Photocatalytic Activity of Cu2S/WO3 and Cu2S/SnO2 Heterostructures for Indoor Air Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heterostructure Development
2.2. Photocatalytic Experiments
2.3. Characterization
3. Results and Discussions
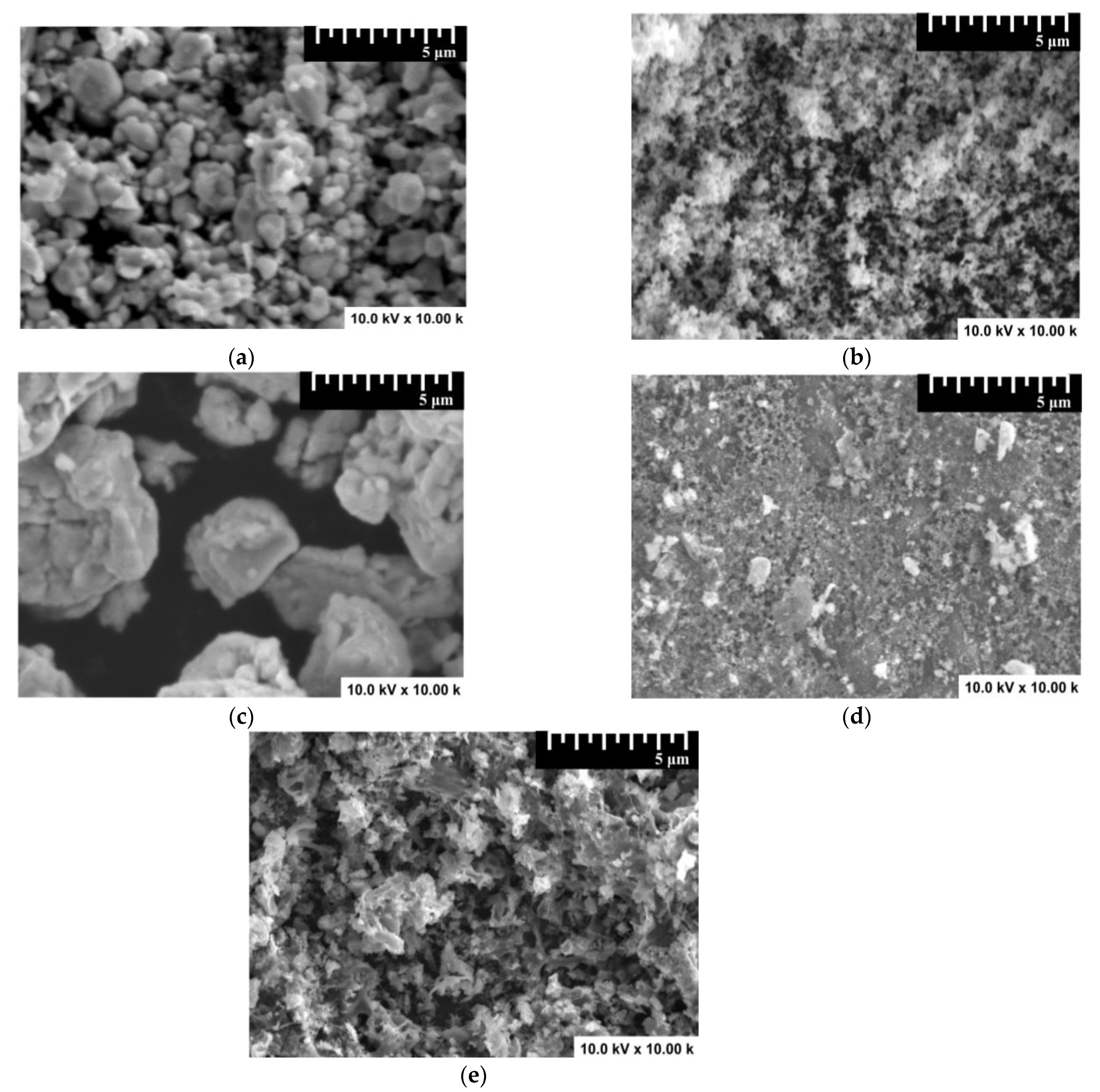
3.1. Composition and Morphology
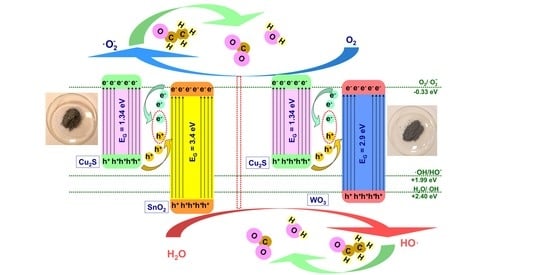
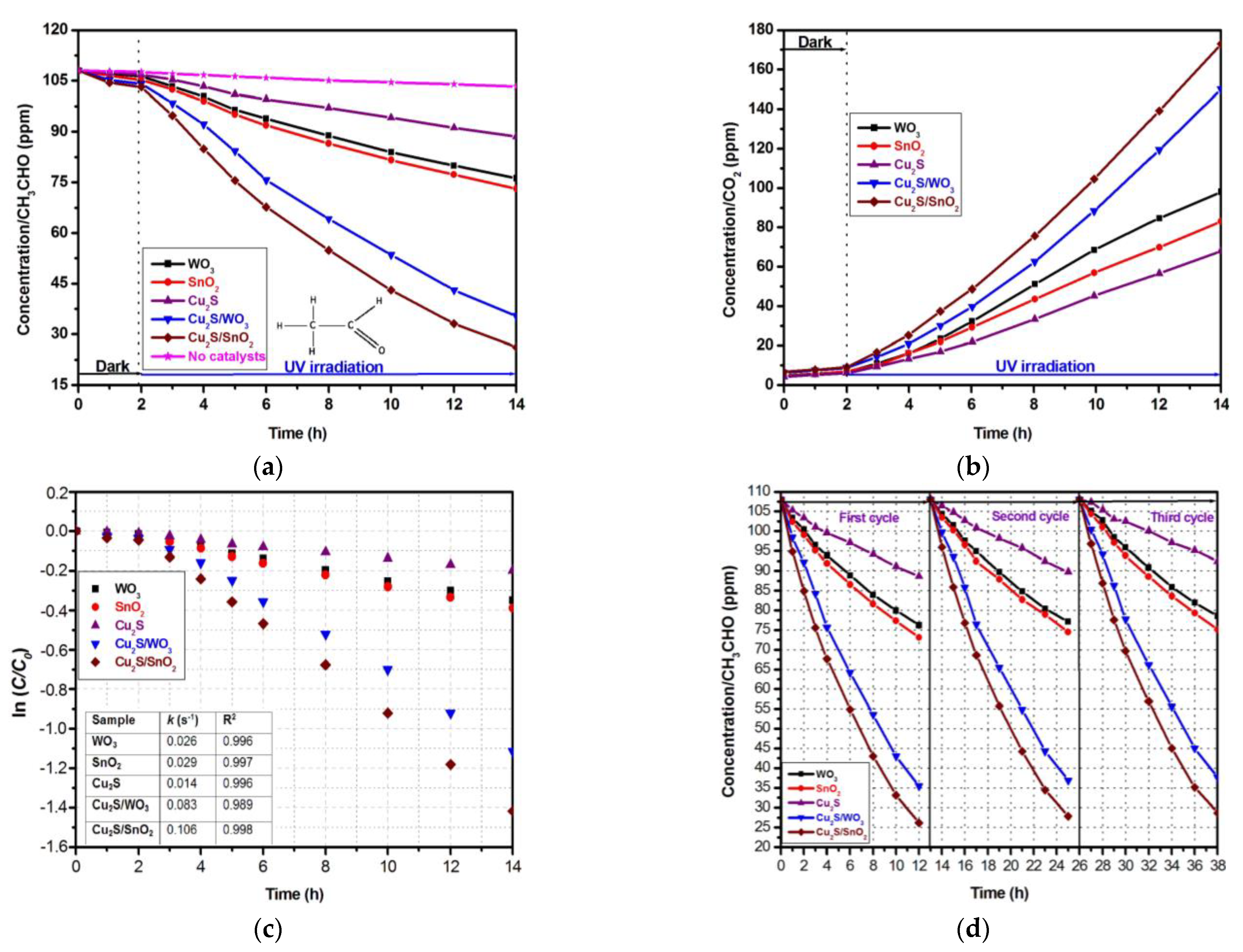
3.2. Photocatalytic Activity and Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, W.; Han, X.; Astorsdotter, J.; Farrauto, R. Catalysts Promoted with Niobium Oxide for Air Pollution Abatement. Catalysts 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Alam, M.S.; Harrison, R.M. Behaviour of traffic emitted semi-volatile and intermediate volatility organic compounds within the urban atmosphere. Sci. Total Environ. 2020, 720, 137470. [Google Scholar] [CrossRef] [PubMed]
- Sanito, R.C.; You, S.J.; Wang, Y.F. Effect of shell powder on removal of metals and volatile organic compounds (VOCs) from resin in an atmospheric-pressure microwave plasma reactor. J. Hazard. Mater. 2020, 394, 122558. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Hou, X.; Xiong, B. Bioelectronic Nose Based on Single-Stranded DNA and Single-Walled Carbon Nanotube to Identify a Major Plant Volatile Organic Compound (p-Ethylphenol) Released by Phytophthora Cactorum Infected Strawberries. Nanomaterials 2020, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Shi, A.; Li, G. Insights into the comprehensive characteristics of volatile organic compounds from multiple cooking emissions and aftertreatment control technologies application. Atmos. Environ. 2020, 240, 117646. [Google Scholar] [CrossRef]
- Qiu, Y.; Ye, N.; Situ, D.; Zuo, S.; Wang, X. Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts. Materials 2019, 12, 728. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, E.; Nadjia, L.; Noureddine, B. SnO2 foam grain-shaped nanoparticles: Synthesis, characterization and UVA light induced photocatalysis. J. Alloy. Compound. 2016, 679, 408–419. [Google Scholar] [CrossRef]
- He, J.; Zou, Z.; Yang, X. Measuring whole-body volatile organic compound emission by humans: A pilot study using an air-tight environmental chamber. Build. Environ. 2019, 153, 101–109. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Tian, S. Interaction of inhalable volatile organic compounds and pulmonary surfactant: Potential hazards of VOCs exposure to lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef]
- Noh, H.L.; Park, Y.K.; Kim, J.H. Colorimetric chemosensor for detection of a volatile organic compound, ethylamine, under versatile conditions: Solution, thin-film, and dyed fabric. Sens. Actuat. B 2019, 301, 127079. [Google Scholar] [CrossRef]
- Dong, N.; Ye, Q.; Chen, M.; Cheng, S.; Kang, T.; Dai, H. Catalytic Oxidation of HCHO over the Sodium-Treated Sepiolite-Supported Rare Earth (La, Eu, Dy, and Tm) Oxide Catalysts. Catalysts 2020, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Ojha, D.P.; Song, J.H.; Kim, H.J. Facile synthesis of graphitic carbon-nitride supported antimony-doped tin oxide nanocomposite and its application for the adsorption of volatile organic compounds. J. Environ. Sci. 2019, 79, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.D.; Allahdadlalouni, M.; Bagheri, H. Reduced graphene oxide–melamine formaldehyde as a highly efficient platform for needle trap microextraction of volatile organic compounds. Microchem. J. 2020, 157, 104932. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Quijo, M.V.; Assaf, E.M. Photocatalytic activity of Nb heterostructure (NaNbO3/Na2Nb4O11) and Nb/clay materials in the degradation of organic compounds. Sol. Energy 2019, 194, 37–46. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B. 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Liu, B.; Long, M.; Yang, J. Interfacial charge behavior modulation in 2D/3D perovskite heterostructure for potential high-performance solar cells. Nano Energy 2019, 59, 715–720. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [Green Version]
- Spanu, D.; Recchia, S.; Altomare, M. Site-selective Pt dewetting on WO3-coated TiO2 nanotube arrays: An electron transfer cascade-based H2 evolution photocatalyst. Appl. Catal. B 2018, 237, 198–205. [Google Scholar] [CrossRef]
- Singhal, S.; Dixit, S.; Shukla, A.K. Self-assembly of the Ag deposited ZnO/carbon nanospheres: A resourceful photocatalyst for efficient photocatalytic degradation of methylene blue dye in water. Adv. Powder Technol. 2018, 29, 3483–3492. [Google Scholar] [CrossRef]
- Li, X.; Peng, T.; Nan, Z. A new efficient visible-light photocatalyst made of SnO2 and cyclized polyacrylonitrile. Mater. Res. Bull. 2018, 97, 517–522. [Google Scholar] [CrossRef]
- Do, J.Y.; Chava, R.K.; Kang, M. Fabrication of core@interface:shell structured CuS@CuInS2:In2S3 particles for highly efficient solar hydrogen production. Appl. Surf. Sci. 2018, 451, 86–98. [Google Scholar] [CrossRef]
- Das, D.; Nandi, P. Ternary ZnCdSO composite photocatalyst for efficient dye degradation under visible light retaining Z-scheme of migration pathways for the photogenerated charge carriers. Sol. Energ. Mat. Sol. C. 2020, 217, 110674. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Popoola, L.T.; Aderibigbe, E.I. Solar photocatalytic degradation of organic pollutants in textile industry wastewater by ZnO/pumice composite photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103907. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Ji, Z. The effect of support on the structure and photocatalytic activity of ternary ZnO-ZnFe2O4/palygorskite composite photocatalysts. Adv. Powder Technol. 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Jiang, Y.; Ni, L. Novel CdIn2S4 nano-octahedra/TiO2 hollow hybrid heterostructure: In-situ synthesis, synergistic effect and enhanced dual-functional photocatalytic activities. Ceram. Int. 2019, 45, 15942–15953. [Google Scholar] [CrossRef]
- Matsunami, D.; Yamanaka, K.; Kojima, K. Comparison of photodegradation of methylene blue using various TiO2 films and WO3 powders under ultraviolet and visible-light irradiation. J. Photochem. Photobiol. A 2019, 369, 106–114. [Google Scholar] [CrossRef]
- Chen, S.; Liu, F.; Liu, C. First-principles calculations and experimental investigation on SnO2@ZnO heterojunction photocatalyst with enhanced photocatalytic performance. J. Colloid Interfac. Sci. 2019, 553, 613–621. [Google Scholar] [CrossRef]
- Meroni, D.; Gasparini, C.; Bianchi, C.L. Ultrasound-assisted synthesis of ZnO photocatalysts for gas phase pollutant remediation: Role of the synthetic parameters and of promotion with WO3. Ultrason. Sonochem. 2020, 66, 105119. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, F.; Swasy, M.I.; Attia, M.F.; Ateia, M.; Encalada, D.; Vizuete, K.; Galeas, S.; Guerrero, V.H.; Debut, A.; et al. Cellulose particles capture aldehyde VOC pollutants. RSC Adv. 2020, 10, 7967–7975. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, Y.S.; Shin, J.W.; Kim, K.H.; Kukkar, D.; Fai Tsang, Y. Potential applications of graphene-based nanomaterials as adsorbent for removal of volatile organic compounds. Environ. Int. 2020, 135, 105356. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Beeldens, A.; Van Langenhove, H. Heterogeneous photocatalytic removal of toluene from air on building materials enriched with TiO2. Build. Environ. 2008, 43, 406–414. [Google Scholar] [CrossRef]
- Seo, H.O.; Park, E.J.; Kim, I.H.; Han, S.W.; Cha, B.J.; Woo, T.G.; Kim, Y.D. Influence of humidity on the photo-catalytic degradation of acetaldehyde over TiO2 surface under UV light irradiation. Catal. Today 2017, 295, 102–109. [Google Scholar] [CrossRef]
- Jeong, M.G.; Park, E.J.; Seo, H.O.; Kim, K.D.; Kim, Y.D.; Lim, D.C. Humidity effect on photocatalytic activity of TiO2 and regeneration of deactivated photocatalysts. Appl. Surf. Sci. 2013, 271, 164–170. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Pawar, A.A.; Kim, H. Ionic liquid based Cu2S@C catalyst for effective coupling of diaryl diselenide with aryl halides under ligand-free conditions. Chem. Eng. J. 2018, 351, 490–497. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Xu, C. Self-optimizing bifunctional CdS/Cu2S with coexistence of light-reduced Cu0 for highly efficient photocatalytic H2 generation under visible-light irradiation. Appl. Catal. B 2017, 217, 30–36. [Google Scholar] [CrossRef]
- Gao, H.; Zhai, C.; Zhu, M. Snowflake-like Cu2S as visible-light-carrier for boosting Pd electrocatalytic ethylene glycol oxidation under visible light irradiation. Electrochim. Acta 2020, 330, 135214. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Shoaib, M. Shape and phase-controlled synthesis of specially designed 2D morphologies of l-cysteine surface capped covellite (CuS) and chalcocite (Cu2S) with excellent photocatalytic properties in the visible spectrum. Appl. Surf. Sci. 2020, 526, 146691. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Chen, W. Cu2S/CIGS core/shell nanowire arrays with epitaxial CIGS growth. Sol. Energ. Mater. Sol. C. 2014, 128, 357–361. [Google Scholar] [CrossRef]
- Fu, Y.; Jin, W. Facile synthesis of core-shell CuS-Cu2S based nanocomposite for the high-performance glucose detection. Mater. Sci. Eng. C 2019, 105, 110120. [Google Scholar] [CrossRef]
- Ghifari, A.; Long, D.X.; Kim, S.; Ma, B.; Hong, J. Transparent Platinum Counter Electrode Prepared by Polyol Reduction for Bifacial, Dye-Sensitized Solar Cells. Nanomaterials 2020, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Bagherian, S.; Zak, A.K. X-ray peak broadening and optical properties analysis of SnO2 nanosheets prepared by sol-gel method. Mater. Sci. Semicon. Proc. 2016, 56, 52–58. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Huang, W. Tuning CuOx-TiO2 interaction and photocatalytic hydrogen production of CuOx/TiO2 photocatalysts via TiO2 morphology engineering. Appl. Surf. Sci. 2019, 473, 500–510. [Google Scholar] [CrossRef]
- Lei, E.; Hu, C.; Liu, Z. Composition, morphology, structure and photocatalytic performances of photocatalysts prepared from titanium potassium oxalate. Solid State Sci. 2019, 88, 36–40. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, X.; Gong, S. Surfactant-assisted synthesis of photocatalysts: Mechanism, synthesis, recent advances and environmental application. Chem. Eng. J. 2019, 372, 429–451. [Google Scholar] [CrossRef]
- Li, L.; Yan, Y.; Zhou, J. Glucose-assisted hydrothermal synthesis of plasmonic Bi deposited nested Bi2O2−xCO3 photocatalysts with enhanced photocatalytic activity. Colloid. Surf. A 2019, 583, 123946. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Chen, W.F.; Lv, Y.R.; Yang, S.Y.; Xu, Y.H. Z-scheme hierarchical Cu2S/Bi2WO6 composites for improved photocatalytic activity of glyphosate degradation under visible light irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]
- Sithole, R.K.; Machogo, L.F.E.; Moloto, N. One-step synthesis of Cu3N, Cu2S and Cu9S5 and photocatalytic degradation of methyl orange and methylene blue. J. Photochem. Photobiol. A 2020, 397, 112577. [Google Scholar] [CrossRef]
- Jo, I.R.; Rajesh, J.A.; Ahn, K.S. Enhanced electrocatalytic activity and electrochemical stability of Cu2S/PbS counter electrode for quantum-dot-sensitized solar cells. Appl. Surf. Sci. 2020, 525, 146643. [Google Scholar] [CrossRef]
- Serinçay, N.; Fellah, M.F. Acetaldehyde adsorption and detection: A density functional theory study on Al-doped graphene. Vacuum 2020, 175, 109279. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Sun, J. Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals. J. Colloid Interface Sci. 2020, 572, 374–383. [Google Scholar] [CrossRef]
- Enesca, A. Enhancing the Photocatalytic Activity of SnO2-TiO2 and ZnO-TiO2 Tandem Structures Toward Indoor Air Decontamination. Front. Chem. 2020, 8, 583270. [Google Scholar] [CrossRef] [PubMed]
- Ichiura, H.; Seike, T.; Kozu, A. Acetaldehyde gas removal by a nylon film–TiO2 composite sheet prepared on a paper surface using interfacial polymerization and electrostatic interactions. Chemosphere 2020, 256, 127143. [Google Scholar] [CrossRef] [PubMed]
- Saqlain, S.; Cha, B.J.; Kim, S.Y.; Sung, J.Y.; Choi, M.C.; Seo, H.O.; Kim, Y.D. Impact of humidity on the removal of volatile organic compounds over Fe loaded TiO2 under visible light irradiation: Insight into photocatalysis mechanism by operando DRIFTS. Mater. Today Commun. 2021, 26, 102119. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, X.; Xie, X.; Lu, G.; Wang, Y.; Lee, S.C.; Sun, J. TiO2/TaS2 with superior charge separation and adsorptive capacity to the photodegradation of gaseous acetaldehyde. Chem. Eng. J. 2020, 379, 122395. [Google Scholar] [CrossRef]
- Gao, C.; Li, J.; Shan, Z.; Huang, F.; Shen, H. Preparation and visible-light photocatalytic activity of In2S3/TiO2 composite. Mater. Chem. Phys. 2010, 122, 183–187. [Google Scholar] [CrossRef]
- Mise, T.; Nakada, T. Low temperature growth and properties of Cu–In–Te based 433 thin films for narrow bandgap solar cells. Thin Solid Films 2010, 518, 5604–5609. [Google Scholar] [CrossRef]
- Iqbal, M.; Wang, Y.; He, T. Interfacial charge kinetics of ZnO/ZnTe heterostructured nanorod arrays for CO2 photoreduction. Electrochim. Acta 2018, 272, 203–211. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B Adv. 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Xiang, Y.; Yu, N.; Cao, L. Simple fabrication of ZnO nanosheets/p-GaN heterostructure and ultraviolet detection. Physica E 2018, 102, 29–32. [Google Scholar] [CrossRef]
- Tian, W.; Wu, H.; Yang, X. Heterostructure based on silver/silver chloride nanocubes loaded titanium dioxide nanofibers: A high-efficient and recyclable visible light-responsive photocatalyst. J. Photochem. Photobiol. A 2018, 350, 122–129. [Google Scholar] [CrossRef]
- Mu, C.; Song, J.; Xiang, J. Facile-synthesized carbonaceous photonic crystals/magnetic particle nanohybrids with heterostructure as an excellent microwave absorber. J. Alloy. Compound. 2018, 741, 814–820. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Yang, P. ZIF-8 derived hexagonal-like α-Fe2O3/ZnO/Au nanoplates with tunable surface heterostructures for superior ethanol gas-sensing performance. Appl. Surf. Sci. 2018, 439, 649–659. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Kuikka, S. Quantifying and predicting ecological and human health risks for binary heavy metal pollution accidents at the watershed scale using Bayesian Networks. Environ. Pollut. 2021, 269, 116125. [Google Scholar] [CrossRef]


| Photocatalyst | Crystallite Size (Å) | ||
|---|---|---|---|
| Cu2S | WO3 | SnO2 | |
| WO3 | - | 93.8 | - |
| SnO2 | - | - | 81.5 |
| Cu2S | 64.7 | - | - |
| Cu2S/WO3 | 82.4 | 92.6 | - |
| Cu2S/SnO2 | 75.9 | - | 82.6 |
| Sample | Elemental Composition (% at) | ||||||
|---|---|---|---|---|---|---|---|
| Cu | Sn | W | O | Oth 1 | S | Sth 1 | |
| WO3 | - | - | 23.2 | 76.8 | 69.6 | - | - |
| SnO2 | - | 31.4 | - | 68.6 | 62.8 | - | - |
| Cu2S | 71.5 | - | - | 3.7 | - | 24.8 | 35.7 |
| Cu2S/WO3 | 17.7 | - | 16.8 | 57.6 | 50.4 | 7.9 | 8.8 |
| Cu2S/SnO2 | 24.8 | - | 23.1 | 41.3 | 46.2 | 10.8 | 12.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Isac, L. Photocatalytic Activity of Cu2S/WO3 and Cu2S/SnO2 Heterostructures for Indoor Air Treatment. Materials 2021, 14, 3656. https://doi.org/10.3390/ma14133656
Enesca A, Isac L. Photocatalytic Activity of Cu2S/WO3 and Cu2S/SnO2 Heterostructures for Indoor Air Treatment. Materials. 2021; 14(13):3656. https://doi.org/10.3390/ma14133656
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Isac. 2021. "Photocatalytic Activity of Cu2S/WO3 and Cu2S/SnO2 Heterostructures for Indoor Air Treatment" Materials 14, no. 13: 3656. https://doi.org/10.3390/ma14133656
APA StyleEnesca, A., & Isac, L. (2021). Photocatalytic Activity of Cu2S/WO3 and Cu2S/SnO2 Heterostructures for Indoor Air Treatment. Materials, 14(13), 3656. https://doi.org/10.3390/ma14133656