Novel Lithium-Ion Capacitor Based on a NiO-rGO Composite
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of GO
2.2. Synthesis of the NiO-rGO Composite
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
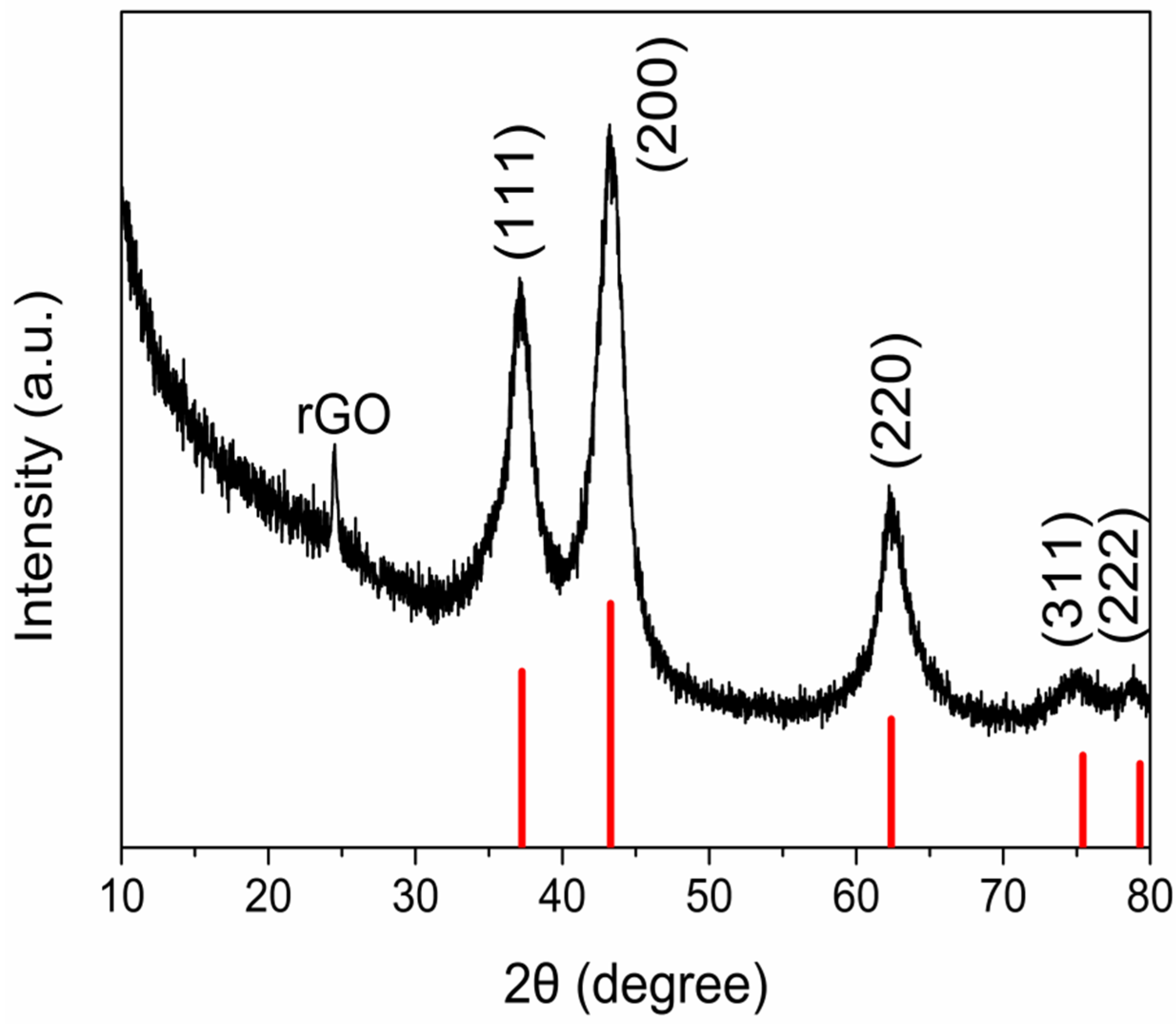
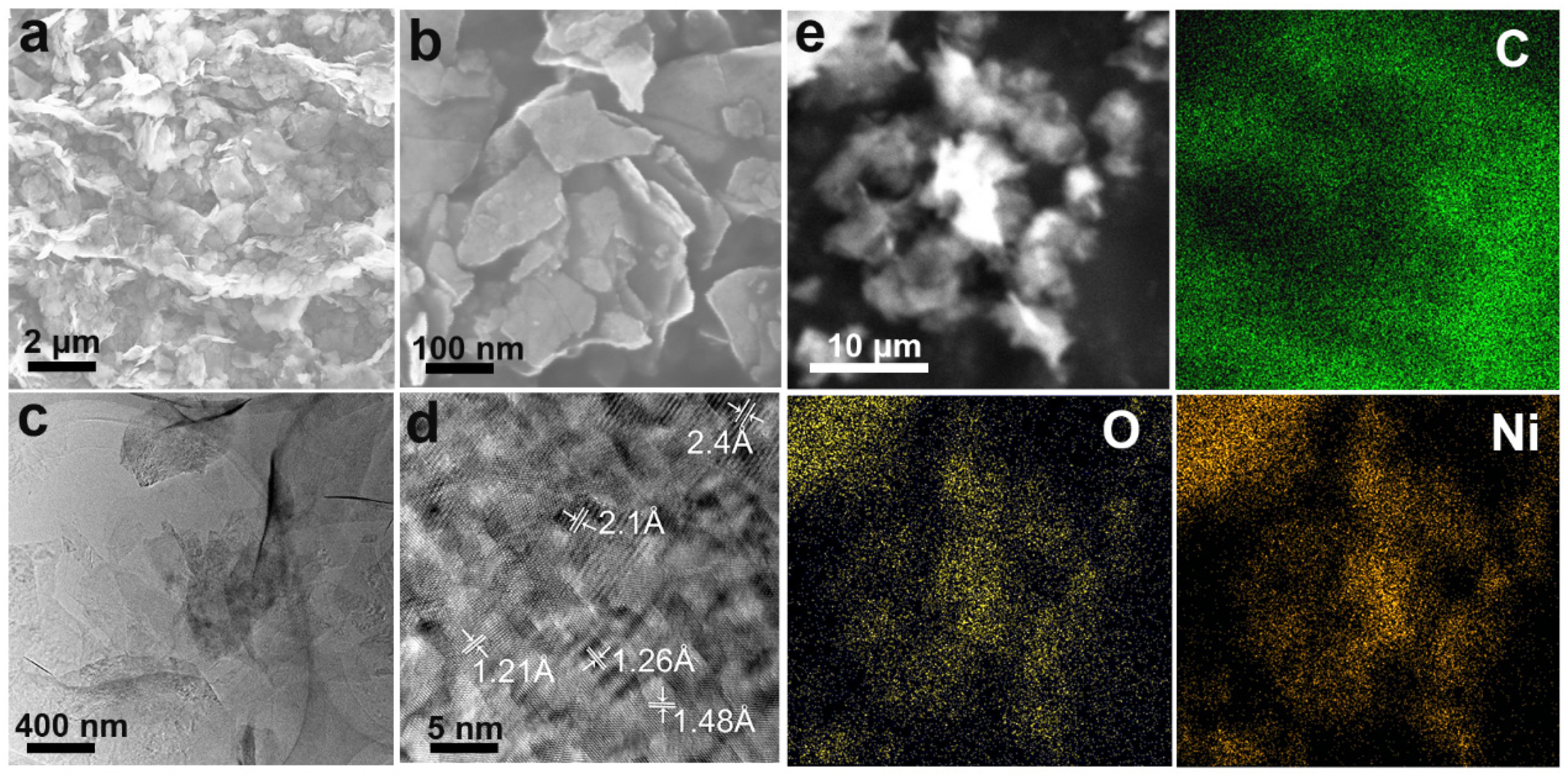
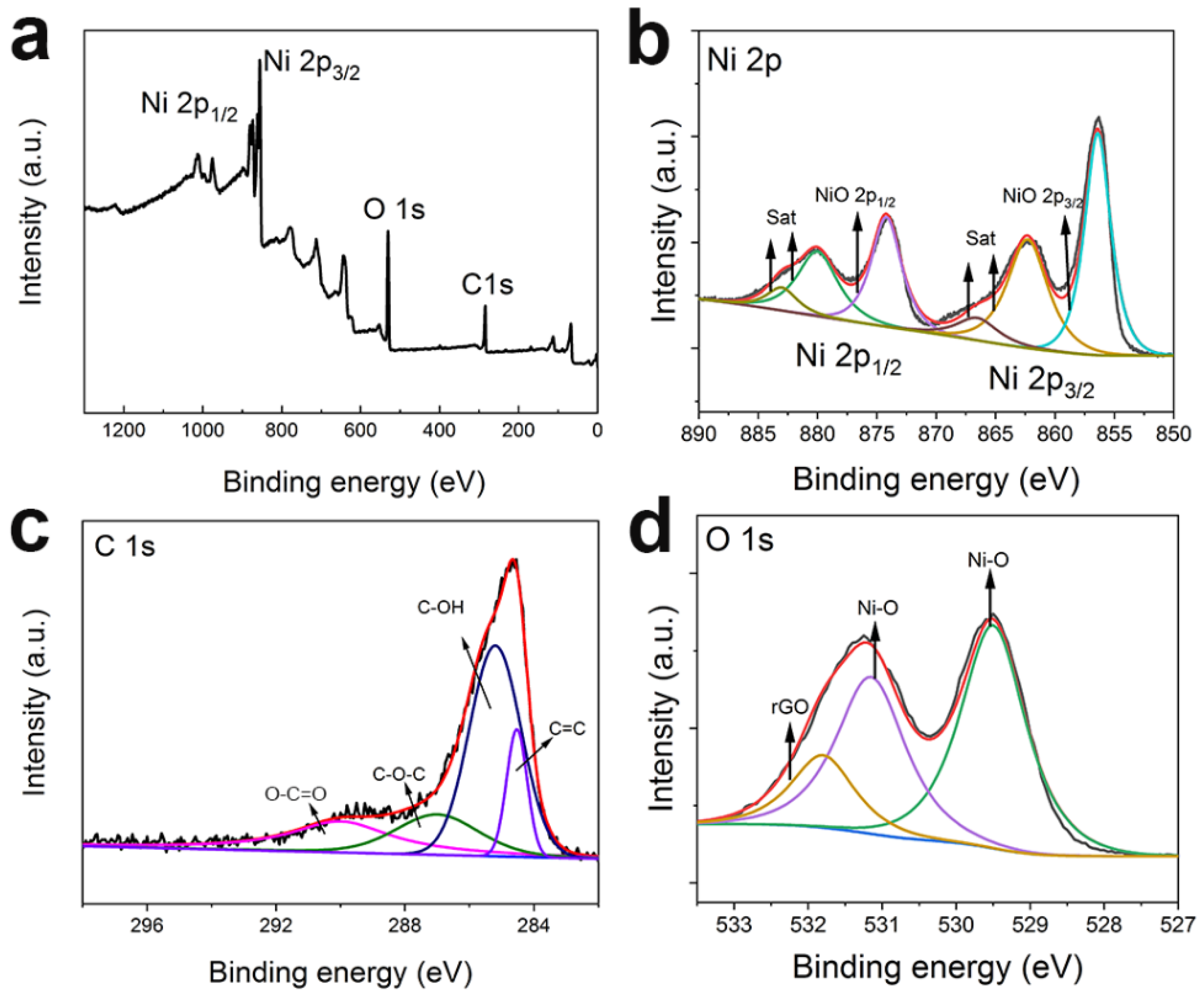
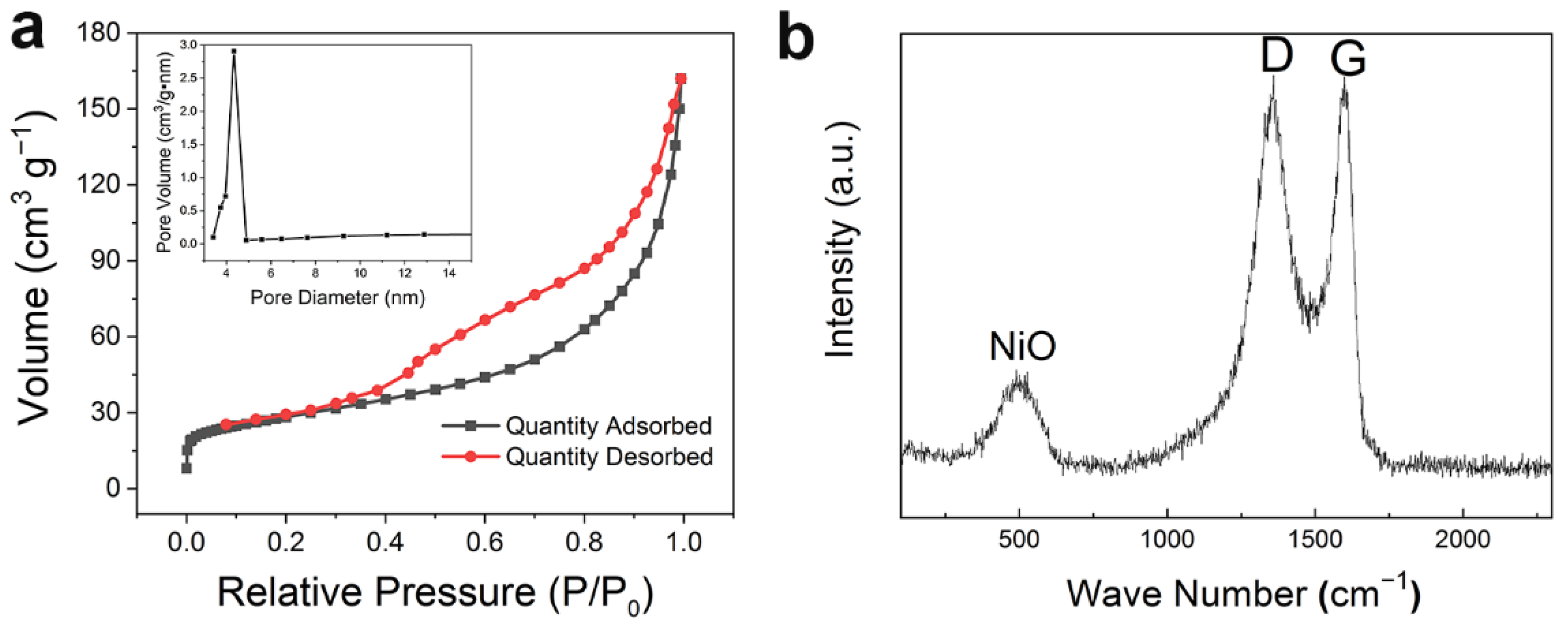
3.1. Material Performance Characterization
3.2. Performance of Half-Cells
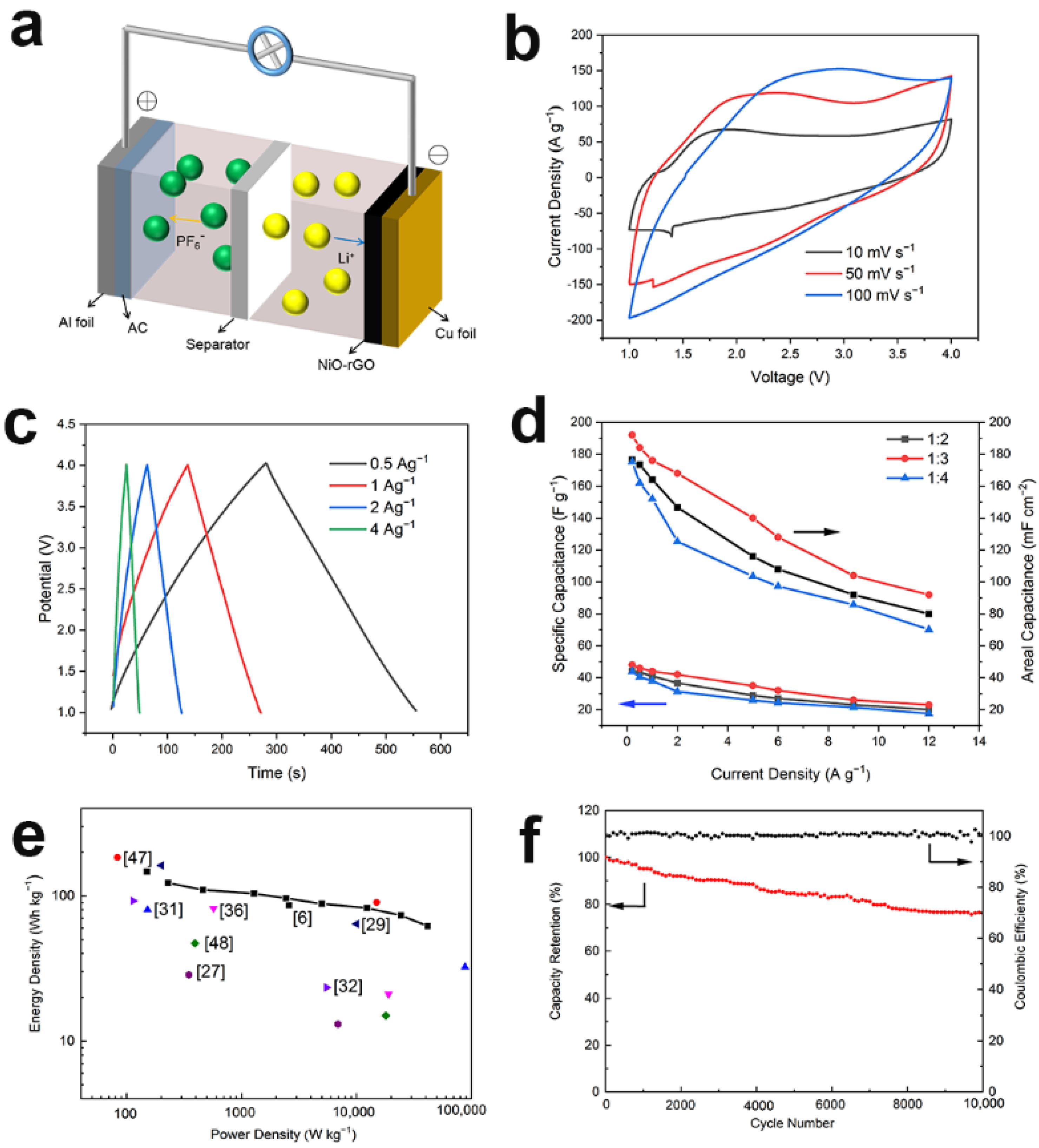
3.3. Electrochemical Performance of LICs
3.4. The Prospect of Industrialization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Liu, C.; He, Z.; Niu, J.; Cheng, Q.; Zhao, Z.; Li, H.; Shi, J.; Wang, H. Two-dimensional SnO2 anchored biomass-derived carbon nanosheet anode for high-performance Li-ion capacitors. RSC Adv. 2021, 11, 10018–10026. [Google Scholar] [CrossRef]
- Luo, X.; Yang, J.; Yan, D.; Wang, W.; Wu, X.; Zhu, Z. MnO2-decorated 3D porous carbon skeleton derived from mollusc shell for high-performance supercapacitor. J. Alloys Compd. 2017, 723, 505–511. [Google Scholar] [CrossRef]
- Pal, B.; Vijayan, B.L.; Krishnan, S.G.; Harilal, M.; Basirun, W.J.; Lowe, A.; Yusoff, M.M.; Jose, R. Hydrothermal syntheses of tungsten doped TiO2 and TiO2/WO3 composite using metal oxide precursors for charge storage applications. J. Alloys Compd. 2018, 740, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, B.L.; Misnon, I.I.; Anilkumar, G.M.; Yang, C.-C.; Jose, R. Void-size-matched hierarchical 3D titania flowers in porous carbon as an electrode for high-density supercapacitive charge storage. J. Alloys Compd. 2021, 858, 157649. [Google Scholar] [CrossRef]
- Lyu, L.; Kang, J.; Seong, K.-D.; Kim, C.W.; Lim, J.; Piao, Y. ZnNiCo hydroxide/graphene-carbon nanotube hydrogel on surface-modified Ni foam as a battery-type electrode for hybrid supercapacitors. J. Alloys Compd. 2021, 872, 159610. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Liu, B.; Ding, Y.; Tang, Y.; Yan, X. One dimensional graphene nanoscroll-wrapped MnO nanoparticles for high-performance lithium ion hybrid capacitors. J. Mater. Chem. A 2021, 9, 6352–6360. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Seenivasan, S.; Lee, H.H.; Jung, H.; Han, J.W.; Kim, D.-H. Interface-modulated uniform outer nanolayer: A category of electrodes of nanolayer-encapsulated core-shell configuration for supercapacitors. Nano Energy 2021, 81, 105667. [Google Scholar] [CrossRef]
- Li, H.; Hu, Z.; Xia, Q.; Zhang, H.; Li, Z.; Wang, H.; Li, X.; Zuo, F.; Zhang, F.; Wang, X.; et al. Operando Magnetometry Probing the Charge Storage Mechanism of CoO Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2006629. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, C.; Wang, X.; Fan, H.J. A High Energy and Power Li-Ion Capacitor Based on a TiO2Nanobelt Array Anode and a Graphene Hydrogel Cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Su, J.; Yang, Z.; Lv, S.; Jin, Z.; Zuo, J. High-Performance Lithium-Ion Capacitors Based on Porosity-Regulated Zirconium Metal−Organic Frameworks. Small 2021, 17, e2005209. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, C.-H.; Gong, H. A High Energy Density Asymmetric Supercapacitor from Nano-architectured Ni(OH)2/Carbon Nanotube Electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Yu, L.; Hu, H.; Bin Wu, H.; Lou, X.W.D. Complex Hollow Nanostructures: Synthesis and Energy-Related Applications. Adv. Mater. 2017, 29, 1604563. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Yu, X.-Y.; Zhang, X.; Lou, X.W. Self-Templated Formation of Uniform NiCo2O4Hollow Spheres with Complex Interior Structures for Lithium-Ion Batteries and Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 1868–1872. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yuan, G.B.; Bin Wu, H.; Lou, X.W. Complex Nanostructures from Materials based on Metal-Organic Frameworks for Electrochemical Energy Storage and Conversion. Adv. Mater. 2017, 29, 1703614. [Google Scholar] [CrossRef]
- Pikul, J.H.; Zhang, H.; Cho, J.; Braun, P.V.; King, W. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Han, P.; Yao, J.; Zhang, S.; Cao, X.; Xiong, J.; Zhang, J.; Cui, G. Nitrogen-doped carbonized polyimide microsphere as a novel anode material for high performance lithium ion capacitors. Electrochimica Acta 2016, 196, 603–610. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, L.; Zhao, S.; Wu, L. Preparation of MnCo2O4@Ni(OH)2Core-Shell Flowers for Asymmetric Supercapacitor Materials with Ultrahigh Specific Capacitance. Adv. Funct. Mater. 2016, 26, 4085–4093. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Gowda, S.R.; Shaijumon, M.; Ajayan, P.M. Hybrid Nanostructures for Energy Storage Applications. Adv. Mater. 2012, 24, 5045–5064. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Cao, L.; Li, J.; Huang, J.; Ma, M.; Cheng, Y.; Wang, C.; Dang, H. Rice crust-like Fe3O4@C/rGO with improved extrinsic pseudocapacitance for high-rate and long-life Li-ion anodes. J. Alloys Compd. 2019, 804, 57–64. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Zhang, P.; Lin, Z.; Yan, X. Fast and Large Lithium Storage in 3D Porous VN Nanowires-Graphene Composite as a Superior Anode Toward High-Performance Hybrid Supercapacitors. Adv. Funct. Mater. 2015, 25, 2270–2278. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.-H.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Shin, W.H.; Jeong, H.M.; Kim, B.G.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Multiwall Carbon Nanotubes for Lithium Storage with Extremely High Capacity. Nano Lett. 2012, 12, 2283–2288. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Tan, H.; Cai, R.; Shi, W.; Li, C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q. MS2(M = Co and Ni) Hollow Spheres with Tunable Interiors for High-Performance Supercapacitors and Photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Li, C.; Sun, X.; Liu, J.; Wang, K.; Ma, Y. High-Performance Lithium-Ion Capacitors Based on CoO-Graphene Composite Anode and Holey Carbon Nanolayer Cathode. ACS Sustain. Chem. Eng. 2019, 7, 11275–11283. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Chang, P.-Y.; Yeh, M.-H.; Doong, R.-A. Ultra-small CoO nanocrystals anchored on reduced graphene oxide for enhanced lithium storage in lithium ion batteries. MRS Commun. 2017, 7, 236–244. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Zhao, X.; Suo, S.; Bai, Y. Novel Lithium-Ion Capacitor Based on a NiO-rGO Composite. Materials 2021, 14, 3586. https://doi.org/10.3390/ma14133586
An Q, Zhao X, Suo S, Bai Y. Novel Lithium-Ion Capacitor Based on a NiO-rGO Composite. Materials. 2021; 14(13):3586. https://doi.org/10.3390/ma14133586
Chicago/Turabian StyleAn, Qi, Xingru Zhao, Shuangfu Suo, and Yuzhu Bai. 2021. "Novel Lithium-Ion Capacitor Based on a NiO-rGO Composite" Materials 14, no. 13: 3586. https://doi.org/10.3390/ma14133586
APA StyleAn, Q., Zhao, X., Suo, S., & Bai, Y. (2021). Novel Lithium-Ion Capacitor Based on a NiO-rGO Composite. Materials, 14(13), 3586. https://doi.org/10.3390/ma14133586





