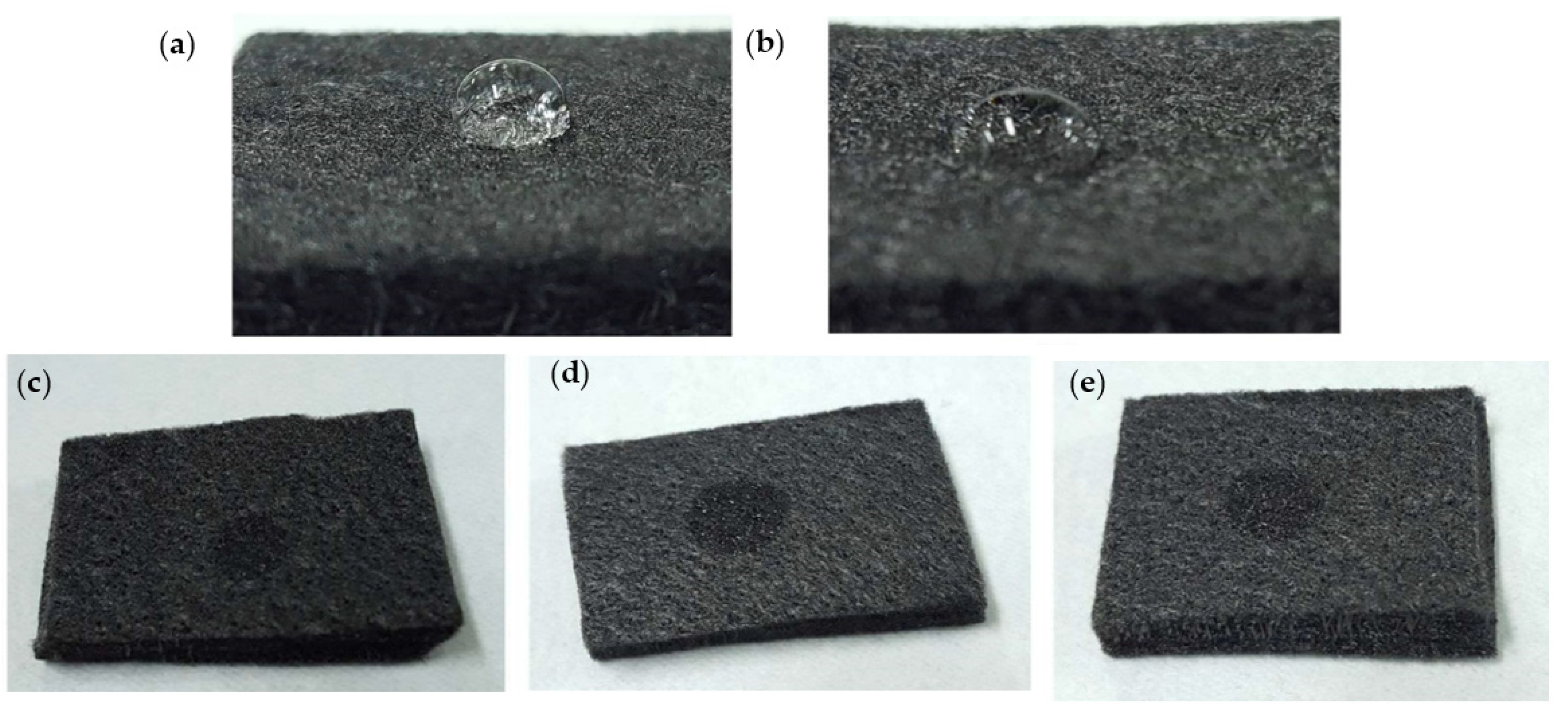
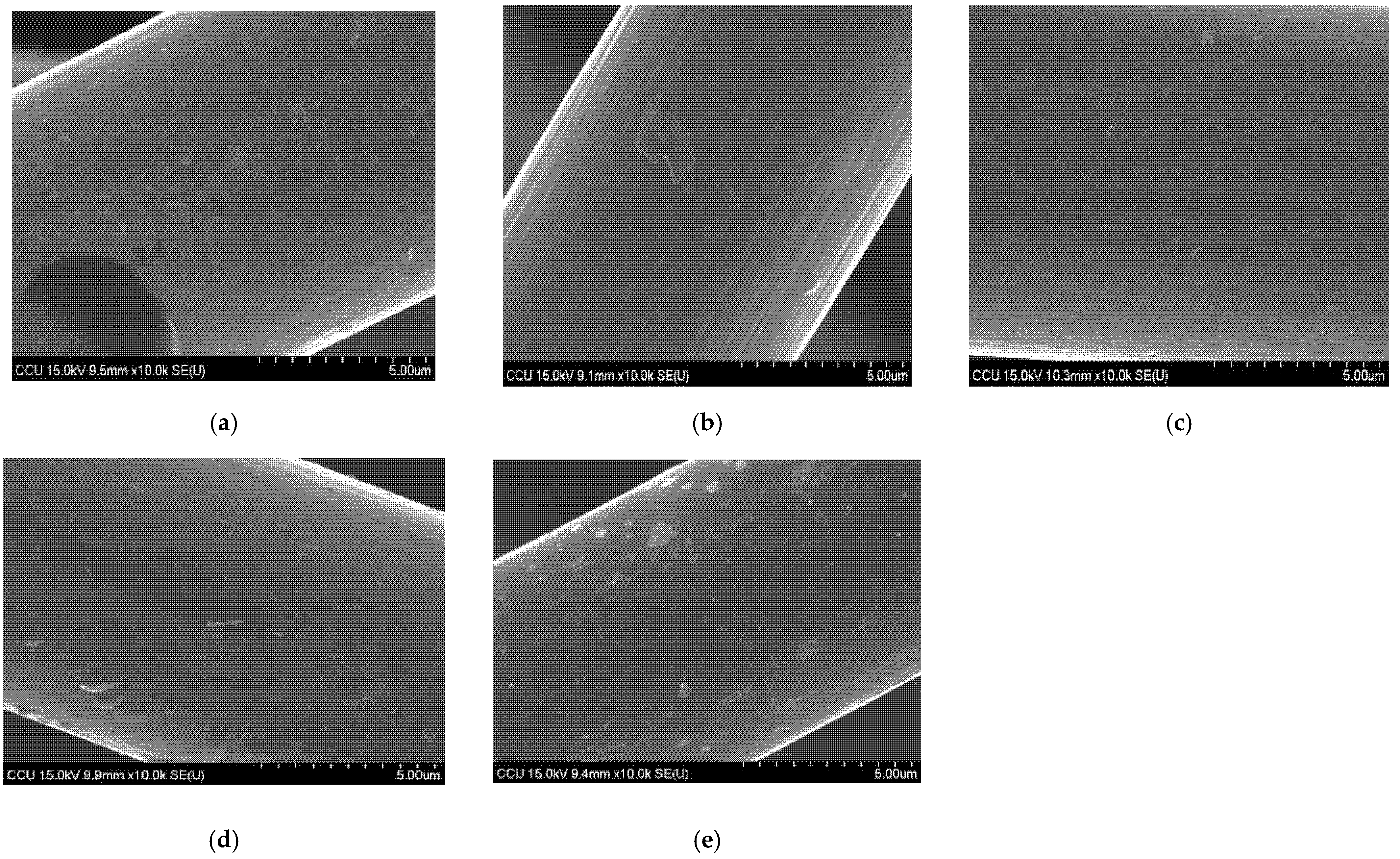
3.1. Characteristics of GF Electrodes
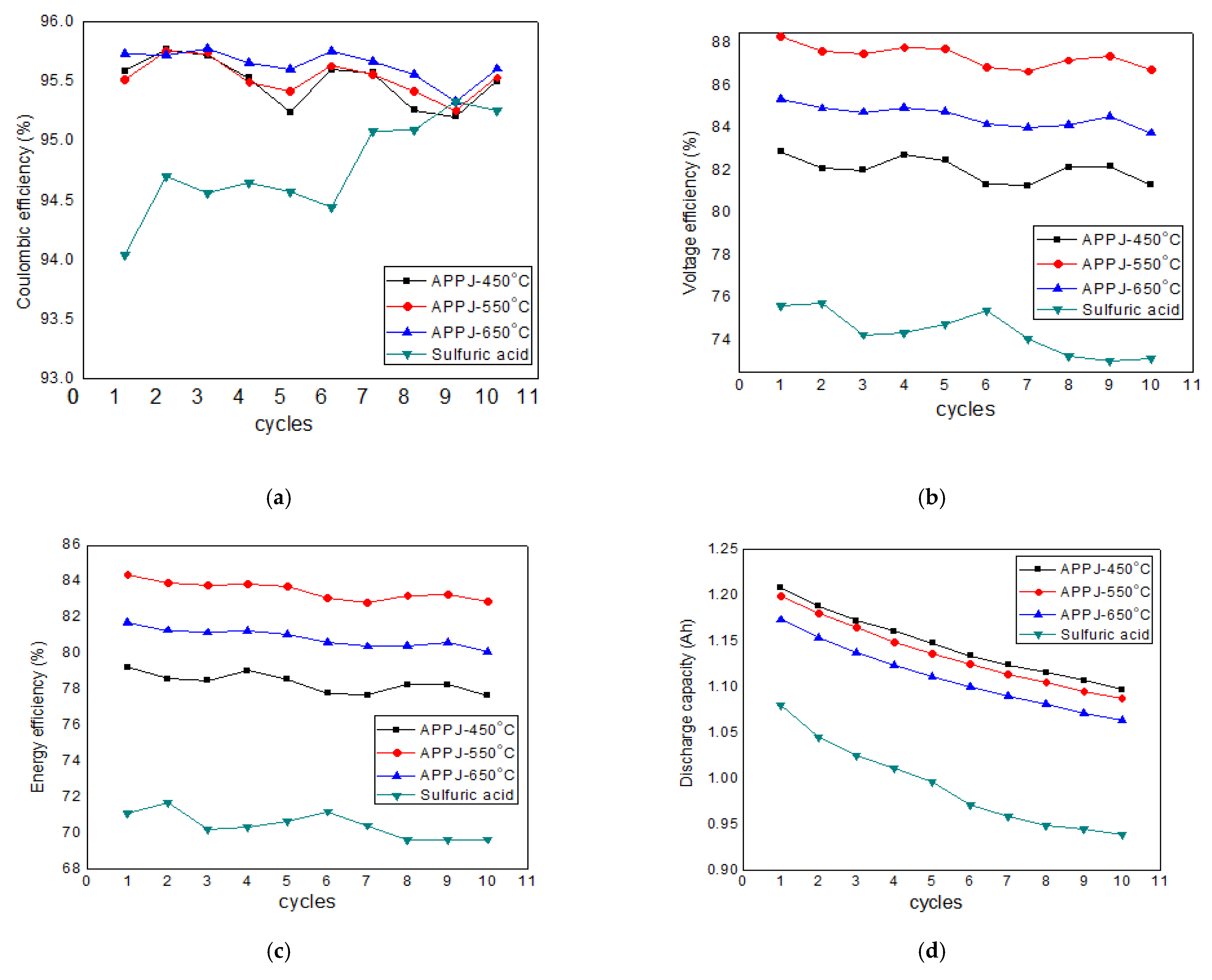
3.2. Effect of Treatment Methods on Efficiency and Discharge Capacity
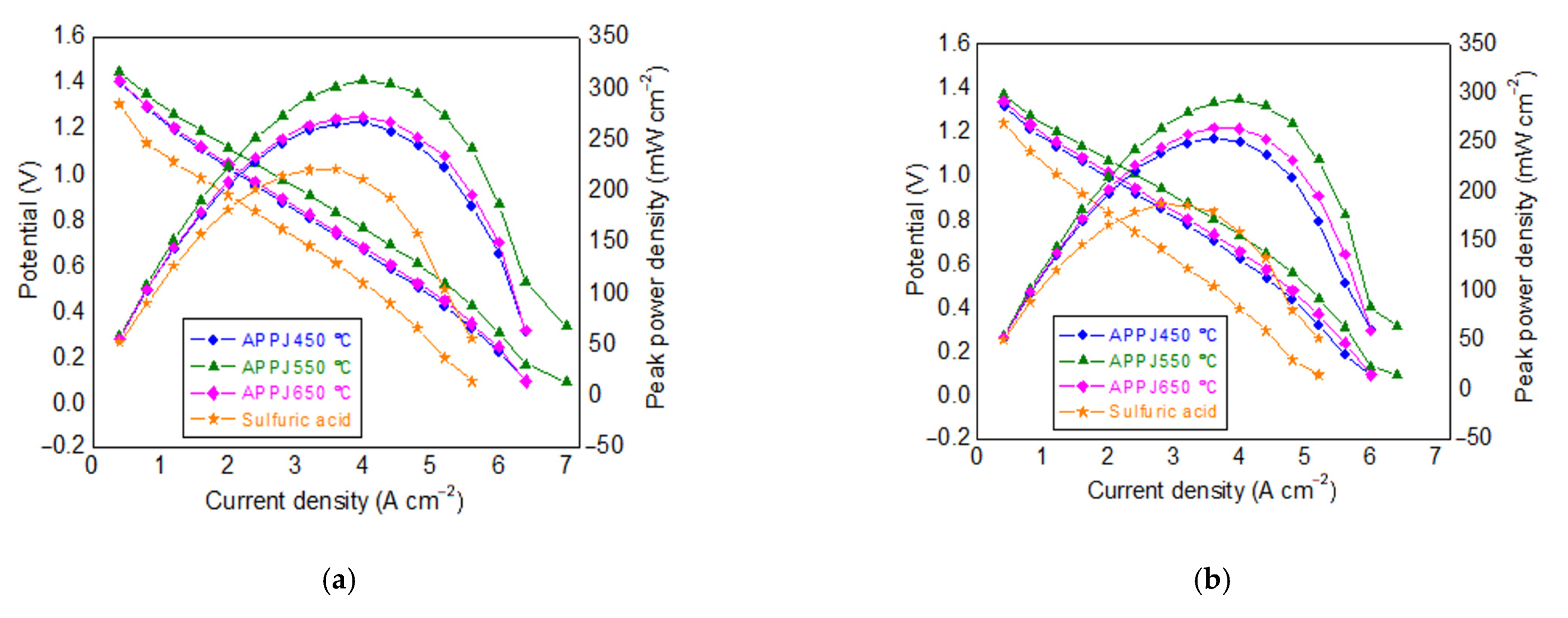
3.3. Effect of Treatment Methods on Limiting Current Density
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Badrinarayanan, R.; Tseng, K.J.; Soong, B.H.; Wei, Z. Modelling and control of vanadium redox flow battery for profile based charging applications. Energy 2017, 141, 1479–1488. [Google Scholar] [CrossRef]
- Wei, Z.; Meng, S.; Tseng, K.J.; Lim, T.M.; Soong, B.H.; Skyllas-Kazacos, M. An adaptive model for vanadium redox flow battery and its application for online peak power estimation. J. Power Sources 2017, 344, 195–207. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhang, J. (Eds.) Redox Flow Batteries: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Weber, S.; Peters, J.F.; Baumann, M.; Weil, M. Life Cycle assessment of a vanadium redox flow battery. Environ. Sci. Technol. 2018, 52, 10864–10873. [Google Scholar] [CrossRef]
- Gencten, M.; Sahin, Y. A critical review on progress of the electrode materials of vanadium redox flow battery. Inter. J. Energy Res. 2020, 44, 7903–7923. [Google Scholar] [CrossRef]
- Satola, B. Review—Bipolar plates for the vanadium redox flow battery. J. Electrochem. Soc. 2021, 168, 060503. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Platt, A.; Zhao, N.; Wang, H.; Li, H.; Fatih, K.; Jang, D. A review of all-vanadium redox flow battery durability: Degradation mechanisms and mitigation strategies. Inter. J. Energy Res. 2019, 43, 6599–6638. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium redox flow batteries: A review oriented to fluid-dynamic optimization. Energies 2021, 14, 176. [Google Scholar] [CrossRef]
- Schnucklake, M.; Cheng, M.; Maleki, M.; Roth, C. A mini-review on decorating, templating of commercial and electrospinning of new porous carbon electrodes for vanadium redox flow batteries. J. Phys. Mater. 2021, 4, 032007. [Google Scholar] [CrossRef]
- Su, X.; Yang, L.; Zhou, Y.; Lin, Y.; Yu, S. Developments of electrodes for vanadium redox flow battery. Energy Storage Sci. Tech. 2019, 8, 65–74. [Google Scholar]
- Chang, T.-C.; Zhang, J.-P.; Fuh, Y.-K. Electrical, mechanical and morphological properties of compressed carbon felt electrodes in vanadium redox flow battery. J. Power Sources 2014, 245, 66–75. [Google Scholar] [CrossRef]
- Park, S.-K.; Shim, J.; Yang, J.H.; Jin, C.-S.; Lee, B.S.; Lee, Y.-S.; Shin, K.-H.; Jeon, J.-D. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.G.; Jiang, Z.Y.; Yang, W.W. Experimental study on the performance of a vanadium redox flow battery with non-uniformly compressed carbon felt electrode. App. Energy 2018, 213, 293–305. [Google Scholar] [CrossRef]
- Ghimire, P.C.; Bhattarai, A.; Schweiss, R.; Scherer, G.G.; Wai, N.; Yan, Q. A comprehensive study of electrode compression effects in all vanadium redox flow batteries including locally resolved measurements. App. Energy 2018, 230, 974–982. [Google Scholar]
- Hsieh, C.-L.; Tsai, P.-H.; Hsu, N.-Y.; Chen, Y.-S. Effect of compression ratio of graphite felts on the performance of an all-vanadium redox flow battery. Energies 2019, 12, 313. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Shen, Y.; Xi, J.; Qiu, X.; Chen, L. ZrO2-nanoparticle-modified graphite felt: Bifunctional effects on vanadium flow batteries. ACS Appl. Mater. Interfaces 2016, 8, 15369–15378. [Google Scholar] [CrossRef]
- Hu, G.; Jing, M.; Wang, D.-W.; Sun, Z.; Xu, C.; Ren, W.; Cheng, H.-M.; Yan, C.; Fan, X.; Li, F. A gradient bi-functional graphene-based modified electrode for vanadium redox flow batteries. Energy Storage Mater. 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Pezeshki, A.M.; Clement, J.T.; Veith, G.M.; Zawodzinski, T.A.; Mench, M.M. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation. J. Power Sources 2015, 294, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-Z.; Liao, W.-Y.; Hsieh, W.-Y.; Hsu, C.-C.; Chen, Y.-S. All-vanadium redox flow batteries with graphite felt electrodes treated by atmospheric pressure plasma jets. J. Power Sources 2015, 274, 894–898. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: A review. Food Eng. Rev. 2014, 7, 82–108. [Google Scholar] [CrossRef]
- Lee, C.-C.; Wan, T.-H.; Hsu, C.-C.; Cheng, I.C.; Chen, J.-Z. Atmospheric-pressure plasma jet processed Pt/ZnO composites and its application as counter-electrodes for dye-sensitized solar cells. Appl. Surf. Sci. 2018, 436, 690–696. [Google Scholar] [CrossRef]
- Kusano, Y. Atmospheric pressure plasma processing for polymer adhesion: A review. J. Adhes. 2014, 90, 755–777. [Google Scholar] [CrossRef]
- Wu, T.-J.; Chou, C.-Y.; Hsu, C.-M.; Hsu, C.-C.; Chen, J.-Z.; Cheng, I.C. Ultrafast synthesis of continuous Au thin films from chloroauric acid solution using an atmospheric pressure plasma jet. RSC Adv. 2015, 5, 99654–99657. [Google Scholar]
- Wang, T.; Moon, S.J.; Hwang, D.-S.; Park, H.; Lee, J.; Kim, S.; Lee, Y.M.; Kim, S. Selective ion transport for a vanadium redox flow battery (VRFB) in nano-crack regulated proton exchange membranes. J. Membr. Sci. 2019, 583, 16–22. [Google Scholar] [CrossRef]
- Lin, C.-H.; Zhuang, Y.-D.; Tsai, D.-G.; Wei, H.-J.; Liu, T.-Y. Performance enhancement of vanadium redox flow battery by treated carbon felt electrodes of polyacrylonitrile using atmospheric pressure plasma. Polymers 2020, 12, 1372. [Google Scholar]
- Chen, S.-Y.; Kuo, Y.-L.; Wang, Y.-M.; Hsu, W.-M.; Chien, T.-H.; Lin, C.-F.; Kuo, C.-H.; Okino, A.; Chiang, T.-C. Atmospheric pressure tornado plasma jet of polydopamine coating on graphite felt for improving electrochemical performance in vanadium redox flow batteries. Catalysts 2021, 627, 11. [Google Scholar]
- Hsu, Y.-W.; Jang, Y.-J.; Wu, C.-Y.; Hsu, C.-C. Downstream characterization of an atmospheric pressure pulsed arc jet. Plasma Chem. Plasma Proces. 2010, 30, 363–372. [Google Scholar] [CrossRef]
- Hsu, A.; Chien, H.-H.; Liao, C.-Y.; Lee, C.-C.; Tsai, J.-H.; Hsu, C.-C.; Cheng, I.C.; Chen, J.-Z. Scan-mode atmospheric-pressure plasma jet processed reduced graphene oxides for quasi-solid-state gel-electrolyte supercapacitors. Coatings 2018, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-Z.; Hsu, C.-C.; Wang, C.; Liao, W.-Y.; Wu, C.-H.; Wu, T.-J.; Liu, H.-W.; Chang, H.; Lien, S.-T.; Li, H.-C.; et al. Rapid atmospheric-pressure-plasma-jet processed porous materials for energy harvesting and storage devices. Coatings 2015, 5, 26–38. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process Polym. 2018, e1700234. [Google Scholar] [CrossRef]
- Ng, S.W.; Slikboer, E.; Dickenson, A.; Walsh, J.L.; Lu, P.; Boehm, D.; Bourke, P. Characterization of an atmospheric pressure air plasma device under different modes of operation and their impact on the liquid chemistry. J. App. Phys. 2021, 129, 123303. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Skyllas-Kazacos, M. Chemical modification of graphite electrode materials for vanadium redox flow battery application-Part II. Acid treatment. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Duman, B.; Ficicilar, B. Acid modified graphite felt cathode electrode for low temperature H2/Br2 redox flow battery. Res. Eng. Struct. Mater. 2020, 6, 375–384. [Google Scholar] [CrossRef]
- Gao, F.; Li, X.; Zhang, Y.; Huang, C.; Zhang, W. Electrocatalytic activity of modified graphite felt in five anthraquinone derivative solutions for redox flow batterie. ACS Omega 2019, 4, 13721–13732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Huang, K.; Liu, S.; Tan, N.; Chen, L. Characteristics of graphite felt electrode electrochemically oxidized for vanadium redox battery application. Trans. Nonferrous Met. Soc. China 2007, 17, 195–199. [Google Scholar] [CrossRef]
- Tien, H.-W.; Huang, Y.-L.; Yang, S.-Y.; Wang, J.-Y.; Ma, C.-C.M. The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon 2011, 49, 1550–1560. [Google Scholar]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical conductivity of conductive carbon blacks: Influence of surface chemistry and topology. App. Surface Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, M.-S.; Kim, Y.-J.; Kim, J.H.; Dou, S.X.; Skyllas-Kazacos, M. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J. Mater. Chem. A 2015, 3, 16913–16933. [Google Scholar] [CrossRef]
- Bellani, S.; Najafi, L.; Prato, M.; Oropesa-Nuñez, R.; Martín-García, B.; Gagliani, L.; Mantero, E.; Marasco, L.; Bianca, G.; Zappia, M.I.; et al. Graphene-based electrodes in a vanadium redox flow battery produced by rapid low-pressure combined gas plasma treatments. Chem. Mater. 2021, 33, 4106–4121. [Google Scholar] [CrossRef]
- Ngamsai, K.; Arpornwichanop, A. Investigating the air oxidation of V(II) ions in a vanadium redox flow battery. J. Power Sources 2015, 295, 292–298. [Google Scholar] [CrossRef]
- Dixon, D.; Babu, D.J.; Bhaskar, A.; Bruns, H.-M.; Schneider, J.J.; Scheiba, F.; Ehrenberg, H. Tuning the performance of vanadium redox flow batteries by modifying the structural defects of the carbon felt electrode. Beilstein J. Nanotechnol. 2019, 10, 1698–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, E.-M.; Berger, B.; Komsiyska, L. Improvement of the performance of graphite felt electrodes for vanadium-redox-flow-batteries by plasma treatment. Int. J. Renew. Energy Dev. 2014, 3, 7–12. [Google Scholar] [CrossRef]
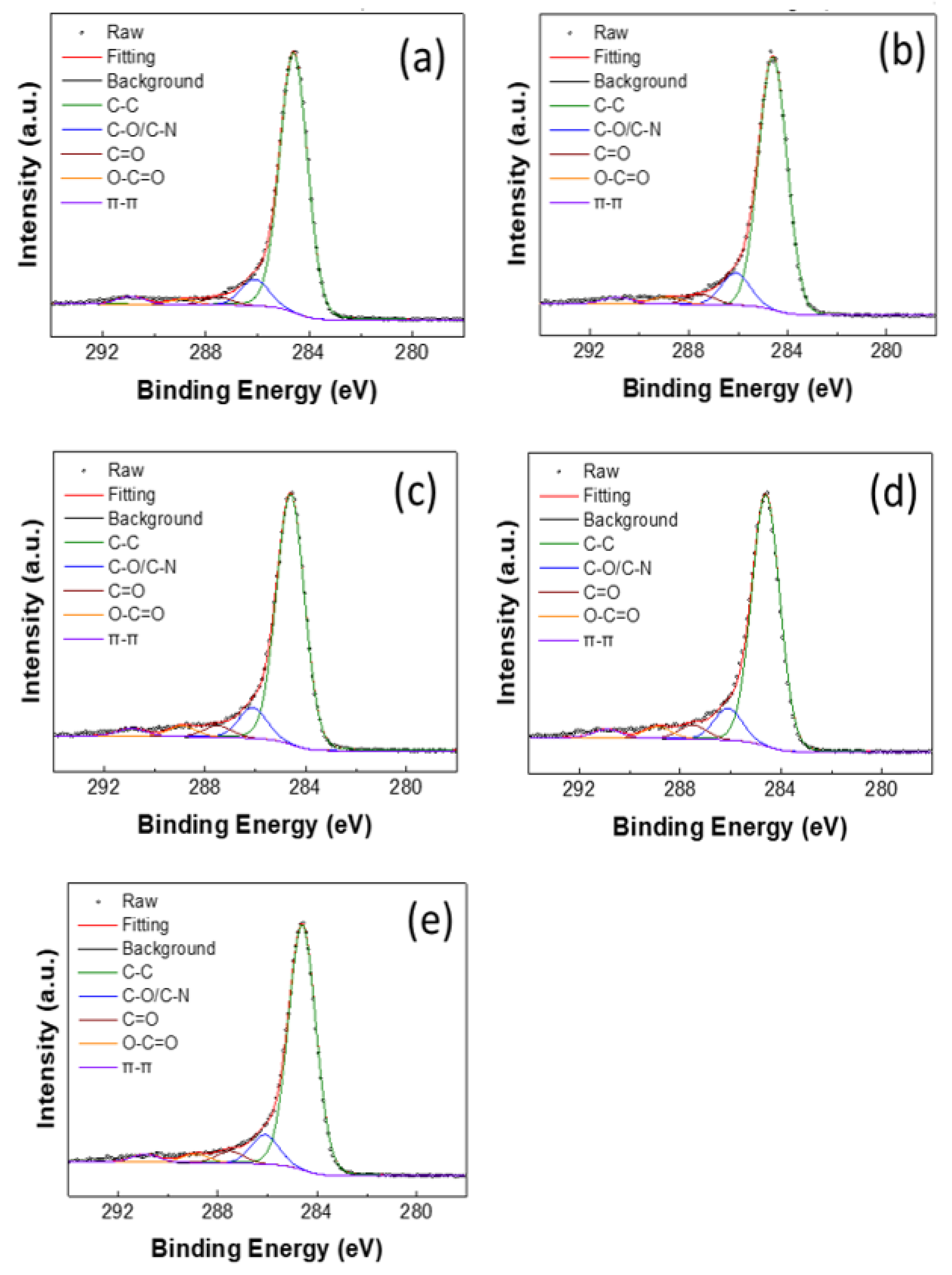
| AtomicRatio (%) | C–C | C–O/C–N | C=O | –COOH | π–π |
|---|---|---|---|---|---|
| As deposited | 84.8 | 8.6 | 2.5 | 1.7 | 2.4 |
| H2SO4-treated | 82.0 | 10.4 | 3.2 | 2.3 | 2.1 |
| APPJ 450 °C | 80.5 | 9.9 | 3.7 | 3.4 | 2.4 |
| APPJ 550 °C | 79.1 | 10 | 4.4 | 3.7 | 2.8 |
| APPJ 650 °C | 80.9 | 9.7 | 4.0 | 2.9 | 2.5 |
| Electrode Material with Treatment Method | CE (%) | VE (%) | EE (%) | Ref. |
|---|---|---|---|---|
| H2SO4-treated GF (0.04) | ~94.5 | ~76.0 | 72.0 | This work |
| APPJ 450 °C-treated GF (0.04) | ~95.5 | ~82.0 | 79.0 | This work |
| APPJ 550 °C-treated GF (0.04) | ~95.5 | ~88.0 | 83.5 | This work |
| APPJ 650 °C-treated GF (0.04) | ~95.5 | ~85.0 | 81.0 | This work |
| APPJ-treated polydopamine coated GF (0.04) | 85.2 | 93.8 | 79.9 | [28] |
| APPJ-treated CF (jet speed 5 mm·s−1; single scan) (0.12) | – | – | ~84.2 | [27] |
| APPJ-treated CF (jet speed 5 mm·s−1; single scan) (0.14) | – | – | ~82.8 | [27] |
| APPJ-treated CF (jet speed 5 mm·s−1; single scan) (0.16) | 97.0 | 79.9 | ~77.6 | [27] |
| APPJ-treated CF (jet speed 10 mm·s−1; single scan) (0.12) | – | – | ~80.0 | [27] |
| APPJ-treated CF (jet speed 5 mm·s−1; double scan) (0.14) | – | – | ~81.7 | [27] |
| APPJ-treated CF (jet speed 5 mm·s−1; triple scan) (0.14) | – | – | ~81.9 | [27] |
| Low-pressure plasma-treated graphene-incorporated GF (0.05) | ~97.0 | ~92.5 | 90.8 | [42] |
| Low-pressure plasma-treated graphene-incorporated GF (0.80) | ~98.0 | <70.0 | <75.0 | [44] |
| Scan mode APPJ-treated GF (0.04) | 92.5 | 88.0 | 81.4 | [21] |
| Spot mode APPJ-treated GF (0.04) | 92.4 | 89.4 | 82.7 | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirabovornwisut, T.; Singh, B.; Chutimasakul, A.; Chang, J.-H.; Chen, J.-Z.; Arpornwichanop, A.; Chen, Y.-S. Characteristics of Graphite Felt Electrodes Treated by Atmospheric Pressure Plasma Jets for an All-Vanadium Redox Flow Battery. Materials 2021, 14, 3847. https://doi.org/10.3390/ma14143847
Jirabovornwisut T, Singh B, Chutimasakul A, Chang J-H, Chen J-Z, Arpornwichanop A, Chen Y-S. Characteristics of Graphite Felt Electrodes Treated by Atmospheric Pressure Plasma Jets for an All-Vanadium Redox Flow Battery. Materials. 2021; 14(14):3847. https://doi.org/10.3390/ma14143847
Chicago/Turabian StyleJirabovornwisut, Tossaporn, Bhupendra Singh, Apisada Chutimasakul, Jung-Hsien Chang, Jian-Zhang Chen, Amornchai Arpornwichanop, and Yong-Song Chen. 2021. "Characteristics of Graphite Felt Electrodes Treated by Atmospheric Pressure Plasma Jets for an All-Vanadium Redox Flow Battery" Materials 14, no. 14: 3847. https://doi.org/10.3390/ma14143847
APA StyleJirabovornwisut, T., Singh, B., Chutimasakul, A., Chang, J.-H., Chen, J.-Z., Arpornwichanop, A., & Chen, Y.-S. (2021). Characteristics of Graphite Felt Electrodes Treated by Atmospheric Pressure Plasma Jets for an All-Vanadium Redox Flow Battery. Materials, 14(14), 3847. https://doi.org/10.3390/ma14143847









