Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth
Abstract
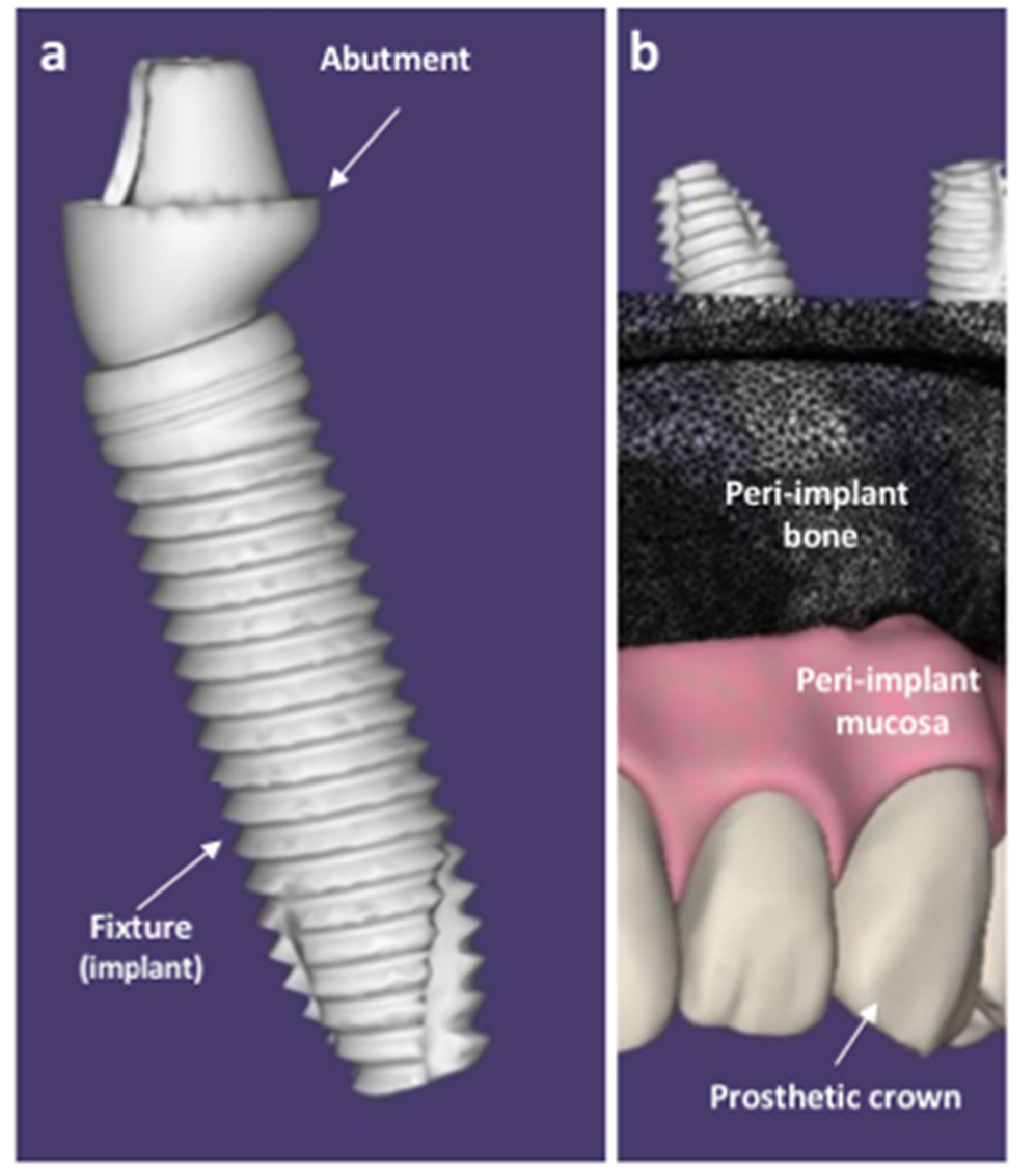
:1. Introduction
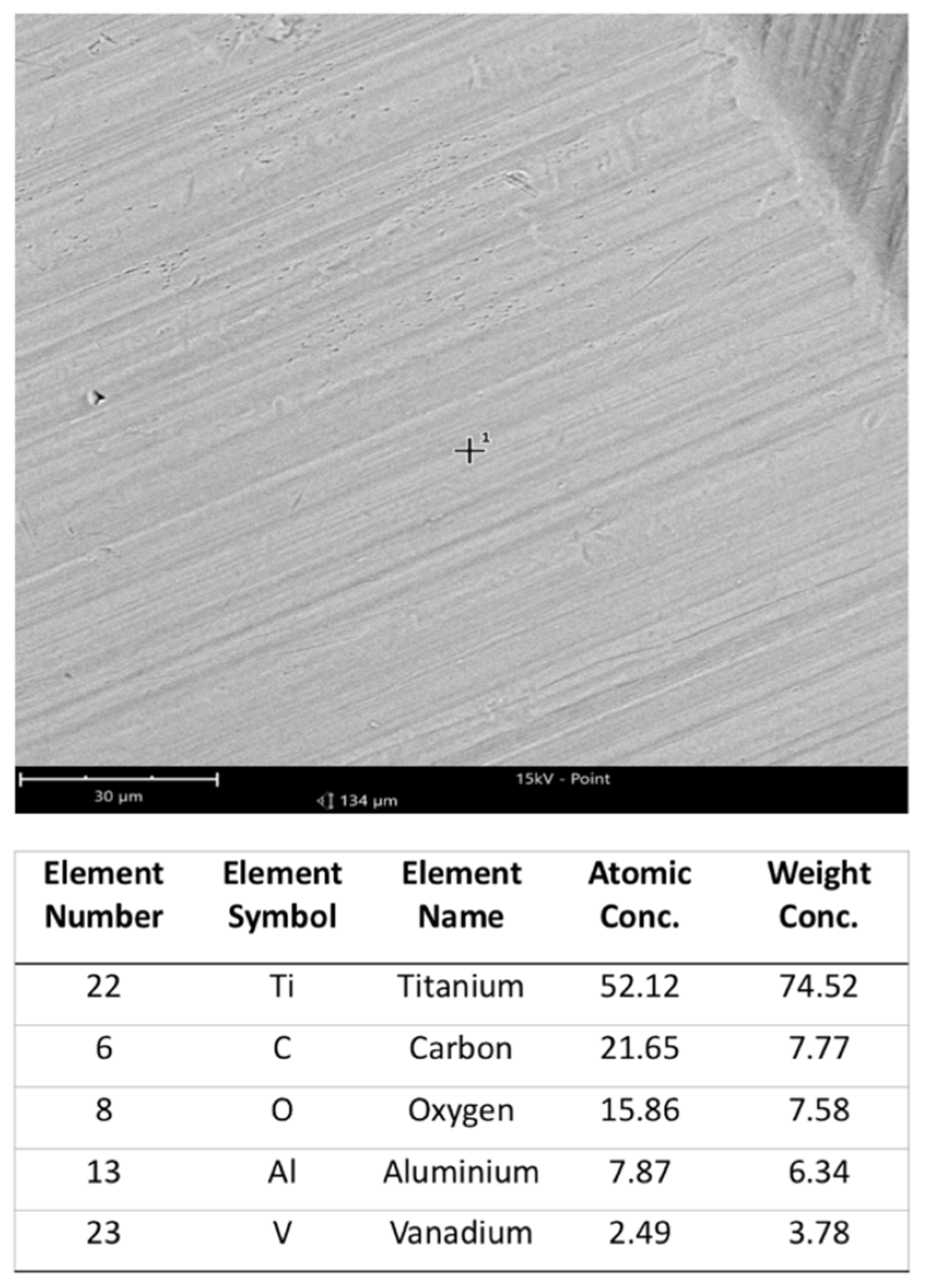
2. Materials and Methods
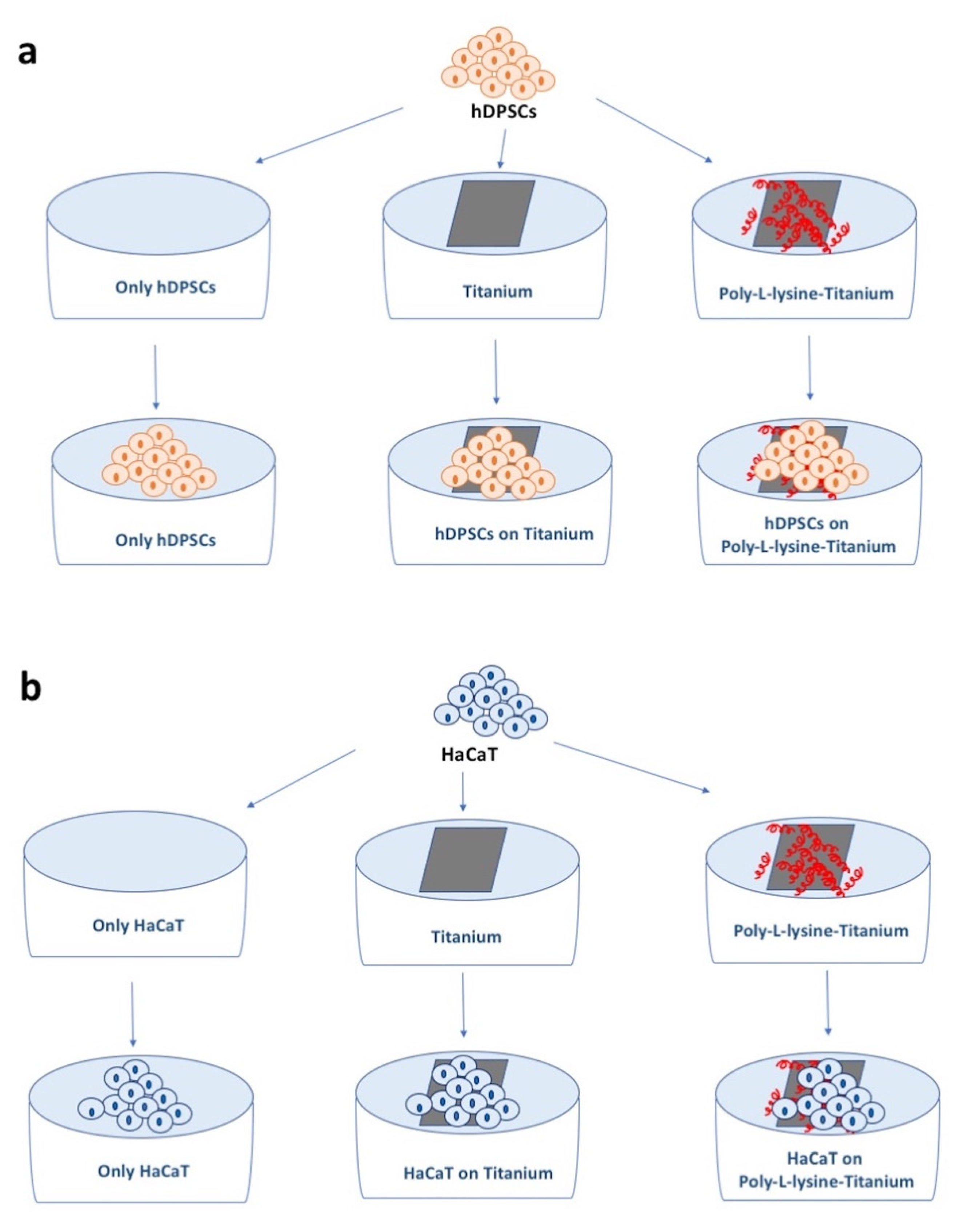
2.1. hDPSCs Culture and Growth Curve
MTT Analyses
2.2. HaCaT Cells Culture and Growth Curve
2.3. Statistical Analyses
3. Results
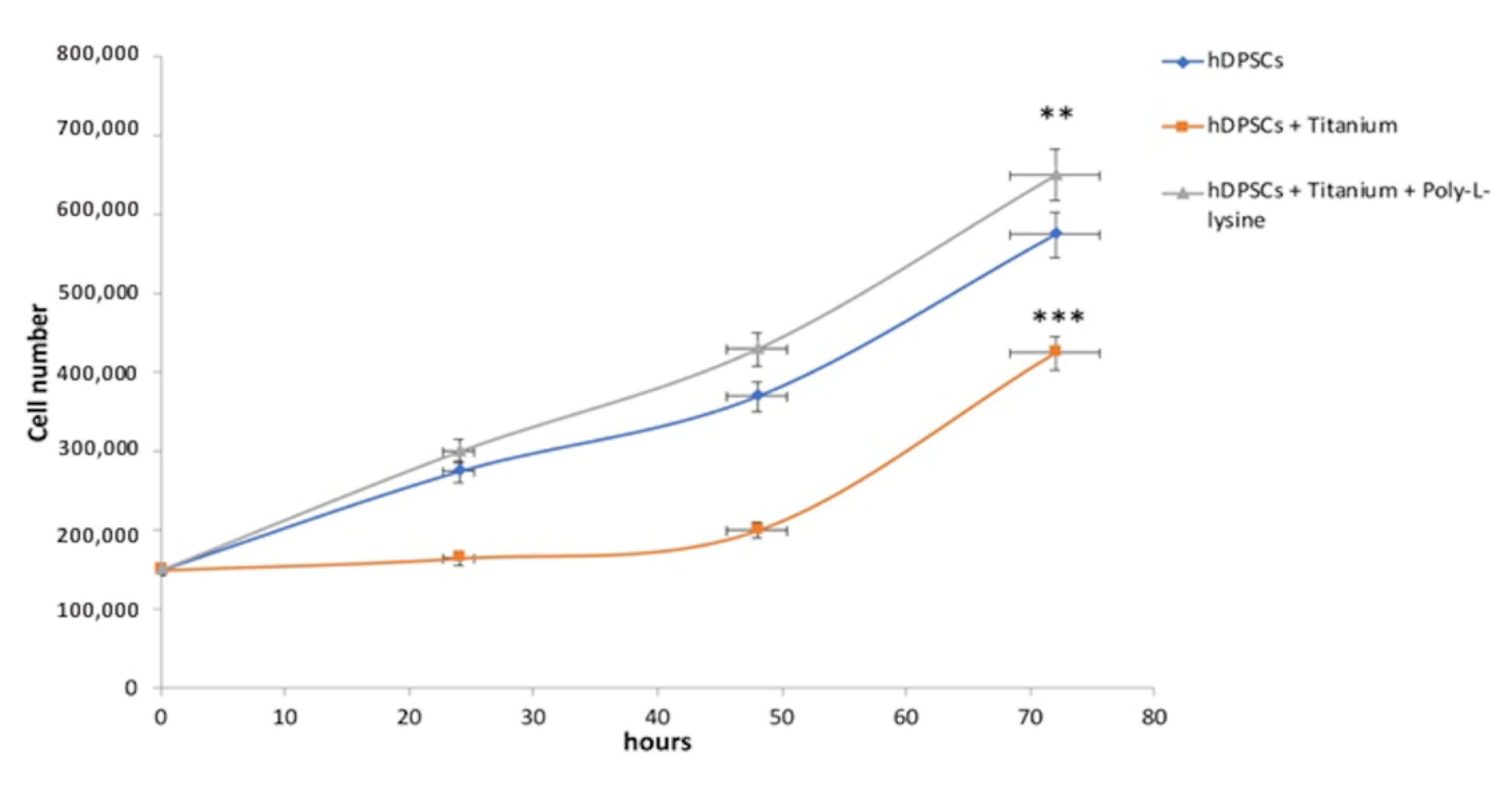
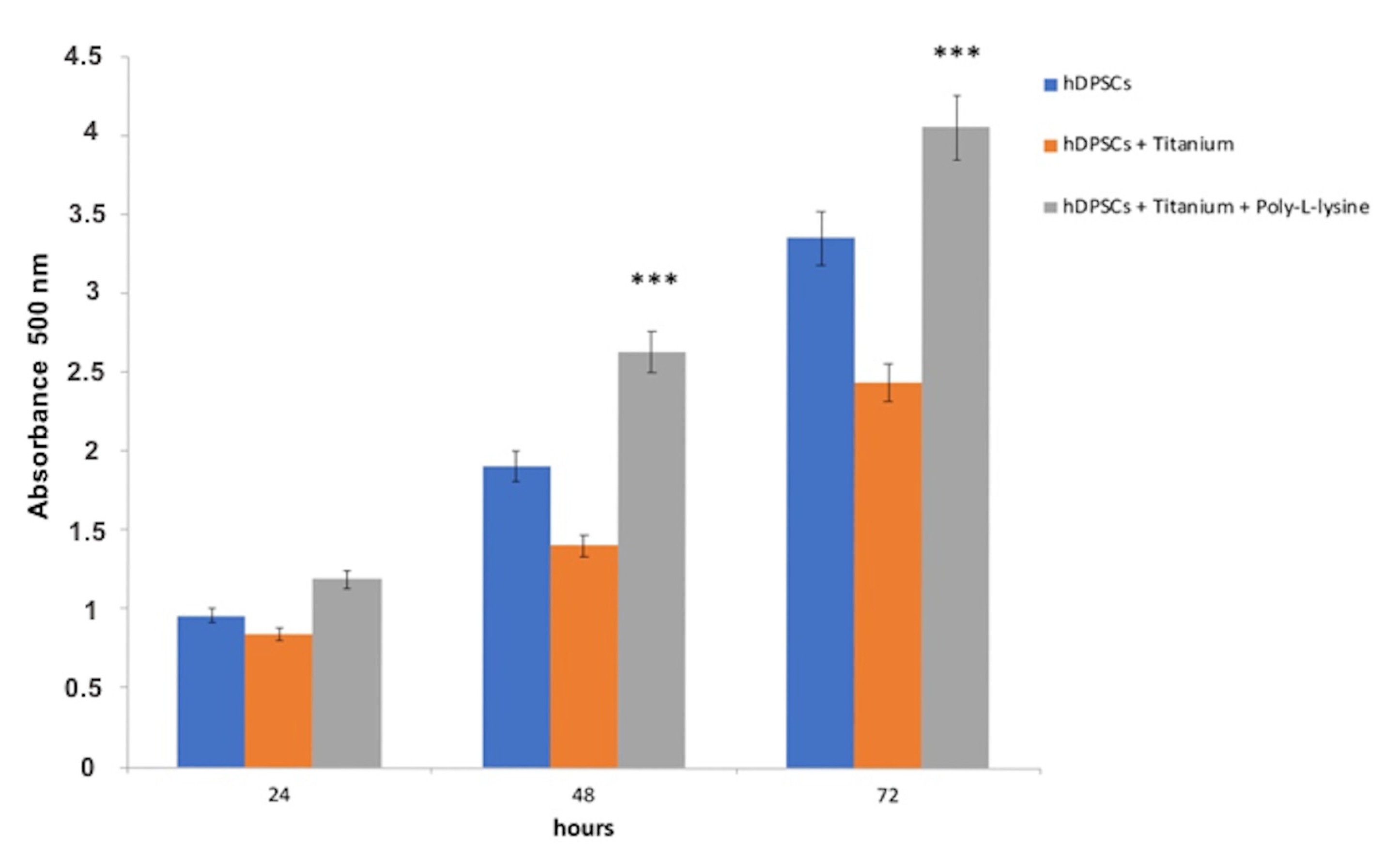
3.1. hDPSCs Growth Curves Analyses
3.2. MTT Evaluation in hDPSCs
3.3. HaCaT Viability and Proliferation: Mucoproliferative Effects of Titanium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majkowska-Marzec, B.; Tęczar, P.; Bartmański, M.; Bartosewicz, B.; Jankiewicz, B.J. Mechanical and Corrosion Properties of Laser Surface-Treated Ti13Nb13Zr Alloy with MWCNTs Coatings. Materials 2020, 13, 3991. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Marquez, D.; Delmar, Y.; Sun, S.; Stewart, R.A. Exploring Macroporosity of Additively Manufactured Titanium Metamaterials for Bone Regeneration with Quality by Design: A Systematic Literature Review. Materials 2020, 13, 4794. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant primary stability with an osteocondensation drilling protocol in different density polyurethane blocks. Comput. Methods Biomech. Biomed. Engin. 2021, 24, 14–20. [Google Scholar] [CrossRef]
- Inchingolo, F.; Paracchini, L.; DE Angelis, F.; Cielo, A.; Orefici, A.; Spitaleri, D.; Santacroce, L.; Gheno, E.; Palermo, A. Biomechanical behaviour of a jawbone loaded with a prosthetic system supported by monophasic and biphasic implants. Oral Implantol. 2017, 9, 65–70. [Google Scholar] [CrossRef]
- Bavetta, G.; Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, G.; Gargiulo Isacco, C.; Scarano, A.; De Vito, D.; et al. A Retrospective Study on Insertion Torque and Implant Stability Quotient (ISQ) as Stability Parameters for Immediate Loading of Implants in Fresh Extraction Sockets. Biomed. Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef] [Green Version]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1997, 16, 1–132. [Google Scholar]
- Annunziata, M.; Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Front. Oral Biol. 2015, 17, 62–77. [Google Scholar]
- Pawelec, K.; White, A.A.; Best, S.M. Properties and characterization of bone repair materials. In Woodhead Publishing Series in Biomaterials, Bone Repair Biomaterials, 2nd ed.; Pawelec, K.M., Planell, J.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 65–102. [Google Scholar]
- Antonelli, A.; Bennardo, F.; Brancaccio, Y.; Barone, S.; Femiano, F.; Nucci, L.; Minervini, G.; Fortunato, L.; Attanasio, F.; Giudice, A. Can Bone Compaction Improve Primary Implant Stability? An In Vitro Comparative Study with Osseodensification Technique. Appl. Sci. 2020, 10, 8623. [Google Scholar] [CrossRef]
- Zemtsova, E.; Arbenin, A.; Valiev, R.; Smirnov, V. Modern techniques of surface geometry modification for the implants based on titanium and its alloys used for improvement of the biomedical characteristics. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–145. [Google Scholar]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Garaicoa, J.L.; Bates, A.M.; Avila-Ortiz, G.; Brogden, K.A. Antimicrobial Prosthetic Surfaces in the Oral Cavity-A Perspective on Creative Approaches. Microorganisms 2020, 8, 1247. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [Green Version]
- Barrere, F.; Layrolle, P.; van Blitterswijk, C.; de Groot, K. Biomimetic coatings on titanium: A crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2001, 12, 529–534. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Spoerke, E.D.; Stupp, S.I. Synthesis of a poly(L-lysine)-calcium phosphate hybrid on titanium surfaces for enhanced bioactivity. Biomaterials 2005, 26, 5120–5129. [Google Scholar] [CrossRef]
- Galli, D.; Benedetti, L.; Bongio, M.; Maliardi, V.; Silvani, G.; Ceccarelli, G.; Ronzoni, F.; Conte, S.; Benazzo, F.; Graziano, A.; et al. In vitro osteoblastic differentiation of human mesenchymal stem cells and human dental pulp stem cells on poly-L-lysine-treated titanium-6-aluminium-4-vanadium. J. Biomed. Mater. Res. A 2011, 97, 118–126. [Google Scholar] [CrossRef]
- Varoni, E.; Canciani, E.; Palazzo, B.; Varasano, V.; Chevallier, P.; Petrizzi, L.; Dellavia, C.; Mantovani, D.; Rimondini, L. Effect of Poly-L-Lysine coating on titanium osseointegration: From characterization to in vivo studies. J. Oral Implantol. 2015, 41, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Di Benedetto, A.; De Vito, D.; Scarano, A.; Scacco, S.; Perillo, L.; Posa, F.; Dipalma, G.; Paduano, F.; Contaldo, M.; et al. Stemness genes expression in naïve vs. osteodifferentiated human dental-derived stem cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2916–2923. [Google Scholar]
- Ballini, A.; Cantore, S.; Scacco, S.; Perillo, L.; Scarano, A.; Aityan, S.K.; Contaldo, M.; Cd Nguyen, K.; Santacroce, L.; Syed, J.; et al. A comparative study on different stemness gene expression between dental pulp stem cells vs. dental bud stem cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1626–1633. [Google Scholar]
- Di Benedetto, A.; Brunetti, G.; Posa, F.; Ballini, A.; Grassi, F.R.; Colaianni, G.; Colucci, S.; Rossi, E.; Cavalcanti-Adam, E.A.; Lo Muzio, L.; et al. Osteogenic differentiation of mesenchymal stem cells from dental bud: Role of integrins and cadherins. Stem Cell Res. 2015, 15, 618–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naddeo, P.; Laino, L.; La Noce, M.; Piattelli, A.; De Rosa, A.; Iezzi, G.; Laino, G.; Paino, F.; Papaccio, G.; Tirino, V. Surface biocompatibility of differently textured titanium implants with mesenchymal stem cells. Dent. Mater. 2015, 31, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Paino, F.; La Noce, M.; Giuliani, A.; De Rosa, A.; Mazzoni, S.; Laino, L.; Amler, E.; Papaccio, G.; Desiderio, V.; Tirino, V. Human DPSCs fabricate vascularized woven bone tissue: A new tool in bone tissue engineering. Clin. Sci. 2017, 131, 699–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangano, C.; Paino, F.; d’Aquino, R.; De Rosa, A.; Iezzi, G.; Piattelli, A.; Laino, L.; Mitsiadis, T.; Desiderio, V.; Mangano, F.; et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocomplex. PLoS ONE 2011, 6, e18721. [Google Scholar] [CrossRef] [PubMed]
- Laino, L.; La Noce, M.; Fiorillo, L.; Cervino, G.; Nucci, L.; Russo, D.; Herford, A.S.; Crimi, S.; Bianchi, A.; Biondi, A.; et al. Dental Pulp Stem Cells on Implant Surface: An In Vitro Study. BioMed Res. Int. 2021, 2021, 3582342. [Google Scholar] [CrossRef]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34, 1–10. [Google Scholar] [PubMed]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology 2000 2018, 76, 116–130. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Impl. Res. 2018, 29, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine gene polymorphisms associate with microbiogical agents in periodontal disease: Our experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Ballini, A.; De Vito, D.; Martelli, F.S.; Georgakopoulos, I.; Almasri, M.; Dibello, V.; Altini, V.; Farronato, G.; Dipalma, G.; et al. Characterization of human apical papilla-derived stem cells. J. Biol. Regul. Homeost. Agents 2017, 31, 901–910. [Google Scholar] [PubMed]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and growth factors in oral squamous cell carcinoma: Useful source of dental-derived stem cells to develop a steroidogenic model in new clinical strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar] [PubMed]
- Cosentino, C.; Di Domenico, M.; Porcellini, A.; Cuozzo, C.; De Gregorio, G.; Santillo, M.R.; Agnese, S.; Di Stasio, R.; Feliciello, A.; Migliaccio, A.; et al. p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 2007, 26, 2095–2103. [Google Scholar] [CrossRef] [Green Version]
- Donini, C.F.; Di Zazzo, E.; Zuchegna, C.; Di Domenico, M.; D’Inzeo, S.; Nicolussi, A.; Avvedimento, E.V.; Coppa, A.; Porcellini, A. The p85α regulatory subunit of PI3K mediates cAMP-PKA and retinoic acid biological effects on MCF7 cell growth and migration. Int. J. Oncol. 2012, 40, 1627–1635. [Google Scholar]
- Boccellino, M.; Di Domenico, M.; Donniacuo, M.; Bitti, G.; Gritti, G.; Ambrosio, P.; Quagliuolo, L.; Rinaldi, B. AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress. PLoS ONE 2018, 13, e0202297. [Google Scholar] [CrossRef] [Green Version]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef]
- D’Angelo, S.; La Porta, R.; Napolitano, M.; Galletti, P.; Quagliuolo, L.; Boccellino, M. Effect of Annurca apple polyphenols on human HaCaT keratinocytes proliferation. J. Med. Food 2012, 15, 1024–1031. [Google Scholar] [CrossRef]
- Tyagi, N.; Bhardwaj, A.; Srivastava, S.K.; Arora, S.; Marimuthu, S.; Deshmukh, S.K.; Singh, A.P.; Carter, J.E.; Singh, S. Development and Characterization of a Novel in vitro Progression Model for UVB-Induced Skin Carcinogenesis. Sci. Rep. 2015, 5, 13894. [Google Scholar] [CrossRef] [Green Version]
- Renò, F.; Rizzi, M.; Cannas, M. Gelatin-based anionic hydrogel as biocompatible substrate for human keratinocyte growth. J. Mater. Sci. Mater. Med. 2012, 23, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Cui, W.; Wang, X.; Lu, X.; Zhang, L.; Li, X.; Li, W.; Zhang, W.; Chen, J. Poly-l-lysine/Sodium Alginate Coating Loading Nanosilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 5, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Huang, Q.; Patil, A.B.; Hu, J.; Fan, L.; Yang, Y.; Duan, H.; Dong, X.; Lin, C. Layer-by-layer immobilizing of polydopamine-assisted ε-polylysine and gum Arabic on titanium: Tailoring of antibacterial and osteogenic properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110690. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Tang, Y.L.; Pang, X.; Zheng, M.; Tang, Y.J.; Liang, X.H. The maintenance of an oral epithelial barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.J.; Chen, L. Epithelial–Mesenchymal Interactions as a Working Concept for Oral Mucosa Regeneration. Tissue Eng. Part B Rev. 2011, 17, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Di Domenico, M.; Carratelli, C.R.; Mazzola, N.; Paolillo, R. Induction of proinflammatory cytokines in human osteoblastic cells by Chlamydia pneumoniae. Cytokine 2011, 56, 450–457. [Google Scholar] [CrossRef]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Lajolo, C.; Rupe, C.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Serpico, R. The Oral Microbiota Changes in Orthodontic Patients and Effects on Oral Health: An Overview. J. Clin. Med. 2021, 10, 780. [Google Scholar] [CrossRef]
- Rizzo, A.; Losacco, A.; Carratelli, C.R.; Domenico, M.D.; Bevilacqua, N. Lactobacillus plantarum reduces Streptococcus pyogenes virulence by modulating the IL-17, IL-23 and Toll-like receptor 2/4 expressions in human epithelial cells. Int. Immunopharmacol. 2013, 17, 453–461. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Inchingolo, F.; Dipalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1323–1328. [Google Scholar] [PubMed]
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; Dipalma, G.; Inchingolo, F.; Saini, R. Clinical results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar] [PubMed]
- Papi, P.; Letizia, C.; Pilloni, A.; Petramala, L.; Saracino, V.; Rosella, D.; Pompa, G. Peri-implant diseases and metabolic syndrome components: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 866–875. [Google Scholar]
- Boccellino, M.; Giuberti, G.; Quagliuolo, L.; Marra, M.; D’Alessandro, A.M.; Fujita, H.; Giovane, A.; Abbruzzese, A.; Caraglia, M. Apoptosis induced by interferon-alpha and antagonized by EGF is regulated by caspase-3-mediated cleavage of gelsolin in human epidermoid cancer cells. J. Cell Physiol. 2004, 201, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattoni, F.; Teté, G.; Calloni, A.M.; Manazza, F.; Gastaldi, G.; Capparè, P. Milled versus moulded mock-ups based on the superimposition of 3D meshes from digital oral impressions: A comparative in vitro study in the aesthetic area. BMC Oral Health 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Lucchese, A.; Gentile, E.; Zulli, C.; Petruzzi, M.; Lauritano, D.; Amato, M.R.; Esposito, P.; Riegler, G.; Serpico, R. Evaluation of the intraepithelial papillary capillary loops in benign and malignant oral lesions by in vivo Virtual Chromoendoscopic Magnification: A preliminary study. J. Biol. Regul. Homeost. Agents 2017, 31, 11–22. [Google Scholar]
- Contaldo, M.; Lauritano, D.; Carinci, F.; Romano, A.; Di Stasio, D.; Lajolo, C.; Della Vella, F.; Serpico, R.; Lucchese, A. Intraoral confocal microscopy of suspicious oral lesions: A prospective case series. Int. J. Dermatol. 2020, 59, 82–90. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; della Vella, F.; Lauritano, D.; Serpico, R.; Santoro, R.; Lucchese, A. Real Time In Vivo Confocal Microscopic Analysis of the Enamel Remineralization by Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP): A Clinical Proof-of-Concept Study. Appl. Sci. 2020, 10, 4155. [Google Scholar] [CrossRef]
- Gentile, E.; Di Stasio, D.; Santoro, R.; Contaldo, M.; Salerno, C.; Serpico, R.; Lucchese, A. In vivo microstructural analysis of enamel in permanent and deciduous teeth. Ultrastruct. Pathol. 2015, 39, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Camerlingo, C.; d’Apuzzo, F.; Grassia, V.; Perillo, L.; Lepore, M. Micro-Raman spectroscopy for monitoring changes in periodontal ligaments and gingival crevicular fluid. Sensors 2014, 14, 22552–22563. [Google Scholar] [CrossRef] [Green Version]
- Boccellino, M.; Quagliuolo, L.; Verde, A.; La Porta, R.; Crispi, S.; Piccolo, M.T.; Vitiello, A.; Baldi, A.; Signorile, P.G. In vitro model of stromal and epithelial immortalized endometriotic cells. J. Cell Biochem. 2012, 113, 1292–1301. [Google Scholar] [CrossRef]
- Pedata, P.; Boccellino, M.; La Porta, R.; Napolitano, M.; Minutolo, P.; Sgro, L.A.; Zei, F.; Sannolo, N.; Quagliuolo, L. Interaction between combustion-generated organic nanoparticles and biological systems: In vitro study of cell toxicity and apoptosis in human keratinocytes. Nanotoxicology 2012, 6, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Milillo, L.; Marinosci, F.; Lo Muzio, L.; Serpico, R.; Antonaci, S. Altered expression of integrins and basement membrane proteins in malignant and pre-malignant lesions of oral mucosa. J. Biol. Regul. Homeost. Agents 2001, 15, 375–380. [Google Scholar] [PubMed]
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial Activity of Indolicidin-Coated Silver Nanoparticles in Oral Disease. Appl. Sci. 2020, 10, 1837. [Google Scholar] [CrossRef] [Green Version]
- Tetè, G.; Cisternino, L.; Giorgio, G.; Sacchi, L.; Montemezzi, P.; Sannino, G. Immediate versus delayed loading of post-extraction implants in the aesthetic zone: A prospective longitudinal study with 4-year follow-up. J. Biol. Regul. Homeost. Agents 2020, 34, 19–25. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735. https://doi.org/10.3390/ma14133735
Contaldo M, De Rosa A, Nucci L, Ballini A, Malacrinò D, La Noce M, Inchingolo F, Xhajanka E, Ferati K, Bexheti-Ferati A, et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials. 2021; 14(13):3735. https://doi.org/10.3390/ma14133735
Chicago/Turabian StyleContaldo, Maria, Alfredo De Rosa, Ludovica Nucci, Andrea Ballini, Davide Malacrinò, Marcella La Noce, Francesco Inchingolo, Edit Xhajanka, Kenan Ferati, Arberesha Bexheti-Ferati, and et al. 2021. "Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth" Materials 14, no. 13: 3735. https://doi.org/10.3390/ma14133735
APA StyleContaldo, M., De Rosa, A., Nucci, L., Ballini, A., Malacrinò, D., La Noce, M., Inchingolo, F., Xhajanka, E., Ferati, K., Bexheti-Ferati, A., Feola, A., & Di Domenico, M. (2021). Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials, 14(13), 3735. https://doi.org/10.3390/ma14133735











