Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Glasses
2.2. Characterization of Glasses
3. Results
3.1. XRD, TEM and Physical Properties
3.2. IR Spectra
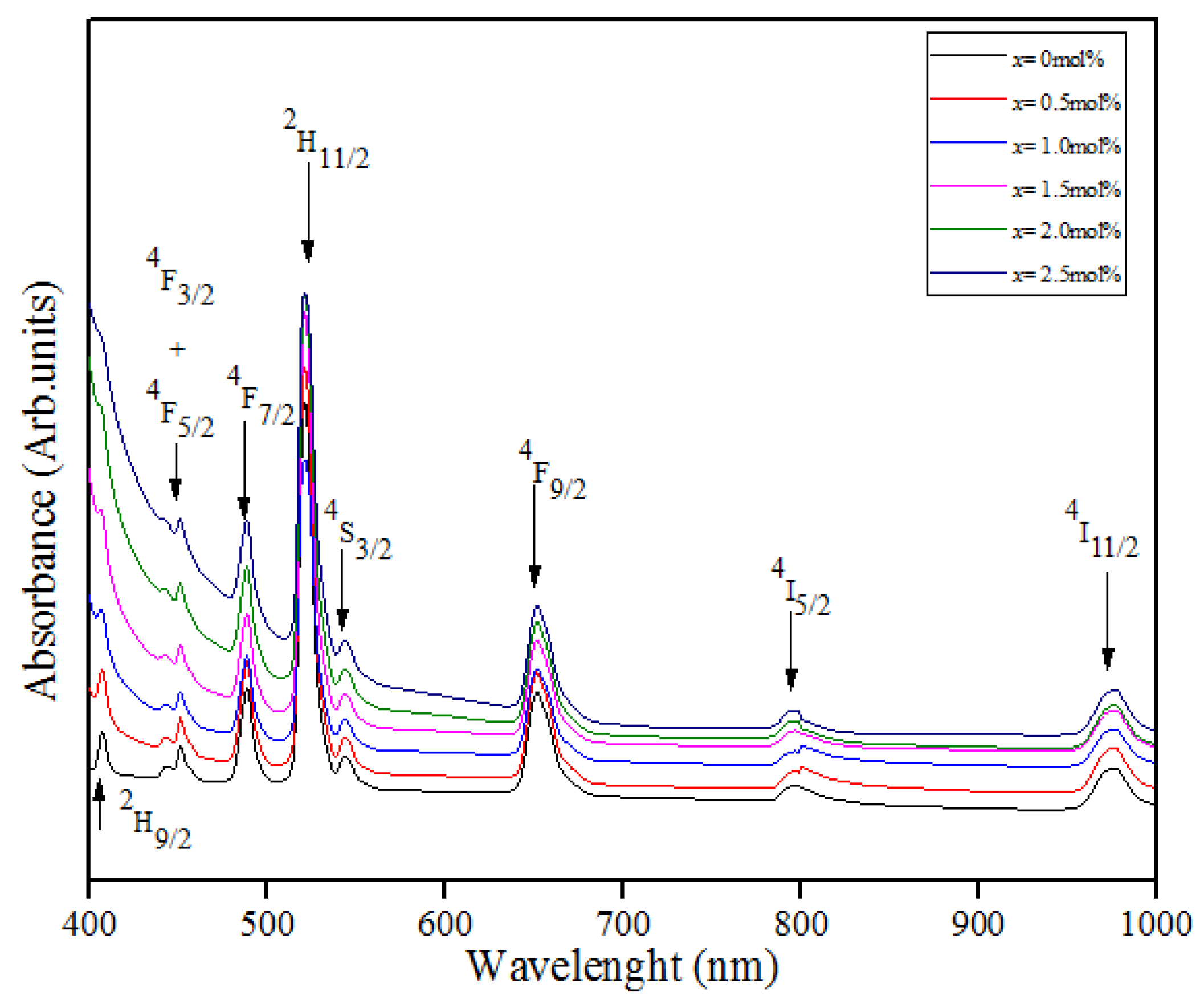
3.3. UV–Vis Properties
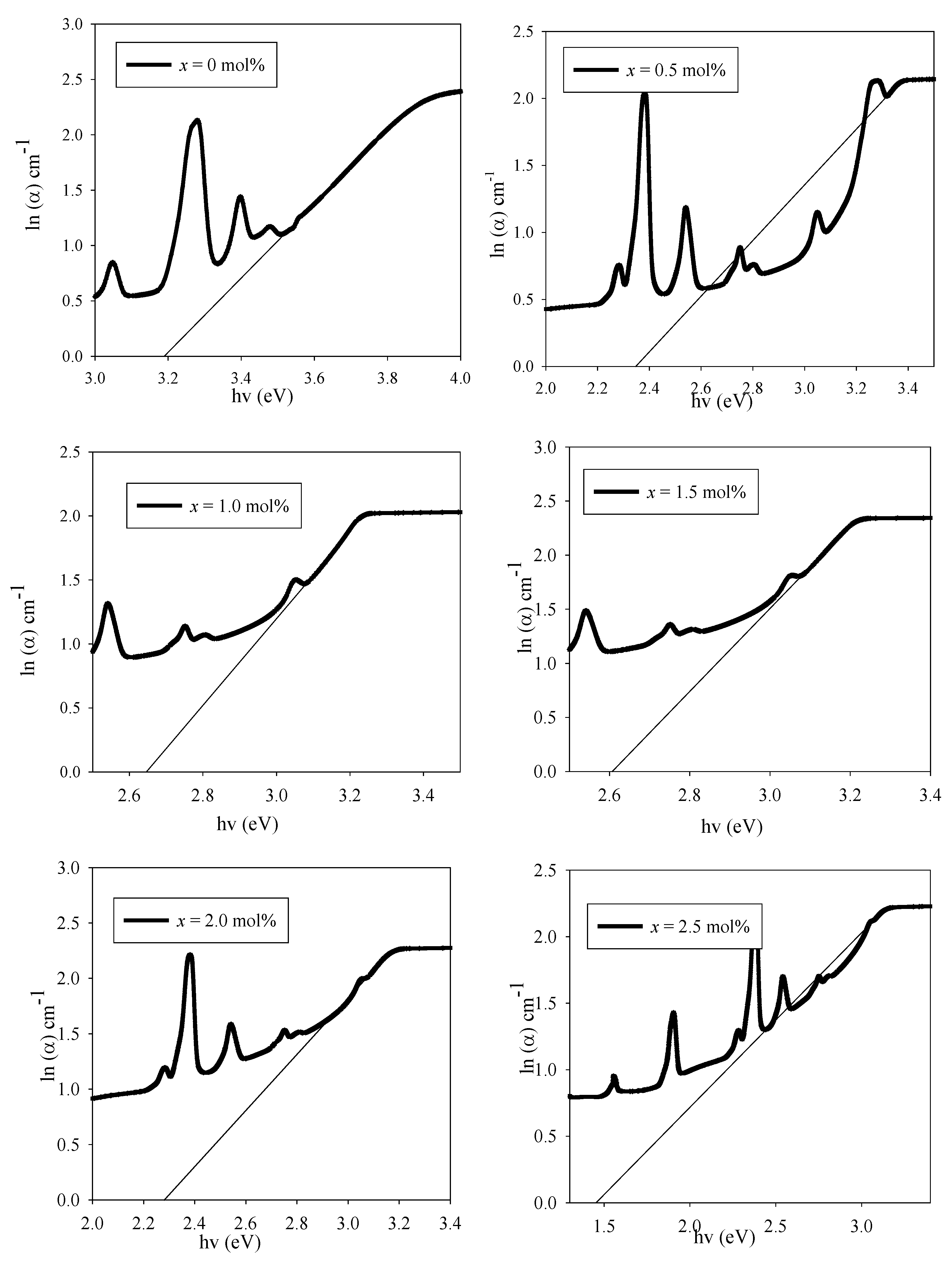
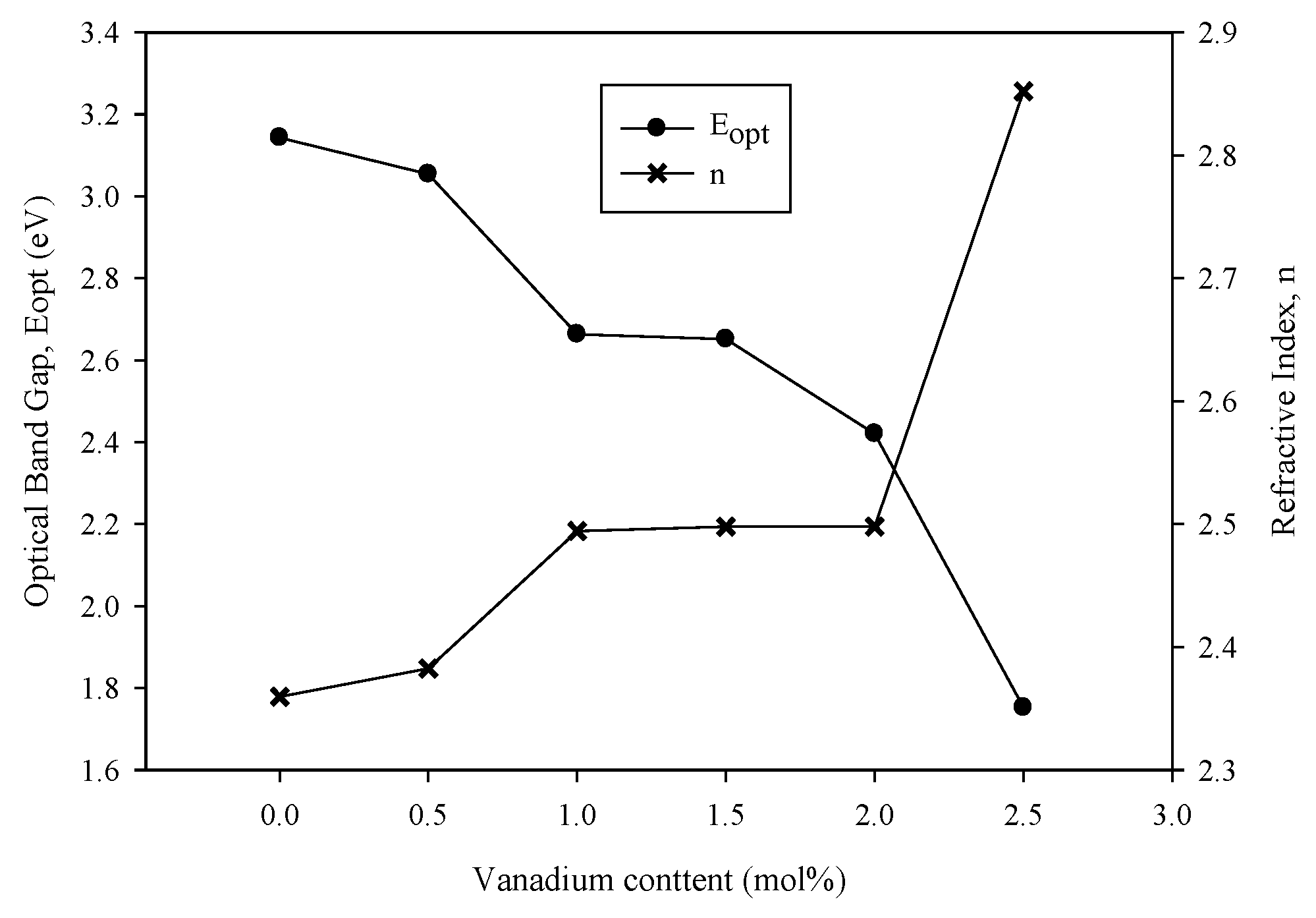
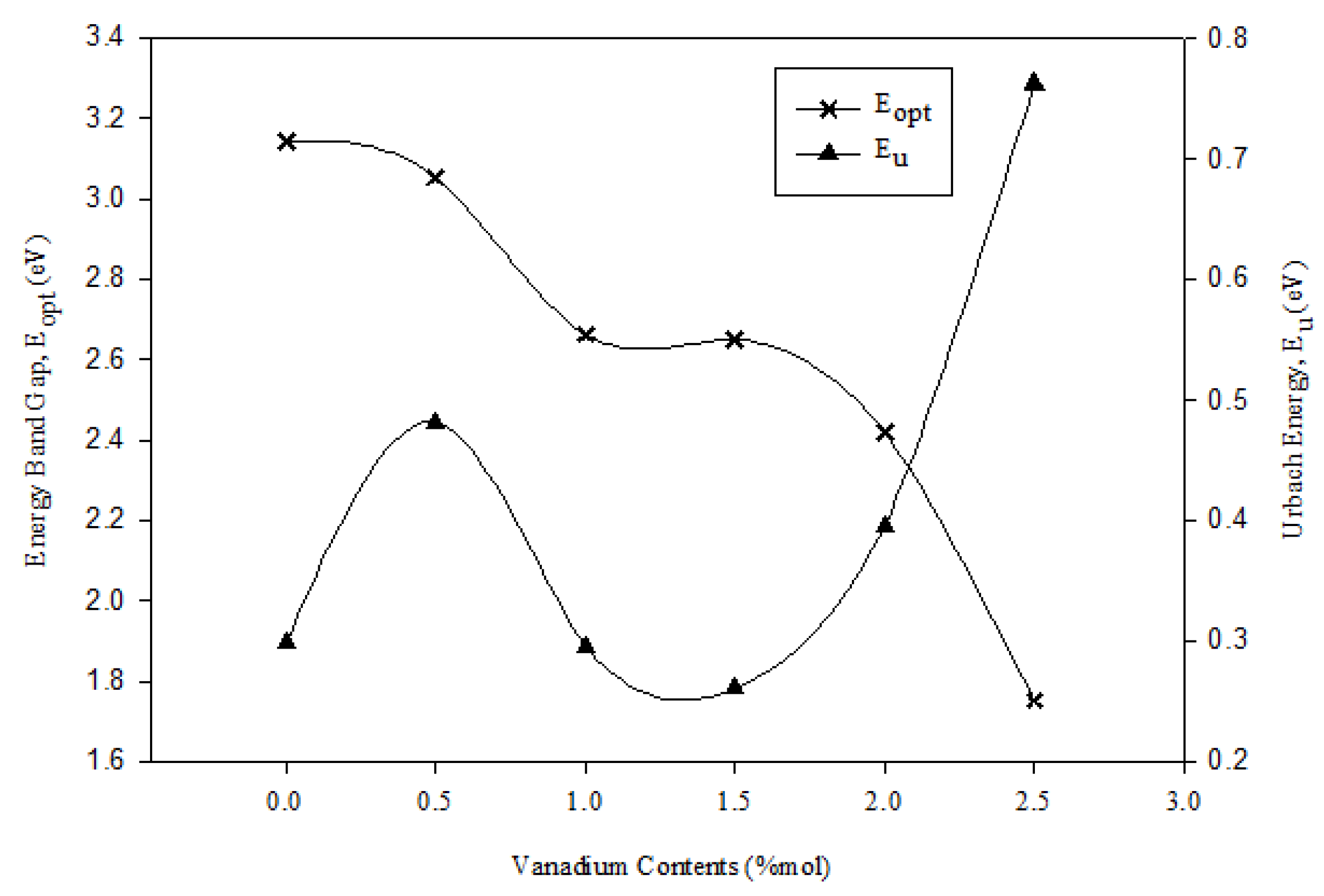
3.4. Optical Properties
3.5. Judd–Ofelt Analysis
3.6. Photoluminescence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvaraju, K.; Marimuthu, K. Structural and spectroscopic studies on concentration dependent Er3þ doped boro–tellurite glasses. J. Lumin. 2012, 132, 1171–1178. [Google Scholar] [CrossRef]
- de Almeida, R.; Davinson, M.; Kassab, L.R.P. Eu3+ luminescence in tellurite glasses with gold nanostructures. Opt. Commun. 2012, 218, 108–112. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Ahlawat, N.; Agarwal, A. Influence of Bi2O3 on thermal, structural and dielectric properties of lithium zinc bismuth borate glasses. J. Alloys Compd. 2014, 597, 110–118. [Google Scholar] [CrossRef]
- Pascuta, P.; Borodi, G.; Culea, E. Influence of europium ions on structure and crystallization properties of bismuth borate glasses and glass ceramics. J. Non-Cryst. Solids 2008, 354, 5475–5479. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Yahya, A.K. Effects of V2O5 on elastic, structural, and optical properties of mixed ionic–electronic 20Na2O–20CaO–(60−x) B2O3–xV2O5 glasses. Ionics. 2018, 24, 2647–2664. [Google Scholar] [CrossRef]
- Syahidah, N.; Yahya, A.K.; Abd–Shukor, R.; Kumar, M. Anomalous elastic behaviour of xSrO–10PbO–(90–x) B2O3 glass system. J. Non-Cryst. Solids 2016, 444, 55–63. [Google Scholar]
- Kabi, S.; Ghosh, A. Mixed glass former effect in AgI doped silver borophosphate glasses. Solid State Ion. 2013, 90, 3–6. [Google Scholar] [CrossRef]
- Narayana, C. Electronic and ionic conductivity studies on microwave synthesized glasses containing. Integr. Med. Res. 2016, 205, 2–7. [Google Scholar] [CrossRef][Green Version]
- Kosuge, K. The phase diagram and phase transition of the V2O3-V2O5, system. J. Phys. Chem. Solids 1967, 28, 1613–1621. [Google Scholar] [CrossRef]
- Guo, P.; Biegler, Z.; Back, T.; Sarangan, A. Vanadium dioxide phase change thin films produced by thermal oxidation of metallic vanadium. Thin Solid Films 2020, 707, 138117. [Google Scholar] [CrossRef]
- Saad, M.; Stambouli, W.; Mohamed, S.A.; Elhouichet, H. Ag nanoparticles induced luminescence enhancement of Eu3+ doped phosphate glasses Moufida. J. Alloys Compd. 2016, 2017, 1–29. [Google Scholar] [CrossRef]
- Kaky, K.M.; Lakshminarayana, G.; Baki, S.O.; Lira, A.; Caldi, U.; Meza-Rocha, A.N.; Falcony, C.; Kityk, I.V.; Taufiq-Yap, Y.H.; Halimah, M.K.; et al. Structural and optical studies of Er3þ–doped alkali/alkaline oxide containing zinc boro–aluminosilicate glasses for 1.5 mm optical amplifier applications. Opt. Mater. 2017, 69, 401–419. [Google Scholar] [CrossRef]
- Neeraja, K.; Rao, T.G.V.M.; Kumar, A.R.; Veeraiah, N.; Reddy, M.R. Influence of modifier oxide on Spectroscopic properties of Ho3+: V4+ co–doped Na2O–SiO2–ZrO2 glasses. J. Alloys Compd. 2014, 586, 159–168. [Google Scholar] [CrossRef]
- Tang, G.; Wen, X.; Qian, Q.; Zhu, T.; Liu, W. Efficient 2.0 mm emission in Er3þ/Ho3þ co–doped barium gallo–germanate glasses under different excitations for mid-infrared laser. J. Alloys Compd. 2016, 664, 19–24. [Google Scholar] [CrossRef]
- Neeraja, K.; Rao, T.G.V.M.; Kumar, A.R.; Lakshmi, V.U.; Veeraiah, N.; Reddy, M.R. Spectroscopic properties of Sm3+ and V4+ ions in Na2O–SiO2–ZrO2 glasses. Mol. Struct. 2013, 1055, 339–348. [Google Scholar] [CrossRef]
- Awang, A.; Ghoshal, S.K.; Sahar, M.R.; Dousti, M.R.; Amjad, R.J.; Nawaz, F. Enhanced spectroscopic properties and Judd–Ofelt parameters of Er–doped tellurite glass: Effect of gold nanoparticles. Curr. Appl. Phys. 2013, 13, 1813–1818. [Google Scholar] [CrossRef]
- Farouk, M.; Samir, A.; Metawe, F.; Elokr, M. Optical absorption and structural studies of bismuth borate glasses containing Er3+ ions. J. Non-Cry. Solids 2013, 371–372, 14–21. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Saddeek, Y.B.; El-Latif, L.A. Ultrasonic studies on alkali borate tungstate glasses. J. Phys. Chem. Solids 2009, 70, 173–179. [Google Scholar] [CrossRef]
- Kundu, V.; Dhiman, R.L.; Maan, A.S.; Goyal, D.R. Structural and Physical Properties of Fe2O3–B2O3–V2O5 Glasses. Adv. Condens. Matter Phys. 2008, 2008, 937054. [Google Scholar] [CrossRef]
- Broglia, G.; Mugoni, C.; Du, J.; Siligardi, C.; Montorsi, M. Lithium vanado–phosphate glasses: Structure and dynamics properties studied by molecular dynamics simulations. J. Non-Cryst. Solids 2014, 403, 53–61. [Google Scholar] [CrossRef]
- Amjad, R.J.; Dousti, M.R.; Sahar, M.R. Spectroscopic investigation and Judd–Ofelt analysis of silver nanoparticles embedded Er3+–doped tellurite glass. Curr. Appl. Phys. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Jiang, Z.; Wen, G.; Luo, Y.; Zhang, X.; Liu, Q.; Liang, A. A new silver nanorod SPR probe for detection of trace benzoyl peroxide. Sci. Rep. 2014, 4, 5323. [Google Scholar] [CrossRef]
- Rajaramakrishna, R.; Karuthedath, S.; Anavekar, R.V.; Jain, H. Nonlinear optical studies of lead lanthanum borate glass doped with Au nanoparticles. J. Non-Cryst. Solids 2012, 358, 1667–1672. [Google Scholar] [CrossRef]
- Maulud, M.F.; Yahya, A.K. Influence of germanate anomaly on elastic, structural, and optical properties lead–germanate glasses. Int. J. Mater. Res. 2016, 107, 1136–1146. [Google Scholar] [CrossRef]
- Opelt, G.S. Intensities of crystal spectra of rare–earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare–earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Maaoui, A.; Haouari, M.; Zaaboub, Z.; Fraj, I.; Saidi, F.; Ouada, H.B. Concentration effects on the optical spectroscopic properties of Er3+—doped TeO2–Nb2O5–ZnO vitreous system. J. Alloys Compd. 2016, 663, 395–406. [Google Scholar] [CrossRef]
- Abdedou, N.; Djouama, T.; Chalal, M.; Poulain, M.; Capoen, B.; Mahiou, R. Synthesis and characterization of Er3+/Cu+–codoped fluorophosphate glasses. J. Alloys Compd. 2019, 790, 248–256. [Google Scholar] [CrossRef]
- Yusof, N.N.; Ghoshal, S.K.; Azlan, M.N. Optical Properties of Titania Nanoparticles Embedded Er3+–Doped Tellurite Glass: Judd–Ofelt Analysis. J. Alloys Compd. 2017, 724, 1–22. [Google Scholar] [CrossRef]
- Rajagukguk, J.; Sinaga, B.; Kaewkhao, J. Structural and spectroscopic properties of Er3+ doped sodium lithium borate glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117342. [Google Scholar] [CrossRef]
- Vijayaa, N.; Babu, P.; Venkatramu, V.; Jayasankara, C.K.; León-Luis, S.F.; Rodríguez-Mendozad, U.R.; Martínd, I.R.; Lavínd, V. Optical characterization of Er3+–doped zinc fluorophosphate glasses for optical temperature sensors. Sens. Actuators B Chem. 2013, 186, 156–164. [Google Scholar] [CrossRef]
- León-Luis, S.F.; Rodríguez-Mendoza, U.R.; Haro-González, P.; Martín, I.R.; Lavín, V. Role of the host matrix on the thermal sensitivity of Er3+ luminescence in optical temperature sensors. Sens. Actuators B Chem. 2012, 174, 176–186. [Google Scholar] [CrossRef]
- Noorazlan, A.M.; Kamari, H.M.; Baki, S.O.; Mohamad, D.W. Green Emission of Tellurite Based Glass Containing Erbium Oxide Nanoparticles. J. Nanomater. 2017, 724, 1083–1092. [Google Scholar] [CrossRef]
- Sardar, D.K.; Gruber, J.B.; Zandi, B.; Hutchinson, J.A.; Trussell, C.W. Judd–Ofelt analysis of the Er3+(4F11) absorption intensities in phosphate glass: Er3+, Yb3+. J. Appl. Phys. 2003, 93, 2041–2046. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Ferid, M.; Barthou, C. Judd–Ofelt analysis and improvement of thermal and optical properties of tellurite glasses by adding P2O5. J. Lumin. 2010, 130, 2394–2401. [Google Scholar] [CrossRef]
- Rayappan, I.A.; Marimuthu, K. Structural and luminescence behavior of the Er3+ doped alkali fluoroborate glasses. J. Non-Cryst. Solids 2013, 367, 43–50. [Google Scholar] [CrossRef]
- Baig, H.N.; Saluja, J.K.; Dhoble, S.J. Synthesis and characterization of erbium–doped YAlO3 phosphor. Luminescence 2016, 31, 401–406. [Google Scholar] [CrossRef]
- Olvera-R, O.; Chamé-F, K.; Sánchez-Mondragón, J.J.; Cisneros, M.T.; Ojeda, M.L.; Velásquez, C. Effect of the trivalent erbium concentration on the luminescent properties of TiO2 Er3+ composites. Comput. Y Sist. 2019, 23, 89–94. [Google Scholar] [CrossRef]
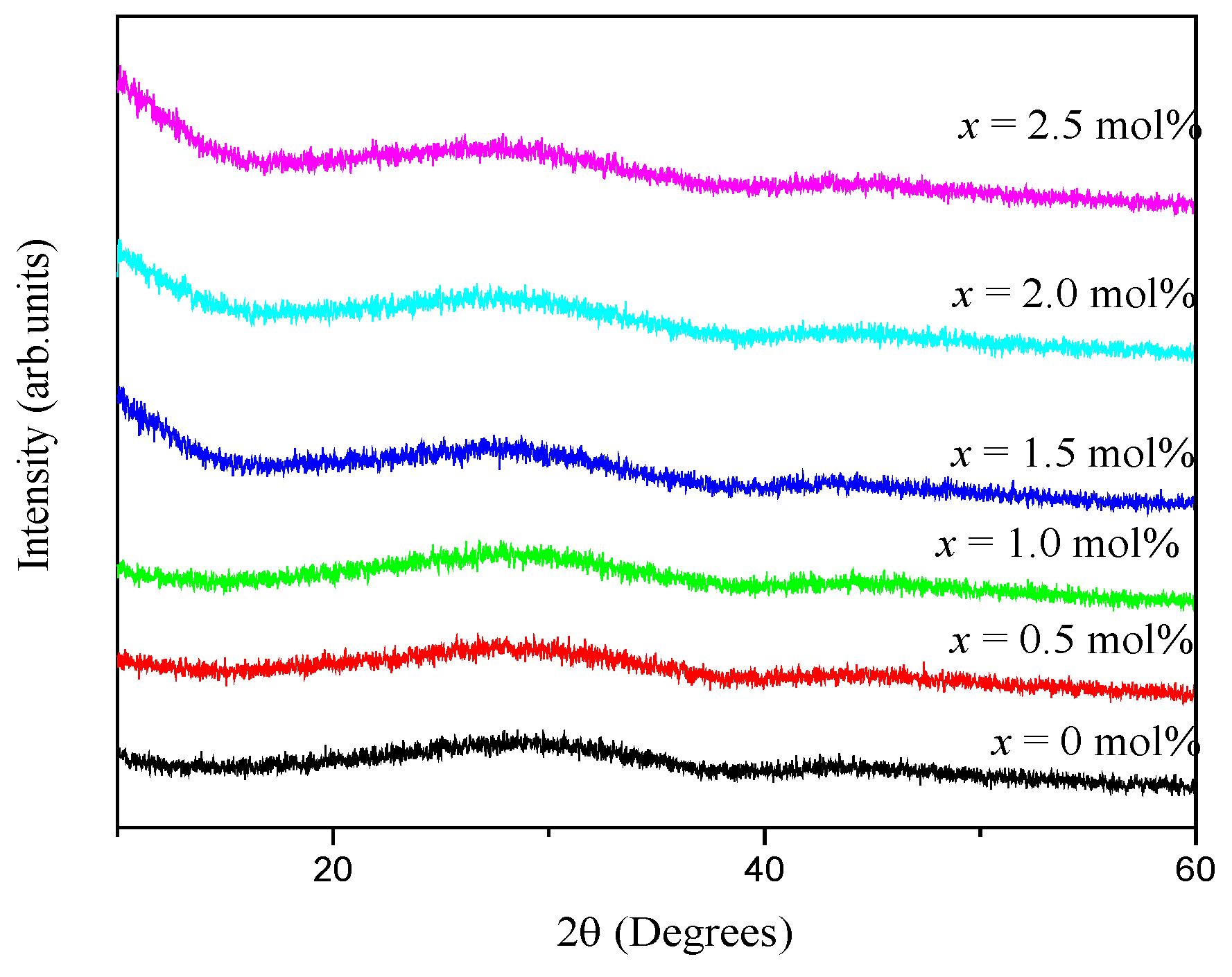
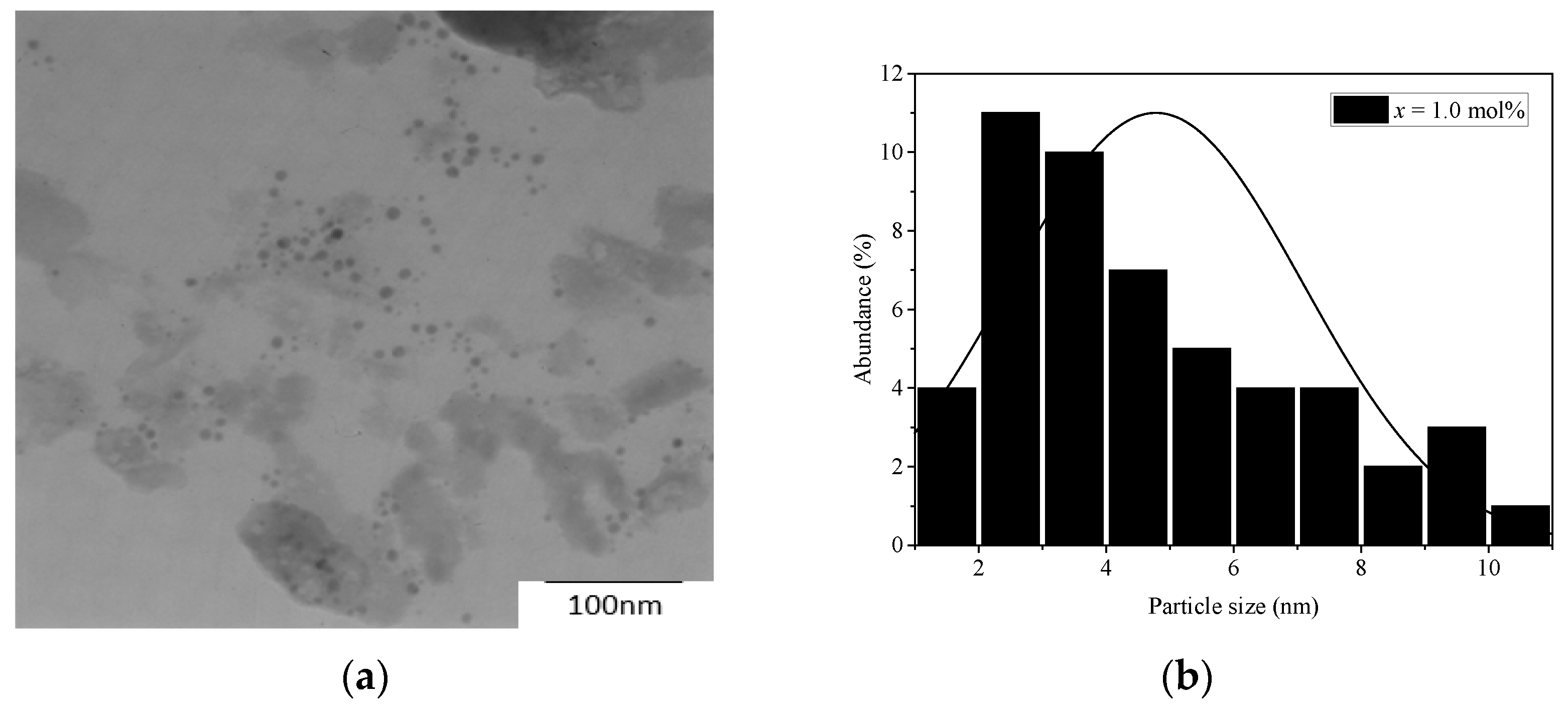
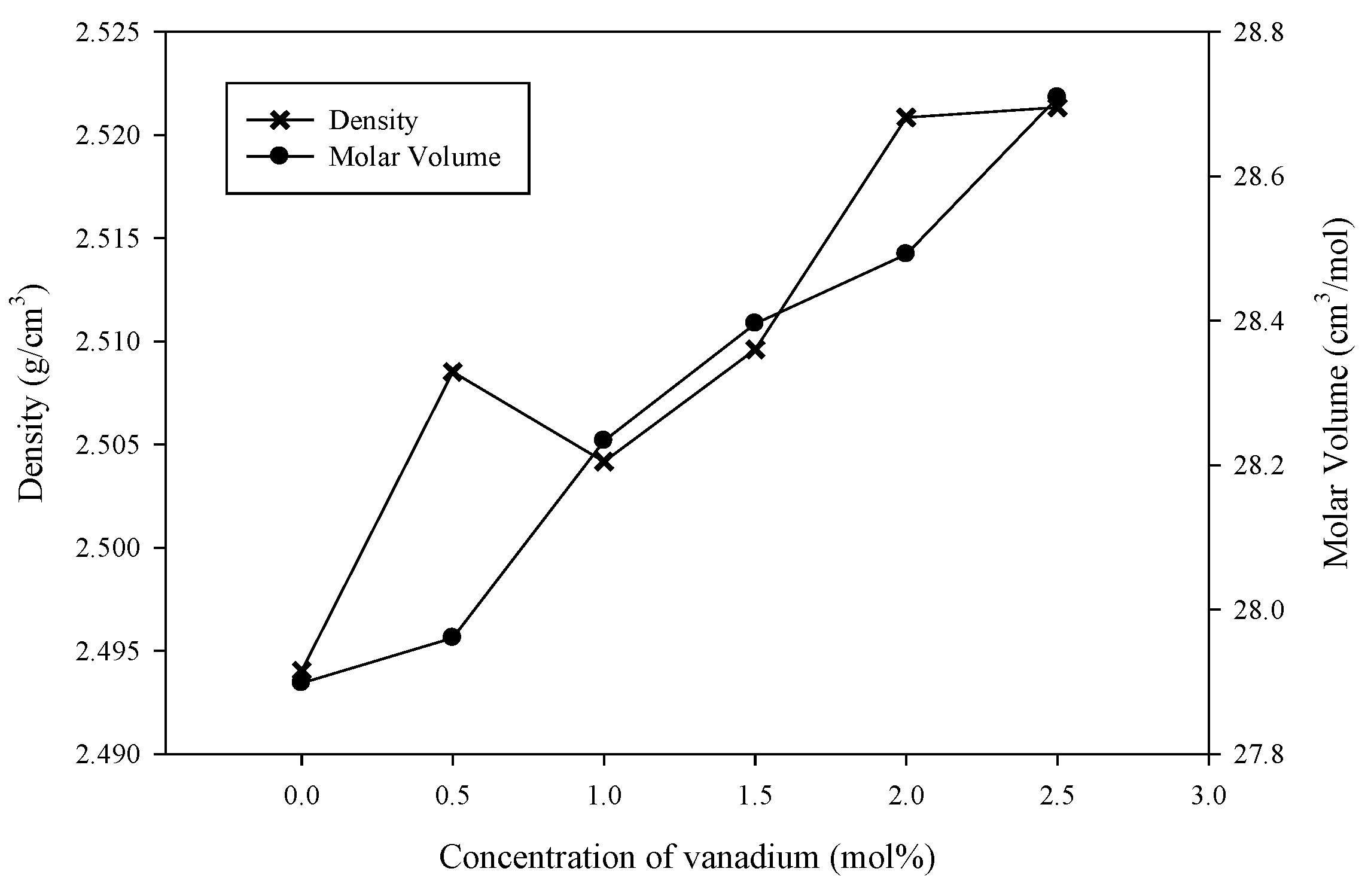
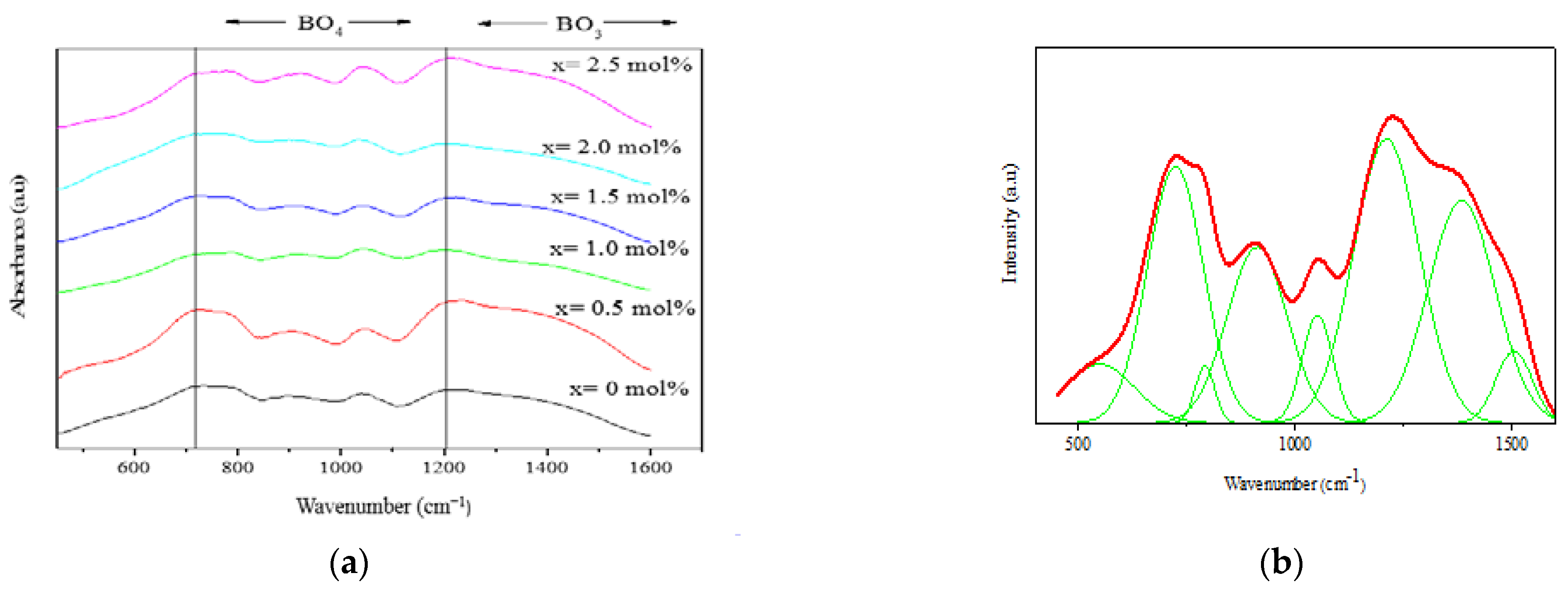


| Samples (mol%) | Density (g cm−3) | Molar Volume (cm3 mol−1) |
|---|---|---|
| x = 0 | 2.494 | 27.898 |
| x = 0.5 | 2.507 | 27.960 |
| x = 1.0 | 2.504 | 28.233 |
| x = 1.5 | 2.509 | 28.395 |
| x = 2.0 | 2.520 | 28.492 |
| x = 2.5 | 2.521 | 28.709 |
| x (mol%) | Eopt (eV) | EU (eV) | n |
|---|---|---|---|
| 0 | 3.143 | 0.298 | 2.360 |
| 0.5 | 3.054 | 0.482 | 2.383 |
| 1.0 | 2.663 | 0.296 | 2.494 |
| 1.5 | 2.651 | 0.261 | 2.498 |
| 2.0 | 2.421 | 0.395 | 2.498 |
| 2.5 | 1.752 | 0.762 | 2.852 |
| Transition 4I15/2→ | Glass Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x = 0 mol% | x = 0.5 mol% | x = 1.0 mol% | x = 1.5 mol% | x = 2.0 mol% | x = 2.5 mol% | |||||||
| fcal | fexp | fcal | fexp | fcal | fexp | fcal | fexp | fcal | fexp | fcal | fexp | |
| 4I11/2 | 7.95 | 6.99 | 7.65 | 7.32 | 6.83 | 6.81 | 8.05 | 7.32 | 8.12 | 7.93 | 7.78 | 7.7 |
| 4I9/2 | 5.95 | 4.86 | 6.31 | 6.02 | 5.81 | 6.24 | 6.09 | 5.1 | 6.38 | 4.96 | 6.13 | 4.63 |
| 4F9/2 | 3.27 | 3.27 | 3.36 | 3.35 | 3.08 | 3.05 | 3.3 | 3.3 | 3.42 | 3.46 | 3.29 | 3.35 |
| 4S3/2 | 6.28 | 4.92 | 5.96 | 4.29 | 5.41 | 4.02 | 6.08 | 5.22 | 6.23 | 4.55 | 6.06 | 4.41 |
| 2H11/2 | 1.03 | 1.03 | 1.05 | 1.05 | 9.07 | 9.07 | 1.19 | 1.19 | 1.15 | 1.15 | 1.06 | 1.06 |
| 4F7/2 | 2.78 | 2.95 | 2.73 | 2.85 | 2.49 | 2.58 | 2.73 | 2.85 | 2.82 | 2.87 | 2.73 | 2.76 |
| rms | 1.53 | 1.22 | 1.03 | 1.10 | 1.33 | 2.86 | ||||||
| Glass Samples | Ω2 cm2 | Ω4 cm2 | Ω6 cm2 | Trends of Ωλ | χ = Ω4/Ω6 | Ref. |
|---|---|---|---|---|---|---|
| x = 0 mol% | 3.19 × 10−20 | 1.44 × 10−20 | 8.45 × 10−21 | Ω2 > Ω4 > Ω6 | 1.708 | This work |
| x = 0.5 mol% | 3.16 × 10−20 | 1.51 × 10−20 | 7.87 × 10−21 | Ω2 > Ω4 > Ω6 | 1.924 | This work |
| x = 1.0 mol% | 2.43 × 10−20 | 1.27 × 10−20 | 6.53 × 10−21 | Ω2 > Ω4 > Ω6 | 1.951 | This work |
| x = 1.5 mol% | 3.38 × 10−20 | 1.32 × 10−20 | 7.30 × 10−21 | Ω2 > Ω4 > Ω6 | 1.812 | This work |
| x = 2.0 mol% | 3.21 × 10−20 | 1.39 × 10−20 | 7.49 × 10−21 | Ω2 > Ω4 > Ω6 | 1.850 | This work |
| x = 2.5 mol% | 2.22 × 10−20 | 1.00 × 10−20 | 5.50 × 10−21 | Ω2 > Ω4 > Ω6 | 1.826 | This work |
| LiBEr5 | 4.39 × 10−20 | 3.22 × 10−20 | 5.50 × 10−21 | Ω2 > Ω4 > Ω6 | 5.85 | [17] |
| BLNEr | 3.35 × 10−20 | 1.34 × 10−20 | 7.89 × 10−21 | Ω2 > Ω4 > Ω6 | 1.69 | [22] |
| Trans. | Para. | x = 0 mol% | x = 0.5 mol% | x = 1.0 mol% | x = 1.5 mol% | x = 2.0 mol% | x = 2.5 mol% |
|---|---|---|---|---|---|---|---|
| 4I15/2 →4I11/2 | A (s−1) | 350.450 | 345.077 | 342.610 | 399.333 | 402.134 | 510.599 |
| βR (%) | 88.284 | 87.946 | 86.740 | 87.979 | 88.033 | 86.711 | |
| τ (ms) | 0.285 | 0.290 | 0.292 | 0.250 | 0.249 | 0.196 | |
| 4I15/2 → 4I9/2 | A (s−1) | 388.305 | 417.475 | 421.780 | 446.074 | 466.749 | 585.557 |
| βR (%) | 89.351 | 89.291 | 89.174 | 89.322 | 89.362 | 89.197 | |
| τ (ms) | 0.258 | 0.240 | 0.237 | 0.224 | 0.214 | 0.171 | |
| 4I15/2 → 4F9/2 | A (s−1) | 3528.567 | 3702.558 | 3723.815 | 3982.301 | 4127.788 | 5193.279 |
| βR (%) | 80.950 | 80.958 | 80.897 | 80.927 | 80.874 | 80.792 | |
| τ (ms) | 0.028 | 0.027 | 0.027 | 0.025 | 0.024 | 0.019 | |
| 4I15/2 → 4S3/2 | A (s−1) | 1104.706 | 1069.927 | 1066.042 | 1198.596 | 1229.809 | 1561.382 |
| βR (%) | 71.196 | 71.162 | 71.074 | 71.145 | 71.160 | 71.060 | |
| τ (ms) | 0.091 | 0.093 | 0.094 | 0.083 | 0.081 | 0.064 | |
| 4I15/2 → 2H11/2 | A (s−1) | 14239.160 | 14798.610 | 13983.960 | 18283.850 | 17738.210 | 21451.450 |
| βR (%) | 99.182 | 99.190 | 99.017 | 99.245 | 99.221 | 99.041 | |
| τ (ms) | 0.007 | 0.007 | 0.007 | 0.005 | 0.006 | 0.005 | |
| 4I15/2 → 4F7/2 | A (s−1) | 4326.441 | 4339.731 | 4338.381 | 4773.069 | 4928.462 | 6225.356 |
| βR (%) | 99.924 | 99.922 | 99.911 | 99.918 | 99.921 | 99.907 | |
| τ (ms) | 0.023 | 0.023 | 0.023 | 0.021 | 0.020 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaini, N.A.; Mohamed, S.N.; Mohamed, Z. Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles. Materials 2021, 14, 3710. https://doi.org/10.3390/ma14133710
Zaini NA, Mohamed SN, Mohamed Z. Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles. Materials. 2021; 14(13):3710. https://doi.org/10.3390/ma14133710
Chicago/Turabian StyleZaini, Nur Adyani, Syafawati Nadiah Mohamed, and Zakiah Mohamed. 2021. "Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles" Materials 14, no. 13: 3710. https://doi.org/10.3390/ma14133710
APA StyleZaini, N. A., Mohamed, S. N., & Mohamed, Z. (2021). Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles. Materials, 14(13), 3710. https://doi.org/10.3390/ma14133710





