Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Loaded with Carboplatin Enhance Cytotoxicity on Human Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Graphical Nanovector Functionalized with HA and Loaded with CPT
2.2. Characterization of cCNT and oxCNT-HA-CPT
2.3. Establishment of Cell Lines
- (a)
- HeLa cell line: Cells derived from adenocarcinoma of the human cervix from a 31-year-old black female patient. They are adherent cells, of epithelial morphology and positive for keratin by immunoperoxidase staining. They have been reported to contain human papillomavirus type 18 sequences, as well as express low levels of p53 and normal levels of pRB. The cells were incubated in 1× Advanced DMEM medium supplemented with 4% v/v fetal bovine serum, 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere (ATCC®, CCL-2 ™) [30].
- (b)
- MDA-MB-231 cell line: Cells derived from human mammary adenocarcinoma from a 51-year-old Caucasian female patient. They are adherent cells, of epithelial morphology and express the WNT7B oncogene. They express EGF and transforming growth factor-alpha (TGF-α). Cells were incubated in 1× Advanced DMEM medium supplemented with 4% v / v fetal bovine serum, 1% v / v penicillin/streptomycin at 37 °C in an atmosphere with 5% CO2 (ATCC®, HTB-26 ™) [31].
- (c)
- NIH-3T3 cell line: Mouse embryonic fibroblasts. They are adherent cells with a tapered morphology. Suitable for transfections, however, in this study they were used to demonstrate susceptibility to oxCNT-HA-CPT. The cells were incubated in 1× Advanced DMEM medium, supplemented with 4% v/v fetal bovine serum, 1% v/v penicillin/streptomycin at 37 °C in an atmosphere with 5% CO2 (ATCC®, CRL-1658 ™) [32].
2.4. Immunofluorescence for the Determination of CD44 Receptor
2.5. Analysis of Cells Viability with 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Colorimetric Assay
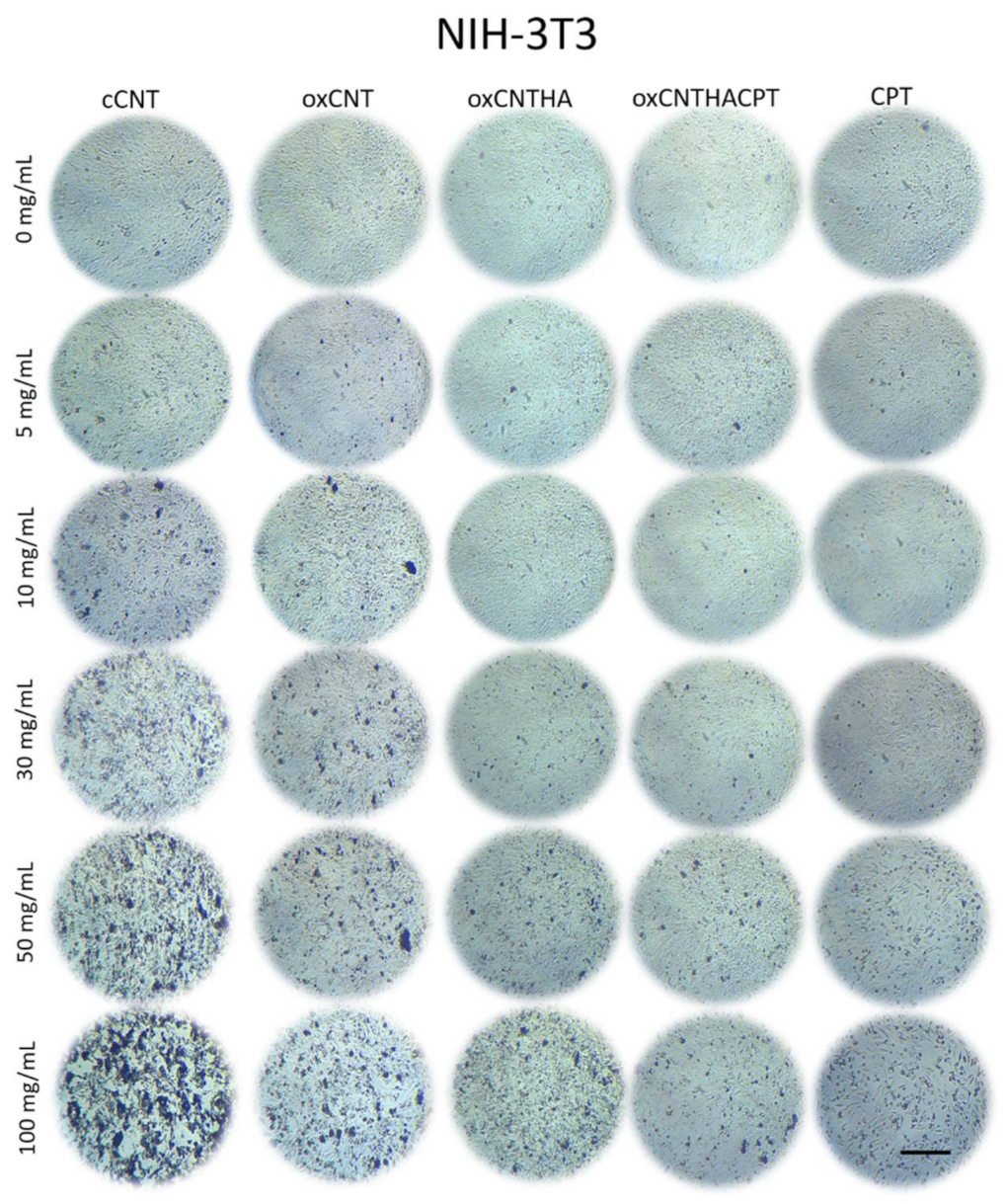
2.6. Nuclei Contrast Assay with DAPI
2.7. Comparative Assay of the oxCNT-HA-CPT Nanovector vs. CPT Chemotherapy
3. Results
3.1. Oxidation and Functionalization Induce Changes in the cCNTs Walls
3.2. HeLa and MDA-MB-231 Tumor Cells Show Higher Expression of the CD44 Receptor than Non-Tumor NIH-3T3
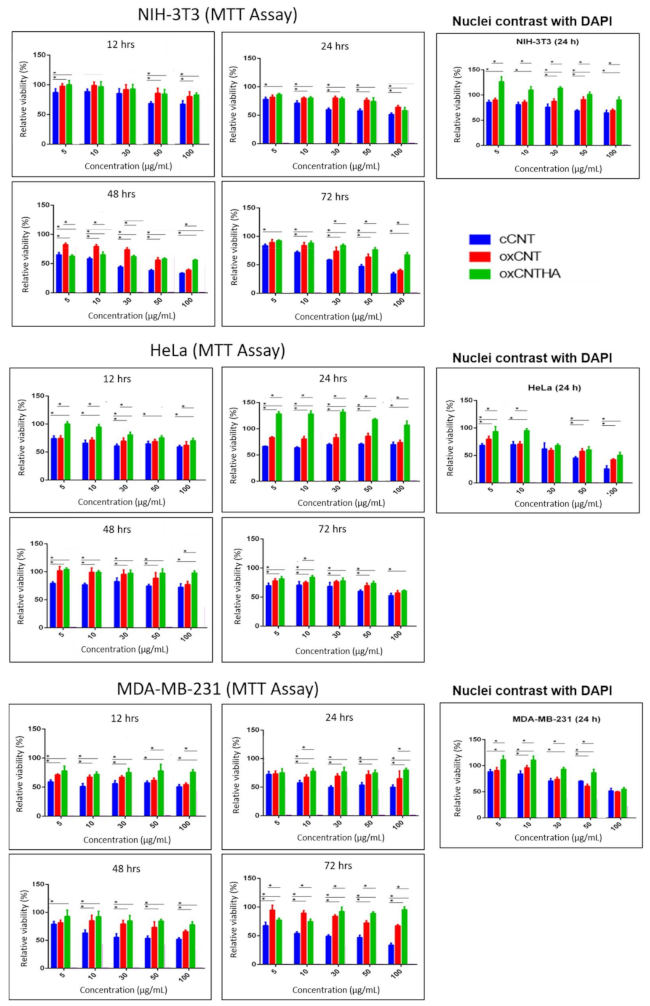
3.3. The Nanodevice Shows a Greater Cytotoxic Effect vs Tumor Cells and Less against Non-Tumor Cells
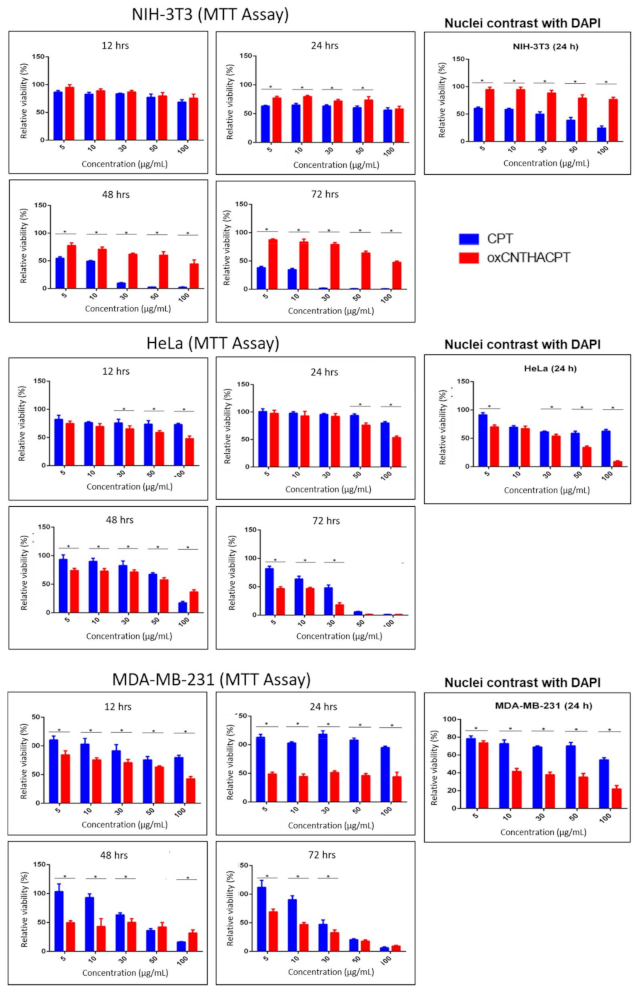
3.4. The oxCNTs-HA-CPT Nanovector Exhibits a Potential Cytotoxic Activity Compared to CPT in Tumor Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nation Cancer Institute. Types of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 16 December 2019).
- World Health Organization. Cancer. 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 17 December 2019).
- Alam, A.; Farooq, U.; Singh, R.; Dubey, V.; Kumar, S.; Kumari, R.; Naik, K.K.; Tripathi, B.; Dhar, K. Chemotherapy Treatment and Strategy Schemes: A Review. Open Access J. Toxicol. 2018, 2, 55. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.; Wheate, N. The Side Effects of Platinum-based Chemotherapy Drugs: A Review for Chemists. R. Soc. Chem. 2018, 47. [Google Scholar] [CrossRef]
- Wilson, T.R.; Longley, D.B.; Johnston, P.G. Chemoresistance in solid tumors. Ann. Oncol. 2006, 17, x315–x324. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Kostarelos, K.; Bianco, A.; Prato, M. The winding road for carbon nanotubes in nanomedicine. Mater. Today 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Vázquez, E.; Prato, M. Functionalization of carbon nanotubes for applications in materials science and nanomedicine. Pure Appl. Chem. 2010, 82, 853–861. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Contreras-Torres, F.F.; Basiuk, E.V.; Heredia, A.; Basiuk, V.A. Deposition of silver nanoparticles onto human serum albumin-functionalized multiwalled carbon nanotubes. Can. J. Chem. Eng. 2013, 91, 264–270. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Contreras-Torres, F.F.; Basiuk, E.V.; Alvarez-Zauco, E.; Heredia, A.; Basiuk, V.A. Aggregation of human serum albumin on graphite and single-walled carbon nanotubes as studied by scanning probe microscopies. J. Nanosci. Nanotechnol. 2011, 11, 5491–5498. [Google Scholar] [CrossRef]
- Salas-Trevino, D.; Saucedo-Cardenas, O.; de Jesus Loera-Arias, M.; De Casas-Ortiz, E.G.; Rodriguez-Rocha, H.; Garcia-Garcia, A.; Montes-de-Oca-Luna, R.; Soto-Dominguez, A. Carbon nanotubes: An alternative for platinum-based drug delivery systems. J. Balk. Union Oncol. 2017, 23, 541–549. [Google Scholar]
- Ochoa-Olmos, O.E.; León-Domínguez, J.A.; Contreras-Torres, F.F.; Sanchez-Nieto, S.; Basiuk, E.V.; Dinkova, T.D. Transformation of plant cell suspension cultures with amine-fuctionalized multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2016, 16, 7461–7471. [Google Scholar] [CrossRef]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasizing tumors. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Oberdöster, E.; Oberdöster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A.; Nabi, F. Nanotoxicity: Dimensional and morphological concerns. Adv. Phys. Chem. 2011. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.H.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol. 2012, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health. Sep. 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Xue, Z.; Xie, J.; Dong, Y.; Ma, Z.; Sun, X.; Kebebe Borga, D.; Liu, Z.; Li, J. Toxicity of Carbon Nanotubes as Anti-Tumor Drug Carriers. Int. J. Nanomed. 2019, 14, 10179–10194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Tavabe, K.R.; Yavar, M.; Kabir, S.; Akbary, P.; Aminikhoei, Z. Toxicity effects of multi-walled carbon nanotubes (MWCNTs) nanomaterial on the common carp (Cyprinus carpio L. 1758) in laboratory conditions. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2020, 237, 108832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Ursini, C.L.; Cavallo, D.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Buresti, G.; Casciardi, S.; Tombolini, F.; Bellucci, S.; Iavicoli, S. Comparative cyto-genotoxicity assessment of functionalized and pristine multiwalled carbon nanotubes on human lung epithelial cells. Toxicol. Vitr. 2012, 26, 831–840. [Google Scholar] [CrossRef]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef]
- Balas, M.; Constanda, S.; Duma-Voiculet, A.; Prodana, M.; Hermenean, A.; Pop, S.; Demetrescu, I.; Dinischiotu, A. Fabrication and toxicity characterization of a hybrid material based on oxidized and aminated MWCNT loaded with carboplatin. Toxicol Vitr. 2016, 37, 189–200. [Google Scholar] [CrossRef]
- Sharma, S.; Naskar, S.; Kuotsu, K. Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: Synthesis, characterization, and in vitro toxicity in human breast cancer. Carbon Lett. 2020, 30, 435–447. [Google Scholar] [CrossRef]
- Salas-Treviño, D.; Saucedo-Cárdenas, O.; Loera-Arias, M.D.J.; Rodríguez-Rocha, H.; García-García, A.; Montes-de-Oca-Luna, R.; Piña-Mendoza, E.I.; Contreras-Torres, F.F.; García-Rivas, G.; Soto-Domínguez, A. Hyaluronate functionalized multi-wall carbon nanotubes filled with carboplatin as a novel drug nanocarrier against murine lung cancer cells. Nanomaterials 2019, 9, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Type Culture Collection. HeLa (ATCC® CCL-2™). Available online: https://www.atcc.org/products/all/CCL-2.aspx#characteristics (accessed on 13 March 2020).
- American Type Culture Collection. MDA-MB-231 (ATCC® HTB-26™). 2020. Available online: https://www.atcc.org/products/all/HTB 26.aspx#generalinformation (accessed on 13 March 2020).
- American Type Culture Collection. NIH/3T3 (ATCC® CRL-1658™). 2020. Available online: https://www.atcc.org/products/all/CRL-1658.aspx (accessed on 13 March 2020).
- Abcam 2020. MTT Assay Kit (Cell Proliferation) ab211091. Available online: https://www.abcam.com/mtt-assay-kit-cell-proliferation-ab211091.html (accessed on 14 April 2020).
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. BioMed Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [Green Version]
- Jurj, A.; Braicu, C.; Pop, L.-A.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, V.; Aschberger, K. Carbon nanotubes: Potential medical applications and safety concerns. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 371–386. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, J.; Luo, L.; Yang, J.; Chen, J.I.E.; Li, B.I.N.; Shen, K. Identification of a cancer stem cell-like side population in the HeLa human cervical carcinoma cell line. Oncol. Lett. 2013, 6, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Oki, A.; Carson, L.; Adams, L.; Neelgund, G.; Soboyejo, N.; Regisford, G.; Stewart, M.; Hibbert, K.; Beharie, G.; et al. Thermal stability of functionalized carbon nanotubes studied by in-situ transmission electron microscopy. Chem. Phys. Lett. 2011, 513, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-González, C.A.; Salas-Treviño, D.; Contreras-Torres, F.F.; Loera-Arias, M.d.J.; Gómez-Tristán, C.A.; Piña-Mendoza, E.I.; García-Rivas, G.d.J.; Guillén-Meléndez, G.A.; Montes-de-Oca-Luna, R.; Saucedo-Cárdenas, O.; et al. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Loaded with Carboplatin Enhance Cytotoxicity on Human Cancer Cell Lines. Materials 2021, 14, 3622. https://doi.org/10.3390/ma14133622
Leyva-González CA, Salas-Treviño D, Contreras-Torres FF, Loera-Arias MdJ, Gómez-Tristán CA, Piña-Mendoza EI, García-Rivas GdJ, Guillén-Meléndez GA, Montes-de-Oca-Luna R, Saucedo-Cárdenas O, et al. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Loaded with Carboplatin Enhance Cytotoxicity on Human Cancer Cell Lines. Materials. 2021; 14(13):3622. https://doi.org/10.3390/ma14133622
Chicago/Turabian StyleLeyva-González, César Adrián, Daniel Salas-Treviño, Flavio Fernando Contreras-Torres, María de Jesús Loera-Arias, Christian Alexis Gómez-Tristán, Edgar Iván Piña-Mendoza, Gerardo de Jesús García-Rivas, Gloria Arely Guillén-Meléndez, Roberto Montes-de-Oca-Luna, Odila Saucedo-Cárdenas, and et al. 2021. "Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Loaded with Carboplatin Enhance Cytotoxicity on Human Cancer Cell Lines" Materials 14, no. 13: 3622. https://doi.org/10.3390/ma14133622
APA StyleLeyva-González, C. A., Salas-Treviño, D., Contreras-Torres, F. F., Loera-Arias, M. d. J., Gómez-Tristán, C. A., Piña-Mendoza, E. I., García-Rivas, G. d. J., Guillén-Meléndez, G. A., Montes-de-Oca-Luna, R., Saucedo-Cárdenas, O., & Soto-Domínguez, A. (2021). Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Loaded with Carboplatin Enhance Cytotoxicity on Human Cancer Cell Lines. Materials, 14(13), 3622. https://doi.org/10.3390/ma14133622








