Preparation of Colored Microcapsule Phase Change Materials with Colored SiO2 Shell for Thermal Energy Storage and Their Application in Latex Paint Coating
Abstract
:1. Introduction
2. Material and Reagents
2.1. Methods
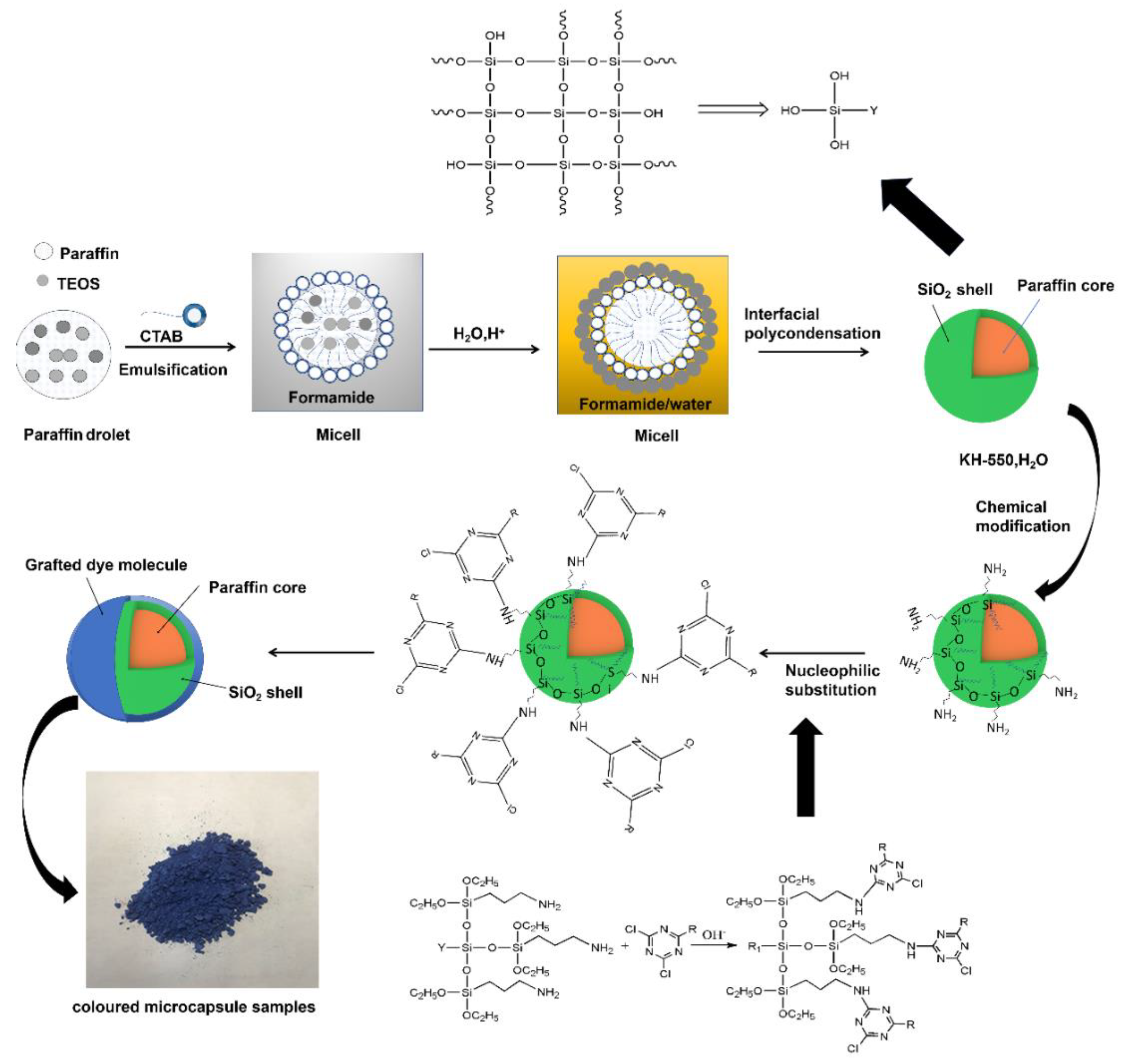
2.1.1. Synthesis of the Paraffin@SiO2 Microcapsules
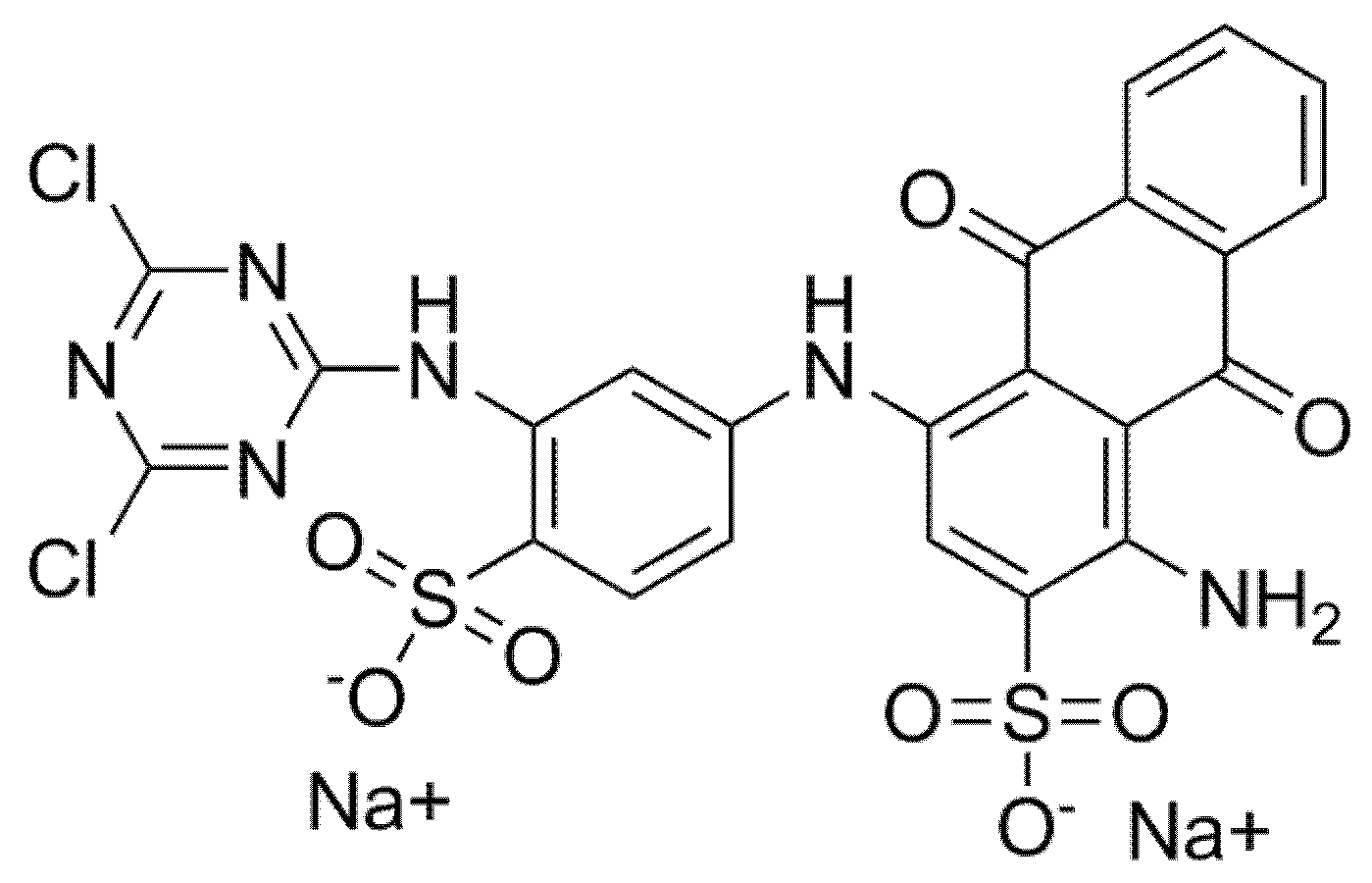
2.1.2. Synthesis of Colored Microcapsules
2.1.3. Preparation of Temperature-Regulating Coating
2.2. Characterizations
3. Results and Discussion
3.1. Synthetic Strategy and Formation Mechanisms
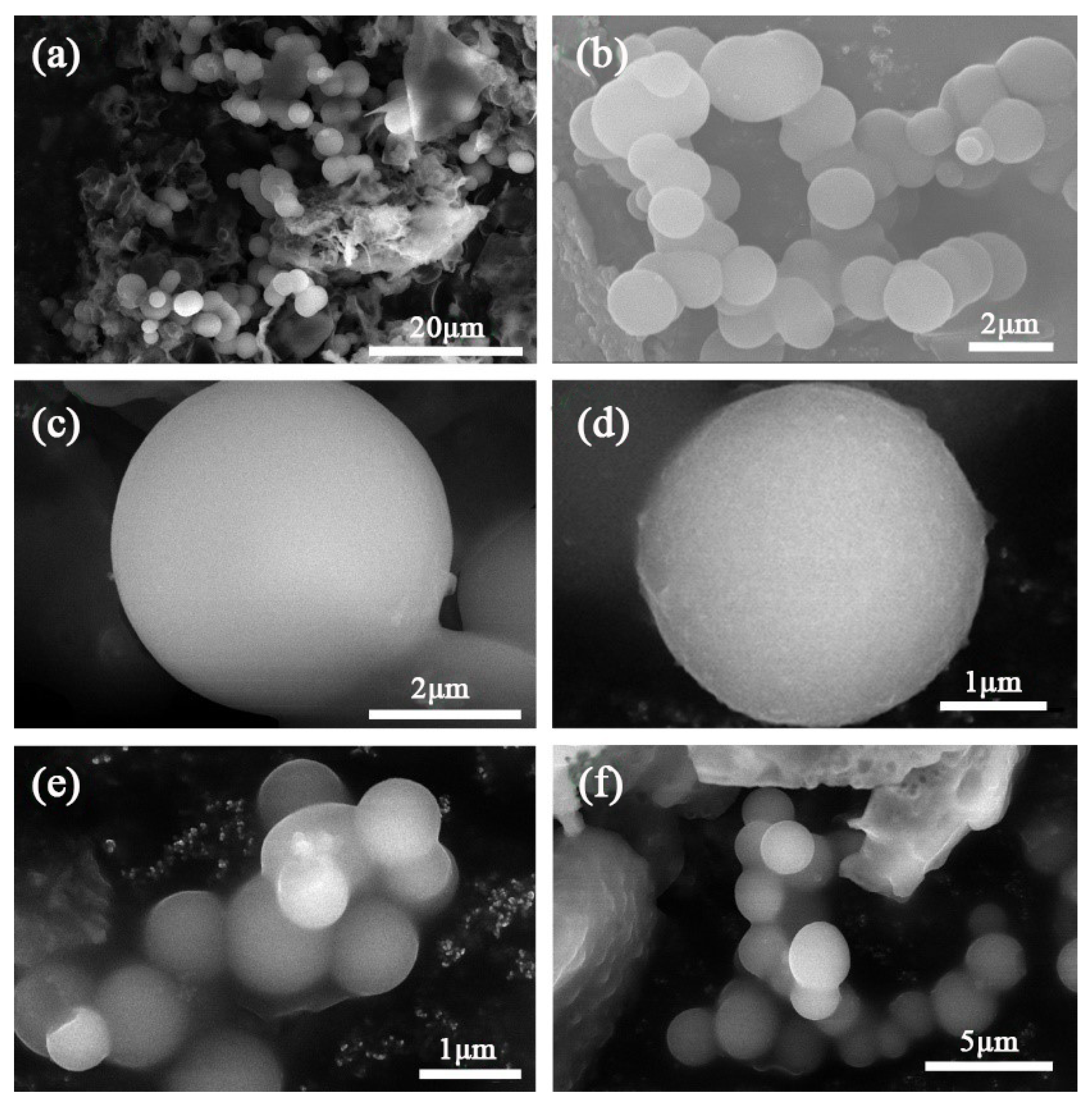
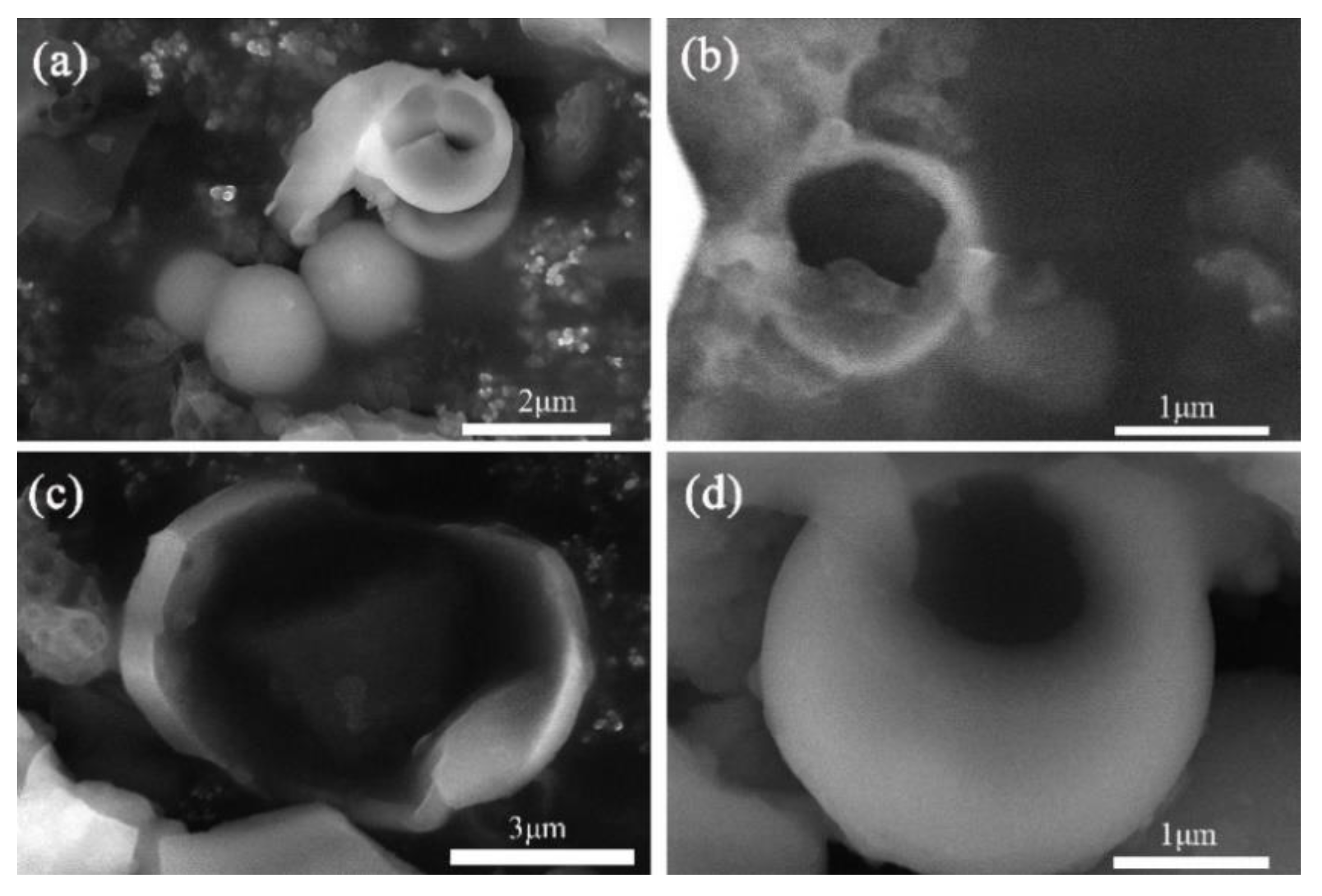
3.2. Chemistry and Crystal Structure of Microcapsules
3.3. Chemical Composition and Crystal Structure
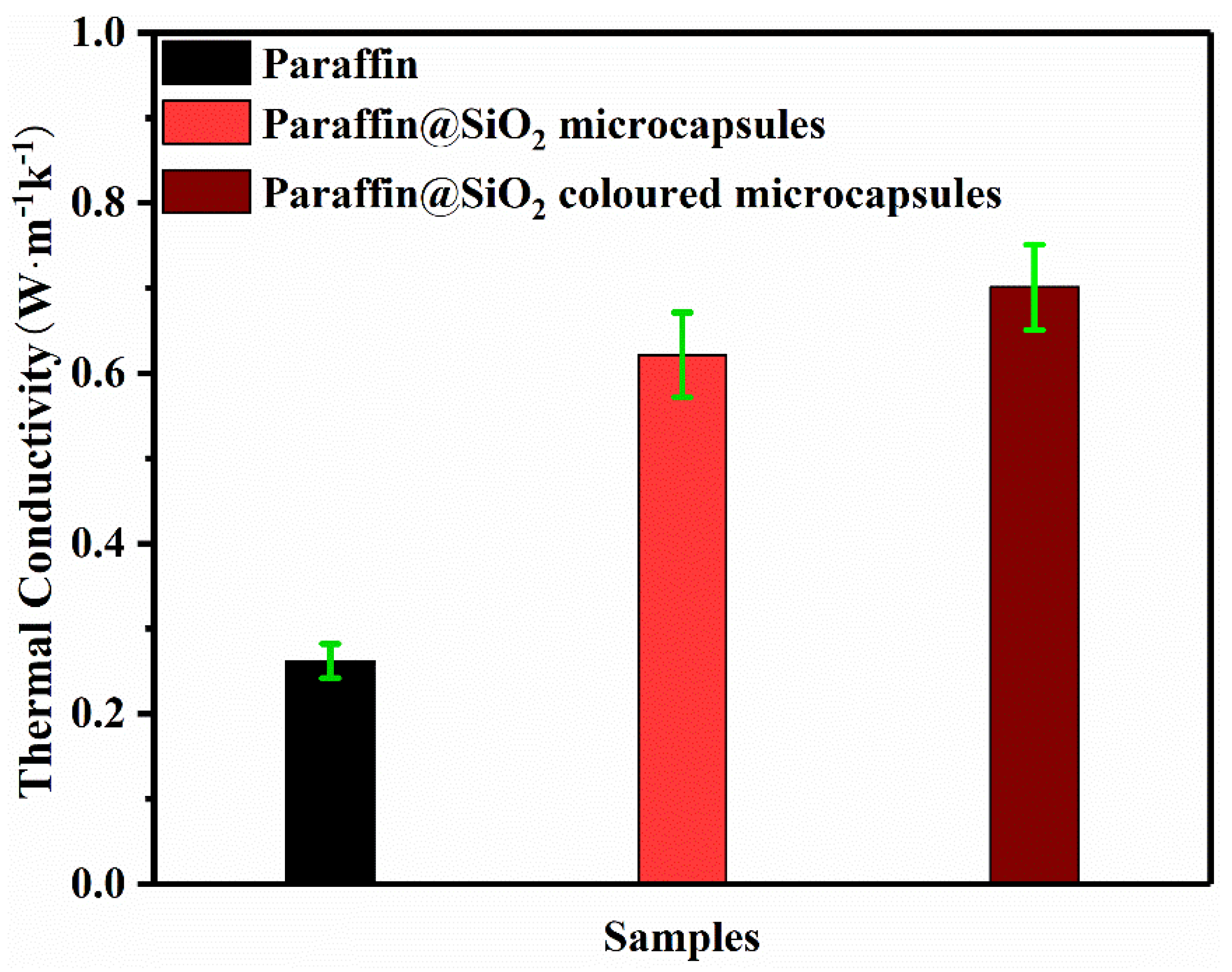
3.4. Thermal Conductivity
3.5. Phase Transition Characteristics and Thermal Performance Analysis
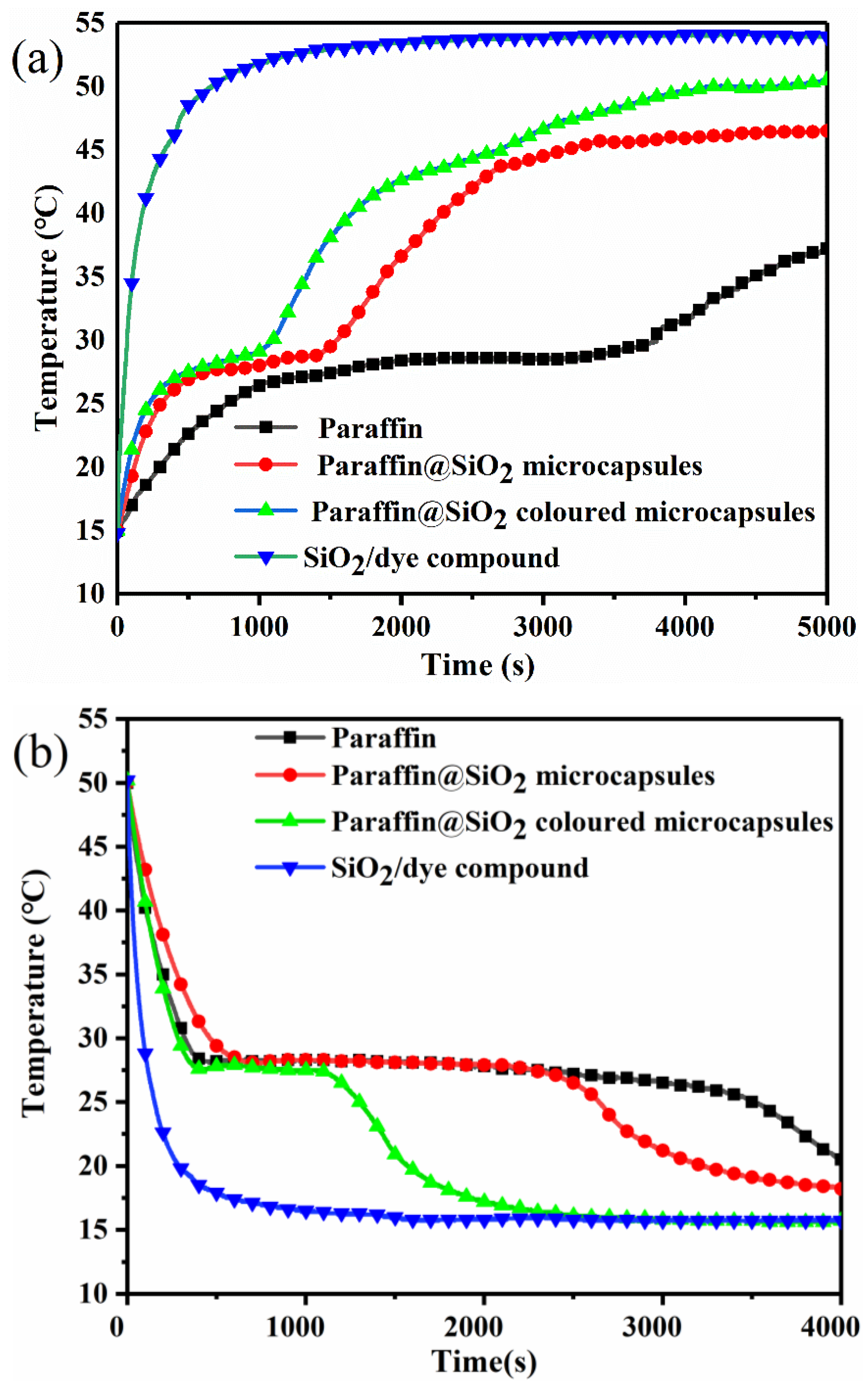
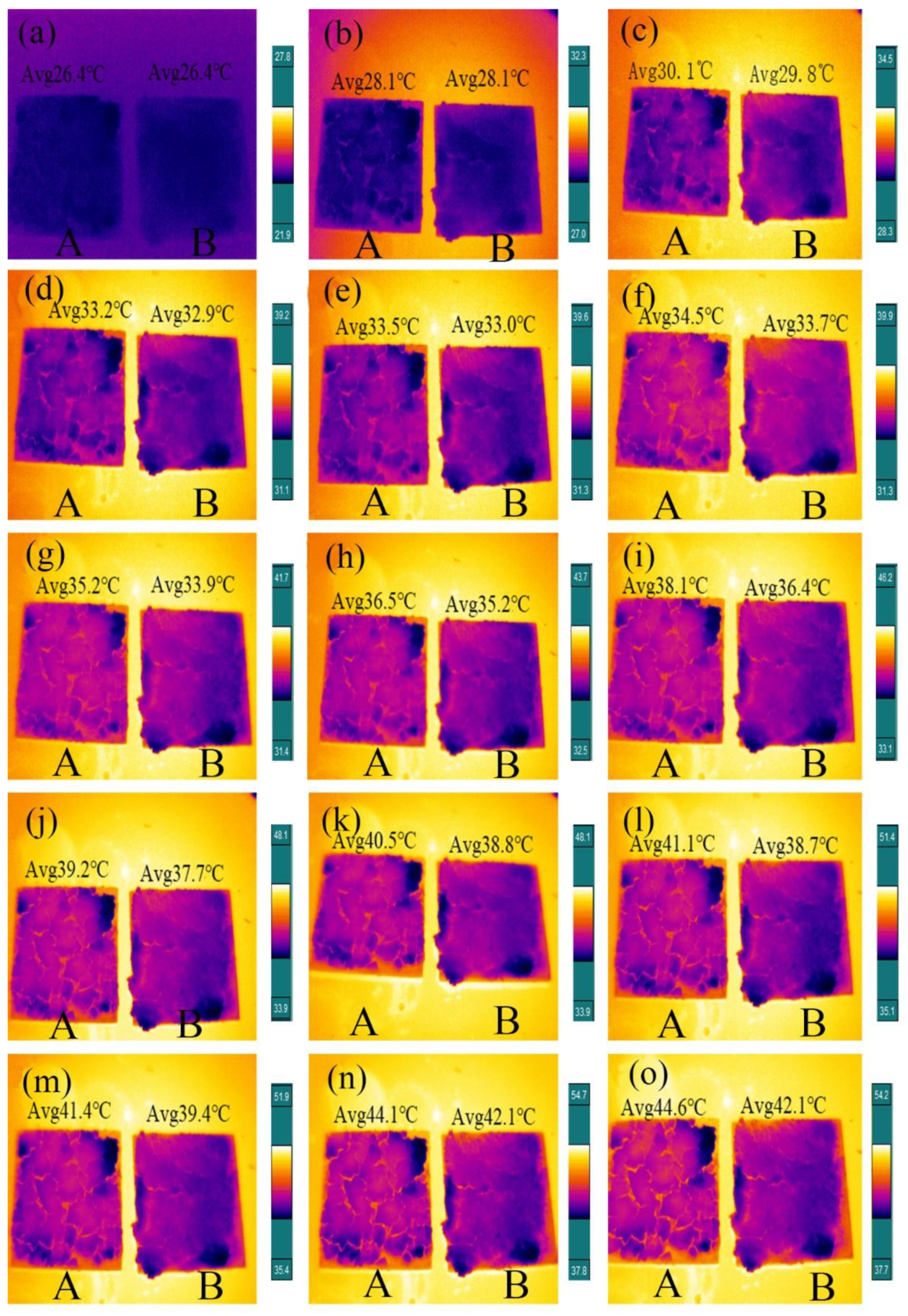
3.6. Temperature-Regulating Performance and Solar Energy Thermal Storage Capacity
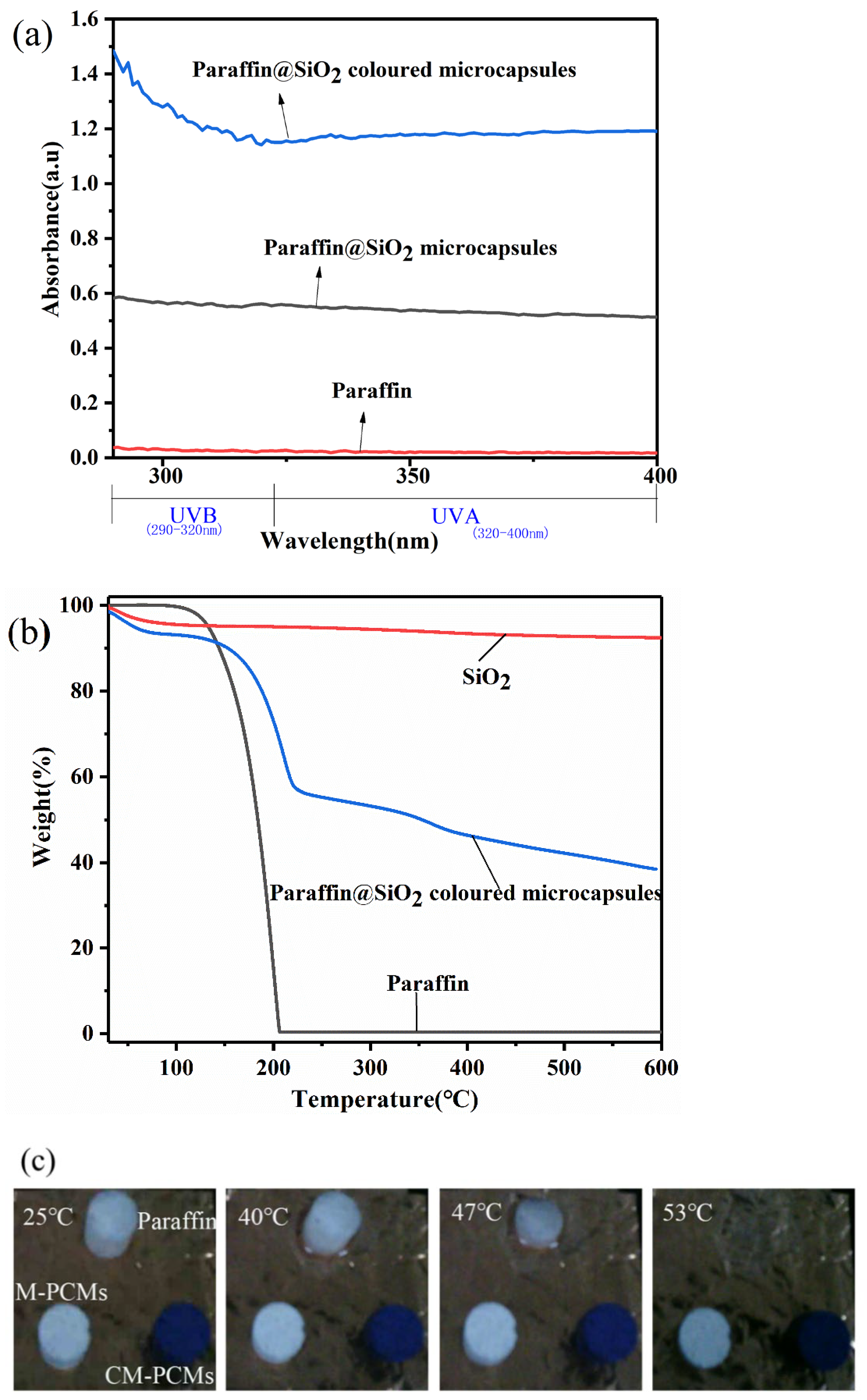
3.7. UV Protection Property and Thermal Stability
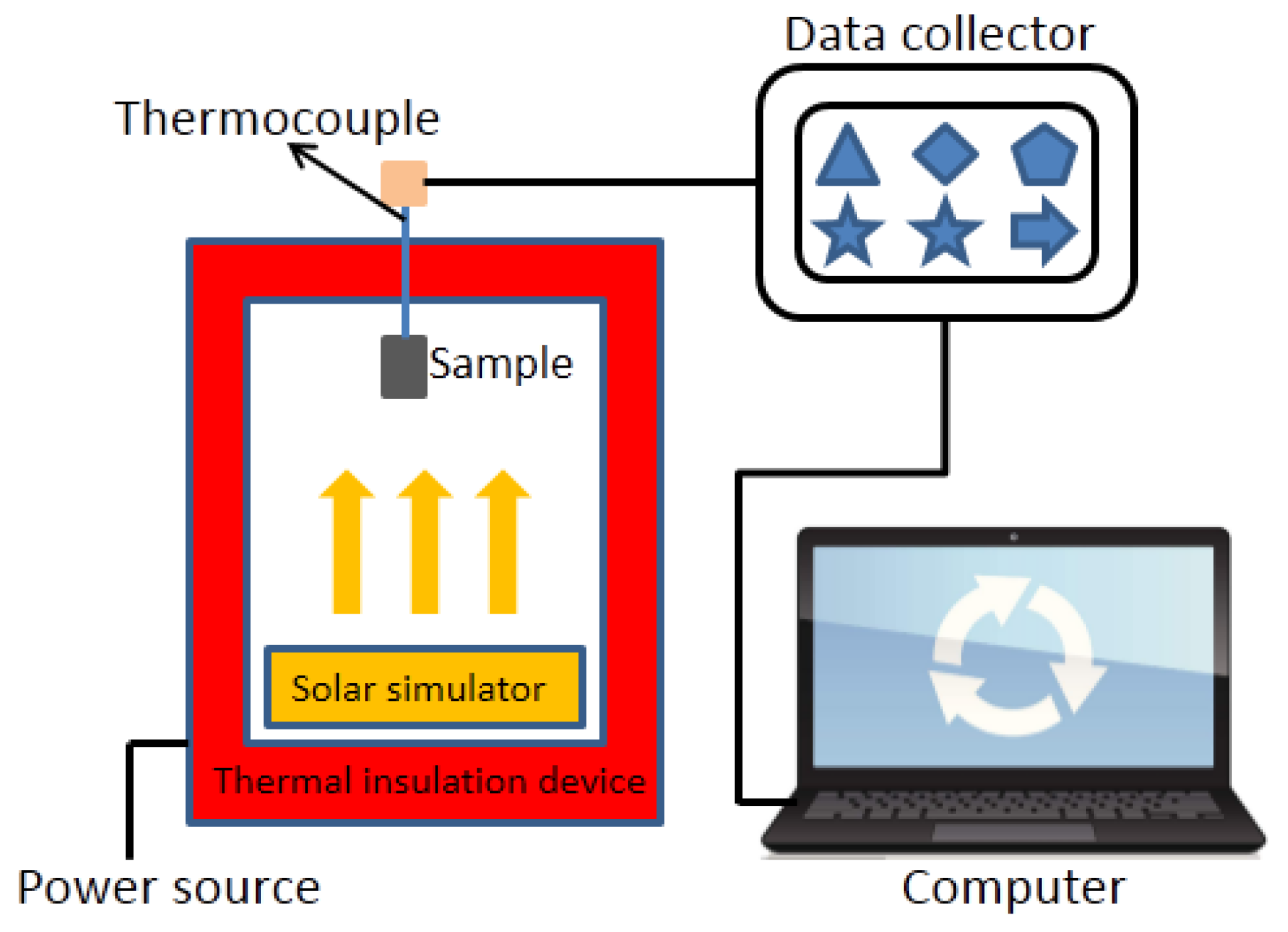
3.8. Temperature Control Performance Testing of Internal Wall Insulation Thermal Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bejan, A.S.; Catalina, T. The Implementation of Phase Changing Materials in Energy-efficient Buildings. Case Study: EFdeN Project. Energy Procedia 2016, 85, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2020. [Google Scholar] [CrossRef]
- Vukadinović, A.; Radosavljević, J.; Đorđević, A. Energy performance impact of using phase-change materials in thermal storage walls of detached residential buildings with a sunspace. Sol. Energy 2020, 206, 228–244. [Google Scholar] [CrossRef]
- Jankowski, N.R.; McCluskey, F.P. A review of phase change materials for vehicle component thermal buffering. Appl. Energy 2014, 113, 1525–1561. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Aydın, A.A. In situ preparation and characterization of encapsulated high-chain fatty acid ester-based phase change material (PCM) in poly(urethane-urea) by using amino alcohol. Chem. Eng. J. 2013, 231, 477–483. [Google Scholar] [CrossRef]
- Fortuniak, W.; Slomkowski, S.; Chojnowski, J.; Kurjata, J.; Tracz, A.; Mizerska, U. Synthesis of a paraffin phase change material microencapsulated in a siloxane polymer, original contribution. Colloid Polym. Sci. 2013, 291, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Silva, L.; Rodríguez, J.F.; Romero, A.; Borreguero, A.M.; Carmona, M.; Sánchez, P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerisation. Chem. Eng. J. 2010, 216–222. [Google Scholar] [CrossRef]
- George, M.; Pandey, A.K.; Rahim, N.A.; Tyagi, V.V.; Shahabuddin, S.; Saidur, R. A novel polyaniline (PANI)/ paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol. Energy 2020, 204, 448–458. [Google Scholar] [CrossRef]
- Luo, R.; Wang, S.; Wang, T.; Zhu, C.; Nomura, T.; Akiyama, T. Fabrication of paraffin@SiO2 shape-stabilized composite phase change material via chemical precipitation method for building energy conservation. Energy Build. 2015, 108, 373–380. [Google Scholar] [CrossRef]
- Saihi, D.; Vroman, I.; Giraud, S.; Bourbigot, S. Microencapsulation of ammonium phosphate with a polyurethane shell part I: Coacervation technique. React. Funct. Polym. 2005, 64, 127–138. [Google Scholar] [CrossRef]
- Liang, C.; Lingling, X.; Hongbo, S.; Zhibin, Z. Microencapsulation of butyl stearate as a phase change material by interfacial polycondensation in a polyurea system. Energy Convers. Manag. 2009, 50, 723–729. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Carmona, M.; Sanchez, M.L.; Valverde, J.L.; Rodriguez, J.F. Improvement of the thermal behaviour of gypsum blocks by the incorporation of microcapsules containing PCMS obtained by suspension polymerization with an optimal core/coating mass ratio. Appl. Therm. Eng. 2010, 30, 1164–1169. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. The manufacture of microencapsulated phase change materials suitable for the design of thermally enhanced fabrics. Thermochim. Acta 2007, 452, 149–160. [Google Scholar] [CrossRef]
- Bayés-García, L.; Ventolà, L.; Cordobilla, R.; Benages, R.; Calvet, T.; Cuevas-Diarte, M.A. Phase Change Materials (PCM) microcapsules with different shell compositions: Preparation, characterization and thermal stability. Sol. Energy Mater. Sol. Cells 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-change characteristics and thermal performance of form-stable n -alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Self-Assembly Synthesis of Microencapsulated n-Eicosane Phase-Change Materials with Crystalline-Phase-Controllable Calcium Carbonate Shell. Energy Fuels 2014, 28, 3519–3529. [Google Scholar] [CrossRef]
- Pan, L.; Tao, Q.; Zhang, S.; Wang, S.; Zhang, J.; Wang, S.; Wang, Z.; Zhang, Z. Preparation, characterization and thermal properties of micro-encapsulated phase change materials. Sol. Energy Mater. Sol. Cells 2012, 98, 66–70. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Wu, D. Fabrication of multifunctional microcapsules containing n-eicosane core and zinc oxide shell for low-temperature energy storage, photocatalysis, and antibiosis. Energy Convers. Manag. 2015, 106, 873–885. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Wang, X.; Wu, D. Fabrication of microencapsulated phase change materials based on n-octadecane core and silica shell through interfacial polycondensation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 104–117. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, L.; Fang, G.; Shan, F. Synthesis and Characterization of Microencapsulated Paraffin Microcapsules as Shape-Stabilized Thermal Energy Storage Materials. Nanoscale Microscale Thermophys. Eng. 2013, 17, 112–123. [Google Scholar] [CrossRef]
- Chang, C.C.; Tsai, Y.L.; Chiu, J.J.; Chen, H. Preparation of phase change materials microcapsules by using PMMA network-silica hybrid shell via sol-gel process. J. Appl. Polym. Sci. 2009, 112, 1850–1857. [Google Scholar] [CrossRef]
- Li, W.; Song, G.; Li, S.; Yao, Y.; Tang, G. Preparation and characterization of novel MicroPCMs (microencapsulated phase-change materials) with hybrid shells via the polymerization of two alkoxy silanes. Energy 2014, 70, 298–306. [Google Scholar] [CrossRef]
- Karkri, M.; Lachheb, M.; Albouchi, F.; Nasrallah, S.B.; Krupa, I. Thermal properties of smart microencapsulated paraffin/plaster composites for the thermal regulation of buildings. Energy Build. 2015, 88, 183–192. [Google Scholar] [CrossRef]
- Dinçalp, H.; Yavuz, S.; Haklı, Ö.; Zafer, C.; Özsoy, C.; Durucasu, I.; Içli, S. Optical and photovoltaic properties of salicylaldimine-based azo ligands. J. Photochem. Photobiol. A Chem. 2010, 210, 8–16. [Google Scholar] [CrossRef]
- Klančnik, M.; Gorenšek, M. Kinetics of Hydrolysis of Monofunctional and Bifunctional Monochloro-s-triazine Reactive Dyes. Dyes Pigments 1997, 337–350. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Xie, K.; Pei, L.; Hou, A. Synthesis of novel green reactive dyes and relationship between their structures and printing properties. Dyes Pigments 2020, 174. [Google Scholar] [CrossRef]
- Lei, J.; Kumarasamy, K.; Zingre, K.T.; Yang, J.; Wan, M.P.; Yang, E.-H. Cool colored coating and phase change materials as complementary cooling strategies for building cooling load reduction in tropics. Appl. Energy 2017, 190, 57–63. [Google Scholar] [CrossRef]
- Soudian, S.; Berardi, U.; Laschuk, N. Development and thermal-optical characterization of a cementitious plaster with phase change materials and thermochromic paint. Sol. Energy 2020, 205, 282–291. [Google Scholar] [CrossRef]
- Jal, P.K.; Patel, S.; Mishra, B.K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 1005–1028. [Google Scholar] [CrossRef]
- Mutneja, R.; Singh, R.; Kaur, V.; Wagler, J.; Kroke, E. Development of new precursors for immobilizing dyes onto silica surfaces. Dyes Pigments 2014, 108, 41–49. [Google Scholar] [CrossRef]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of organic molecules on silica surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Przybylska, A.; Kurc, B.; Ciesielczyk, F. Hybrid pigments preparation via adsorption of C.I. Mordant Red 3 on both unmodified and aminosilaneefunctionalised silica supports. Dyes Pigments 2011, 89, 127–136. [Google Scholar] [CrossRef]
- Harris, R.G.; Wells, J.D.; Johnson, B.B. Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2001, 180, 131–140. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Tailoring of bifunctional microencapsulated phase change materials with CdS/SiO2 double-layered shell for solar photocatalysis and solar thermal energy storage. Appl. Therm. Eng. 2018, 134, 603–614. [Google Scholar] [CrossRef]
- Yan, D.; Tang, B.; Zhang, S. Preparation and performances of sunlight-induced phase change PEG/SiO2-dye composite for solar energy conversion and storage. Sol. Energy Mater. Sol. Cells 2020, 215. [Google Scholar] [CrossRef]




| Parameter | Phase State | Result |
|---|---|---|
| Melting area (°C) | - | 26–30 |
| solidification area (°C) | - | 27–23 |
| Density (g/cm3) | Solid phase | 0.862 |
| Liquid phase | 0.850 | |
| Specific Heat capacity (kJ/Kg·K) | Solid phase | 2.14 |
| Liquid phase | 2.02 | |
| Thermal Conductivity (W/m·K) | Solid phase | 0.26 |
| Liquid phase | 0.25 |
| Samples | Preparation Process | Temperature (°C) | Reaction Time (h) | Stirring Speed (r/min) |
|---|---|---|---|---|
| Paraffin | Paraffin/TEOS | 45 | 0.5 | 1000 |
| Microcapsules | Micelle | 45 | 0.5 | 1000 |
| SiO2 shell | 50 | 5 | 500 | |
| Modified microcapsule | KH550 | 40 | 2 | 500 |
| Colored microcapsules | Colored SiO2 shell | 40 | 2 | 300 |
| Type of Equipment | Parameter | Model and Company | Repetitions |
|---|---|---|---|
| Dryer | Temperature | Shanghai Experimental Instrument General Factory (ZK-82B) | - |
| Device for testing the thermal conductivity | Thermal conductivity | Xia tech hot plate thermal conductivity meter (TC-3000) | 5 |
| Time/temperature curve test device | Time/temperature curve | Self-assembled test device | 3 |
| differential scanning calorimeter | Thermal performance | American Waters Corporation(Q20) | - |
| Samples | Crystallization Process | Melting Process | Encapsulation Efficiency (%) | Thermal Conductivity (W·m−1·K−1) | ||
|---|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | |||
| Paraffin | 28.56 | 203.49 | 24.98 | 206.15 | - | 0.2619 |
| Microcapsules | 27.93 | 77.31 | 22.48 | 78.23 | 38 | 0.6215 |
| Colored microcapsules | 27.98 | 60.49 | 23.16 | 59.03 | 30 | 0.7011 |
| Sample Code | Color Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | c* | h* | |
| Microcapsules | 98.45 | −0.08 | −0.14 | 0.16 | 241.41 |
| Colored microcapsules | 54.71 | −6.40 | −25.30 | 26.09 | 255.81 |
| X-Br | 63.76 | −3.23 | −38.76 | 38.89 | 265.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, E.; Wei, Z.; Lian, C.; Zhou, Y.; Gan, S.; Xu, B. Preparation of Colored Microcapsule Phase Change Materials with Colored SiO2 Shell for Thermal Energy Storage and Their Application in Latex Paint Coating. Materials 2021, 14, 4012. https://doi.org/10.3390/ma14144012
Ma E, Wei Z, Lian C, Zhou Y, Gan S, Xu B. Preparation of Colored Microcapsule Phase Change Materials with Colored SiO2 Shell for Thermal Energy Storage and Their Application in Latex Paint Coating. Materials. 2021; 14(14):4012. https://doi.org/10.3390/ma14144012
Chicago/Turabian StyleMa, Enpei, Zhenghuang Wei, Cheng Lian, Yinping Zhou, Shichang Gan, and Bin Xu. 2021. "Preparation of Colored Microcapsule Phase Change Materials with Colored SiO2 Shell for Thermal Energy Storage and Their Application in Latex Paint Coating" Materials 14, no. 14: 4012. https://doi.org/10.3390/ma14144012
APA StyleMa, E., Wei, Z., Lian, C., Zhou, Y., Gan, S., & Xu, B. (2021). Preparation of Colored Microcapsule Phase Change Materials with Colored SiO2 Shell for Thermal Energy Storage and Their Application in Latex Paint Coating. Materials, 14(14), 4012. https://doi.org/10.3390/ma14144012






