Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties
Abstract
:1. Introduction
2. Experimental
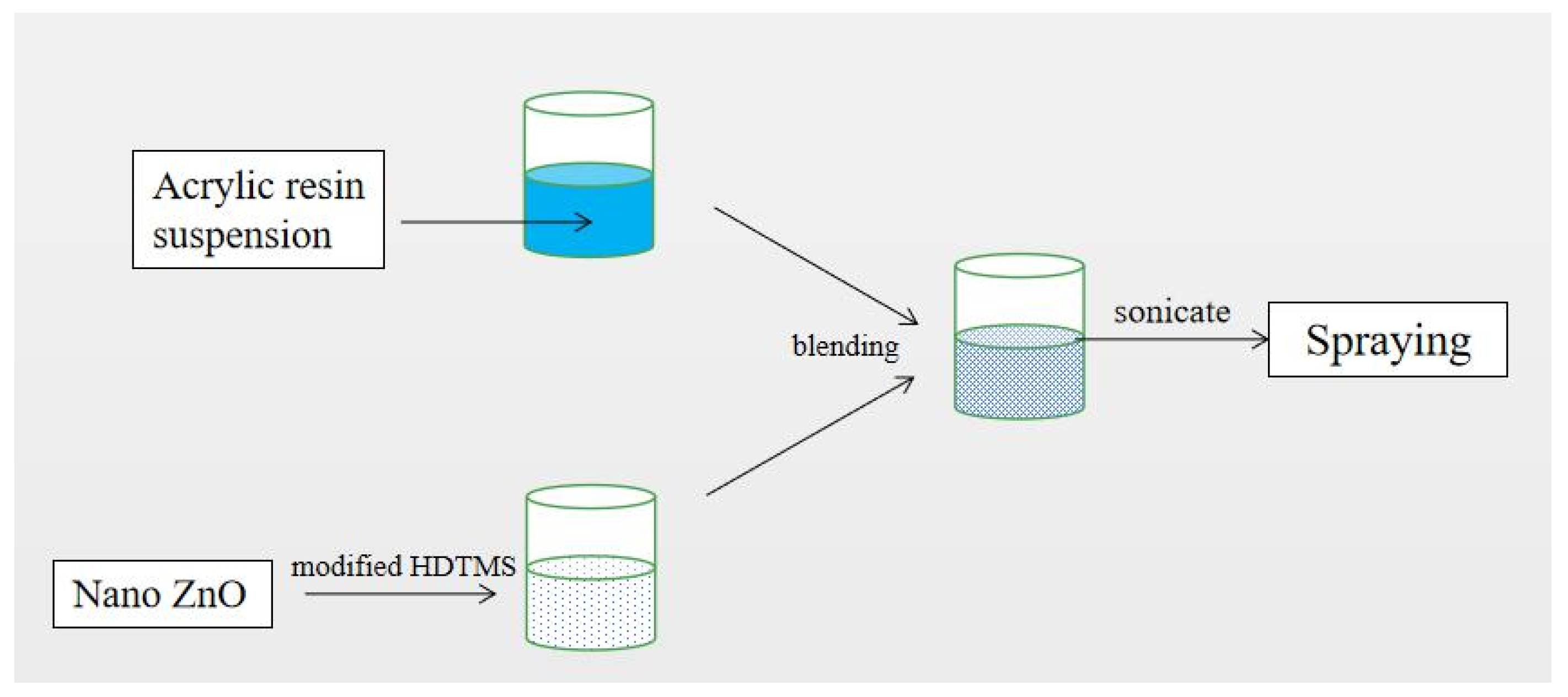
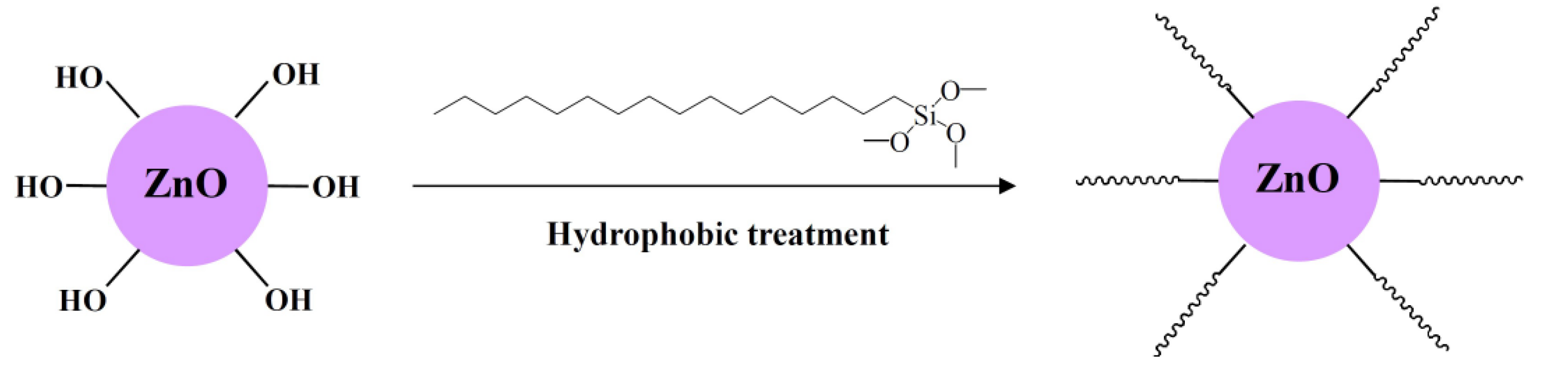
2.1. Preparation of Superhydrophobic Coatings
2.2. Structure and Morphology Testing and Characterization
2.3. Antibacterial Testing
2.3.1. Specimen Preparation and Pretreatment
2.3.2. Bacteria Inoculation
2.3.3. Colony Count
2.3.4. Antibacterial Rate Calculation
3. Results and Discussion
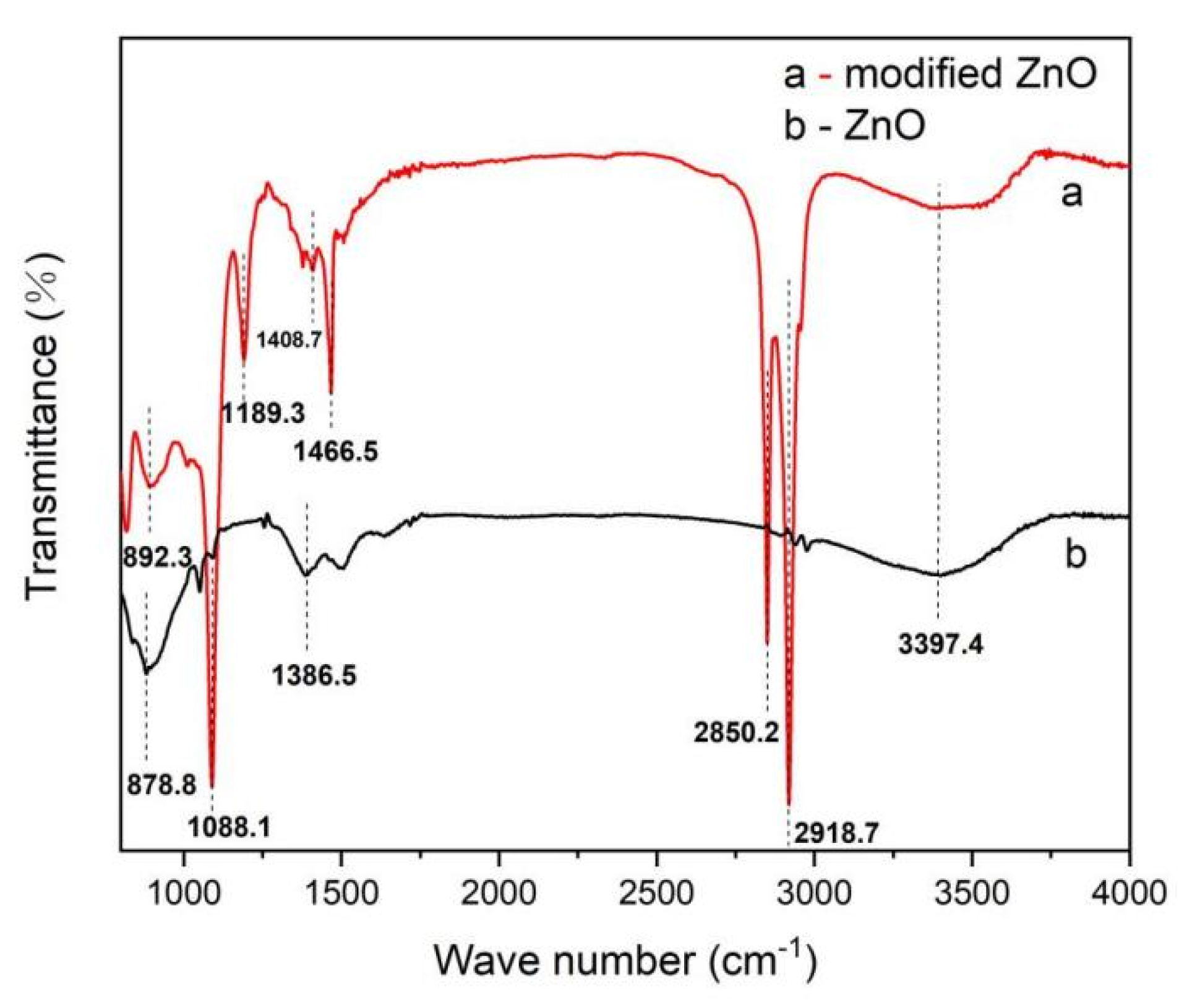
3.1. FTIR Analysis
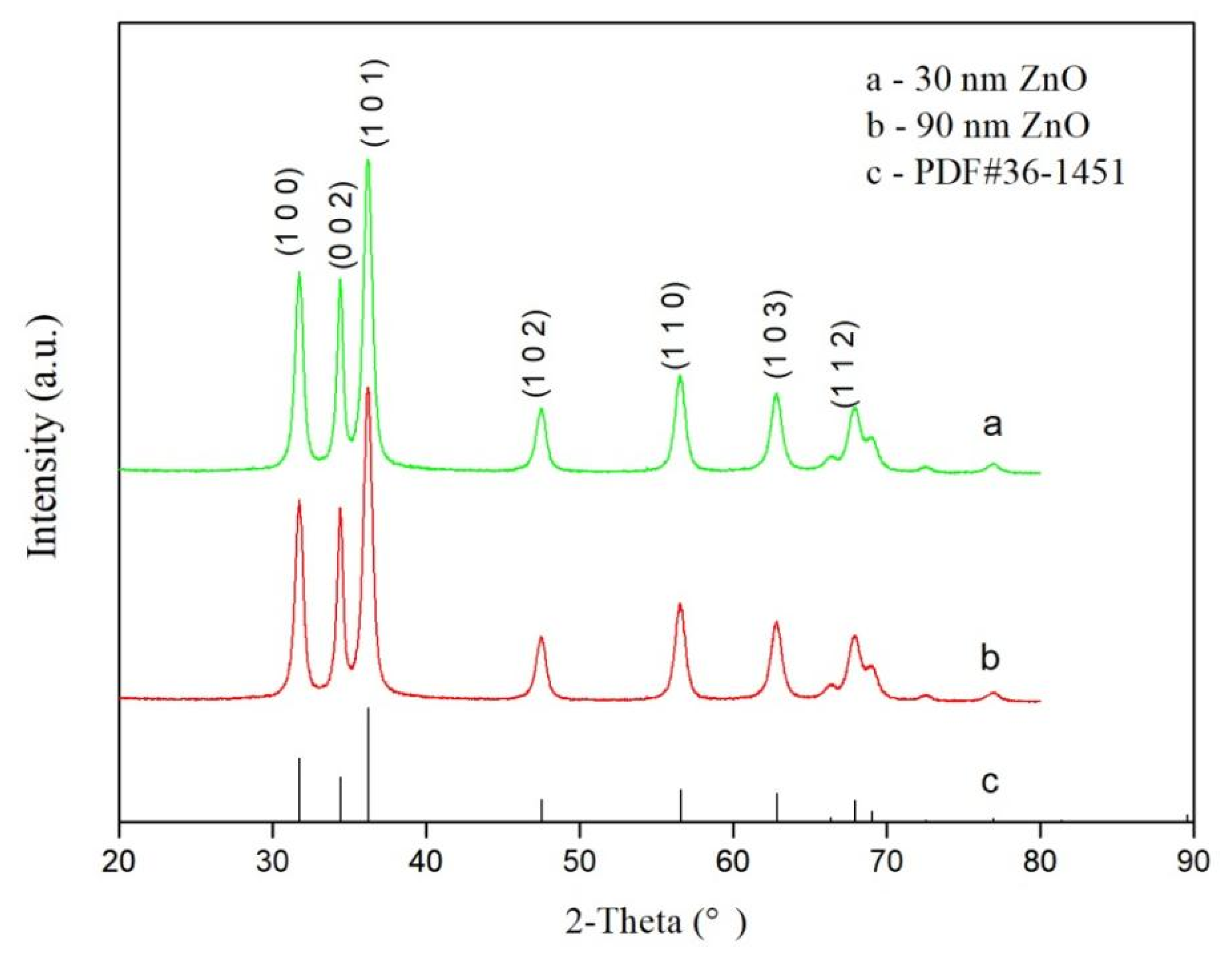
3.2. XRD Analysis
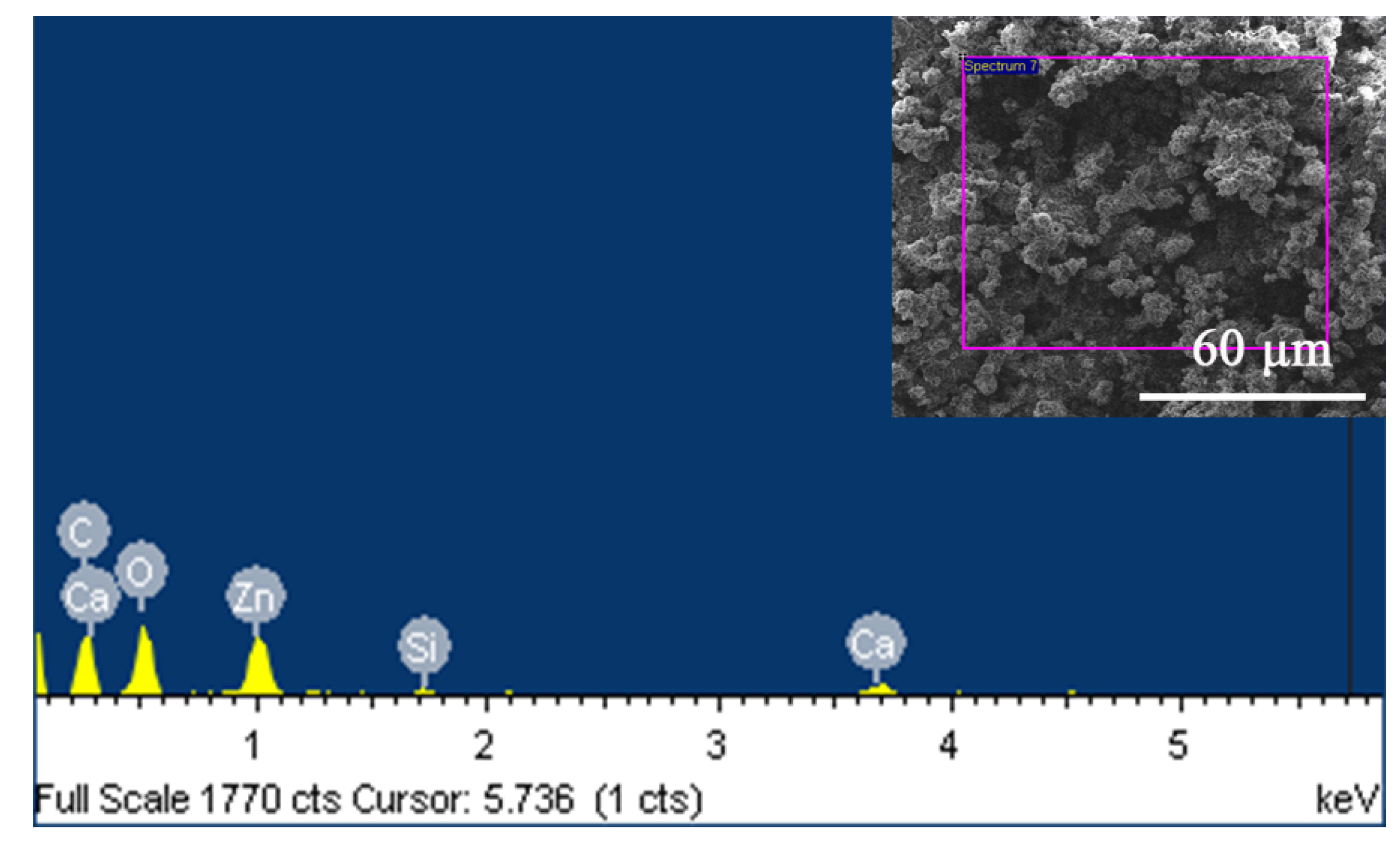
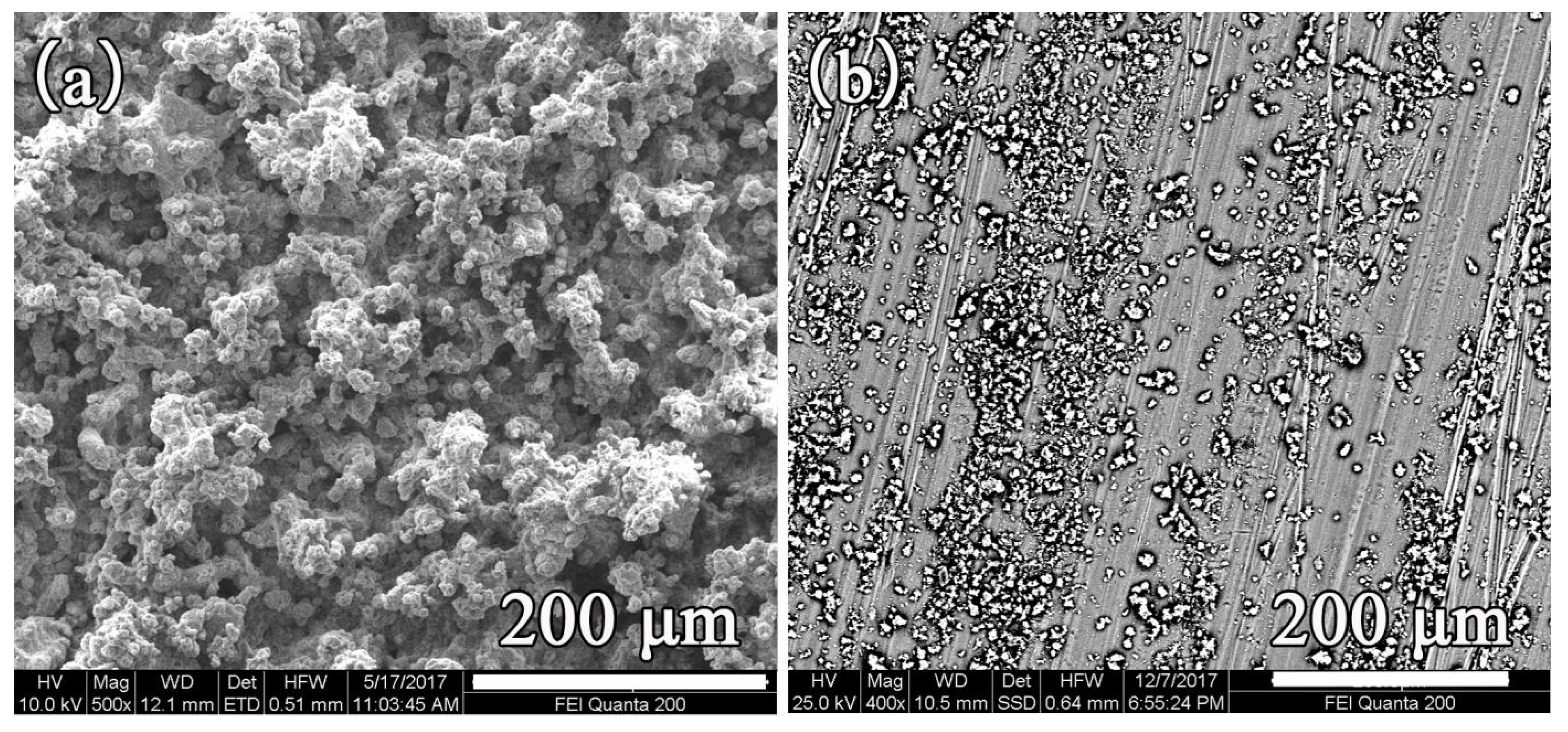
3.3. Morphology and EDS Analysis
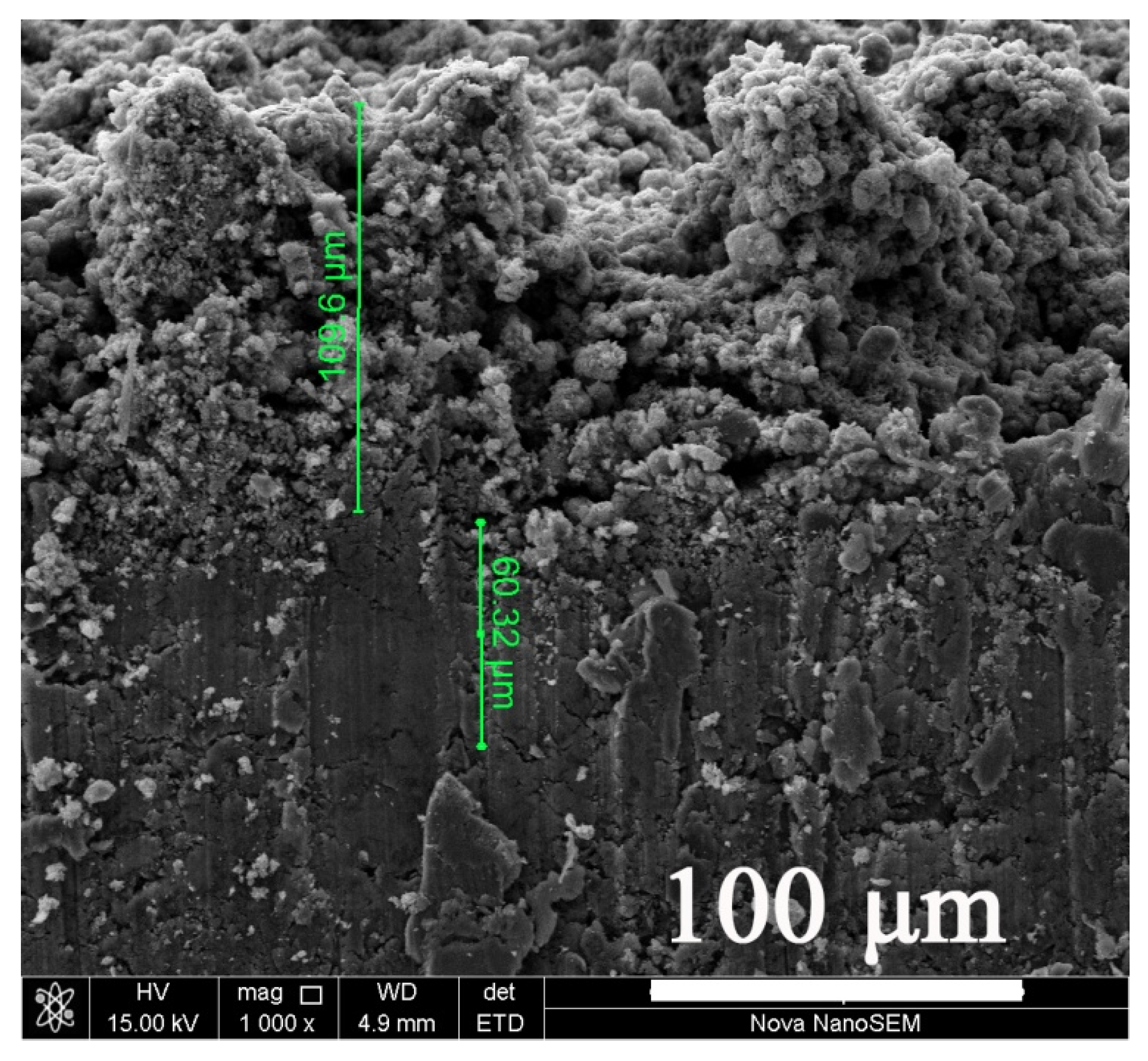
3.4. Cross-Section Morphology
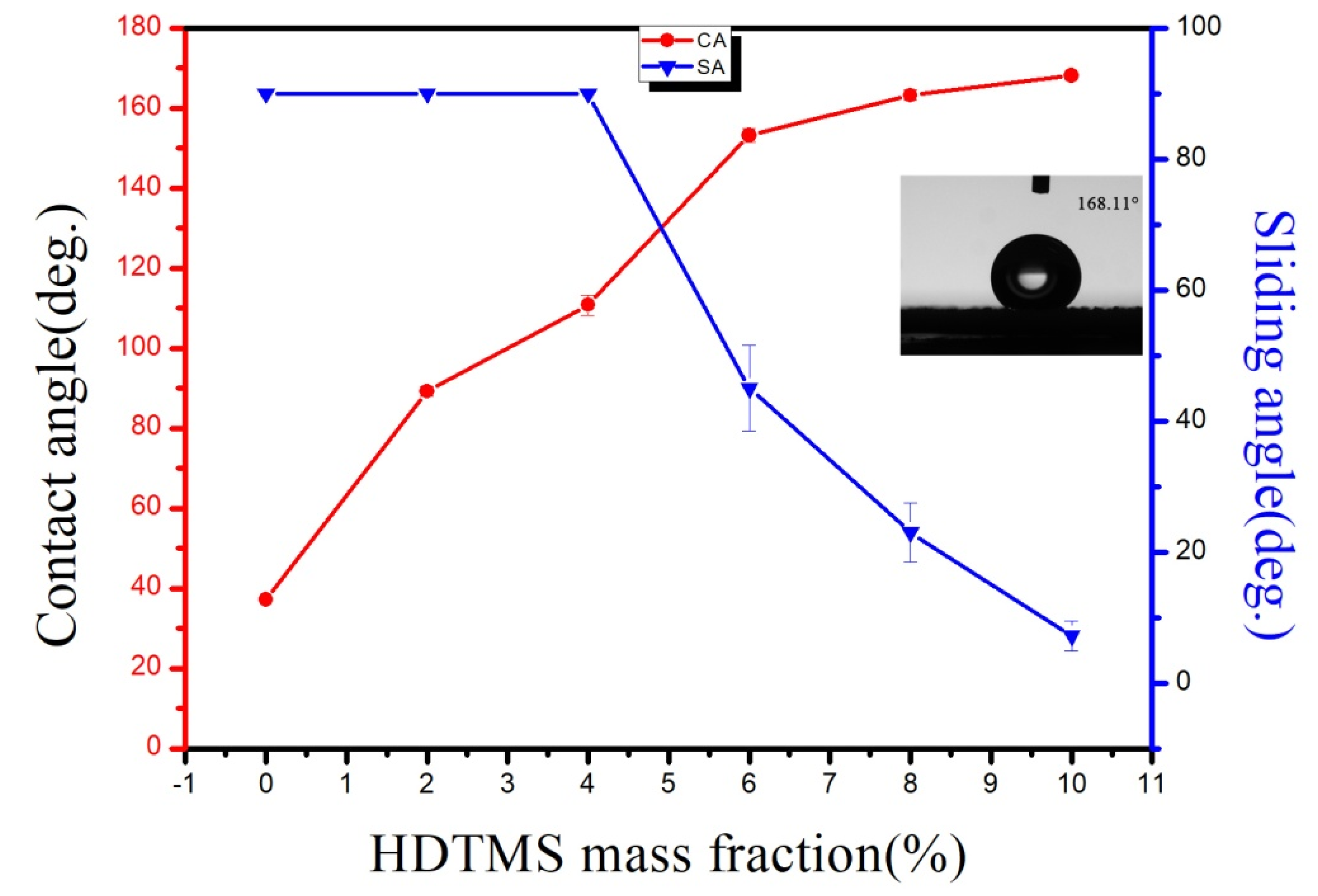
3.5. Effect of Surfactant on Hydrophobicity
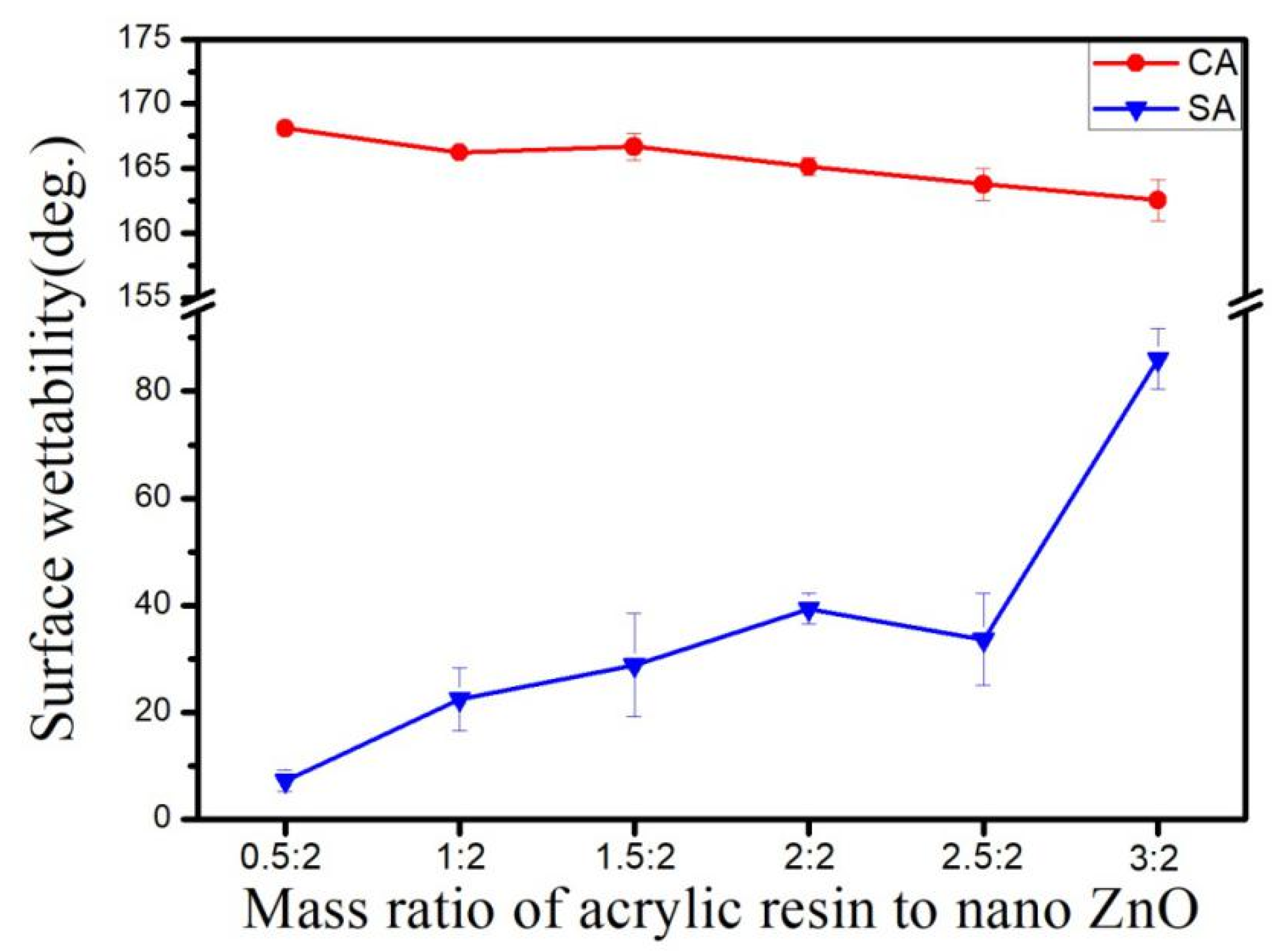
3.6. Effect of Weight Ratio of Acrylic Resin to ZnO on Hydrophobicity
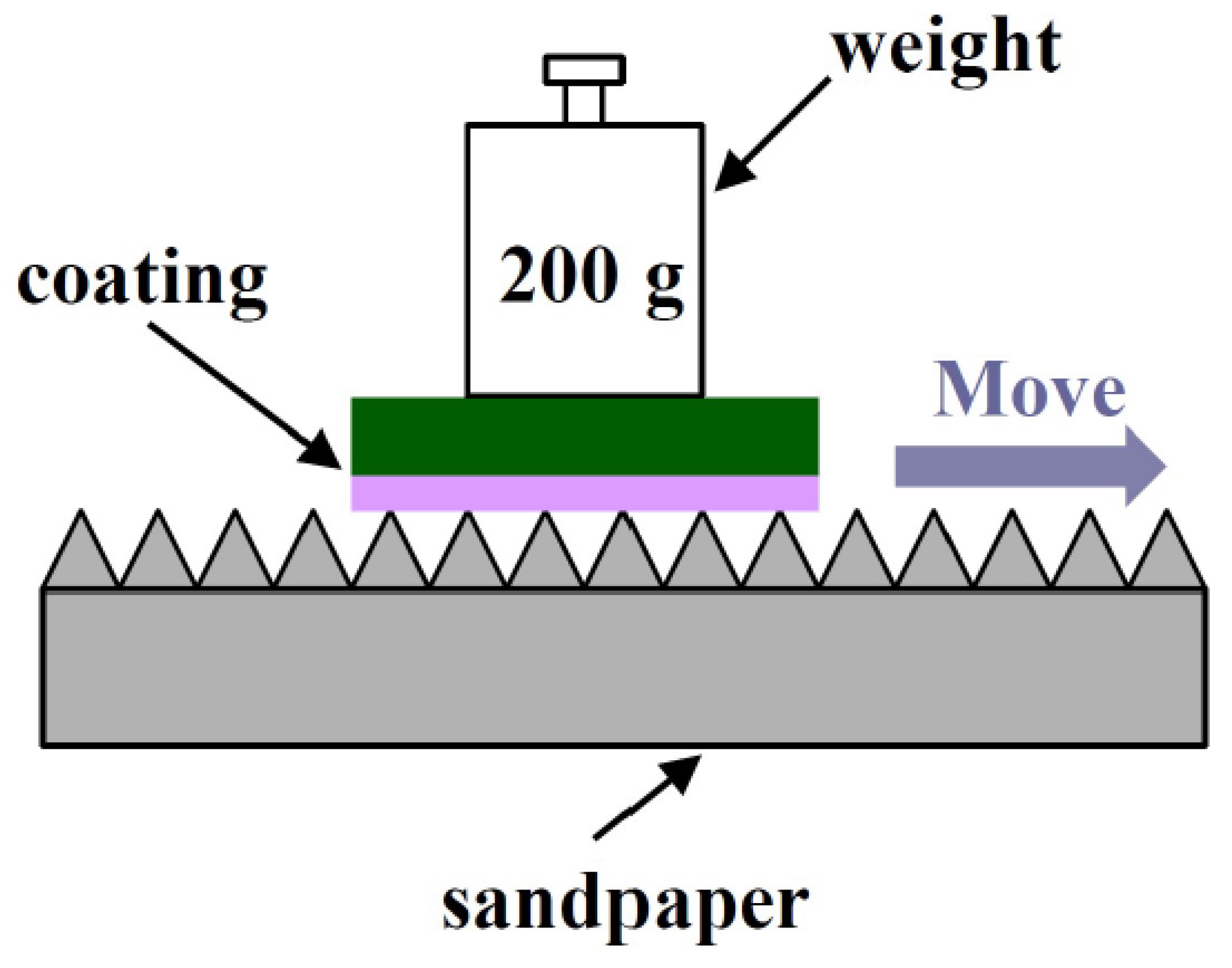
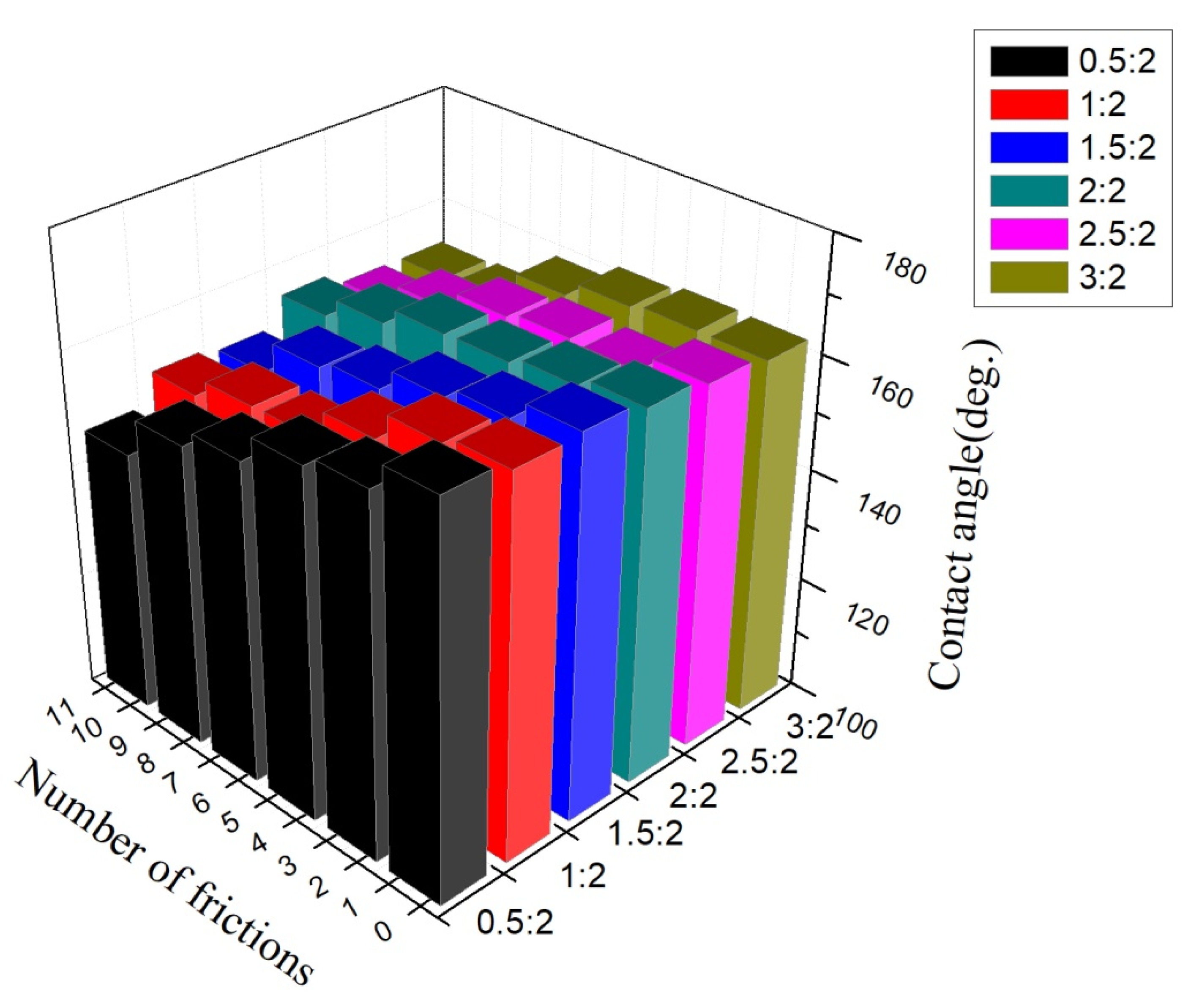
3.7. Wear Resistance
3.8. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhao, W.J.; Xu, J.H.; Mo, M.T.; Peng, S.S.; Zeng, Z.X.; Wu, X.D.; Xue, Q.J. Designing enyironmentally benign modified silica resign coatings with biomimetic textures for antibiofouling. RSC Adv. 2015, 5, 36874–36881. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent progress in antireflection and self-cleaning technology from surface engineering to functional surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Chen, H.; Zhang, J.D.; Zhao, D.J.; Liu, Y.J.; Zhou, X.Y.; Song, L.; Shi, P.; Tang, J.X.; Chen, X. Preparation and characterization of self-cleaning stable superhydrophobic linear low-density polyethylene. Sci. Technol. Adv. Mater. 2008, 9, 045007. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Szunerits, S.; Xu, W.G.; Boukherroub, R. Preparation of superhydrophobic coatings on zinc as effective corrosion barriers. ACS Appl. Mater. Inter. 2009, 1, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.F.; Yin, X.X.; Mu, P.; Wang, Q.T.; Hu, J.J.; Li, J. Slippery liquid-infused porous surface (SLIPS) with superior liquid repellency, anti-corrosion, anti-icing and intensified durability for protecting substrates. Chem. Eng. J. 2020, 401, 126137. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 2011, 21, 4699–4700. [Google Scholar] [CrossRef]
- Hu, Z.F.; Wu, X.M.; Chu, F.Q.; Zhang, X.; Yuan, Z.P. Off-centered droplet impact on single-ridge superhydrophobic surfaces. Exp. Therm. Fluid Sci. 2021, 120, 110245. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 803–814. [Google Scholar] [CrossRef]
- Pernites, R.B.; Santos, C.M.; Maldonado, M.; Ponnapati, R.R.; Rodrigues, D.F.; Advincula, R.C. Tunable protein and bacterial cell adsorption on colloidally templated superhydrophobic polythiophenefilms. Chem. Mater. 2011, 24, 870–880. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.J.; Yin, J.H.; Ming, W.H. Anti-bioadhesion on hierarchically structured, superhydrophobic surfaces. Chem. Commun. 2013, 49, 9191–9193. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, P.V.; Vanithakumari, S.C.; Gopal, J.; Mudali, U.K.; Raj, B. Enhancing corrosion and biofouling resistance through superhydrophobic surface modification. Curr. Sci. India. 2011, 101, 1328–1336. [Google Scholar]
- Wang, Z.H.; Zuilhof, H. Self-healing superhydrophobic fluoropolymer brushes as highly protein-repellent coatings. Langmuir 2016, 32, 6310–6318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Mittal, N.; Sharma, A. Multifunctional mesoporous carbon capsules and their robust coatings for encapsulation of actives: Antimicrobial and anti-bioadhesion functions. ACS Appl. Mater. Interfaces 2017, 9, 19371–19379. [Google Scholar] [CrossRef]
- Greene, G.W.; Ortiz, V.; Pozo-Gonzalo, C.; Moulton, S.E.; Wang, X.; Martin, L.L.; Michalczky, A.; Howlett, P.C. Lubricin antiadhesive coatings exhibit size-selective transport properties that inhibit biofouling of electrode surfaces with minimal loss in electrochemical activity. Adv. Mater. Interfaces 2018, 5, 1701296. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, Y.L.; Fleischer, C.C.; Huang, J.; Lin, R.; Yang, L.; Mao, H. Impact of anti-biofouling surface coatings on the properties of nanomaterials and their biomedical applications. J. Mater. Chem. B 2018, 6, 9–24. [Google Scholar] [CrossRef]
- Fan, L.; Li, B.; Zhang, J. Antibioadhesive superhydrophobic syringe needles inspired by mussels and lotus leafs. Adv. Mater. Interfaces 2015, 2, 1500019. [Google Scholar] [CrossRef]
- Dou, X.Q.; Zhang, D.; Feng, C.L.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Jin, C.F.; Jiang, Y.F.; Niu, T.; Huang, J.G. Cellulose-based material with amphiphobicity to inhibit bacterial adhesion by surface modification. J. Mater. Chem. 2012, 22, 12562–12567. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Zulkarneev, E.R.; Kiseleva, I.A.; Vasiliev, A.L.; Emelyanenko, A.M. Effective antibacterial nanotextured surfaces based on extreme wettability and bacteriophage seeding. ACS Appl. Nano. Mater. 2018, 1, 1348–1359. [Google Scholar]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP nanofibrous mats with antibacterial and anti-biofouling properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Marmur, A. Underwater superhydrophobicity: Theoretical feasibility. Langmuir 2006, 22, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Lei, S.; Ou, J.F.; Xue, M.S.; Li, W. Superhydrophobic surface with excellent mechanical durability and easy repairability. Appl. Surf. Sci. 2013, 276, 397–400. [Google Scholar] [CrossRef]
- Li, C.Q.; Xie, C.; Ou, J.F.; Xue, M.S.; Wang, F.J.; Lei, S.; Fang, X.Z.; Zhou, H.Y.; Li, W. ZnO superhydrophobic coating via convenient spraying and its biofouling resistance. Surf. Interface Anal. 2018, 50, 1278–1285. [Google Scholar] [CrossRef]
- Li, C.Q.; Zhan, Y.L.; Lei, S.; Li, W. Preparations of superhydrophobic surfaces using the one-step spin coating method and characterizations of their anti-icing behavior. Int. J. Mater. Res. 2019, 110, 1135–1141. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Chen, J.M.; Kuo, R.R.; Lin, T.S.; Wu, C.F. Influence of surface roughness on water-and oil-repellent surfaces coated with nanoparticles. Appl. Surf. Sci. 2005, 240, 318–326. [Google Scholar] [CrossRef]
- Tran, N.G.; Chun, D. Green manufacturing of extreme wettability contrast surfaces with superhydrophilic and superhydrophobic patterns on aluminum. J. Mater. Process. Technol. 2021, 297, 117–245. [Google Scholar] [CrossRef]
- Usman, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Gbadamosi, A.O.; El Badawy, T.H.; Said, K.A.M. An overview of superhydrophobic ceramic membrane surface modification for oil-water separation. J. Mater. Res. Technol. 2021, 12, 643–667. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO Nanocatalyst. Sci. Rep. 2018, 8, 3925. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Zhai, J.; Wang, Y.L.; Jiang, L. Self-assembly of amino-functionalized monolayers on silicon surfaces and preparation of superhydrophobic surfaces based on alkanoic acid dual layers and surface roughening. J. Colloid. Interf. Sci. 2006, 298, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Diao, P.; Cai, S. Highly hydrophilic and superhydrophobic ZnO nanorod array films. Thin Solid Film. 2007, 515, 7162–7166. [Google Scholar] [CrossRef]
- Sakai, M.; Kono, H.; Nakajima, A.; Zhang, X.T.; Sakai, H.; Abe, M.; Fujishima, A. Sliding of water droplets on the superhydrophobic surface with ZnO nanorods. Langmuir 2009, 25, 14182–14186. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Zheng, Y.; Yang, Z. Spray fabrication of superhydrophobic coating on aluminum alloy for corrosion mitigation. Mater. Today Commun. 2021, 26, 101828. [Google Scholar] [CrossRef]
- Lee, D.; Park, D.; Shin, K.; Seo, H.M.; Lee, H.; Choi, Y.; Kim, J.W. ZnO nanoparticles-laden cellulose nanofibers-armored Pickering emulsions with improved UV protection and water resistance. J. Ind. Eng. Chem. 2021, 96, 219–225. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Liu, F.; Xu, L.; Zhang, Z.C.; Han, E.H.; Ke, W.; Arukalam, I.O. Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 2019, 127, 239–251. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Z.; Wang, X.Q.; Zhang, Z.; Zhang, Q.; Yan, B.; Wang, Y. A compound of ZnO/PDMS with photocatalytic, self-cleaning and antibacterial properties prepared via two-step method. Appl. Surf. Sci. 2021, 550, 149286. [Google Scholar] [CrossRef]
- Xie, C.; Li, C.Q.; Xie, Y.; Cao, Z.; Li, S.Q.; Zhao, J.S.; Wang, M. ZnO/Acrylic polyurethane nanocomposite superhydrophobic coating on aluminum substrate obtained via spraying and Co-Curing for the control of marine biofouling. Surf. Interfaces 2021, 22, 100833. [Google Scholar] [CrossRef]


| Sample | Colony Counts | Antibacterial Rate |
|---|---|---|
| nano-ZnO/acrylic resin superhydrophobic coating | 0 | 100% |
| Comparison sample (pure acrylic resin coating) | 57 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wang, C.; Li, Z.; Cao, Z.; Xie, Y.; Xue, M.; Zhao, J. Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties. Materials 2021, 14, 3775. https://doi.org/10.3390/ma14143775
Li C, Wang C, Li Z, Cao Z, Xie Y, Xue M, Zhao J. Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties. Materials. 2021; 14(14):3775. https://doi.org/10.3390/ma14143775
Chicago/Turabian StyleLi, Changquan, Chen Wang, Ziang Li, Zhenjun Cao, Yu Xie, Mingshan Xue, and Jinsheng Zhao. 2021. "Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties" Materials 14, no. 14: 3775. https://doi.org/10.3390/ma14143775
APA StyleLi, C., Wang, C., Li, Z., Cao, Z., Xie, Y., Xue, M., & Zhao, J. (2021). Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties. Materials, 14(14), 3775. https://doi.org/10.3390/ma14143775






