Selective Adsorption of Potassium in Seawater by CoHCF Thin Film Electrode and Its Electrochemical Desorption/Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powder-Form MHCFs
2.2. Preparation of CoHCF Thin Film
2.3. K+ Adsorption from Powder-Form MHCFs
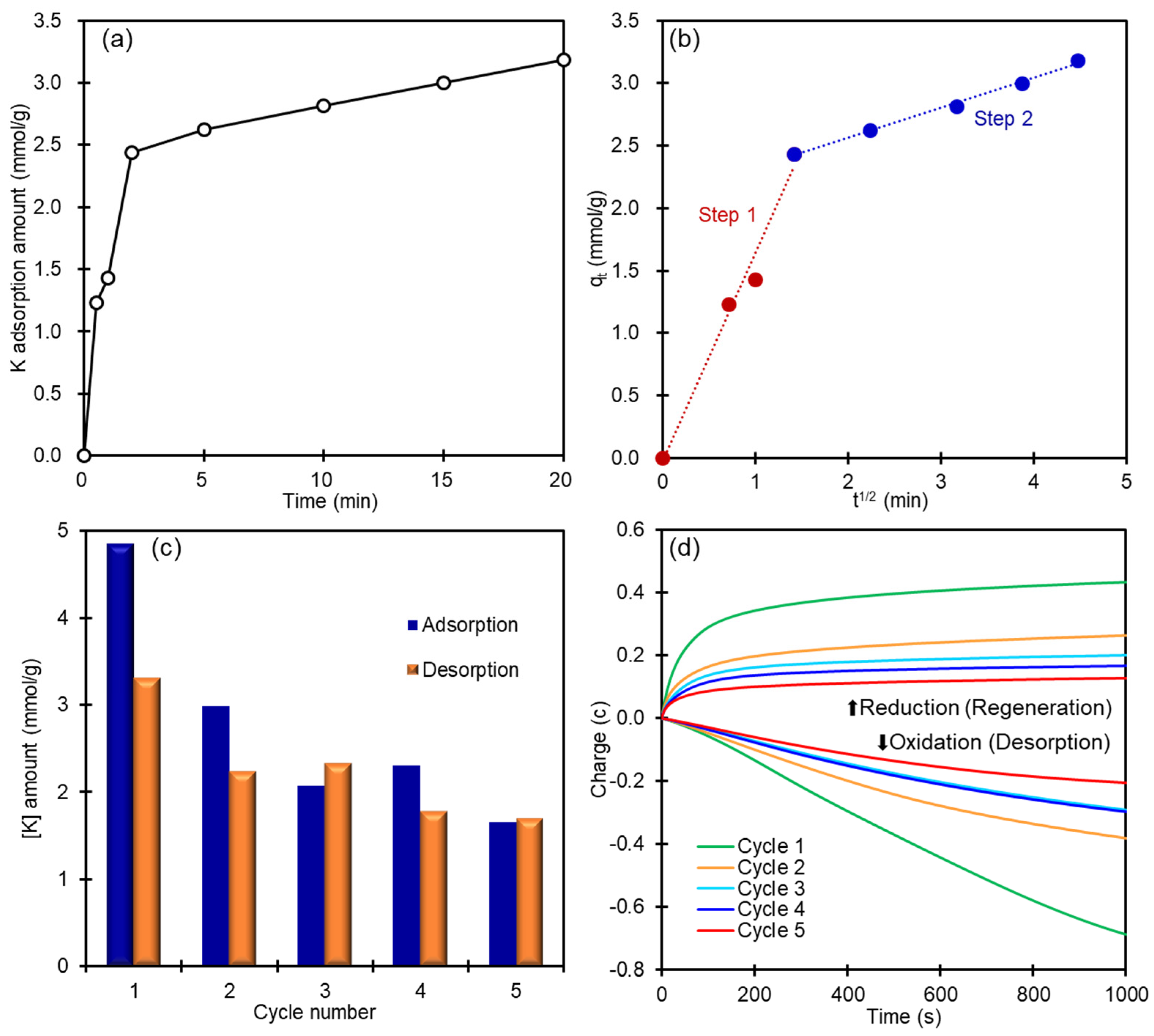
2.4. Adsorption Kinetics and Cycle Experiments for K+ Recovery
2.5. Characterization of the NaCoHCF Thin Film
3. Results and Discussion
3.1. K+ ion Adsorption Using Powder CoHCF
3.2. Characterization of CoHCF Thin Film
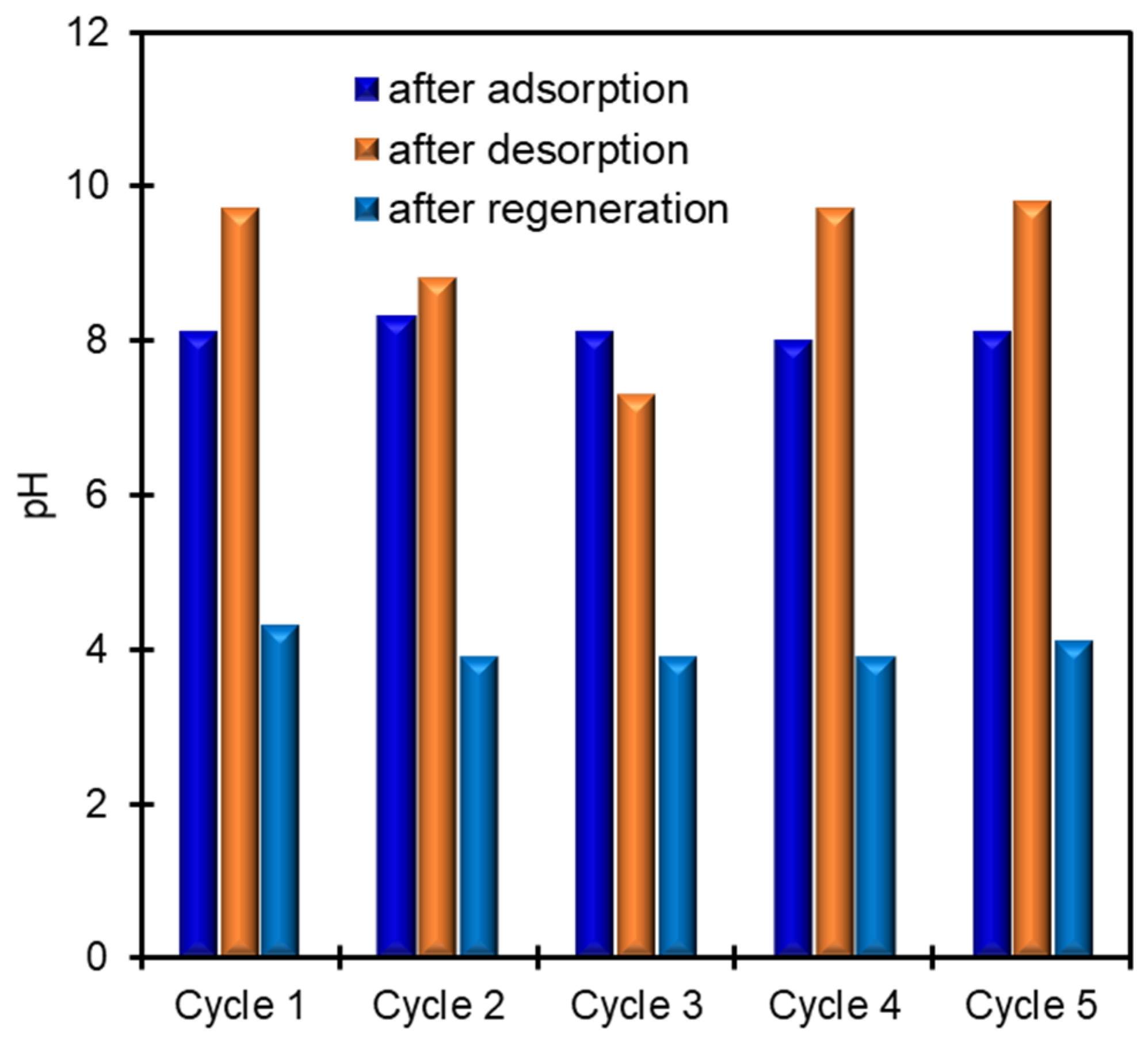
3.3. K+ Recovery from Seawater by CoHCF Thin Film
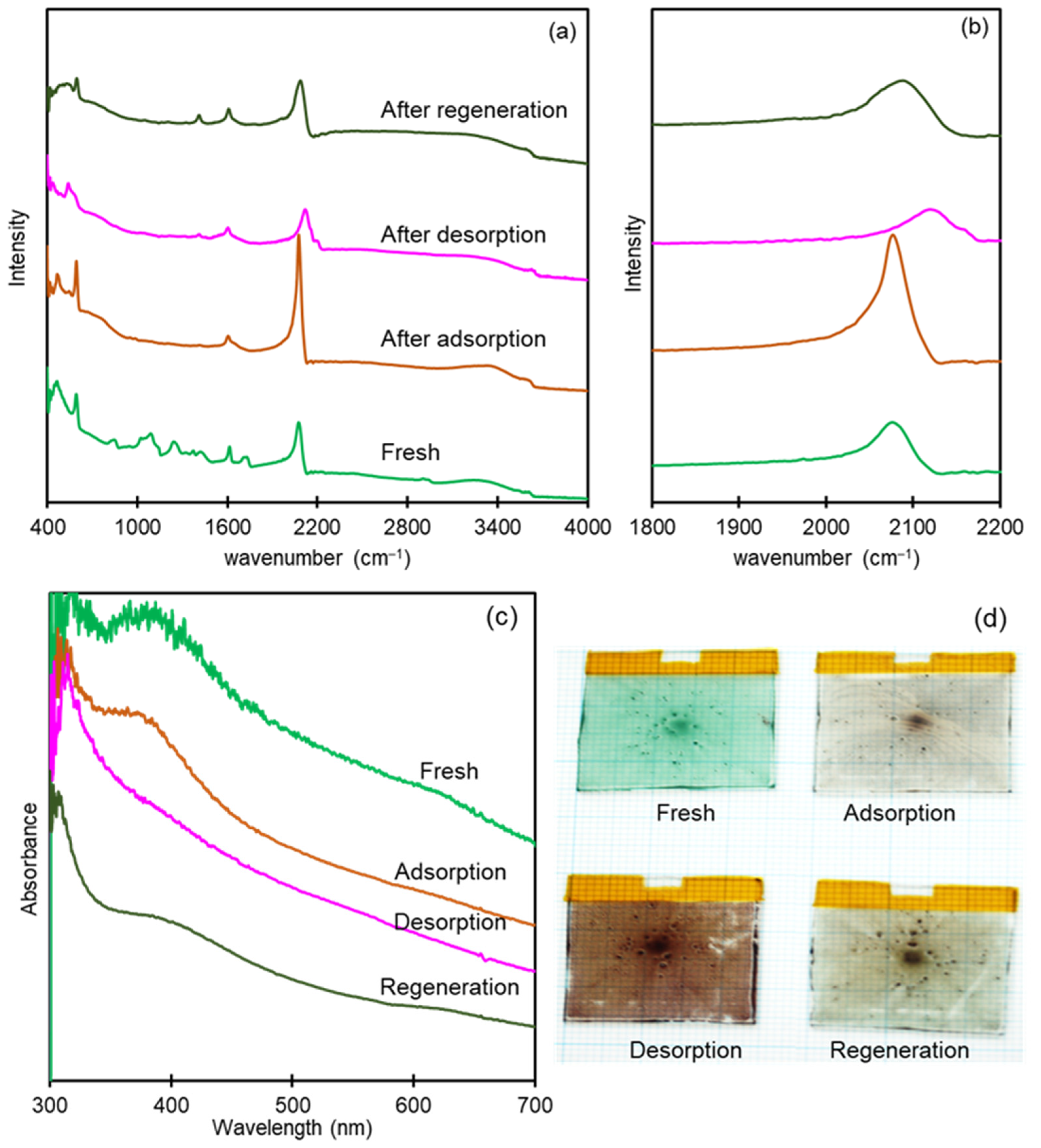
3.4. Spectroelectrochemical Characterization of CoHCF Thin Film
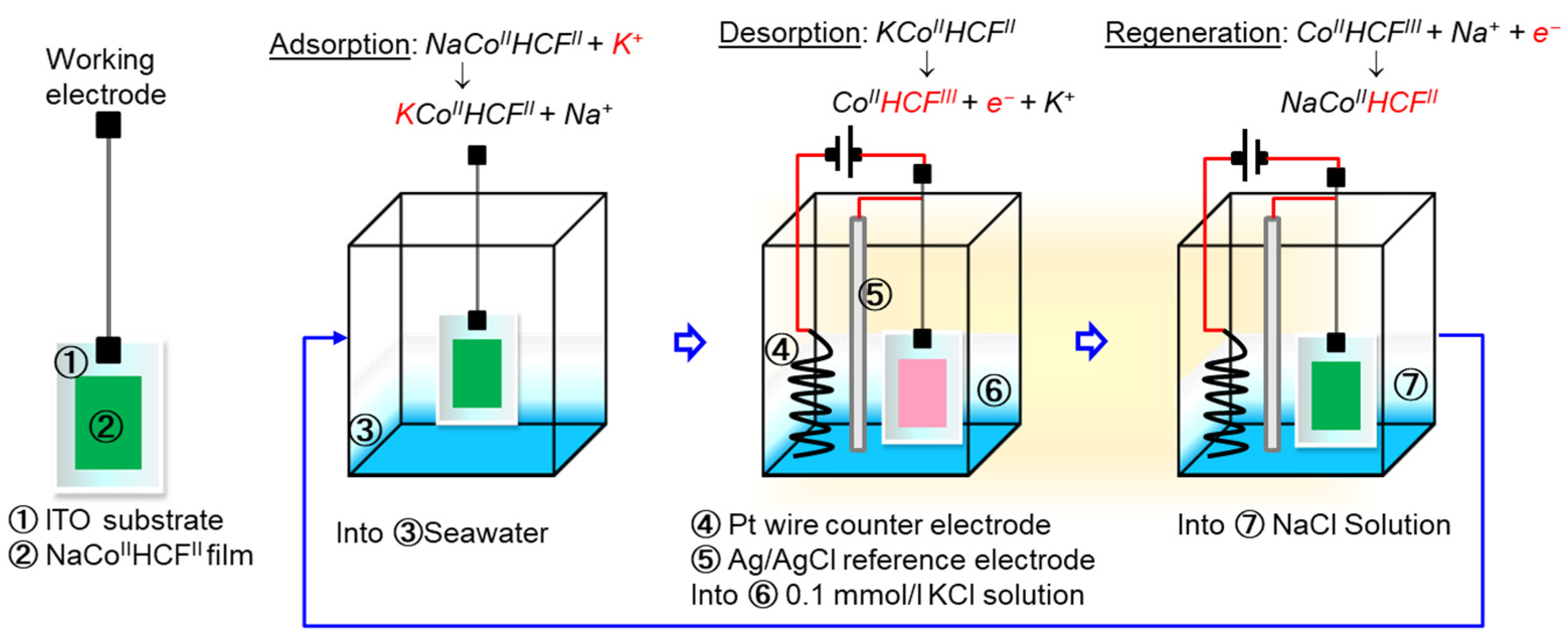
3.5. Mechanism of Electrochemical K+ Recovery and Film Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciceri, D.; De Oliveira, M.; Allanore, A. Potassium fertilizer via hydrothermal alteration of K-feldspar ore. Green Chem. 2017, 19, 5187–5202. [Google Scholar] [CrossRef]
- Hou, J.; Yuan, J.; Xu, J.; Sun, L. Synthesis and characterization of K-phillipsite (K-PHI) membrane for potassium extraction from seawater. Microporous Mesoporous Mater. 2013, 172, 217–221. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Meena, R.S. Potassium Solubilizing Microorganisms for Sustainable Agriculture. Potassium Solubilizing Microorg. Sustain. Agric. 2016, 1–331. [Google Scholar] [CrossRef]
- Acevedo-Morantes, M.; Colón, G.; Realpe, A. Electrolytic removal of nitrate and potassium from wheat leachate using a four compartment electrolytic cell. Desalination 2011, 278, 354–364. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, L.; Li, X.; Park, H.-S. Ammonium and potassium removal for anaerobically digested wastewater using natural clinoptilolite followed by membrane pretreatment. J. Hazard. Mater. 2008, 151, 125–133. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Zheng, M.; Zhang, C.; Xie, T.; Wang, C. The precipitation of magnesium potassium phosphate hexahydrate for P and K recovery from synthetic urine. Water Res. 2015, 80, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-J.; Zhao, Z.-G.; Yu, S.-J.; Guan, Y.-G.; Li, D.; He, X. Using strong acid–cation exchange resin to reduce potassium level in molasses vinasses. Desalination 2012, 286, 210–216. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, V.W.-C.; Fane, A.G. An improved life cycle impact assessment (LCIA) approach for assessing aquatic eco-toxic impact of brine disposal from seawater desalination plants. Desalination 2013, 308, 233–241. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Diallo, M.S.; Kotte, M.R.; Cho, M. Mining Critical Metals and Elements from Seawater: Opportunities and Challenges. Environ. Sci. Technol. 2015, 49, 9390–9399. [Google Scholar] [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2016, 3, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Zhang, A.-B.; Sun, J.; Ye, Y.; Chen, X.-G.; Xia, M.-S. Application of ocean manganese nodules for the adsorption of potassium ions from seawater. Miner. Eng. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Ghara, K.K.; Korat, N.; Bhalodia, D.; Solanki, J.; Maiti, P.; Ghosh, P.K. Production of pure potassium salts directly from sea bittern employing tartaric acid as a benign and recyclable K+ precipitant. RSC Adv. 2014, 4, 34706–34711. [Google Scholar] [CrossRef]
- Maiti, P.; Ghara, K.K.; Ghosh, P.K. A Process of Production of Potassium Ammonium Sulfate Compound Fertilizer in Cost—Effective Manner Directly from Concentrated Sea Bittern. US Patent 20180230065, 16 August 2018. [Google Scholar]
- Yuan, J.; Zhao, Y.; Li, Q.; Ji, Z.; Guo, X. Preparation of potassium ionic sieve membrane and its application on extracting potash from seawater. Sep. Purif. Technol. 2012, 99, 55–60. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Ye, C.; Mao, J.; Ren, Y.; Li, Y.; Lin, Y.; Power, I.M.; Luo, Y. Salt Crystallization Sequences of Nonmarine Brine and Their Application for the Formation of Potassium Deposits. Aquat. Geochem. 2018, 24, 209–229. [Google Scholar] [CrossRef]
- Eringathodi, S.; Agnihotri, P.; Ganguly, B.; Bhatt, P.; Subramanian, P.S.; Paul, P.; Ghosh, P.K. Towards Understanding of the Selective Precipitation of Alkali Metal Cations in Presence of Dipicrylamine Anion. Eur. J. Inorg. Chem. 2005, 2005, 2198–2205. [Google Scholar] [CrossRef]
- Gurbuz, H.; Yavasoglu, N.; Bulutcu, A.N. Recovery of Potassium Salts from Bittern by Potassium Pentaborate Crystallization. Sep. Sci. Technol. 1996, 31, 857–870. [Google Scholar] [CrossRef]
- Ivanov, V.; Timofeevskaja, V.; Gavlina, O.; Gorshkov, V. Dual-temperature reagent-less ion-exchange separations of alkali metal salts on zeolites. Microporous Mesoporous Mater. 2003, 65, 257–265. [Google Scholar] [CrossRef]
- Casadellà, A.; Kuntke, P.; Schaetzle, O.; Loos, K. Clinoptilolite-based mixed matrix membranes for the selective recovery of potassium and ammonium. Water Res. 2016, 90, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-L.; Liu, X.-W.; Fu, R.; Tan, Z.-Y. Magnetic P zeolites: Synthesis, characterization and the behavior in potassium extraction from seawater. Sep. Purif. Technol. 2008, 63, 92–100. [Google Scholar] [CrossRef]
- Hou, J.; Yuan, J.; Shang, R. Synthesis and characterization of zeolite W and its ion-exchange properties to K+ in seawater. Powder Technol. 2012, 226, 222–224. [Google Scholar] [CrossRef]
- Du, X.; Hao, X.; Wang, Z.; Guan, G. Electroactive ion exchange materials: Current status in synthesis, applications and future prospects. J. Mater. Chem. A 2016, 4, 6236–6258. [Google Scholar] [CrossRef]
- Kholoud, E.; Watanabe, H.; Takahashi, A.; Emara, M.M.; Abd-El-Nabey, B.A.; Kurihara, M.; Tajima, K.; Kawamoto, T. Cobalt hexacyanoferrate nanoparticles for wet-processed brown–bleached electrochromic devices with hybridization of high-spin/low-spin phases. J. Mater. Chem. C 2017, 5, 8921–8926. [Google Scholar] [CrossRef]
- Parajuli, D.; Takahashi, A.; Noguchi, H.; Kitajima, A.; Tanaka, H.; Takasaki, M.; Yoshino, K.; Kawamoto, T. Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem. Eng. J. 2016, 283, 1322–1328. [Google Scholar] [CrossRef]
- El-Bahy, S.M.; Fadel, D.A.; El-Bahy, Z.M.; Metwally, A. Rapid and highly efficient cesium removal by newly synthesized carbomer encapsulated potassium copper hexacyanoferrate composite. J. Environ. Chem. Eng. 2018, 6, 1875–1885. [Google Scholar] [CrossRef]
- Jiang, Y.; Minami, K.; Sakurai, K.; Takahashi, A.; Parajuli, D.; Lei, Z.; Zhang, Z.; Kawamoto, T. High-capacity and selective ammonium removal from water using sodium cobalt hexacyanoferrate. RSC Adv. 2018, 8, 34573–34581. [Google Scholar] [CrossRef] [Green Version]
- Kulesza, P.J.; Malik, M.A.; Berrettoni, M.; Giorgetti, M.; Zamponi, S.; Schmidt, R.; Marassi, R. Electrochemical Charging, Countercation Accommodation, and Spectrochemical Identity of Microcrystalline Solid Cobalt Hexacyanoferrate. J. Phys. Chem. B 1998, 102, 1870–1876. [Google Scholar] [CrossRef]
- Li, N.; Li, Z.; Yuan, J.; Hu, J.; Miao, J.; Zhang, Q.; Niu, L.; Song, J. Nickel hexacyanoferrate nanoparticles anchored to multiwalled carbon nanotubes with a grafted poly(4-vinylpyridine) linker for electrically switched ion exchange. Electrochimica Acta 2012, 72, 150–156. [Google Scholar] [CrossRef]
- Nguyen, B.T.T.; Ang, J.Q.; Toh, C.-S. Sensitive detection of potassium ion using Prussian blue nanotube sensor. Electrochem. Commun. 2009, 11, 1861–1864. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt Hexacyanoferrate Nanoparticles as a High-Rate and Ultra-Stable Supercapacitor Electrode Material. ACS Appl. Mater. Interfaces 2014, 6, 11007–11012. [Google Scholar] [CrossRef]
- Chen, R.; Tanaka, H.; Kawamoto, T.; Asai, M.; Fukushima, C.; Na, H.; Kurihara, M.; Watanabe, M.; Arisaka, M.; Nankawa, T. Selective removal of cesium ions from wastewater using copper hexacyanoferrate nanofilms in an electrochemical system. Electrochimica Acta 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Zhang, N. Development of Functional Adsorbent Materials for Wastewater Treatment and Resource Recovery. Available online: http://hdl.handle.net/2241/00158027 (accessed on 15 June 2021).
- Zhang, N.; Kawamoto, T.; Jiang, Y.; Takahashi, A.; Ishizaki, M.; Asai, M.; Kurihara, M.; Zhang, Z.; Lei, Z.; Parajuli, D. Interpretation of the Role of Composition on the Inclusion Efficiency of Monovalent Cations into Cobalt Hexacyanoferrate. Chem. A Eur. J. 2019, 25, 5950–5958. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Minami, N.; Tanaka, H.; Sue, K.; Minami, K.; Parajuli, D.; Lee, K.-M.; Ohkoshi, S.-I.; Kurihara, M.; Kawamoto, T. Efficient synthesis of size-controlled open-framework nanoparticles fabricated with a micro-mixer: Route to the improvement of Cs adsorption performance. Green Chem. 2015, 17, 4228–4233. [Google Scholar] [CrossRef]
- Torad, N.L.; Takahashi, A.; Kawakami, M.; Kawamoto, T.; Tanaka, H. Decontamination of very dilute Cs in seawater by a coagulation–precipitation method using a nanoparticle slurry of copper hexacyanoferrate. Environ. Sci. Water Res. Technol. 2019, 5, 1328–1338. [Google Scholar] [CrossRef]
- Lei, Z.; Cagnetta, G.; Li, X.; Qu, J.; Li, Z.; Zhang, Q.; Huang, J. Enhanced adsorption of potassium nitrate with potassium cation on H 3 PO 4 modified kaolinite and nitrate anion into Mg-Al layered double hydroxide. Appl. Clay Sci. 2018, 154, 10–16. [Google Scholar] [CrossRef]
- Balsamo, M.; Montagnaro, F. Fractal-like Vermeulen Kinetic Equation for the Description of Diffusion-Controlled Adsorption Dynamics. J. Phys. Chem. C 2015, 119, 8781–8785. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Poots, V.J.P.; McKay, G.; Healy, J.J. Removal of Basic Dye from Effluent Using Wood as an Adsorbent. J. Water Pollut. Control Fed. 1978, 926–935. [Google Scholar] [CrossRef]
- Ayrault, S.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.; Loos-Neskovic, C. Sorption Mechanisms of Cesium on CuII2FeII(CN)6and CuII3[FeIII(CN)6]2Hexacyanoferrates and Their Relation to the Crystalline Structure. J. Solid State Chem. 1998, 141, 475–485. [Google Scholar] [CrossRef]
- Matsuda, T.; Kim, J.; Moritomo, Y. Symmetry Switch of Cobalt Ferrocyanide Framework by Alkaline Cation Exchange. J. Am. Chem. Soc. 2010, 132, 12206–12207. [Google Scholar] [CrossRef] [PubMed]


| Component | Concentration (ppm) | Component | Concentration (ppm) |
|---|---|---|---|
| MgCl2·6H2O | 1328.41 | KBr | 33.02 |
| CaCl2·2H2O | 418.47 | H3BO3 | 4.72 |
| SrCl2·6H2O | 13.97 | NaF | 1.64 |
| KCl | 364.23 | NaCl | 9651.06 |
| NaHCO3 | 55.01 | Na2SO4 | 662.64 |
| Plane | Peak 2θ (deg) | FWHM (deg) * | Size (nm) | Average Size (nm) |
|---|---|---|---|---|
| 200 | 17.41 | 0.32 | 249.6 | - |
| 220 | 24.66 | 0.49 | 165.8 | 178.4 |
| 420 | 39.55 | 0.70 | 119.9 | - |
| Cycle Number | Qred/C | Qmeas/C | FE/% |
|---|---|---|---|
| 1 | 0.255 | 0.687 | 37.07 |
| 2 | 0.173 | 0.381 | 45.29 |
| 3 | 0.180 | 0.291 | 61.76 |
| 4 | 0.137 | 0.297 | 46.12 |
| 5 | 0.131 | 0.205 | 63.84 |
| State of the CoHCF Thin Film | x (Na) | y (K) | z (Fe) |
|---|---|---|---|
| Fresh | 0.850 | 0 | 0.650 |
| After adsorption | 0.005 | 0.680 | 0.672 |
| After desorption | 0.003 | 0.220 | 0.643 |
| After regeneration | 0.411 | 0.203 | 0.680 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Kawamoto, T.; Watanabe, H.; Jiang, Y.; Zhang, Z.; Lei, Z.; Parajuli, D. Selective Adsorption of Potassium in Seawater by CoHCF Thin Film Electrode and Its Electrochemical Desorption/Regeneration. Materials 2021, 14, 3592. https://doi.org/10.3390/ma14133592
Zhang N, Kawamoto T, Watanabe H, Jiang Y, Zhang Z, Lei Z, Parajuli D. Selective Adsorption of Potassium in Seawater by CoHCF Thin Film Electrode and Its Electrochemical Desorption/Regeneration. Materials. 2021; 14(13):3592. https://doi.org/10.3390/ma14133592
Chicago/Turabian StyleZhang, Nan, Tohru Kawamoto, Hiroshi Watanabe, Yong Jiang, Zhenya Zhang, Zhongfang Lei, and Durga Parajuli. 2021. "Selective Adsorption of Potassium in Seawater by CoHCF Thin Film Electrode and Its Electrochemical Desorption/Regeneration" Materials 14, no. 13: 3592. https://doi.org/10.3390/ma14133592
APA StyleZhang, N., Kawamoto, T., Watanabe, H., Jiang, Y., Zhang, Z., Lei, Z., & Parajuli, D. (2021). Selective Adsorption of Potassium in Seawater by CoHCF Thin Film Electrode and Its Electrochemical Desorption/Regeneration. Materials, 14(13), 3592. https://doi.org/10.3390/ma14133592








