Hydrogen-Assisted Brittle Fracture Behavior of Low Alloy 30CrMo Steel Based on the Combination of Experimental and Numerical Analyses
Abstract
:1. Introduction
2. Material and Experiment
3. Model Framework
3.1. The First Step: Elastic-Plastic Analysis
3.2. The Second Step: Hydrogen Diffusion Analysis
3.3. The Third Step: Cohesive Analysis
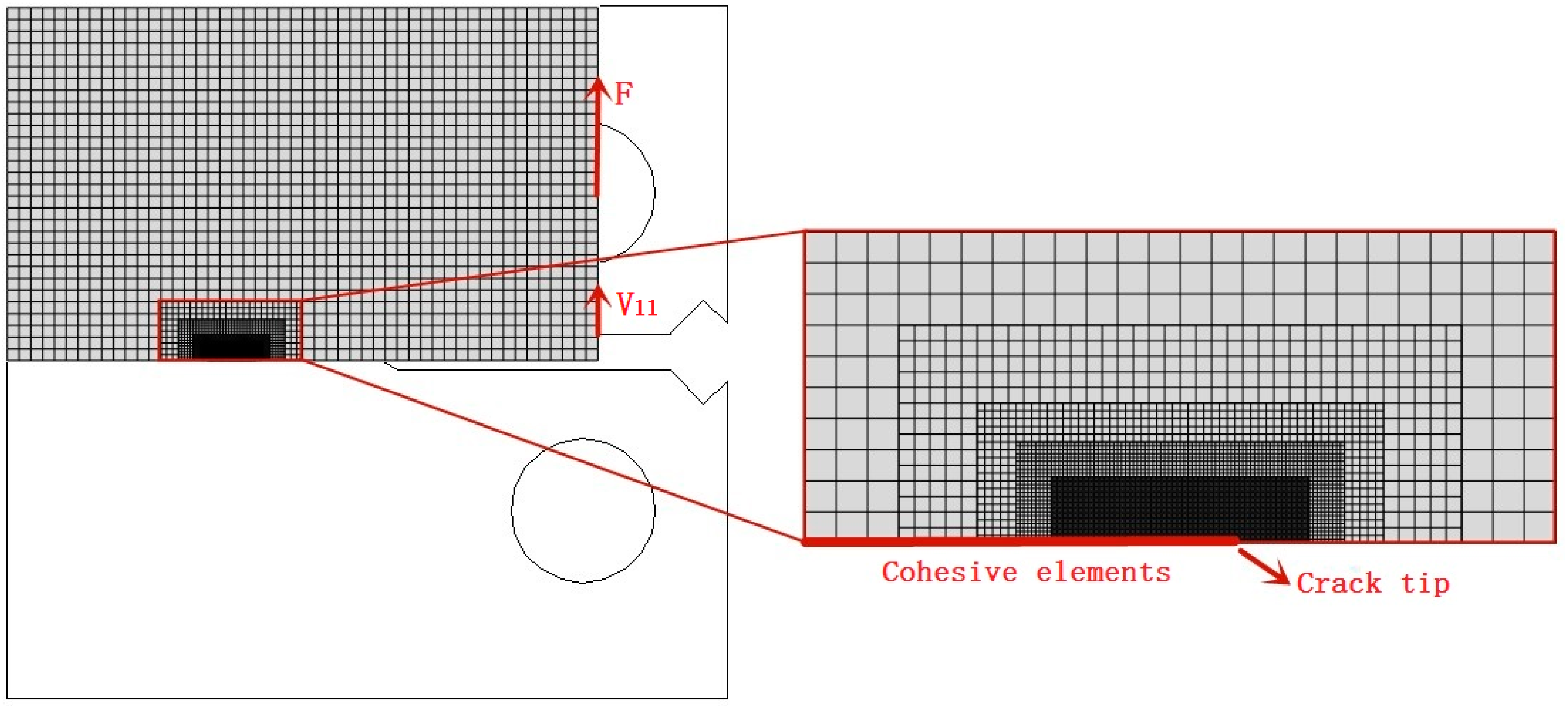
4. Finite Element (FE) Computational Model
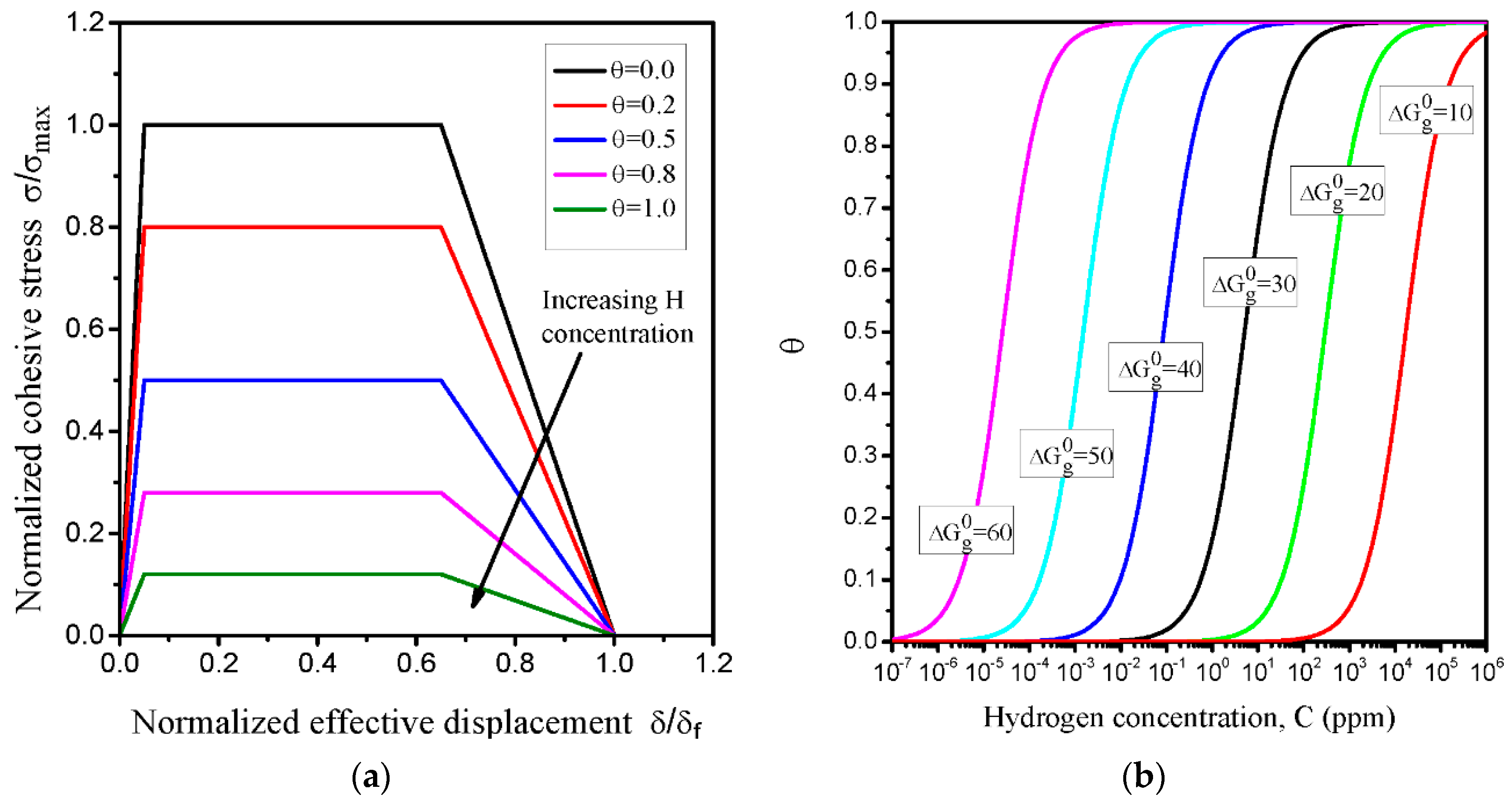
4.1. Selection of Trapezoidal Traction-Separation Law (TSL) Parameters
4.2. Implementing Hydrogen-Influenced TSL
5. Numerical Results and Discussion
6. Conclusions
- From the fracture toughness test, the CTOD-R curves show a decreasing trend with the increase in the hydrogen charging time, which leads to the higher crack propagation and the lower crack growth resistance. This trend is also reflected in the change of fracture micromechanism: MVC is a dominant mode in an uncharged specimen, changing to QC fracture and to QC and IG fracture under hydrogen-charged condition.
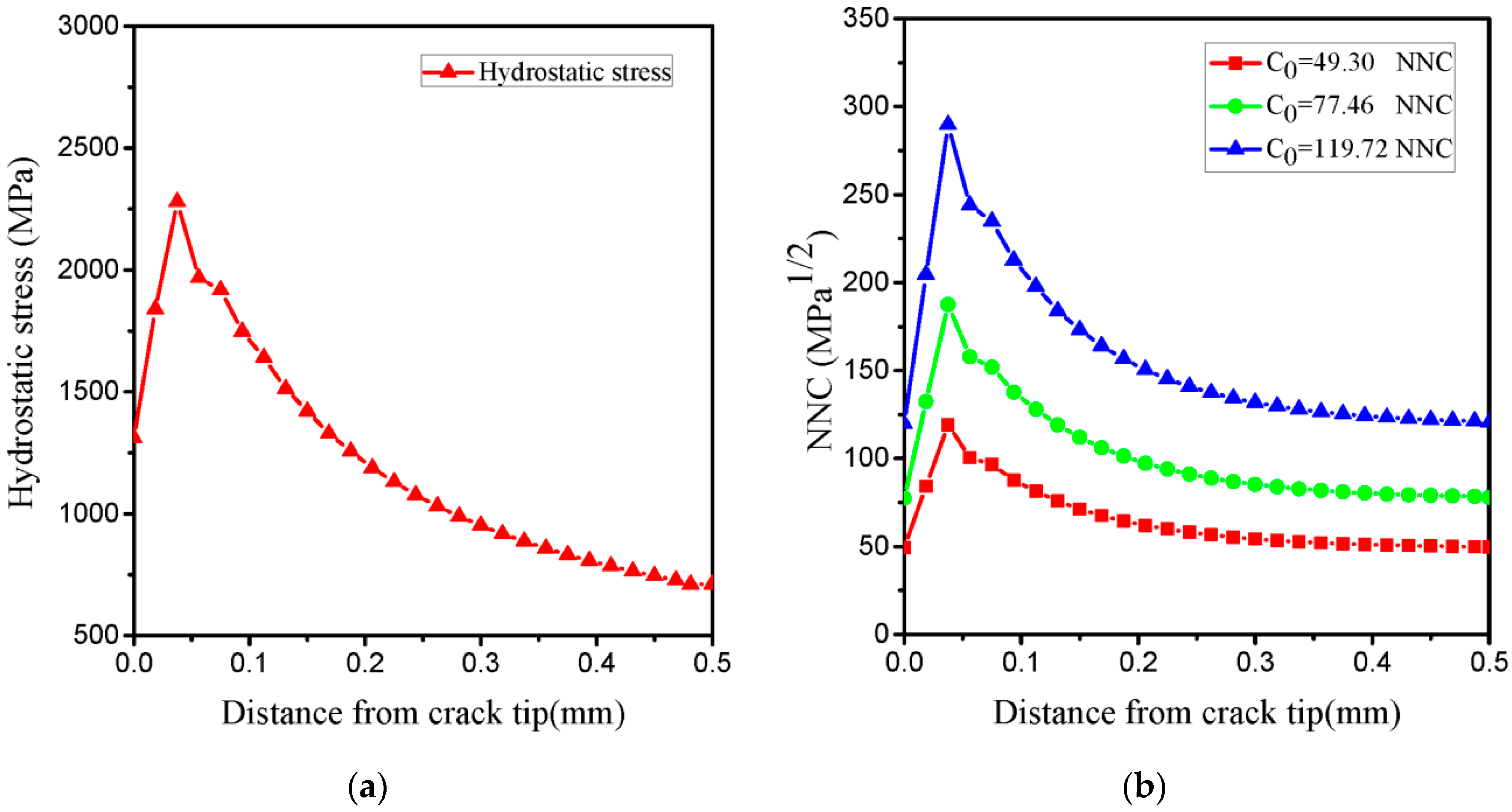
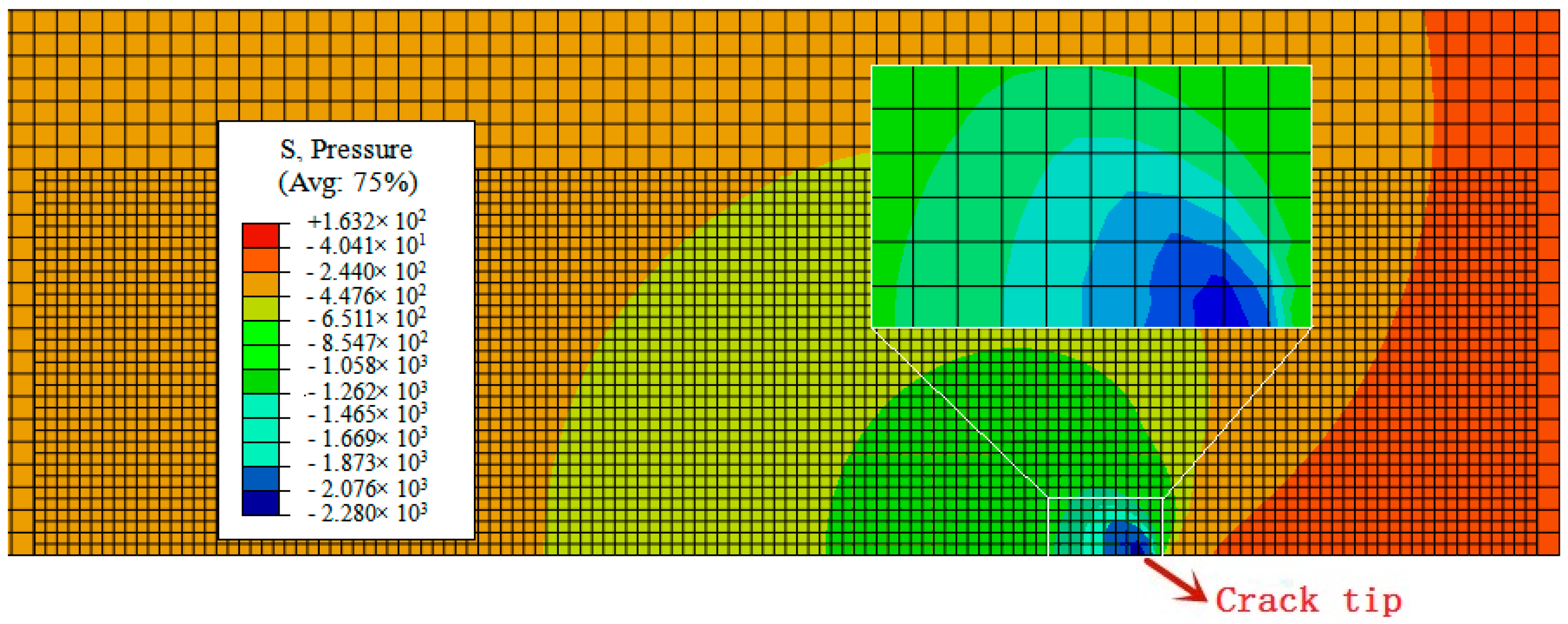
- In our simulation, the peak of hydrogen concentration (NNC) corresponds to the peak of hydrostatic stress, and is formed ahead of the crack tip. This result can explain the experimental phenomena well: hydrogen atoms diffusing ahead of the crack tip, thereby promoting the hydrogen embrittlement indexes.
- The initial TSL parameters from the best fit to the load-displacement and J-R experimental curves without hydrogen were calibrated for the critical cohesive separation of 0.0393 mm and the cohesive strength of 2100 MPa. According to FEM and experimental curves, the initial normalized hydrogen concentrations corresponding to hydrogen-charged specimens for 12, 24 and 36 h were estimated 49.30, 77.46 and 119.72 NNC (corresponding to initial hydrogen concentration in lattice sites of 3.5, 5.5 and 8.5 ppm), respectively.
- The general trend of the computational CTOD-R curves is the same as that obtained from the experimental data but not fully in agreement, the computational CTOD values are slightly higher. Therefore, it is possible for more (HE) mechanisms to work together in order to predict the crack extension resistance correctly. The numerical model can provide design and prediction to calculate hydrogen-assisted fracture behavior prior to extensive laboratory testing, provided that the material properties and properly calibrated TSL parameters are known.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharp, V.J.; Billingham, J.; Stacey, A. Performance of high strength steels used in jack-ups. Mar. Struct. 1999, 12, 349–370. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–216161. [Google Scholar] [CrossRef]
- Shimotomai, M. Heuristic Design of Advanced Martensitic Steels That Are Highly Resistant to Hydrogen Embrittlement by ε-Carbide. Metals 2021, 11, 370. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Determination of the critical hydrogen concentration for delayed fracture of high strength steel by constant load test and numerical calculation. Corros. Sci. 2006, 48, 2189–2202. [Google Scholar] [CrossRef]
- Yatabe, H.; Yamada, K.; Rios de Los, E.R.; Miller, K.J. Formation of hydrogen-assisted intergranular cracks in high strength steels. Fatigue Fract. Eng. Mater. Struct. 1995, 18, 377–384. [Google Scholar] [CrossRef]
- Miresmaeili, R.; Ogino, M.; Nakagawa, T.; Kanayama, H. A coupled elastoplastic-transient hydrogen diffusion analysis to simulate the onset of necking in tension by using the finite element method. Int. J. Hydrogen Energy 2010, 35, 1506–1514. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of low carbon structural steel. Proc. Mater. 2014, 3, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Troiano, A. The role of hydrogen and other interstitials in the mechanical behaviour of metals. Trans. Am. Soc. Met. 1960, 52, 54–80. [Google Scholar]
- Guzmán, A.A.; Jeon, J.; Hartmaier, A.; Janisch, R. Hydrogen Embrittlement at Cleavage Planes and Grain Boundaries in Bcc Iron-Revisiting the First-Principles Cohesive Zone Model. Materials 2020, 13, 5785. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.; Wang, S.; Gross, D.; Nygren, K. Hydrogen embrittlement understood. Met. Mater. Trans. A Phys. 2015, 46, 2323–2341. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.K.; Seok, M.Y.; Choi, I.C.; Lee, Y.H.; Park, S.J.; Ramamurty, U.; Suh, J.Y.; Jang, J. The role of hydrogen in hardening/softening steel: Influence of the charging process. Scr. Mater. 2015, 107, 46–49. [Google Scholar] [CrossRef]
- Liang, Y.; Sofronis, P.; Aravas, N. On the effect of hydrogen on plastic instabilities in metals. Acta Mater. 2003, 51, 2717–2730. [Google Scholar] [CrossRef]
- Stepanova, E.; Grabovetskaya, G.; Syrtanov, M.; Mishin, I. Effect of Hydrogen on the Deformation Behavior and Localization of Plastic Deformation of the Ultrafine-Grained Zr–1Nb Alloy. Metals 2020, 10, 592. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of industrial components: Prediction, prevention and models. Corros. Sci. 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; SijackiZeravcic, V.C.; Rajicic, B.; Miskovic, Z. Towards a unified and practical industrial model for prediction of hydrogen embrittlement and damage in steels. Procedia Struct. Integr. 2016, 2, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Novak, P.; Yuan, R.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. A statistical, physical-based, micro-mechanical model of hydrogen-induced intergranular fracture in steel. J. Mech. Phys. Solids 2010, 58, 105–123. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B.; Ngo, T.D. Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng. Fract. Mech. 2021, 123, 105312. [Google Scholar]
- Zafra, A.; Álvarez, G.; Belzunce, J.; Alegre, J.; Rodríguez, C. Fracture toughness of coarse-grain heat affected zone of quenched and tempered CrMo steels with internal hydrogen: Fracture micromechanisms. Eng. Fract. Mech. 2021, 241, 107433. [Google Scholar] [CrossRef]
- Peral, L.B.; Zafra, A.; Belzunce, J.; Rodríguez, C. Effects of hydrogen on the fracture toughness of CrMo and CrMoV steels quenched and tempered at different temperatures. Int. J. Hydrogen Energy 2019, 44, 3953–3965. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, J.; Akiyama, E.; Wang, Y.F.; Li, Q.Z. Microstructural and crystallographic study of hydrogen-assisted cracking in high strength PSB1080 steel. Int. J. Hydrogen Energy 2018, 43, 17898–17911. [Google Scholar] [CrossRef]
- Wu, W.J.; Wang, Y.F.; Tao, P.; Li, F.X.; Gong, J.M. Cohesive zone modeling of hydrogen-induced delayed intergranular fracture in high strength steels. Results Phys. 2018, 11, 591–598. [Google Scholar] [CrossRef]
- Thomas, R.L.S.; Scully, J.R.; Gangloff, R. Internal hydrogen embrittlement of ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. 2003, 34, 327–344. [Google Scholar] [CrossRef]
- Sofronis, P.; McMeeking, R.M. Numerical analysis of hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1989, 37, 317–350. [Google Scholar] [CrossRef]
- Krom, A.H.M.; Bakker, A.D. Hydrogen trapping models in steel. Met. Mater. Trans. B. 2000, 31, 1475–1482. [Google Scholar] [CrossRef]
- Taha, A.; Sofronis, P. A micromechanics approach to the study of hydrogen transport and embrittlement. Eng. Fract. Mech. 2001, 68, 803–837. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Golahmar, A.; Niordson, C.F. A phase field formulation for hydrogen assisted cracking. Comput. Methods Appl. Mech. Eng. 2018, 342, 742–761. [Google Scholar] [CrossRef] [Green Version]
- Bouhala, L.; Makradi, A.; Belouettar, S.; Younes, A.; Natarajan, S. An XFEM/CZM based inverse method for identification of composite failure parameters. Comput. Struct. 2015, 153, 91–97. [Google Scholar] [CrossRef]
- Rozylo, P. Experimental-numerical study into the stability and failure of compressed thin-walled composite profiles using progressive failure analysis and cohesive zone model. Comput. Struct. 2021, 257, 113303. [Google Scholar] [CrossRef]
- Sung, S.J.; Pan, J.; Korinko, P.S.; Morgan, M.; McWillliams, A. Simulations of fracture tests of uncharged and hydrogen-charged additively manufactured 304 stainless steel specimens using cohesive zone modeling. Eng. Fract. Mech. 2019, 209, 125–146. [Google Scholar] [CrossRef]
- Wu, S.J.; Sung, S.J.; Pan, J.; Lam, P.S.; Morgan, M.; Korinko, P. Modeling of Crack Extensions in Arc-Shaped Specimens of Hydrogen-Charged Austenitic Stainless Steels Using Cohesive Zone Model. Press. Vessel. Pip. Conf. 2018, 7, 15–20. [Google Scholar]
- Yu, H.Y.; Olsen, J.S.; Alvaro, A.; Olden, V.; He, J.Y.; Zhang, Z.L. A uniform hydrogen degradation law for high strength steels. Eng. Fract. Mech. 2016, 157, 56–71. [Google Scholar] [CrossRef]
- Alvaro, A.; Olden, V.; Akselsen, O.M. 3D cohesive modelling of hydrogen embrittlement in the heat affected zone of an X70 pipeline steel. Int. J. Hydrogen Energy 2013, 38, 7539–7549. [Google Scholar] [CrossRef]
- Singh, D.K.; Maiti, S.K.; Bhandakkar, T.K.; Singh Raman, R.K. Efficient approach for cohesive zone based three-dimensional analysis of hydrogen-assisted cracking of a circumferentially notched round tensile specimen. Int. J. Hydrogen Energy 2017, 42, 15943–15955. [Google Scholar] [CrossRef]
- Jiang, D.E.; Carter, E.A. First principles assessment of ideal fracture energies of materials with mobile impurities: Implications for hydrogen embrittlement of metals. Acta Mater. 2004, 52, 4801–4807. [Google Scholar] [CrossRef]
- Serebrinsky, S.; Carter, E.A.; Ortiz, M. A quantum-mechanically informed continuum model of hydrogen embrittlement. J. Mech. Phys. Solids 2004, 52, 2403–2430. [Google Scholar] [CrossRef]
- Sobhaniaragh, B.; Afzalimir, S.H.; Ruggieri, C. Towards the prediction of hydrogen–induced crack growth in high–graded strength steels. Thin-Walled Struct. 2021, 159, 107245. [Google Scholar] [CrossRef]
- Alvaro, A.; Jensen, I.T.; Kheradmand, N.; Løvvik, O.M.; Olden, V. Hydrogen embrittlement in nickel, visited by first principles modeling, cohesive zone simulation and nanomechanical testing. Int. J. Hydrogen Energy 2015, 40, 16892–16900. [Google Scholar] [CrossRef]
- Gobbi, G.; Colombo, C.; Vergani, L. Sensitivity analysis of a 2D cohesive model for hydrogen embrittlement of AISI 4130. Eng. Fract. Mech. 2016, 167, 101–111. [Google Scholar] [CrossRef]
- Jemblie, L.; Olden, V.; Akselsen, O.M. A coupled diffusion and cohesive zone modelling approach for numerically assessing hydrogen embrittlement of steel structures. Int. J. Hydrogen Energy 2017, 42, 11980–11995. [Google Scholar] [CrossRef] [Green Version]
- ASTM. E1820-09: Standard Test Method for Measurement of Fracture Toughness; ASTM: Philadelphia, PA, USA, 2016. [Google Scholar]
- Li, X.F.; Zhang, J.; Chen, J.; Shen, S.; Yang, G.X.; Wang, T.J.; Song, X.L. Effect of aging treatment on hydrogen embrittlement of PH 13-8 Mo martensite stainless steel. Mater. Sci. Eng. A Struct. Mater. 2016, 651, 474–485. [Google Scholar] [CrossRef]
- ISO Technical Committees. ISO12135 International Standard of Unified Method of Test for the Determination of Quasistatic Fracture Toughness; ISO 2002; ISO Technical Committees: Geneva, Switzerland, 2002; pp. 65–67. [Google Scholar]
- Yao, Y.; Cai, L.X.; Bao, C.; Jiang, H. The Fined COD Transform Formula for CT Specimens to Investigate Material Fracture Toughness. AMM 2012, 188, 11–16. [Google Scholar] [CrossRef]
- Scheider, I.; Pfuff, M.; Dietzel, W. Simulation of hydrogen assisted stress corrosion cracking using the cohesive model. Eng. Fract. Mech. 2008, 75, 4283–4291. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Robertson, I.; Sofronis, P. Interpreting hydrogeninduced fracture surfaces in terms of deformation processes:a new approach. Acta Mater. 2011, 59, 3680–3687. [Google Scholar] [CrossRef]
- Toribio, J.; Vergara, D.; Lorenzo, M. Hydrogen effects in multiaxial fracture of cold-drawn pearlitic steel wires. Eng. Fract. Mech. 2017, 174, 243–252. [Google Scholar] [CrossRef]
- Lufrano, J.; Sofronis, P. Enhanced hydrogen concentrations ahead of rounded notches and cracks—Competition between plastic strain and hydrostatic stress. Acta Mater. 1998, 46, 1519–1526. [Google Scholar] [CrossRef]
- Łunarska, E.; Chmiel, J.; Balitskii, A.; Szyszko, M. Hydrogen Diffusion in Metals Assisted by Stress: 2D Numerical Modelling and Analysis of Directionality. Solid State Phenom. 2015, 225, 33–38. [Google Scholar]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, L.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Mater. 2012, 60, 5182–5189. [Google Scholar] [CrossRef]
- Nagao, A.; Martin, M.L.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The effect ofnanosized (Ti, Mo)C precipitates on hydrogen embrittlement of tempered lath martensitic steel. Acta Mater. 2014, 74, 244–254. [Google Scholar] [CrossRef]
- Kumar, B.S.; Kain, V.; Singh, M.; Vishwanadh, B. Influence of hydrogen on mechanical properties and fracture of tempered 13 wt% Cr martensitic stainless steel. Mater. Sci. Eng. A. 2017, 700, 140–151. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R.; Østby, E. Cohesive zone modeling of hydrogen-induced stress cracking in 25% Cr duplex stainless steel. Scr. Mater. 2007, 57, 615–618. [Google Scholar] [CrossRef]
- Dugdale, D. Yielding of steel sheets containing slits. J. Mech. Phys. Solids 1960, 8, 100–104. [Google Scholar] [CrossRef]
- Barenblatt, G. The mathematical theory of equilibrium cracks in brittle fracture. Adv. Appl. Mech. 1962, 7, 55–129. [Google Scholar]
- Hondros, E.; Seah, M. The theory of grain boundary segregation in terms of surface adsorption analogues. Met. Trans. A 1977, 8, 1363–1371. [Google Scholar] [CrossRef]
- Li, H.; Yuan, H.; Li, X.; Karihaloo, B. Assessment of low cycle fatigue crack growth under mixed-mode loading conditions by using a cohesive zone model. Int. J. Fatigue 2015, 75, 39–50. [Google Scholar] [CrossRef]
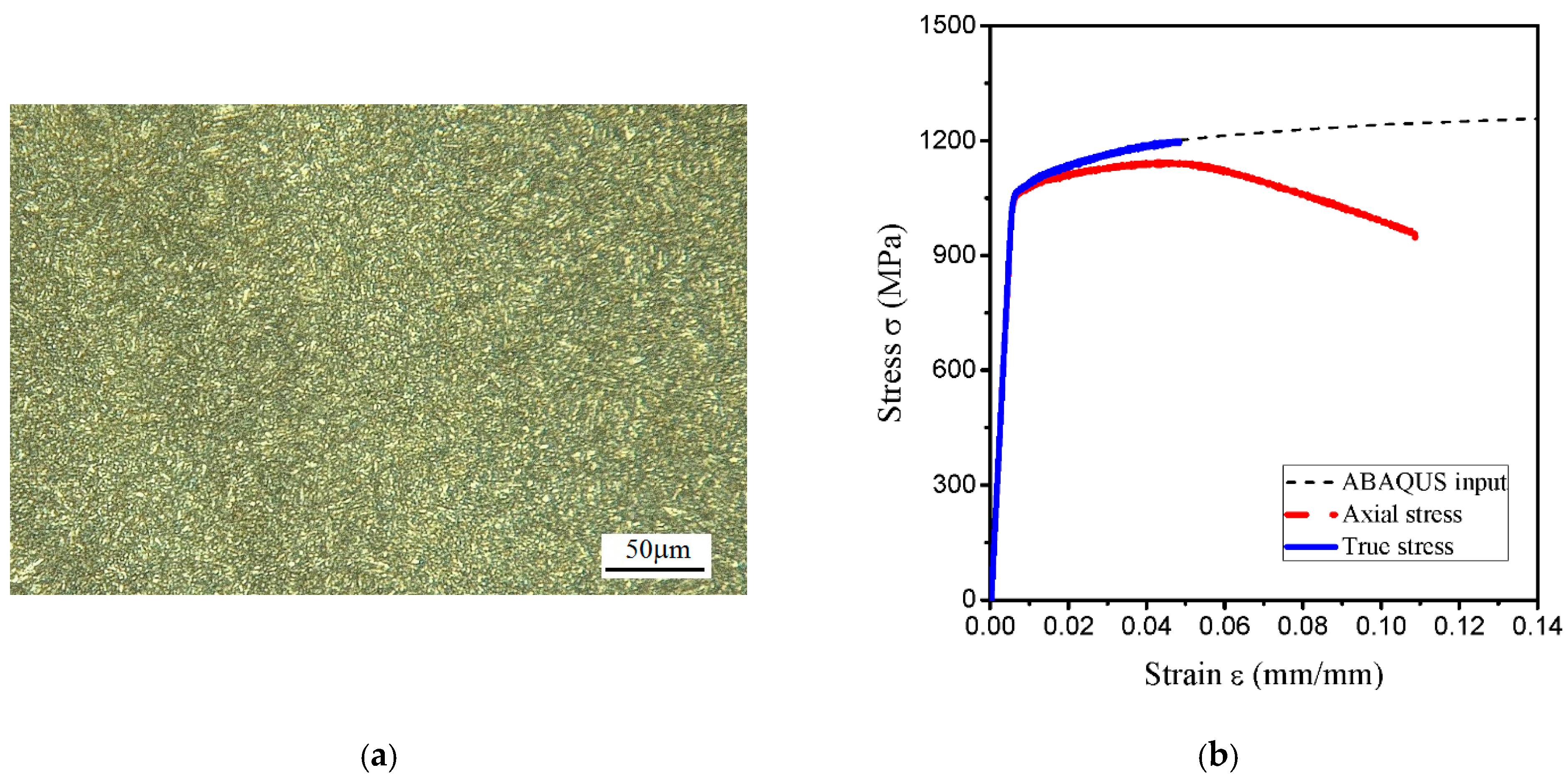

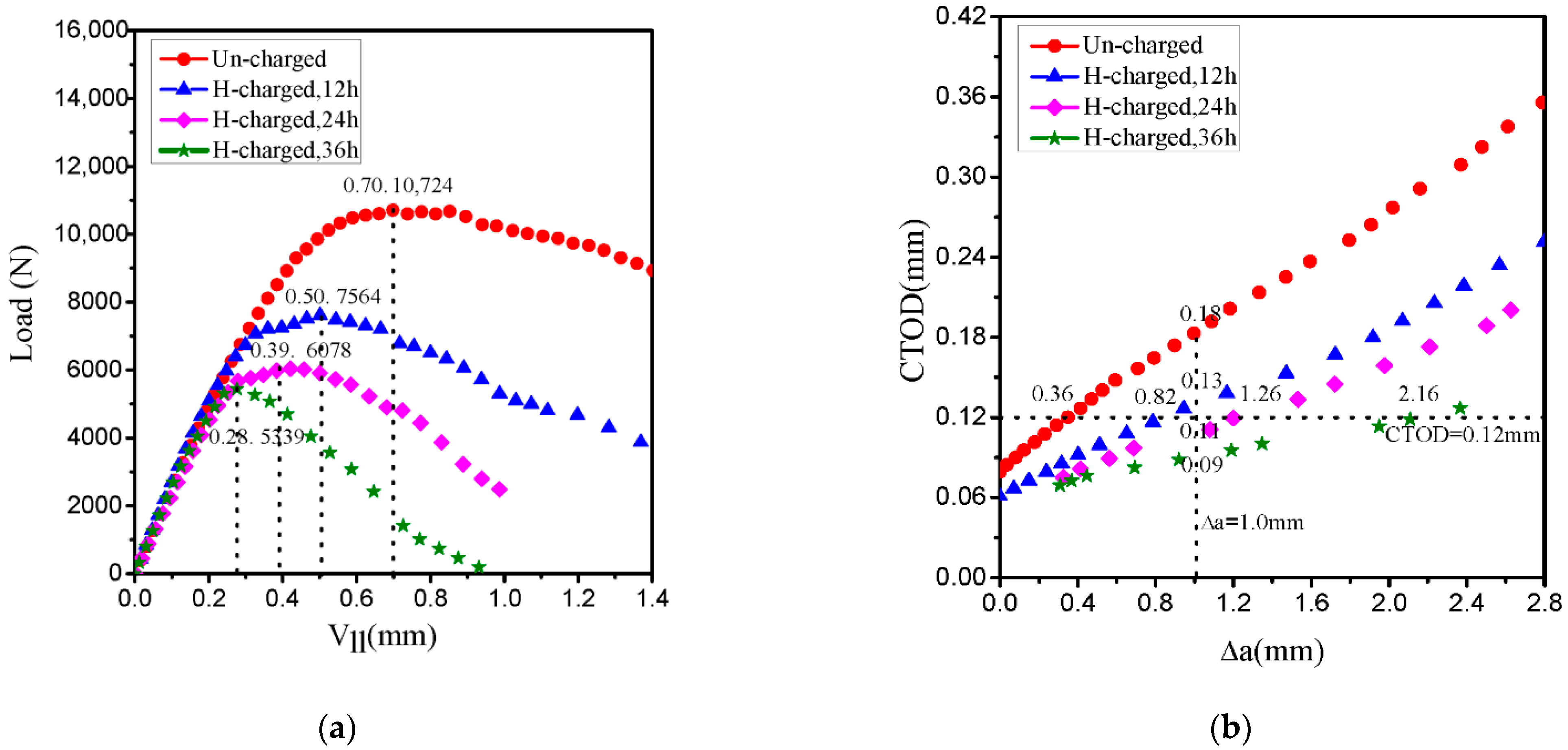
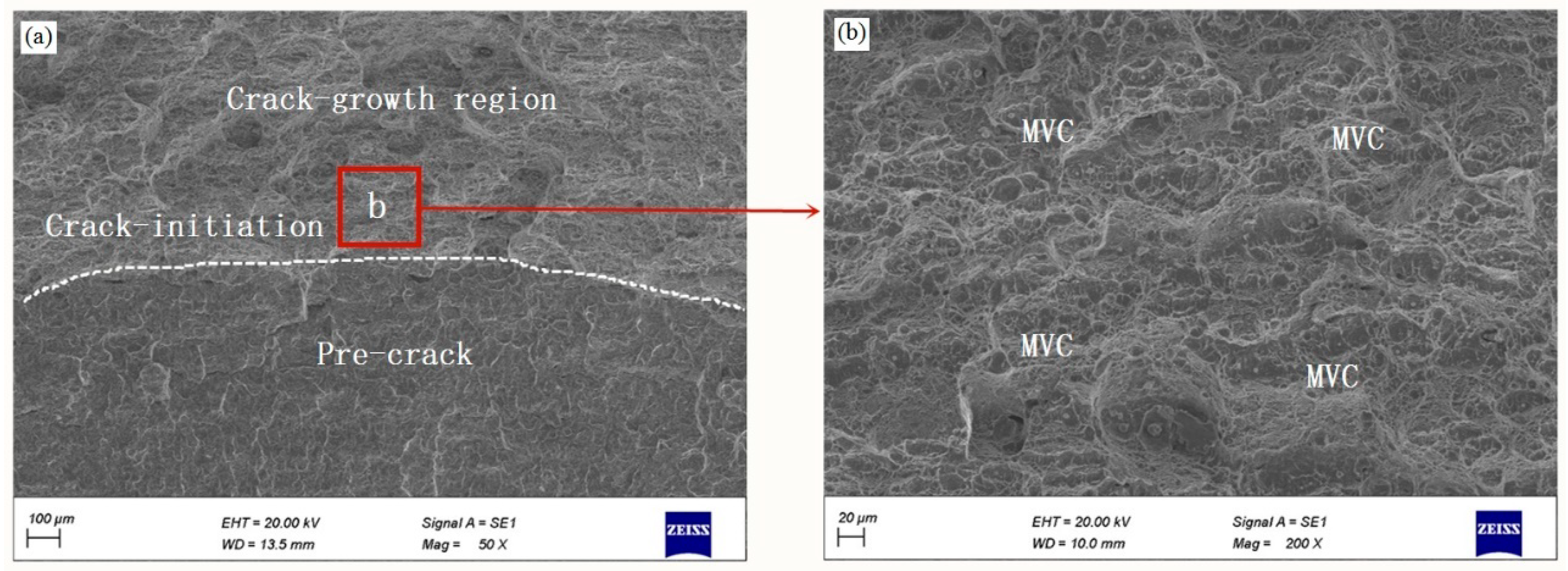

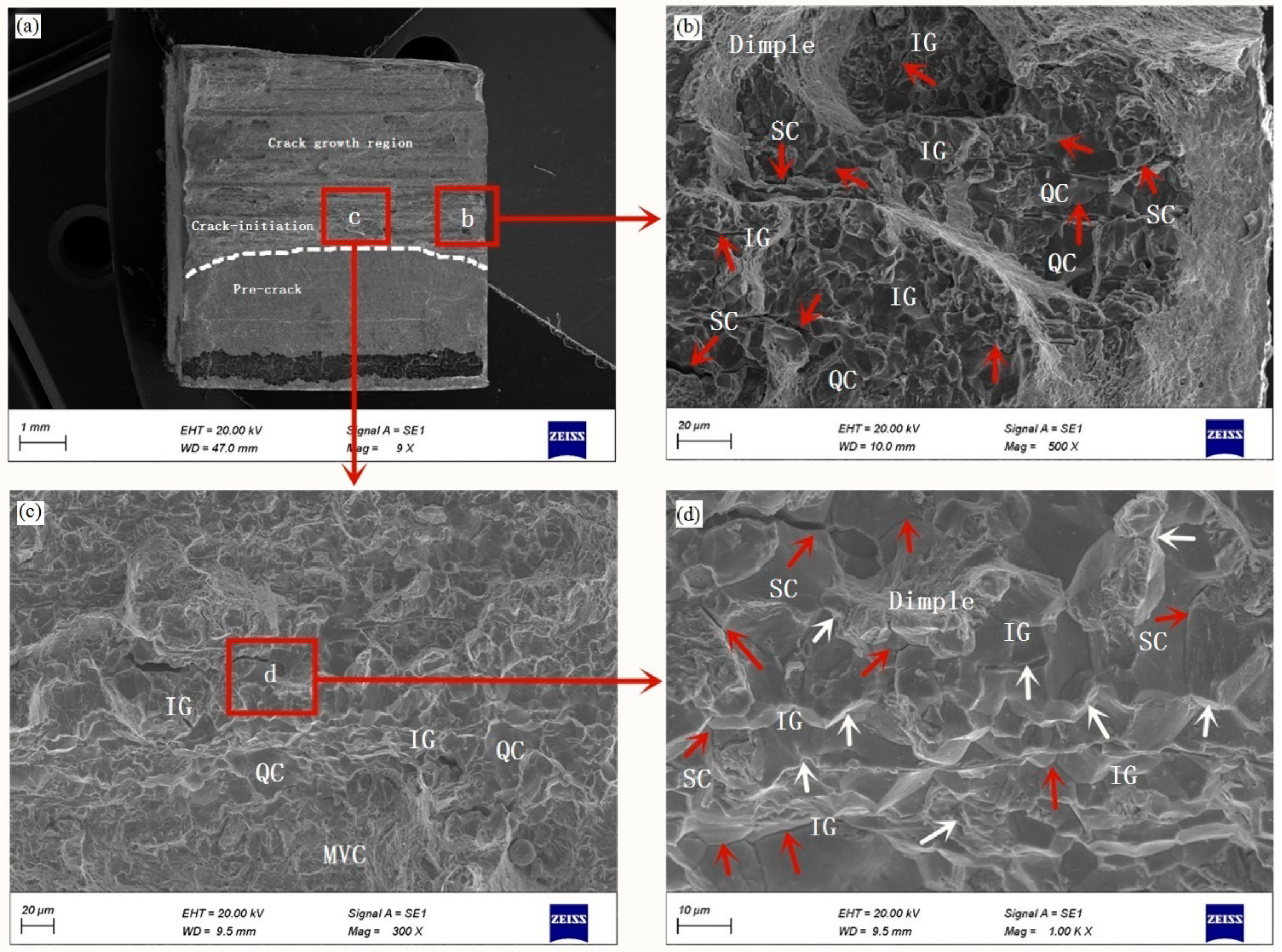


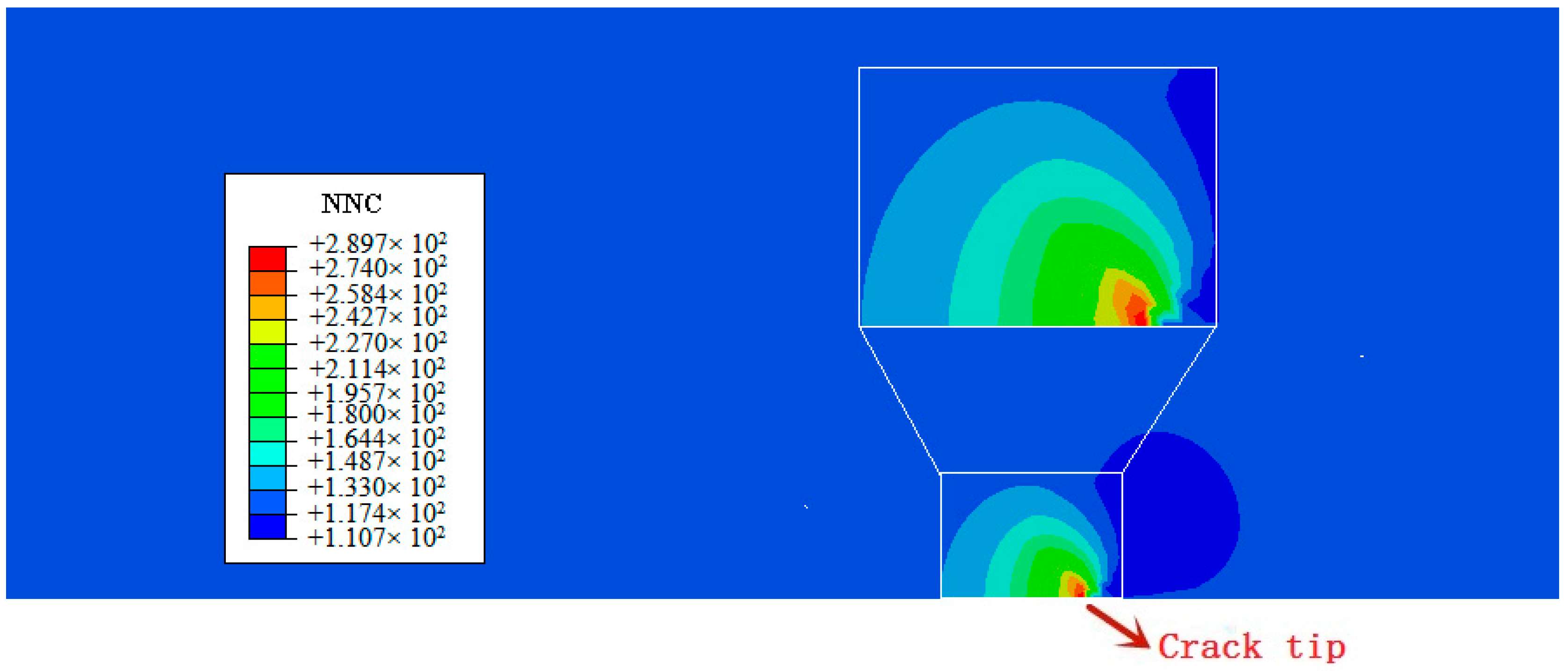
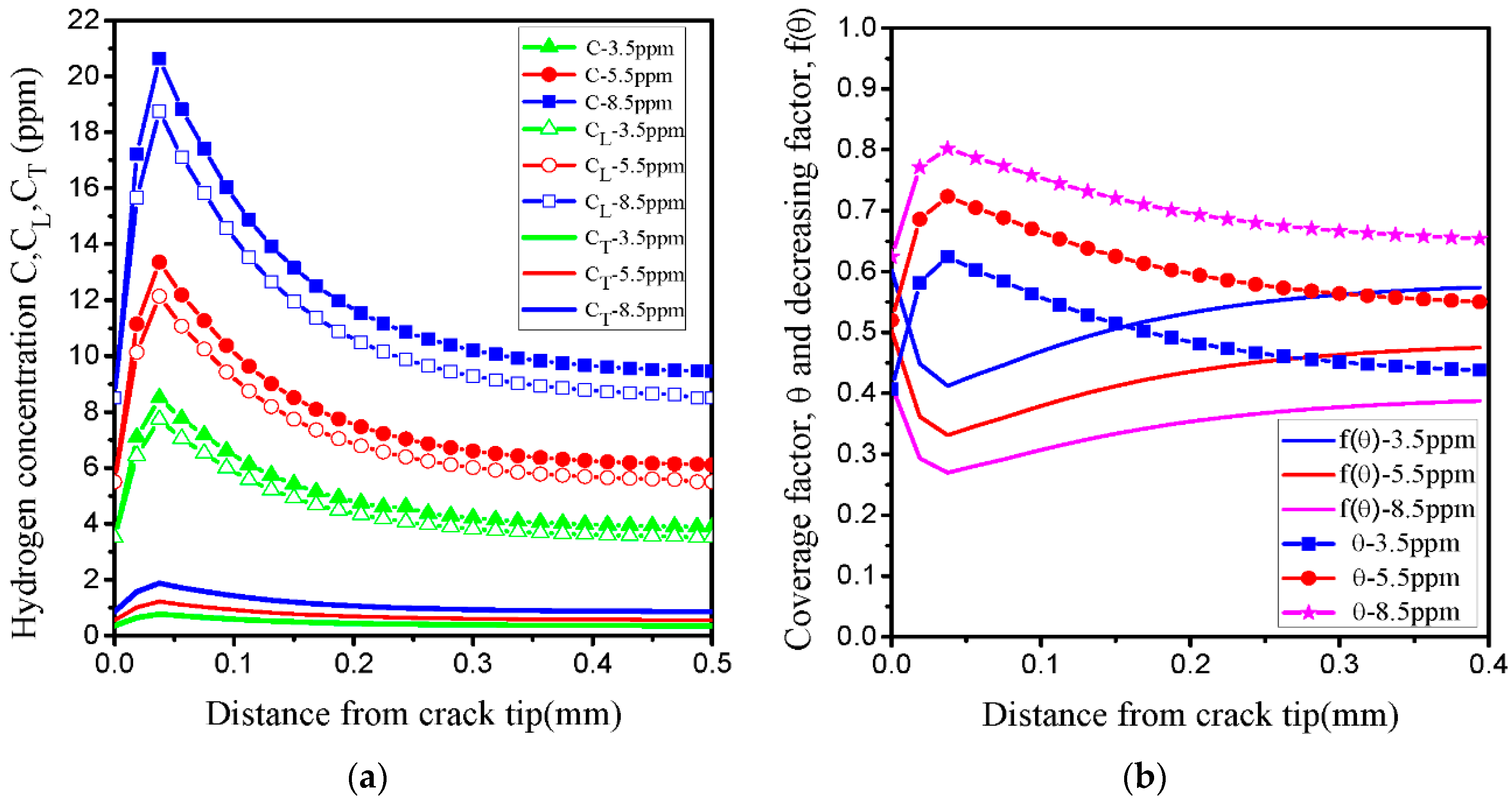
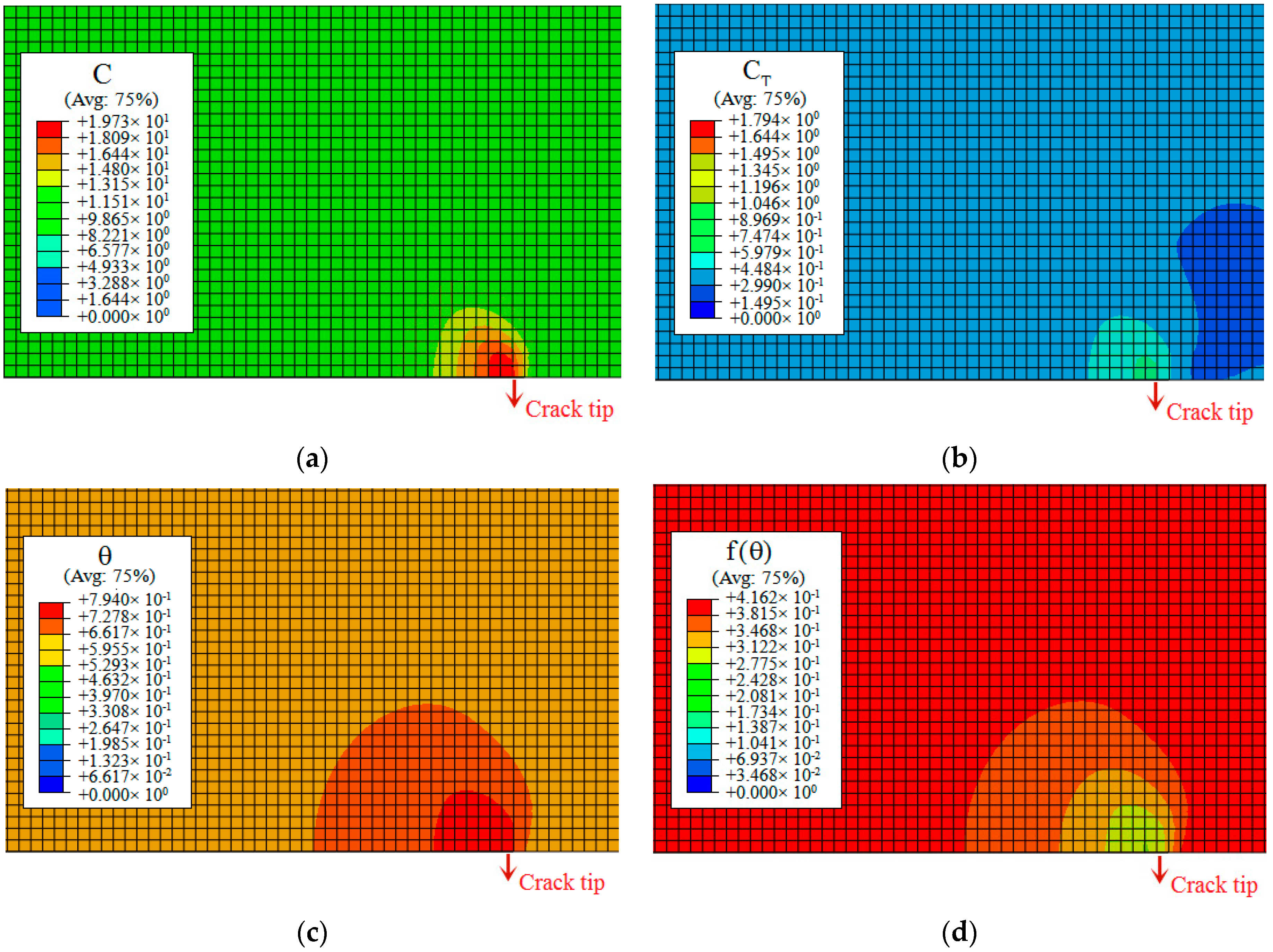

| C | Si | Mn | Cr | Ni | Mo | Cu |
|---|---|---|---|---|---|---|
| 0.31 | 0.25 | 0.48 | 0.91 | 0.02 | 0.17 | 0.03 |
| (MPa) | Plastic-Strain (mm/mm) |
|---|---|
| 1067.2 | 0.003 |
| 1143.6 | 0.015 |
| 1192.9 | 0.04 |
| 1229 | 0.08 |
| 1240.9 | 0.1 |
| 1278.4 | 0.2 |
| 1329.8 | 0.5 |
| 1349.1 | 0.7 |
| 1363.8 | 0.9 |
| 1370 | 1.0 |
| Parameter | Value | Source |
|---|---|---|
| Hydrogen solubility | 0.071 ppm mm N−1/2 | |
| The universal gas constant | 8.314 J K−1 mol−1 | [25] |
| Partial molar volume of hydrogen | 2.0 × 103 mm3 mol−1 | [34] |
| Hydrogen diffusion coefficient | 4.0 × 10−5 mm2 s−1 | [23] |
| The Gibbs free energy | 30 KJ·mol−1 | [37] |
| Temperature | 296 K |
| (MPa) | (mm) | (mm) | (mm) | (MPa) |
|---|---|---|---|---|
| 2100 (2 ) | 0.00197 | 0.0255 | 0.0393 | 1050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, K.; Lu, D.; Zeng, B. Hydrogen-Assisted Brittle Fracture Behavior of Low Alloy 30CrMo Steel Based on the Combination of Experimental and Numerical Analyses. Materials 2021, 14, 3711. https://doi.org/10.3390/ma14133711
Li Y, Zhang K, Lu D, Zeng B. Hydrogen-Assisted Brittle Fracture Behavior of Low Alloy 30CrMo Steel Based on the Combination of Experimental and Numerical Analyses. Materials. 2021; 14(13):3711. https://doi.org/10.3390/ma14133711
Chicago/Turabian StyleLi, Yunlong, Keshi Zhang, Damin Lu, and Bin Zeng. 2021. "Hydrogen-Assisted Brittle Fracture Behavior of Low Alloy 30CrMo Steel Based on the Combination of Experimental and Numerical Analyses" Materials 14, no. 13: 3711. https://doi.org/10.3390/ma14133711
APA StyleLi, Y., Zhang, K., Lu, D., & Zeng, B. (2021). Hydrogen-Assisted Brittle Fracture Behavior of Low Alloy 30CrMo Steel Based on the Combination of Experimental and Numerical Analyses. Materials, 14(13), 3711. https://doi.org/10.3390/ma14133711






