Amorphous–Crystalline Calcium Phosphate Coating Promotes In Vitro Growth of Tumor-Derived Jurkat T Cells Activated by Anti-CD2/CD3/CD28 Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and MAO Treatment Procedures
2.2. CaP Coating Characterization
2.3. Jurkat T Cell Culture
2.4. Jurkat T Cell Viability and Immunophenotype Detection
2.5. Gene Expression in Jurkat T Cells
2.6. Evaluation of Cell Secretion
2.7. Statistical Analysis
3. Results
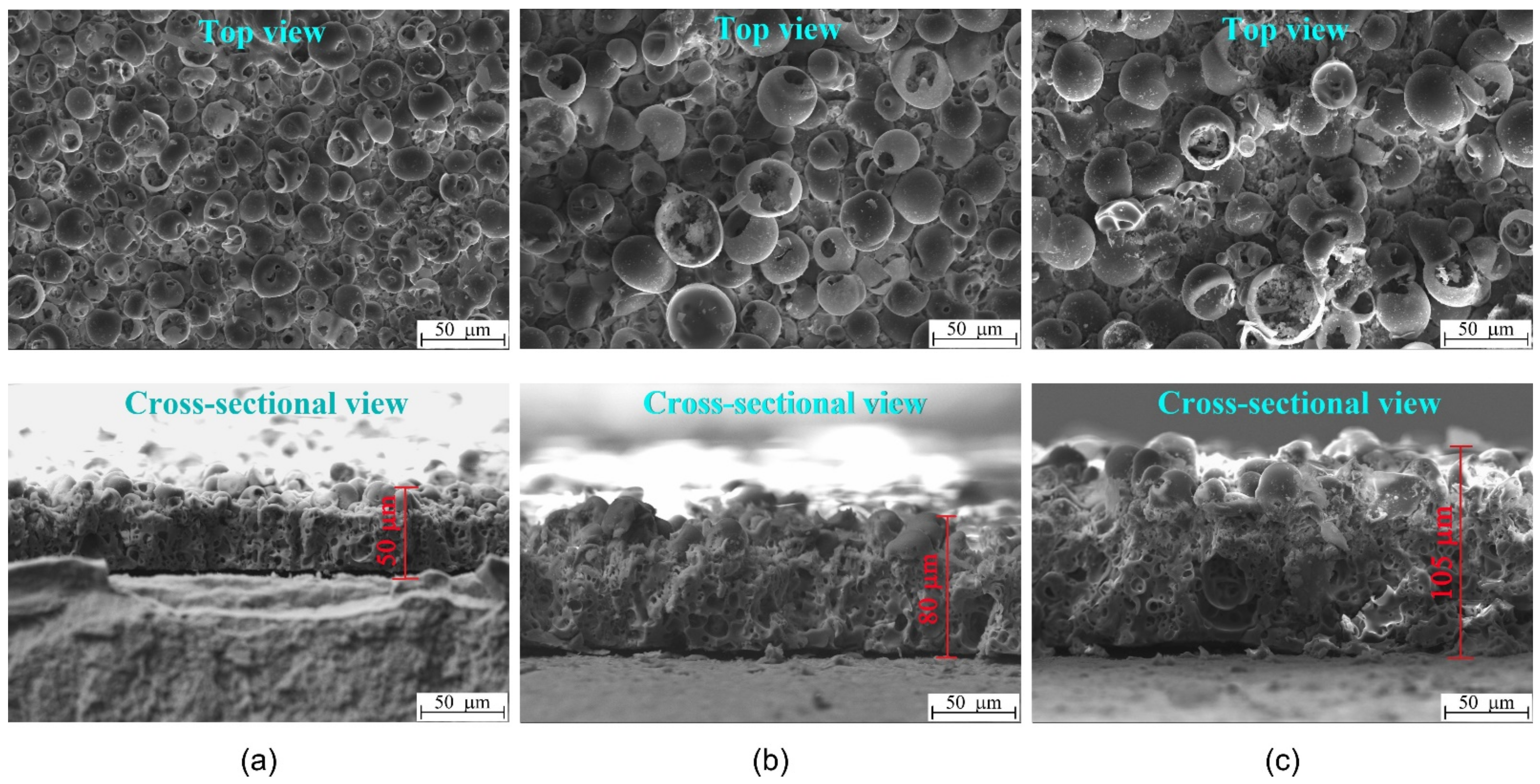
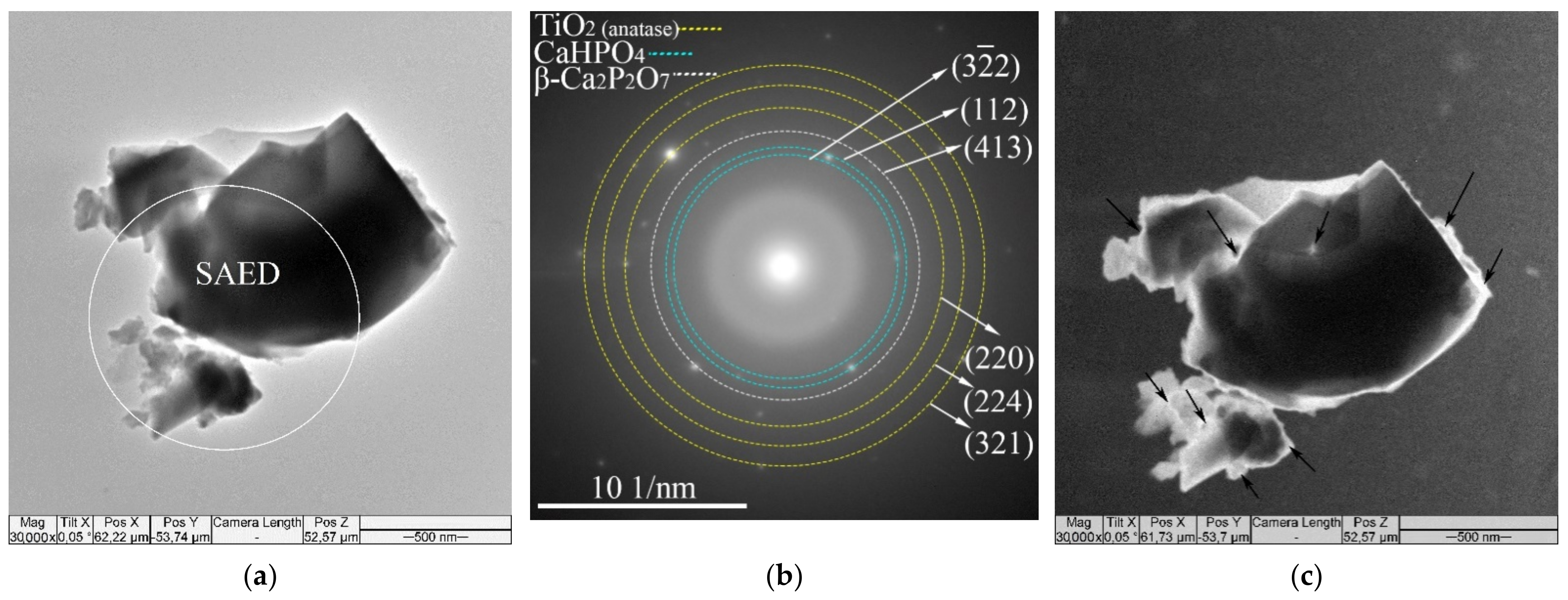
3.1. Characterization of CaP Coatings
3.2. In Vitro Cytological Properties of CaP Coatings
3.2.1. Viability and Immunophenotype of Activated Jurkat T Cells
3.2.2. Secretion Capacity of Activated Jurkat T Cells
3.2.3. hTERTExpression in Activated Jurkat T Cells Contacted with CaP-Coated Ti Substrates
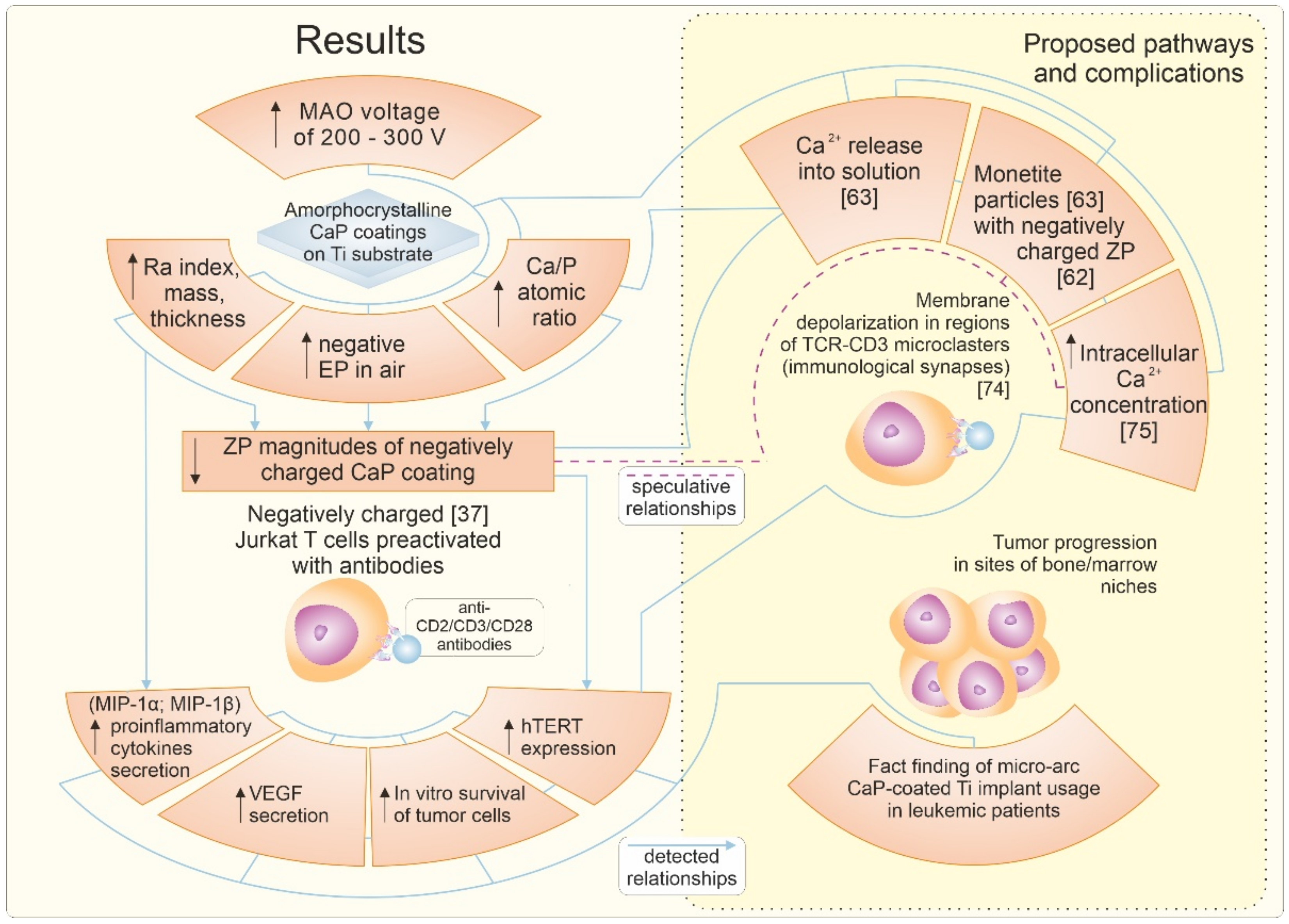
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arjunan, A.; Baroutaji, A.; Praveen, A.S.; Robinson, J.; Wang, C. Classification of biomaterial functionality. In Reference Module in Materials Science and Materials Engineering; Hashmi, M.S.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 0128035811/9780128035818. [Google Scholar] [CrossRef]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive glass and silicate-based ceramic coatings on metallic implants: Open challenge or outdated topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Aguilar-Reyes, A.E.; Flores-Martínez, M.; León-Patiño, C.A.; Nuñéz-Anita, R.E. Synthesis and characterisation of β-TCP/bioglass/zirconia scaffolds. Adv. Appl. Ceram. 2017, 116, 452–461. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics, and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Safronova, T.V.; Selezneva, I.I.; Tikhonova, S.A.; Kiselev, A.S.; Davydova, G.A.; Shatalova, T.B.; Larionov, D.S.; Rau, J.V. Biocompatibility of biphasic α,β-tricalcium phosphate ceramics in vitro. Bioact. Mater. 2020, 5, 423–427. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. Bioactive and inert dental glass-ceramics. J. Biomed. Mater. Res. Part A 2017, 105A, 619–639. [Google Scholar] [CrossRef]
- Machado López, M.M.; Espitia Cabrera, M.I.; Faure, J.; Contreras García, M.E. Electrochemical behavior of 45S5 bioactive ceramic coating on Ti6Al4V alloy for dental applications. IOP Conf. Ser. Mater. Sci. Eng. 2016, 123, 012006. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic review of current dental implant coating materials and novel coating techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef]
- De Bonis, A.; Curcio, M.; Fosca, M.; Cacciotti, I.; Santagata, A.; Teghil, R.; Rau, J.V. RBP1 bioactive glass-ceramic films obtained by pulsed laser deposition. Mater. Lett. 2016, 175, 195–198. [Google Scholar] [CrossRef]
- Costa, R.C.; Souza, J.G.S.; Cordeiro, J.M.; Bertolini, M.; de Avila, E.D.; Landers, R.; Rangel, E.C.; Fortulan, C.A.; Retamal-Valdes, B.; da Cruz, N.C.; et al. Synthesis of bioactive glass-based coating by plasma electrolytic oxidation: Untangling a new deposition pathway toward titanium implant surfaces. J. Colloid Int. Sci. 2020, 579, 680–698. [Google Scholar] [CrossRef]
- Łączka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. Bioactivity and osteoinductivity of glasses and glassceramics and their material determinants. Ceram. Int. 2016, 42, 14313–14325. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Belyavskaya, O.A.; Linders, J.; Loza, K.; Prymak, O.; Mayer, C.; Rau, J.; Epple, M.; Sharkeev, Y.P. Glancing angle deposition of Zn-doped calcium phosphate coatings by RF magnetron sputtering. Coatings 2019, 9, 220. [Google Scholar] [CrossRef]
- Rizwan, M.; Alias, R.; Zaidi, U.Z.; Mahmoodian, R.; Hamd, M. Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: A review. J. Biomed. Mater. Res. Part A 2017, 106, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO coatings design for Mg-Ca alloy for cardiovascular stent and bone regeneration applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the plasma electrolytic oxidation mechanism of titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behavior and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Sharkeev, Y.; Komarova, E.; Sedelnikova, M.; Sun, Z.; Zhu, Q.; Zhang, J.; Tolkacheva, T.; Uvarkin, P. Structure and properties of micro-arc calcium phosphate coatings on pure titanium and Ti–40Nb alloy. Trans. Nonferrous Met. Soc. 2017, 27, 125–133. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Tolkacheva, T.V.; Rau, J.V.; Buyko, E.E.; Ivanov, V.V.; Sheikin, V.V. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact. Mater. 2019, 4, 224–235. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Chebodaeva, V.V.; Tolkacheva, T.V.; Kondranova, A.M.; Zakharenko, A.M.; Bakina, O.V. Effect of the porosity, roughness, wettability and charge of micro-arc coatings on the effciency of doxorubicin delivery and suppression of cancer cells. Coatings 2020, 10, 664. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A review on the dissolution models of calcium apatites. Prog. Cryst. Growth Charact. Mater. 2002, 44, 45–61. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Inorganic chemistry of the dissolution phenomenon: The dissolution mechanism of calcium apatites at the atomic (ionic) level. Comments Inorg. Chem. 1999, 20, 285–299. [Google Scholar] [CrossRef]
- da Rocha, D.N.; de Oliveira Cruz, L.R.; de Campos, J.B.; dos Santos, J.L.; Marçal, R.L.S.B.; Mijares, D.Q.; da Silva, M.H.P. Bioactivity of strontium-monetite coatings for biomedical applications. Ceram. Int. 2019, 45, 7568–7579. [Google Scholar] [CrossRef]
- Sukhodub, L.F.; Sukhodub, L.B.; Simka, W.; Kumeda, M. Hydroxyapatite and brushite coatings on plasma electrolytic oxidized Ti6Al4V alloys obtained by the thermal substrate deposition method. Mater. Lett. 2019, 250, 163–166. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Kumar, A.M.; Babu, R.S.; Rahman, M.M.; Samyn, L.M.; de Barros, A.L.F. Biocompatible hydrophilic brushite coatings on AZX310 and AM50 alloys for orthopaedic implants. J. Mater. Sci. Mater. Med. 2018, 29, 123. [Google Scholar] [CrossRef]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178–5224. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Dekhtyar, Y.; Dvornichenko, M.V.; Karlov, A.V.; Khlusov, I.A.; Polyaka, N.; Sammons, R.; Zaytsev, K.V. Electrically functionalized hydroxyapatite and calcium phosphate surfaces to enhance immobilization and proliferation of osteoblasts in vitro and modulate osteogenesis in vivo. IFMBE Proc. 2009, 25, 245–248. [Google Scholar] [CrossRef]
- Thian, E.S.; Ahmad, Z.; Huang, J.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Bonfield, W.; Best, S.M. The role of surface wettability and surface charge of electrosprayed nanoapatites on the behaviour of osteoblasts. Acta Biomater. 2010, 6, 750–755. [Google Scholar] [CrossRef]
- Ohgaki, M.; Kizuki, T.; Katsura, M.; Yamashita, K. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 366–373. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and osteoinductive surface modifications of biomaterials for bone regeneration: A concise review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, F.; Wang, J.; Chen, X.; Liang, J.; Yang, X.; Zhu, X.; Fan, Y.; Zhang, X. Calcium phosphate altered the cytokine secretion of macrophages and influenced the homing of mesenchymal stem cells. J. Mater. Chem. B 2018, 6, 4765–4774. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Yurova, K.A.; Shunkin, E.O.; Krivosheev, V.V.; Porokhova, E.D.; Sizikova, A.E.; et al. Costimulatory effect of rough calcium phosphate coating and blood mononuclear cells on adipose-derived mesenchymal stem cells in vitro as a model of in vivo tissue repair. Materials 2020, 13, 4398. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Khaziakhmatova, O.G.; Shupletsova, V.V.; Yurova, K.A.; Malashchenko, V.V.; Shunkin, E.O.; Ivanov, P.A.; Komarova, E.G.; Chebodaeva, V.V.; Porokhova, E.D.; et al. Calcium phosphate coating prepared by microarc oxidation affects hTERT expression, molecular presentation, and cytokine secretion in tumor-derived Jurkat T cells. Materials 2020, 13, 4307. [Google Scholar] [CrossRef]
- Ma, Y.; Yamamoto, Y.; Nicovich, P.R.; Goyette, J.; Rossy, J.; Gooding, J.J.; Gaus, K. A FRET sensor enables quantitative measurements of membrane charges in live cells. Nat. Biotechnol. 2017, 35, 363–370. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Melashchenko, E.S.; Khaziakhmatova, O.G.; Yurova, K.A.; Sharkeev, Y.P.; Komarova, E.G.; Sedel’nikova, M.B.; Todosenko, N.M.; Khlusov, I.A. The morphofunctional response of T-lymphocytes to in vitro contact with a calcium phosphate Coating in the presence of a T-cell activator. Cell Tissue Biol. 2021, 15, 51–60. [Google Scholar] [CrossRef]
- Tauzin, S.; Chaigne-Delalande, B.; Selva, E.; Khadra, N.; Daburon, S.; Contin-Bordes, C.; Blanco, P.; Le Seyec, J.; Ducret, T.; Counillon, L.; et al. The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway. PLoS Biol. 2011, 9, e3000521. [Google Scholar] [CrossRef]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Khlusov, I.A.; Prymak, O.; Epple, M. Zn- or Cu-containing CaP-based coatings formed by micro-arc oxidation on titanium and Ti-40Nb alloy: Part I—Microstructure, composition and properties. Materials 2020, 13, 4116. [Google Scholar] [CrossRef]
- Komarova, E.G.; Sharkeev, Y.P.; Sedelnikova, M.B.; Prymak, O.; Epple, M.; Litvinova, L.S.; Shupletsova, V.V.; Malashchenko, V.V.; Yurova, K.A.; Dzyuman, A.N.; et al. Zn- or Cu-containing CaP-based coatings formed by micro-arc oxidation on titanium and Ti-40Nb alloy: Part II—Wettability and biological performance. Materials 2020, 13, 4366. [Google Scholar] [CrossRef]
- Bulina, N.V.; Chaikina, M.V.; Prosanov, I.Y.; Komarova, E.G.; Sedelnikova, M.B.; Sharkeev, Y.P.; Sheikin, V.V. Lanthanum-silicate-substituted apatite synthesized by fast mechanochemical method: Characterization of powders and biocoatings produced by micro-arc oxidation. Mater. Sci. Eng. C 2018, 92, 435–446. [Google Scholar] [CrossRef]
- Eguchi, M. On the permanent electret. Philos. Mag. 1925, 49, 178–192. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Dekhtyar, Y.; Sharkeev, Y.P.; Pichugin, V.F.; Khlusova, M.Y.; Polyaka, N.; Tjulkins, F.; Vendinya, V.; Legostaeva, E.V.; Litvinova, L.S.; et al. Nanoscale electrical potential and roughness of a calcium phosphate surface promotes the osteogenic phenotype of stromal cells. Materials 2018, 11, 978. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Khlusov, I.; Litvinova, L.; Shupletsova, V.; Khaziakhmatova, O.; Melashchenko, E.; Yurova, K.; Leitsin, V.; Khlusova, M.; Pichugin, V.; Sharkeev, Y. Rough titanium oxide coating prepared by micro-arc oxidation causes down-regulation of hTERT expression, molecular presentation, and cytokine secretion in tumor Jurkat T cells. Materials 2018, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.; Yurova, K.; Shupletsova, V.; Khaziakhmatova, O.; Malashchenko, V.; Shunkin, E.; Melashchenko, E.; Todosenko, N.; Khlusova, M.; Sharkeev, Y.; et al. Gene expression regulation and secretory activity of mesenchymal stem cells upon in vitro contact with microarc calcium phosphate coating. Int. J. Mol. Sci. 2020, 21, 7682. [Google Scholar] [CrossRef]
- van den Broek, L.J.; Kroeze, K.L.; Waaijman, T.; Breetveld, M.; Sampat-Sardjoepersad, S.C.; Niessen, F.B.; Middelkoop, E.; Scheper, R.J.; Gibbs, S. Differential response of human adipose tissue-derived mesenchymal stem cells, dermal fibroblasts, and keratinocytes to burn wound exudates: Potential role of skin-specific chemokine CCL27. Tissue Eng. Part A 2014, 20, 197–209. [Google Scholar] [CrossRef]
- Zhdanov, D.D.; Pokrovsky, V.S.; Pokrovskaya, M.V.; Alexandrova, S.S.; Eldarov, M.A.; Grishin, D.V.; Basharov, M.M.; Gladilina, Y.A.; Podobed, O.V.; Sokolov, N.N. Inhibition of telomerase activity and induction of apoptosis by Rhodospirillum rubrum L-asparaginase in cancer Jurkat cell line and normal human CD4+ T lymphocytes. Cancer Med. 2017, 6, 2697–2712. [Google Scholar] [CrossRef] [PubMed]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation—An overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Elliott, S.R. Physics of Amorphous Materials, 2nd ed.; Long-man: London, UK, 1990; p. 481. ISBN 058202160X/978-0582021600. [Google Scholar]
- Dorozhkin, S.V. Amorphous calcium orthophosphates: Nature, chemistry and biomedical applications. Int. J. Mater. Chem. 2012, 2, 19–46. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanodimensional and nanocrystalline calcium orthophosphates. IJCMS 2013, 1, 105–174. [Google Scholar]
- Plohl, O.; Fras Zemljic, L.; Potrč, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Li, C.; Choi, Y.J.; Levin, M.; Kaplan, D.L. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials 2013, 34, 6695–6705. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Ribeiro, C.; Correia, D.M.; Ribeiro, S.; Sencadas, V.; Botelho, G.; Lanceros-Mendez, S. Piezoelectric poly(vinylidene fluoride) microstructure and poling state in active tissue engineering. Eng. Life Sci. 2015, 15, 351–356. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Lee, K.J.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment. Sci. Rep. 2017, 7, 3833. [Google Scholar] [CrossRef]
- Xie, H.; Saito, T.; Hickner, M.A. Zeta potential of ion-conductive membranes by streaming current measurements. Langmuir 2011, 27, 4721–4727. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Kim, C.S.; Pollack, S.R. The effect of phase differences on the time-dependent variation of the zeta potential of hydroxyapatite. J. Biomed. Mater. Res. 1992, 26, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Process of calcification on artificial materials. Z. Kardiol. 2001, 90, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Probst, J.; Thull, R. Surface properties of calcium phosphate particles for self setting bone cements. Biomol. Eng. 2002, 19, 51–55. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Mushtovatova, L.S.; Karpova, M.R.; Sheikinc, V.V.; Litvinova, L.S.; Khlusov, I.A. Zn-, Cu-or Ag-incorporated micro-arc coatings on titanium alloys: Properties and behavior in synthetic biological media. Surf. Coat. Technol. 2019, 369, 52–68. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. An important Calcium Phosphate Compound—Its Synthesis, Properties and Applications in Orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Edmond, V.; Dufour, F.; Poiroux, G.; Shoji, K.; Malleter, M.; Fouqué, A.; Tauzin, S.; Rimokh, R.; Sergent, O.; Penna, A.; et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene 2015, 34, 996–1005. [Google Scholar] [CrossRef]
- Batista, A.; Millán, J.; Mittelbrunn, M.; Sánchez-Madrid, F.; Alonso, M.A. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J. Immunol. 2004, 172, 6709–6714. [Google Scholar] [CrossRef]
- Terui, Y.; Ikeda, M.; Tomizuka, H.; Kasahara, T.; Ohtsuki, T.; Uwai, M.; Mori, M.; Itoh, T.; Tanaka, M.; Yamada, M.; et al. Activated endothelial cells induce apoptosis in leukemic cells by endothelial interleukin-8. Blood 1998, 92, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, L.S.; Shupletsova, V.V.; Dunets, N.A.; Khaziakhmatova, O.G.; Yurova, K.A.; Khlusova, M.Y.; Slepchenko, G.B.; Cherempey, E.G.; Sharkeev, Y.P.; Komarova, E.G.; et al. Imbalance of morphofunctional responses of Jurkat T lymphoblasts at short-term culturing with relief zinc- or copper-containing calcium phosphate coating on titanium. Dokl. Biochem. Biophys. 2017, 472, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Gallin, J.I. Degranulating stimuli decrease the negative surface charge and increase the adhesiveness of human neutrophils. J. Clin. Investig. 1980, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T.; Weiss, A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004, 4, 301–308. [Google Scholar] [CrossRef]
- Wiskocil, R.; Weiss, A.; Imboden, J.; Kamin-Lewis, R.; Stobo, J. Activation of a human T cell line: A two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J. Immunol. 1985, 134, 1599–1603. [Google Scholar]
- Stevens, M.J.; Donato, L.J.; Lower, S.K.; Sahai, N. Oxide-dependent adhesion of the Jurkat line of T lymphocytes. Langmuir 2009, 25, 6270–6278. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, D.; Jiang, Y.H.; Yao, W.J.; Wang, X.J.; Wei, X.C.; Gao, J.; Xie, L.D.; Yan, Z.Y.; Wen, Z.Y.; et al. Influence of expressed TRAIL on biophysical properties of the human leukemic cell line Jurkat. Cell Res. 2004, 14, 161–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alonso-Nocelo, M.; Abellan-Pose, R.; Vidal, A.; Abal, M.; Csaba, N.; Alonso, M.J.; Lopez-Lopez, R.; de la Fuente, M. Selective interaction of PEGylated polyglutamic acid nanocapsules with cancer cells in a 3D model of a metastatic lymph node. J. Nanobiotechnol. 2016, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.K. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Redmann, K.; Jenssen, H.L.; Köhler, H.J. Experimental and functional changes in transmembrane potential and zeta potential of single cultured cells. Exp. Cell Res. 1974, 87, 281–289. [Google Scholar] [CrossRef]
- Yao, C.X.; Lin, T.Y.; Su, Y.L.; Zou, H.; Yan, Z.Y.; Wu, S.M. Inhibitory effects of CuInS2 and CdTe nanoparticles on macrophage cytokine production and phagocytosis in vitro. Enzyme Microb. Technol. 2019, 127, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Das, J.K.; Thirumurugan, A.; Suar, M.; Parashar, S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine 2018, 13, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Minc, N. Electrochemical control of cell and tissue polarity. Annu. Rev. Cell Dev. Biol. 2014, 30, 317–336. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Fernandes de Lima, V.M.; Wiedemann, M.; Klottig, H.; Rahmann, H.; Hanke, W. Exogenous application of gangliosides changes the state of excitability of retinal tissue as demonstrated by retinal spreading depression experiments. Naunyn Schmiedebergs Arch. Pharmacol. 1997, 355, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays 2012, 34, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Tasian, S.K.; Bornhäuser, M.; Rutella, S. Targeting Leukemia Stem Cells in the Bone Marrow Niche. Biomedicines 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Khlusov, I.A.; Litvinova, L.S.; Khlusova, M.Y.; Yurova, K.A. Concept of Hematopoietic and Stromal Niches for Cell-Based Diagnostics and Regenerative Medicine (a Review). Curr. Pharm. Des. 2018, 24, 3034–3054. [Google Scholar] [CrossRef] [PubMed]
- Guerrouahen, B.S.; Al-Hijji, I.; Tabrizi, A.R. Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia. Stem Cells Int. 2011, 2011, 375857. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Scadden, D.T.; Gilliland, D.G. The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood 2009, 114, 1150–1157. [Google Scholar] [CrossRef]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef]
- Delahaye, M.C.; Salem, K.I.; Pelletier, J.; Aurrand-Lions, M.; Mancini, S.J.C. Toward Therapeutic Targeting of Bone Marrow Leukemic Niche Protective Signals in B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 10, 606540. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Shevtsova, N.M.; Khlusova, M.Y. Detection in vitro and quantitative estimation of artificial microterritories which promote osteogenic differentiation and maturation of stromal stem cells. Methods Mol. Biol. 2013, 1035, 103–119. [Google Scholar] [CrossRef]



| hTERT (human telomerase reverse transcriptase) hTERT_for5′-TGACACCTCACCTCACCCAC-3′ hTERT_rev5′-CACTGTCTTCCGCAAGTTCAC-3′ |
| U2AF1L4 (U2 small nuclear RNA auxiliary factor 1 like 4) U2AF1L4_for 5′-CTTCACAACAAGCCGACATTC-3′ U2AF1L4_rev 5′-CAAGGTTGTCGCACACATTC-3′ |
| GFI1 (growth factor independent 1 transcriptional repressor) GFI1_for5′-TGGAGCAGCACAAAGCC-3′ GFI1_rev 5′-GACAGTGTGGATGACCTCTTG-3′ |
| HNRNPLL (heterogeneous nuclear ribonucleoprotein L) HNRNPLL_for5′-CTCTCAATTCAGAATCCGCTTTATC-3′ HNRNPLL_rev 5′- CCATTGCTTGTATCCCATTTCTC-3′ |
| RPLPO (large ribosomal protein) RPLPO_for 5′-GGCGACCTGGAAGTCCAACT-3′ RPLPO_rev 5′-CCATCAGCACCACAGCCTTC-3′ |
| hTERT_probe FAM-5′-ACCCTGGTCCGAGGTGTCCCTGAG-3′-BHQ-1 U2AF1L4_probe FAM-5′-CCAGGAGGTGTTCACAGAACTGCA-3′~BHQ-1 GFI1_probe FAM-5′-CGCAGGAACGGAGCTTTGACTGTA-3′~BHQ-1 HNRPLL_probe FAM-5′-TATGCAACCCTGTTGGCAAAGTGC-3′~BHQ-1 RPLPO_ probe Bgl635-5′-ATCTGCTGCATCTGCTTGGAGCCCA-3′-BHQ-2 |
| Parameters of Bilateral CaP Coating on a Titanium Substrate, n = 3 a | Relative Expression of Genes | |||||
|---|---|---|---|---|---|---|
| Ra, µm | Thickness, µm | Weight, mg | hTERT | U2AF1L4 | GFI1 | HNRNPLL |
| 3.1 (2.5; 4.7) | 53.0 (39.5; 70.5) | 14.5 (10.6; 19.1) | 4.49 b (1.80; 7.01) | −1.0 (−1.36; 4.11) | 1.34 (−2.69; 1.57) | 65.50 (−50.70; 168.39) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharkeev, Y.P.; Komarova, E.G.; Chebodaeva, V.V.; Sedelnikova, M.B.; Zakharenko, A.M.; Golokhvast, K.S.; Litvinova, L.S.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Yurova, K.A.; et al. Amorphous–Crystalline Calcium Phosphate Coating Promotes In Vitro Growth of Tumor-Derived Jurkat T Cells Activated by Anti-CD2/CD3/CD28 Antibodies. Materials 2021, 14, 3693. https://doi.org/10.3390/ma14133693
Sharkeev YP, Komarova EG, Chebodaeva VV, Sedelnikova MB, Zakharenko AM, Golokhvast KS, Litvinova LS, Khaziakhmatova OG, Malashchenko VV, Yurova KA, et al. Amorphous–Crystalline Calcium Phosphate Coating Promotes In Vitro Growth of Tumor-Derived Jurkat T Cells Activated by Anti-CD2/CD3/CD28 Antibodies. Materials. 2021; 14(13):3693. https://doi.org/10.3390/ma14133693
Chicago/Turabian StyleSharkeev, Yurii P., Ekaterina G. Komarova, Valentina V. Chebodaeva, Mariya B. Sedelnikova, Aleksandr M. Zakharenko, Kirill S. Golokhvast, Larisa S. Litvinova, Olga G. Khaziakhmatova, Vladimir V. Malashchenko, Kristina A. Yurova, and et al. 2021. "Amorphous–Crystalline Calcium Phosphate Coating Promotes In Vitro Growth of Tumor-Derived Jurkat T Cells Activated by Anti-CD2/CD3/CD28 Antibodies" Materials 14, no. 13: 3693. https://doi.org/10.3390/ma14133693
APA StyleSharkeev, Y. P., Komarova, E. G., Chebodaeva, V. V., Sedelnikova, M. B., Zakharenko, A. M., Golokhvast, K. S., Litvinova, L. S., Khaziakhmatova, O. G., Malashchenko, V. V., Yurova, K. A., Gazatova, N. D., Kozlov, I. G., Khlusova, M. Y., Zaitsev, K. V., & Khlusov, I. A. (2021). Amorphous–Crystalline Calcium Phosphate Coating Promotes In Vitro Growth of Tumor-Derived Jurkat T Cells Activated by Anti-CD2/CD3/CD28 Antibodies. Materials, 14(13), 3693. https://doi.org/10.3390/ma14133693










