A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes
Abstract
:1. Introduction
- possess high ionic conductivities (around 10−3–10−4 S/cm at room temperature);
- negligible electronic conductivity;
- have high ionic transference number;
- have high mechanical and chemical stability;
- and possess a high Electrochemical Stability Window (ESW).
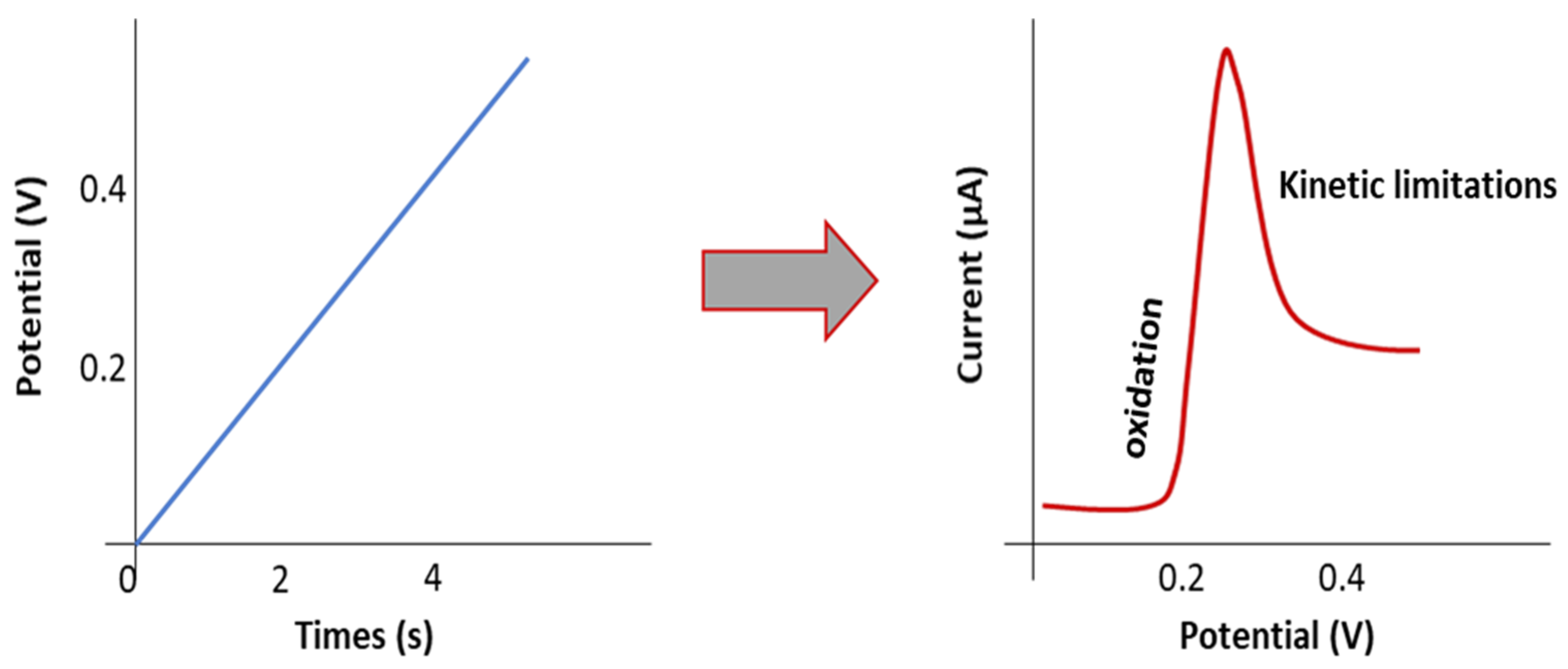
2. ESW Evaluation: Overview of the Recent Literature on SPE and SCE with Typical ESW Measurements by LSV/CV Methods
3. Toward a More Specific and Better Evaluation of the ESW
3.1. Cell Configuration
3.2. Other Methods: Improved Setups for the ESW Evaluation
3.3. Final Validation Tests
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Liao, K.; Ran, R.; Shao, Z. Recent Advances in Filler Engineering of Polymer Electrolytes for Solid-State Li-Ion Batteries: A Review. Energy Fuels 2020, 34, 9189–9207. [Google Scholar] [CrossRef]
- Ohno, S.; Bernges, T.; Buchheim, J.; Duchardt, M.; Hatz, A.-K.; Kraft, M.A.; Kwak, H.; Santhosha, A.L.; Liu, Z.; Minafra, N.; et al. How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Lett. 2020, 5, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef] [Green Version]
- Fenton, D.; Parker, J.; Wright, P. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical conductivity in ionic complexes of poly (ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Armand, M.; Chabagno, J.; Duclot, M. Poly-ethers as solid electrolytes. In Fast Ion Transport in Solids: Electrodes, and Electrolytes. Proceedings of the International Conference on Fast Ion Transport in Solids, Electrodes, and Electrolytes, Lake Geneva, WI, USA, 21–25 May 1979; Vashishta, P., Mundy, J.N., Shenoy, G.K., Eds.; North Holland: Amsterdam, The Netherlands, 1979; pp. 131–136. ISBN 0444003533. [Google Scholar]
- Michael, M.; Jacob, M.; Prabaharan, S.; Radhakrishna, S. Enhanced lithium ion transport in PEO-based solid polymer electrolytes employing a novel class of plasticizers. Solid State Ion. 1997, 98, 167–174. [Google Scholar] [CrossRef]
- Gorecki, W.; Jeannin, M.; Belorizky, E.; Roux, C.; Armand, M. Physical properties of solid polymer electrolyte PEO(LiTFSI) complexes. J. Phys. Condens. Matter 1995, 7, 6823–6832. [Google Scholar] [CrossRef]
- Walker, C.W.; Salomon, M. Improvement of Ionic Conductivity in Plasticized PEO-Based Solid Polymer Electrolytes. J. Electrochem. Soc. 1993, 140, 3409–3412. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, H.; Feng, W.; Zhou, Z.; Armand, M.; Nie, J. Lithium (fluorosulfonyl)(pentafluoroethylsulfonyl) imide/poly (ethylene oxide) polymer electrolyte: Physical and electrochemical properties. Solid State Ion. 2019, 338, 161–167. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, Y.-C.; Park, M.-S.; Kim, D.-W. Solid polymer electrolyte supported by porous polymer membrane for all-solid-state lithium batteries. J. Membr. Sci. 2020, 603, 117995. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Thapaliya, B.P.; Sun, X.-G.; Ding, F.; Liu, X.; Zhang, J.; Dai, S. In situ polymerized succinonitrile-based solid polymer electrolytes for lithium ion batteries. Solid State Ion. 2020, 345, 115159. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Rousseau, D.M.; Strekis, C.A.; Stanzione, J.F. Optimizing conductivity and cationic transport in crosslinked solid polymer electrolytes. Solid State Ion. 2020, 345, 115161. [Google Scholar] [CrossRef]
- Yuan, H.; Luan, J.; Yang, Z.; Zhang, J.; Wu, Y.; Lu, Z.; Liu, H. Single Lithium-Ion Conducting Solid Polymer Electrolyte with Superior Electrochemical Stability and Interfacial Compatibility for Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 7249–7256. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Mo, J.; Yan, H.; Xu, L.; Peng, P.; Li, J.; Jiang, B.; Chu, L.; Li, M. Comprehensively-upgraded polymer electrolytes by multifunctional aramid nanofibers for stable all-solid-state Li-ion batteries. Nano Energy 2020, 69, 104398. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Y.; Zhao, Y.; Sun, Q.; Luo, J.; Zhao, F.; Lin, X.; Li, X.; Li, R.; Zhang, L.; et al. Engineering the conductive carbon/PEO interface to stabilize solid polymer electrolytes for all-solid-state high voltage LiCoO2 batteries. J. Mater. Chem. A 2019, 8, 2769–2776. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, J.; Yu, T.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Nickel phosphate nanorod-enhanced polyethylene oxide-based composite polymer electrolytes for solid-state lithium batteries. J. Colloid Interface Sci. 2020, 565, 110–118. [Google Scholar] [CrossRef]
- Cholant, C.M.; Krüger, L.; Balboni, R.D.C.; Rodrigues, M.P.; Tavares, F.C.; Peres, L.L.; Flores, W.H.; Gündel, A.; Pawlicka, A.; Avellaneda, C.O. Synthesis and characterization of solid polymer electrolyte based on poly(vinyl alcohol)/gum Arabic/LiClO4. Ionics 2020, 26, 2941–2948. [Google Scholar] [CrossRef]
- Bergfelt, A.; Hernández, G.; Mogensen, R.; Lacey, M.J.; Mindemark, J.; Brandell, D.; Bowden, T.M. Mechanically Robust Yet Highly Conductive Diblock Copolymer Solid Polymer Electrolyte for Ambient Temperature Battery Applications. ACS Appl. Polym. Mater. 2019, 2, 939–948. [Google Scholar] [CrossRef]
- Jo, Y.H.; Li, S.; Zuo, C.; Zhang, Y.; Gan, H.; Li, S.; Yu, L.; He, D.; Xie, X.; Xue, Z. Self-Healing Solid Polymer Electrolyte Facilitated by a Dynamic Cross-Linked Polymer Matrix for Lithium-Ion Batteries. Macromolecules 2020, 53, 1024–1032. [Google Scholar] [CrossRef]
- Ben Youcef, H.; Orayech, B.; del Amo, J.M.L.; Bonilla, F.; Shanmukaraj, D.; Armand, M. Functionalized cellulose as quasi single-ion conductors in polymer electrolyte for all-solid–state Li/Na and Li S batteries. Solid State Ion. 2020, 345, 115168. [Google Scholar] [CrossRef]
- Khani, H.; Kalami, S.; Goodenough, J.B. Micropores-in-macroporous gel polymer electrolytes for alkali metal batteries. Sustain. Energy Fuels 2019, 4, 177–189. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Y.; Liu, A.; Li, W.; An, M.; Bai, Y.; Chen, G. Polymer electrolyte with dual functional groups designed via theoretical calculation for all-solid-state lithium batteries. J. Power Sources 2020, 450, 227614. [Google Scholar] [CrossRef]
- Wu, N.; Chien, P.-H.; Li, Y.; Dolocan, A.; Xu, H.; Xu, B.; Grundish, N.S.; Jin, H.; Hu, Y.-Y.; Goodenough, J.B. Fast Li+ Conduction Mechanism and Interfacial Chemistry of a NASICON/Polymer Composite Electrolyte. J. Am. Chem. Soc. 2020, 142, 2497–2505. [Google Scholar] [CrossRef]
- Meng, N.; Zhang, H.; Lianli, S.; Lian, F. Salt-with-Salt, a novel strategy to design the flexible solid electrolyte membrane for highly safe lithium metal batteries. J. Membr. Sci. 2020, 597, 117768. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, J.; Zhang, F.; Zhang, H. Crosslinked poly(ethylene oxide)-based membrane electrolyte consisting of polyhedral oligomeric silsesquioxane nanocages for all-solid-state lithium ion batteries. J. Power Sources 2020, 449, 227541. [Google Scholar] [CrossRef]
- Tian, X.; Yang, P.; Yi, Y.; Liu, P.; Wang, T.; Shu, C.; Qu, L.; Tang, W.; Zhang, Y.; Li, M.; et al. Self-healing and high stretchable polymer electrolytes based on ionic bonds with high conductivity for lithium batteries. J. Power Sources 2020, 450, 227629. [Google Scholar] [CrossRef]
- Cha, J.H.; Didwal, P.N.; Kim, J.M.; Chang, D.R.; Park, C.-J. Poly(ethylene oxide)-based composite solid polymer electrolyte containing Li7La3Zr2O12 and poly(ethylene glycol) dimethyl ether. J. Membr. Sci. 2020, 595, 117538. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Tang, W.; Song, S.; Yao, J.; Wen, Z.; Lu, L.; Savilov, S.V.; Hu, N.; Molenda, J. Preparation of Nanocomposite Polymer Electrolyte via In Situ Synthesis of SiO2 Nanoparticles in PEO. Nanomaterials 2020, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, Z.; Liu, D.; Gao, Y.; Wang, Y.; Bu, H.-T.; Li, M.; Zhang, Y.; Gao, G.; Ding, S. A composite solid polymer electrolyte incorporating MnO2 nanosheets with reinforced mechanical properties and electrochemical stability for lithium metal batteries. J. Mater. Chem. A 2020, 8, 2021–2032. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Han, D.; Xiao, M.; Sun, L.; Meng, Y. Lithium (4-styrenesulfonyl) (trifluoromethanesulfonyl) imide based single-ion polymer electrolyte with superior battery performance. Energy Storage Mater. 2020, 24, 579–587. [Google Scholar] [CrossRef]
- Cao, X.; Cheng, J.; Zhang, X.; Zhou, D.; Tong, Y. Composite Polymer Electrolyte based on Liquid Crystalline Copolymer with High-temperature Stability and Bendability for All-solid-state Lithium-ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 677–695. [Google Scholar] [CrossRef]
- Zhang, Z.; You, J.; Zhang, S.; Wang, C.; Zhou, Y.; Li, J.; Huang, L.; Sun, S. Metal Organic Framework Nanorod Doped Solid Polymer Electrolyte with Decreased Crystallinity for High-Performance All-Solid-State Lithium Batteries. ChemElectroChem. 2019, 7, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Gao, H.; Hang, J.; Li, C.; Liu, P. MOF-derived ionic conductor enhancing polymer electrolytes with superior electrochemical performances for all solid lithium metal batteries. J. Membr. Sci. 2020, 598, 117800. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R. Dielectric relaxation, Li-ion transport, electrochemical, and structural behaviour of PEO/PVDF/LiClO4/TiO2/PC-based plasticized nanocomposite solid polymer electrolyte films. Compos. Commun. 2020, 17, 182–191. [Google Scholar] [CrossRef]
- Ouhib, F.; Meabe, L.; Mahmoud, A.; Grignard, B.; Thomassin, J.-M.; Boschini, F.; Zhu, H.; Forsyth, M.; Mecerreyes, D.; Detrembleur, C. Influence of the Cyclic versus Linear Carbonate Segments in the Properties and Performance of CO2-Sourced Polymer Electrolytes for Lithium Batteries. ACS Appl. Polym. Mater. 2020, 2, 922–931. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Z.; Zhou, Q.; Shangguan, X.; Du, X.; Dong, S.; Qiao, L.; Huang, S.; Liu, X.; Tang, K.; et al. A supramolecular interaction strategy enabling high-performance all solid state electrolyte of lithium metal batteries. Energy Storage Mater. 2020, 25, 756–763. [Google Scholar] [CrossRef]
- Li, J.; Hu, R.; Zhou, H.; Tao, S.; Wang, Y. Nano-SiO2@PMMA-doped composite polymer PVDF-HFP/PMMA/PEO electrolyte for lithium metal batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 2708–2719. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, J.-F.; Yu, Z.-Y.; Guo, X. Composite polymer electrolytes reinforced by two-dimensional layer-double-hydroxide nanosheets for dendrite-free lithium batteries. Solid State Ion. 2020, 347, 115275. [Google Scholar] [CrossRef]
- Ryu, H.-M.; Kim, M.Y.; Jung, H.Y.; Lim, J.S.; Kim, Y.A.; Kim, H.-S. Fabrication and electrochemical behavior of thin composite solid electrolyte for all-solid lithium batteries. Ionics 2020, 26, 2863–2874. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, H.; Zheng, H.; Sun, Y.; Liang, X.; Xiang, H. Nonflammable hybrid solid electrolyte membrane for a solid-state lithium battery compatible with conventional porous electrodes. J. Membr. Sci. 2020, 603, 117820. [Google Scholar] [CrossRef]
- Li, A.; Liao, X.; Zhang, H.; Shi, L.; Wang, P.; Cheng, Q.; Borovilas, J.; Li, Z.; Huang, W.; Fu, Z.; et al. Nacre-Inspired Composite Electrolytes for Load-Bearing Solid-State Lithium-Metal Batteries. Adv. Mater. 2020, 32, e1905517. [Google Scholar] [CrossRef]
- Ai, S.; Wang, T.; Li, T.; Wan, Y.; Xu, X.; Lu, H.; Qu, T.; Luo, S.; Jiang, J.; Yu, X.; et al. A Chitosan/Poly(ethylene oxide)-Based Hybrid Polymer Composite Electrolyte Suitable for Solid-State Lithium Metal Batteries. ChemistrySelect 2020, 5, 2878–2885. [Google Scholar] [CrossRef]
- Bi, J.; Mu, D.; Wu, B.; Fu, J.; Yang, H.; Mu, G.; Zhang, L.; Wu, F. A hybrid solid electrolyte Li0.33La0.557TiO3/poly(acylonitrile) membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries. J. Mater. Chem. A 2020, 8, 706–713. [Google Scholar] [CrossRef]
- Pareek, T.; Dwivedi, S.; Ahmad, S.A.; Badole, M.; Kumar, S. Effect of NASICON-type LiSnZr(PO4)3 ceramic filler on the ionic conductivity and electrochemical behavior of PVDF based composite electrolyte. J. Alloys Compd. 2020, 824, 153991. [Google Scholar] [CrossRef]
- Xuan, C.; Gao, S.; Wang, Y.; You, Q.; Liu, X.; Liu, J.; Xu, R.; Yang, K.; Cheng, S.; Liu, Z.; et al. In-situ generation of high performance thiol-conjugated solid polymer electrolytes via reliable thiol-acrylate click chemistry. J. Power Sources 2020, 456, 228024. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P. Predominantly chain segmental relaxation dependent ionic conductivity of multiphase semicrystalline PVDF/PEO/LiClO4 solid polymer electrolytes. Electrochim. Acta 2020, 338, 135890. [Google Scholar] [CrossRef]
- Meabe, L.; Goujon, N.; Li, C.; Armand, M.; Forsyth, M.; Mecerreyes, D. Single-Ion Conducting Poly(Ethylene Oxide Carbonate) as Solid Polymer Electrolyte for Lithium Batteries. Batter. Supercaps. 2020, 3, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Ao, X.; Lei, Z.; Wang, J.; Deng, Y.; Wang, C. Hollow nanotubular clay composited comb-like methoxy poly(ethylene glycol) acrylate polymer as solid polymer electrolyte for lithium metal batteries. Electrochim. Acta 2020, 340, 135995. [Google Scholar] [CrossRef]
- Grewal, M.S.; Tanaka, M.; Kawakami, H. Fabrication and characterizations of soft and flexible Poly(dimethylsiloxane)-incorporated network polymer electrolyte membranes. Polymer 2020, 186, 122045. [Google Scholar] [CrossRef]
- Choudhury, S. (Ed.) A Highly Reversible Room-Temperature Lithium Metal Battery Based on Cross-Linked Hairy Nanoparticles. In Rational Design of Nanostructured Polymer Electrolytes and Solid–Liquid Interphases for Lithium Batteries; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–57. [Google Scholar]
- Liu, L.; Chu, L.; Jiang, B.; Li, M. Li1.4Al0.4Ti1.6(PO4)3 nanoparticle-reinforced solid polymer electrolytes for all-solid-state lithium batteries. Solid State Ion. 2019, 331, 89–95. [Google Scholar] [CrossRef]
- Sasikumar, M.; Jagadeesan, A.; Raja, M.; Krishna, R.H.; Sivakumar, P. The effects of PVAc on surface morphological and electrochemical performance of P(VdF-HFP)-based blend solid polymer electrolytes for lithium ion-battery applications. Ionics 2018, 25, 2171–2181. [Google Scholar] [CrossRef]
- Grewal, M.S.; Tanaka, M.; Kawakami, H. Free-standing polydimethylsiloxane-based cross-linked network solid polymer electrolytes for future lithium ion battery applications. Electrochim. Acta 2019, 307, 148–156. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, Q.; Smith, D.M.; Li, C.Y. Plasticized Hybrid Network Solid Polymer Electrolytes for Lithium-Metal Batteries. Adv. Mater. Interfaces 2019, 6, 1801445. [Google Scholar] [CrossRef]
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J. Energy Storage 2019, 26, 100947. [Google Scholar] [CrossRef]
- He, K.-Q.; Zha, J.-W.; Du, P.; Cheng, S.H.-S.; Liu, C.; Dang, Z.-M.; Li, R.K.Y. Tailored high cycling performance in a solid polymer electrolyte with perovskite-type Li0.33La0.557TiO3 nanofibers for all-solid-state lithium ion batteries. Dalton Trans. 2019, 48, 3263–3269. [Google Scholar] [CrossRef]
- Meabe, L.; Huynh, T.V.; Mantione, D.; Porcarelli, L.; Li, C.; O’Dell, L.; Sardon, H.; Armand, M.; Forsyth, M.; Mecerreyes, D. UV-cross-linked poly(ethylene oxide carbonate) as free standing solid polymer electrolyte for lithium batteries. Electrochim. Acta 2019, 302, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Li, Y.; Feng, Y.; Peng, C.; Li, Z.; Feng, W. A solid-state single-ion polymer electrolyte with ultrahigh ionic conductivity for dendrite-free lithium metal batteries. Energy Storage Mater. 2019, 19, 401–407. [Google Scholar] [CrossRef]
- Deka, J.R.; Saikia, D.; Lou, G.-W.; Lin, C.-H.; Fang, J.; Yang, Y.-C.; Kao, H.-M. Design, synthesis and characterization of polysiloxane and polyetherdiamine based comb-shaped hybrid solid polymer electrolytes for applications in electrochemical devices. Mater. Res. Bull. 2019, 109, 72–81. [Google Scholar] [CrossRef]
- Imholt, L.; Dörr, T.S.; Zhang, P.; Ibing, L.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Sources 2019, 409, 148–158. [Google Scholar] [CrossRef]
- Ma, Q.; Chakrabarti, A.; Mei, X.; Yue, Z.; Dunya, H.; Filler, R.; Mandal, B.K. New oligoether plasticizers for poly(ethylene oxide)-based solid polymer electrolytes. Ionics 2018, 25, 1633–1643. [Google Scholar] [CrossRef]
- Na Choi, B.; Yang, J.H.; Kim, Y.S.; Chung, C.-H. Effect of morphological change of copper-oxide fillers on the performance of solid polymer electrolytes for lithium-metal polymer batteries. RSC Adv. 2019, 9, 21760–21770. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Tu, Z.; Nijamudheen, A.; Zachman, M.J.; Stalin, S.; Deng, Y.; Zhao, Q.; Vu, D.; Kourkoutis, L.F.; Mendoza-Cortes, J.L.; et al. Stabilizing polymer electrolytes in high-voltage lithium batteries. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Feng, W.; Zhen, Y.; Zhao, P.; Cai, Z.; Li, L. Effects of the shapes of BaTiO3 nanofillers on PEO-based electrolytes for all-solid-state lithium-ion batteries. Ionics 2019, 25, 1471–1480. [Google Scholar] [CrossRef]
- Nair, J.R.; Shaji, I.; Ehteshami, N.; Thum, A.; Diddens, D.; Heuer, A.; Winter, M. Solid Polymer Electrolytes for Lithium Metal Battery via Thermally Induced Cationic Ring-Opening Polymerization (CROP) with an Insight into the Reaction Mechanism. Chem. Mater. 2019, 31, 3118–3133. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Feng, X.; Zhang, Z.; Liu, P. High electrochemical performance poly(ethylene oxide)/2,4-toluene diisocyante/polyethylene glycol as electrolytes for all-solid-state lithium batteries. J. Membr. Sci. 2019, 587, 117179. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Z.-Q.; Yan, W.; Ma, X.; Sun, D.; Jin, Y.; Chen, X.; He, K. Solid Polymer Electrolyte Based on Polymerized Ionic Liquid for High Performance All-Solid-State Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 4675–4683. [Google Scholar] [CrossRef]
- Niu, C.; Liu, J.; Chen, G.; Liu, C.; Qian, T.; Zhang, J.; Cao, B.; Shang, W.; Chen, Y.; Han, J.; et al. Anion-regulated solid polymer electrolyte enhances the stable deposition of lithium ion for lithium metal batteries. J. Power Sources 2019, 417, 70–75. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Q.; Zhao, C.; Gao, X.; Adair, K.R.; Liu, Y.; Luo, J.; Lin, X.; Liang, J.; Huang, H.; et al. High-areal-capacity all-solid-state lithium batteries enabled by rational design of fast ion transport channels in vertically-aligned composite polymer electrodes. Nano Energy 2019, 61, 567–575. [Google Scholar] [CrossRef]
- Lu, Y.; He, K.-W.; Zhang, S.-J.; Zhou, Y.-X.; Wang, Z.-B. UV-curable-based plastic crystal polymer electrolyte for high-performance all-solid-state Li-ion batteries. Ionics 2019, 25, 1607–1615. [Google Scholar] [CrossRef]
- Huang, S.; Cui, Z.; Qiao, L.; Xu, G.; Zhang, J.; Tang, K.; Liu, X.; Wang, Q.; Zhou, X.; Zhang, B.; et al. An in-situ polymerized solid polymer electrolyte enables excellent interfacial compatibility in lithium batteries. Electrochim. Acta 2019, 299, 820–827. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Shen, F.; Zhao, B.; Han, X. Holey graphene oxide as filler to improve electrochemical performance of solid polymer electrolytes. Mater. Express 2019, 9, 1055–1061. [Google Scholar] [CrossRef]
- Zhang, N.; He, J.; Han, W.; Wang, Y. Composite solid electrolyte PEO/SN/LiAlO2 for a solid-state lithium battery. J. Mater. Sci. 2019, 54, 9603–9612. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y. Tailored Solid Polymer Electrolytes by Montmorillonite with High Ionic Conductivity for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lu, W.; Cong, L.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; Xie, H.; Liu, J. Cross-linking network based on Poly(ethylene oxide): Solid polymer electrolyte for room temperature lithium battery. J. Power Sources 2019, 420, 63–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Feng, W.; Zhen, Y.; Zhao, P.; Li, L.; Cai, Z. The effects of the size and content of BaTiO3 nanoparticles on solid polymer electrolytes for all-solid-state lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 749–758. [Google Scholar] [CrossRef]
- Rangasamy, V.S.; Thayumanasundaram, S.; Locquet, J.-P. Solid polymer electrolytes with poly(vinyl alcohol) and piperidinium based ionic liquid for Li-ion batteries. Solid State Ion. 2019, 333, 76–82. [Google Scholar] [CrossRef]
- Wu, N.; Shi, Y.; Lang, S.; Zhou, J.; Liang, J.; Wang, W.; Tan, S.; Yin, Y.; Wen, R.; Guo, Y. Self-Healable Solid Polymeric Electrolytes for Stable and Flexible Lithium Metal Batteries. Angew. Chem. Int. Ed. 2019, 58, 18146–18149. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Inafune, Y.; Tanaka, M.; Mochizuki, Y.; Matsumoto, F.; Kawakami, H. Development of all-solid-state battery based on lithium ion conductive polymer nanofiber framework. J. Power Sources 2019, 423, 255–262. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Zhang, Q.; Hou, C.; Shi, Q.; Wang, H. A highly ionic conductive poly(methyl methacrylate) composite electrolyte with garnet-typed Li6.75La3Zr1.75Nb0.25O12 nanowires. Chem. Eng. J. 2019, 375, 121922. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Miller, J.D. Advanced Nanoclay-Based Nanocomposite Solid Polymer Electrolyte for Lithium Iron Phosphate Batteries. ACS Appl. Mater. Interfaces 2019, 11, 8954–8960. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Jiao, X.; Song, Z.; Sadd, M.; Xu, X.; Matic, A.; Xiong, S.; Song, J. Enhanced ionic conductivity and interface stability of hybrid solid-state polymer electrolyte for rechargeable lithium metal batteries. Energy Storage Mater. 2019, 23, 105–111. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Pan, Q.; Li, C.Y. Polymerized Ionic Liquid-Containing Interpenetrating Network Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 34904–34912. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, H.; Qie, B.; Cheng, Q.; Li, A.; Borovilas, J.; Xu, B.; Shi, C.; Jin, T.; Liao, X.; et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte. Nano Energy 2019, 60, 205–212. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Dou, Q.; Wong, K.W.; Ng, K.M. Li7La3Zr2O12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. J. Mater. Chem. A 2019, 7, 3391–3398. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Shi, L.; Lu, S.; Sun, Z.; Shi, Y.; Wu, H.; Zhang, Y.; Ding, S. Poly(ionic liquid)-polyethylene oxide semi-interpenetrating polymer network solid electrolyte for safe lithium metal batteries. Chem. Eng. J. 2019, 375, 121925. [Google Scholar] [CrossRef]
- Sun, Y.; Zhan, X.; Hu, J.; Wang, Y.; Gao, S.; Shen, Y.; Cheng, Y.-T. Improving Ionic Conductivity with Bimodal-Sized Li7La3Zr2O12 Fillers for Composite Polymer Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 12467–12475. [Google Scholar] [CrossRef]
- Jo, Y.H.; Zhou, B.; Jiang, K.; Li, S.; Zuo, C.; Gan, H.; He, D.; Zhou, X.; Xue, Z. Self-healing and shape-memory solid polymer electrolytes with high mechanical strength facilitated by a poly(vinyl alcohol) matrix. Polym. Chem. 2019, 10, 6561–6569. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, N.; Liu, J.; Cong, L.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; Xie, H.; et al. Synthesis and interface stability of polystyrene-poly(ethylene glycol)-polystyrene triblock copolymer as solid-state electrolyte for lithium-metal batteries. J. Power Sources 2019, 428, 93–104. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, B.; Wang, J.; Zuo, C.; He, D.; Xie, X.; Xue, Z. PEO-based electrolytes blended with star polymers with precisely imprinted polymeric pseudo-crown ether cavities for alkali metal ion batteries. J. Membr. Sci. 2019, 576, 182–189. [Google Scholar] [CrossRef]
- Raut, P.; Li, S.; Chen, Y.-M.; Zhu, Y.; Jana, S.C. Strong and Flexible Composite Solid Polymer Electrolyte Membranes for Li-Ion Batteries. ACS Omega 2019, 4, 18203–18209. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Q.; Qi, F.; Zhang, J.; Liu, K.; Li, J.; Chen, W.; Du, Y.; Jin, Y.; Liang, Y.; et al. Epoxy containing solid polymer electrolyte for lithium ion battery. Electrochim. Acta 2019, 318, 302–313. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, H.; Wei, Z.; Wang, J.; Yao, X.; Chen, S. Preparation of new composite polymer electrolyte for long cycling all-solid-state lithium battery. Ionics 2019, 25, 907–916. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Ma, J.; Sun, X.; Zhou, X.; Cui, G. Selectively Wetted Rigid–Flexible Coupling Polymer Electrolyte Enabling Superior Stability and Compatibility of High-Voltage Lithium Metal Batteries. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Wang, W.; Fang, Z.; Zhao, M.; Peng, Y.; Zhang, J.; Guan, S. Solid polymer electrolytes based on the composite of PEO–LiFSI and organic ionic plastic crystal. Chem. Phys. Lett. 2020, 747, 137335. [Google Scholar] [CrossRef]
- Gao, J.; Shao, Q.; Chen, J. Lithiated Nafion-garnet ceramic composite electrolyte membrane for solid-state lithium metal battery. J. Energy Chem. 2020, 46, 237–247. [Google Scholar] [CrossRef]
- Tang, S.; Lan, Q.; Xu, L.; Liang, J.; Lou, P.; Liu, C.; Mai, L.; Cao, Y.-C.; Cheng, S. A novel cross-linked nanocomposite solid-state electrolyte with super flexibility and performance for lithium metal battery. Nano Energy 2020, 71, 104600. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Xiaokaiti, P.; Yoshida, A.; Abudula, A.; Guan, G. A sandwich-type composite polymer electrolyte for all-solid-state lithium metal batteries with high areal capacity and cycling stability. J. Membr. Sci. 2020, 596, 117739. [Google Scholar] [CrossRef]
- Peng, X.; Huang, K.; Song, S.; Wu, F.; Xiang, Y.; Zhang, X. Garnet-Polymer Composite Electrolytes with High Li+ Conductivity and Transference Number via Well-Fused Grain Boundaries in Microporous Frameworks. ChemElectroChem 2020, 7, 2389–2394. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Y.; Liang, Y.; Andrews, J.; Dong, H.; Yuan, M.; Ding, W.; Banerjee, S.; Ardebili, H.; Robertson, M.L.; et al. Chemically inert covalently networked triazole-based solid polymer electrolytes for stable all-solid-state lithium batteries. J. Mater. Chem. A 2019, 7, 19691–19695. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhang, S.; Shi, L.; Wu, H.; Bu, H.; Ding, S. g-C3N4 nanosheets enhanced solid polymer electrolytes with excellent electrochemical performance, mechanical properties, and thermal stability. J. Mater. Chem. A 2019, 7, 11069–11076. [Google Scholar] [CrossRef]
- Yang, K.; Liao, Z.; Zhang, Z.; Yang, L.; Hirano, S.-I. Ionic plastic crystal-polymeric ionic liquid solid-state electrolytes with high ionic conductivity for lithium ion batteries. Mater. Lett. 2019, 236, 554–557. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liang, Y.; Dong, H.; Hao, F.; Wang, A.; Zhu, Y.; Cui, X.; Yao, Y. Hyperbranched PEO-Based Hyperstar Solid Polymer Electrolytes with Simultaneous Improvement of Ion Transport and Mechanical Strength. ACS Appl. Energy Mater. 2019, 2, 1608–1615. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Cui, Z.; Shangguan, X.; Zhang, H.; Zhang, J.; Tang, K.; Li, L.; Zhou, X.; Cui, G. A fluorinated polycarbonate based all solid state polymer electrolyte for lithium metal batteries. Electrochim. Acta 2020, 337, 135843. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, T.; Chu, X.; Su, H.; Wang, Z.; Chen, N.; Gu, B.; Zhang, H.; Deng, W.; Zhang, H.; et al. Strong Lewis Acid–Base and Weak Hydrogen Bond Synergistically Enhancing Ionic Conductivity of Poly(ethylene oxide)@SiO2 Electrolytes for a High Rate Capability Li-Metal Battery. ACS Appl. Mater. Interfaces 2020, 12, 10341–10349. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tong, R.-A.; Zhang, J.; Chen, L.; Wang, C.-A. Blending Poly(ethylene oxide) and Li6.4La3Zr1.4Ta0.6O12 by Haake Rheomixer without any solvent: A low-cost manufacture method for mass production of composite polymer electrolyte. J. Power Sources 2020, 451, 227797. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Dittmer, A.J.; Wilson, B.E.; Hu, J.; Stein, A.; Buhlmann, P. Unbiased Quantification of the Electrochemical Stability Limits of Electrolytes and Ionic Liquids. J. Electrochem. Soc. 2015, 162, A2250–A2258. [Google Scholar] [CrossRef]
- Subramania, A.; Sundaram, N.T.K.; Kumar, G.V.; Vasudevan, T. New polymer electrolyte based on (PVA–PAN) blend for Li-ion battery applications. Ionics 2006, 12, 175–178. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Shin, C.; Baek, D.-H.; Choi, J.-W.; Manuel, J.; Heo, M.-Y.; Ahn, J.-H.; Nah, C. Preparation and electrochemical characterization of polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile blend/composite membranes for lithium batteries. J. Power Sources 2010, 195, 6088–6094. [Google Scholar] [CrossRef]
- Du, Y.-L.; Wen, T.-C. The feasibility study of composite electrolytes comprising thermoplastic polyurethane and poly(ethylene oxide). Mater. Chem. Phys. 2001, 71, 62–69. [Google Scholar] [CrossRef]
- Shin, J.-H.; Passerini, S. Effect of fillers on the electrochemical and interfacial properties of PEO–LiN(SO2CF2CF3)2 polymer electrolytes. Electrochim. Acta 2004, 49, 1605–1612. [Google Scholar] [CrossRef]
- Dong, X.; Yao, J.; Zhu, W.; Huang, X.; Kuai, X.; Tang, J.; Li, X.; Dai, S.; Shen, L.; Yang, R.; et al. Enhanced high-voltage cycling stability of Ni-rich cathode materials via the self-assembly of Mn-rich shells. J. Mater. Chem. A 2019, 7, 20262–20273. [Google Scholar] [CrossRef]
- Qiu, Q.-Q.; Shadike, Z.; Wang, Q.-C.; Yue, X.-Y.; Li, X.; Yuan, S.-S.; Fang, F.; Wu, X.-J.; Hunt, A.; Waluyo, I.; et al. Improving the Electrochemical Performance and Structural Stability of the LiNi0.8Co0.15Al0.05O2 Cathode Material at High-Voltage Charging through Ti Substitution. ACS Appl. Mater. Interfaces 2019, 11, 23213–23221. [Google Scholar] [CrossRef]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward Reliable Values of Electrochemical Stability Limits for Electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Streipert, B.; Röser, S.; Wagner, R.; Laskovic, I.C.; Winter, M. Determining oxidative stability of battery electrolytes: Validity of common electrochemical stability window (ESW) data and alternative strategies. Phys. Chem. Chem. Phys. 2017, 19, 16078–16086. [Google Scholar] [CrossRef]
- Hallinan, D.T.; Rausch, A.; McGill, B. An electrochemical approach to measuring oxidative stability of solid polymer electrolytes for lithium batteries. Chem. Eng. Sci. 2016, 154, 34–41. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Nair, J.; Laskovic, I.C.; Winter, M.; Kasnatscheew, J. Poly(Ethylene Oxide)-based Electrolyte for Solid-State-Lithium-Batteries with High Voltage Positive Electrodes: Evaluating the Role of Electrolyte Oxidation in Rapid Cell Failure. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dewald, G.F.; Ohno, S.; Kraft, M.A.; Koerver, R.; Till, P.; Vargas-Barbosa, N.M.; Janek, J.; Zeier, W.G. Experimental Assessment of the Practical Oxidative Stability of Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2019, 31, 8328–8337. [Google Scholar] [CrossRef]
- Han, F.; Zhu, Y.; He, X.; Mo, Y.; Wang, C. Electrochemical Stability of Li10GeP2S12 and Li7La3Zr2O12 Solid Electrolytes. Adv. Energy Mater. 2016, 6, 1501590. [Google Scholar] [CrossRef]
- Benabed, Y.; Rioux, M.; Rousselot, S.; Hautier, G.; Dollé, M. Assessing the Electrochemical Stability Window of NASICON-Type Solid Electrolytes. Front. Energy Res. 2021, 9. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z. A New Class of Ionically Conducting Fluorinated Ether Electrolytes with High Electrochemical Stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, S.; Huang, M.; Qiao, B.; Shigenobu, K.; Giordano, L.; Lopez, J.; Tatara, R.; Ueno, K.; Dokko, K.; et al. Molecularly Tunable Polyanions for Single-Ion Conductors and Poly(solvate ionic liquids). Chem. Mater. 2021, 33, 524–534. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Tenhaeff, W.E. Determining the Absolute Anodic Stability Threshold of Polymer Electrolytes: A Capacity-Based Electrochemical Method. Chem. Mater. 2021, 33, 1927–1934. [Google Scholar] [CrossRef]
- Binninger, T.; Marcolongo, A.; Mottet, M.; Weber, V.; Laino, T. Comparison of computational methods for the electrochemical stability window of solid-state electrolyte materials. J. Mater. Chem. A 2020, 8, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Venkatram, S.; Kim, C.; Batra, R.; Chandrasekaran, A.; Ramprasad, R. Electrochemical Stability Window of Polymeric Electrolytes. Chem. Mater. 2019, 31, 4598–4604. [Google Scholar] [CrossRef]
- Schwietert, T.K.; Arszelewska, V.A.; Wang, C.; Yu, C.; Vasileiadis, A.; De Klerk, N.J.J.; Hageman, J.; Hupfer, T.; Kerkamm, I.; Xu, Y.; et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 2020, 19, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.; Yu, S.; Williams, L.; Schmidt, R.D.; Garcia-Mendez, R.; Wolfenstine, J.; Allen, J.; Kioupakis, E.; Siegel, D.; Sakamoto, J. Electrochemical Window of the Li-Ion Solid Electrolyte Li7La3Zr2O12. ACS Energy Lett. 2017, 2, 462–468. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, T.; Richards, W.D.; Li, J.; Kim, J.C.; Bo, S.-H.; Ceder, G. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci. 2017, 10, 1150–1166. [Google Scholar] [CrossRef] [Green Version]
- Dahn, J.R.; Burns, J.C.; Stevens, D.A. Importance of Coulombic Efficiency Measurements in R&D Efforts to Obtain Long-Lived Li-Ion Batteries. Electrochem. Soc. Interface 2016, 25, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Becking, J.; Gröbmeyer, A.; Kolek, M.; Rodehorst, U.; Schulze, S.; Winter, M.; Bieker, P.; Stan, M.C. Lithium-Metal Foil Surface Modification: An Effective Method to Improve the Cycling Performance of Lithium-Metal Batteries. Adv. Mater. Interfaces 2017, 4, 1700166. [Google Scholar] [CrossRef]
- Storelli, A.; Rousselot, S.; Alzate-Carvajal, N.; Pelé, V.; Dolle, M. On the Importance of Li Metal Morphology on the Cycling of Lithium Metal Polymer Cells. J. Electrochem. Soc. 2021, 168, 040505. [Google Scholar] [CrossRef]
- Rynne, O.; Dubarry, M.; Molson, C.; Nicolas, E.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Exploiting Materials to Their Full Potential, a Li-Ion Battery Electrode Formulation Optimization Study. ACS Appl. Energy Mater. 2020, 3, 2935–2948. [Google Scholar] [CrossRef]
- Lou, S.; Liu, Q.; Zhang, F.; Liu, Q.; Yu, Z.; Mu, T.; Zhao, Y.; Borovilas, J.; Chen, Y.; Ge, M.; et al. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Banerjee, A.; Wang, X.; Fang, C.; Wu, E.A.; Meng, Y.S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chem. Rev. 2020, 120, 6878–6933. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.S.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]


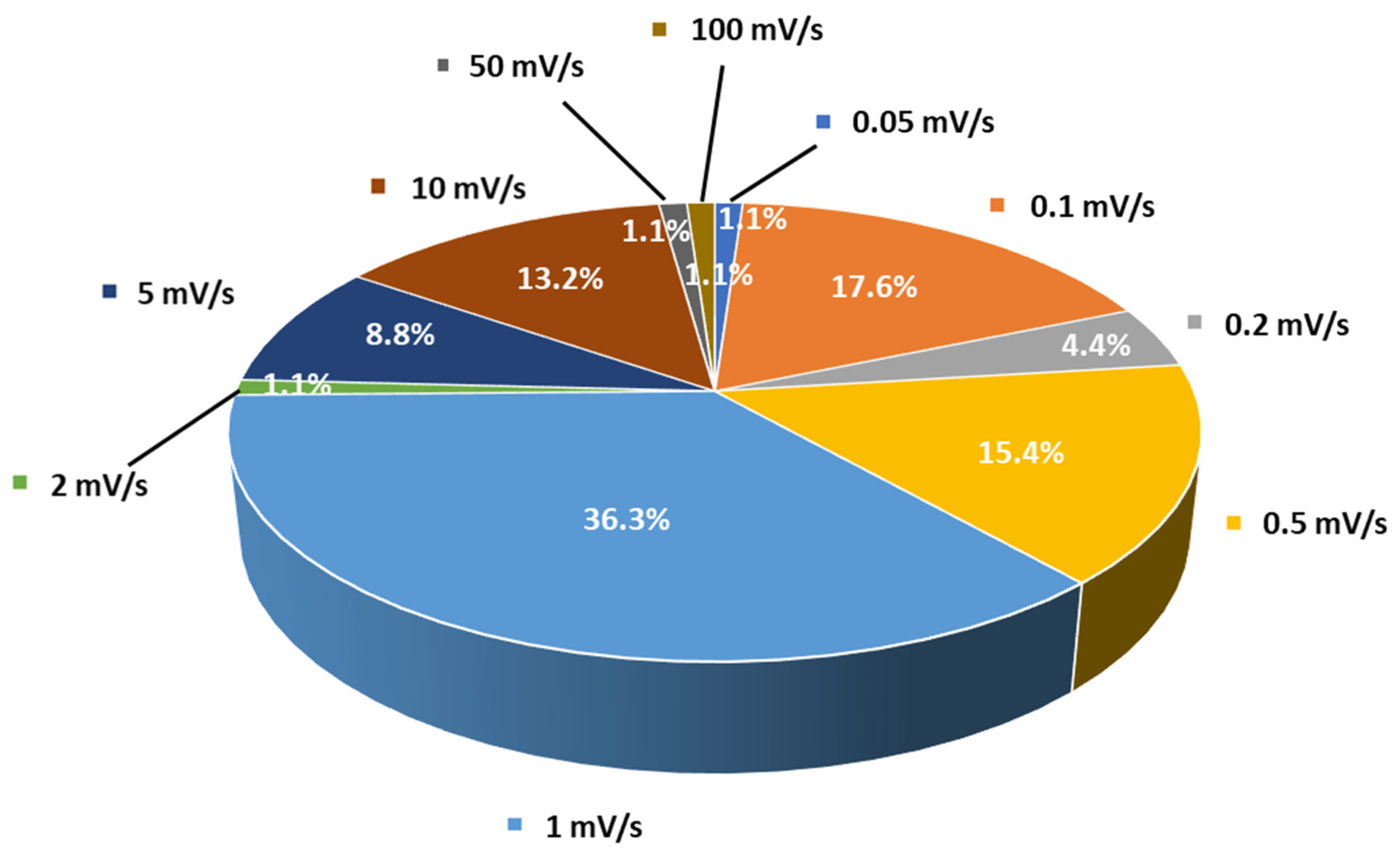
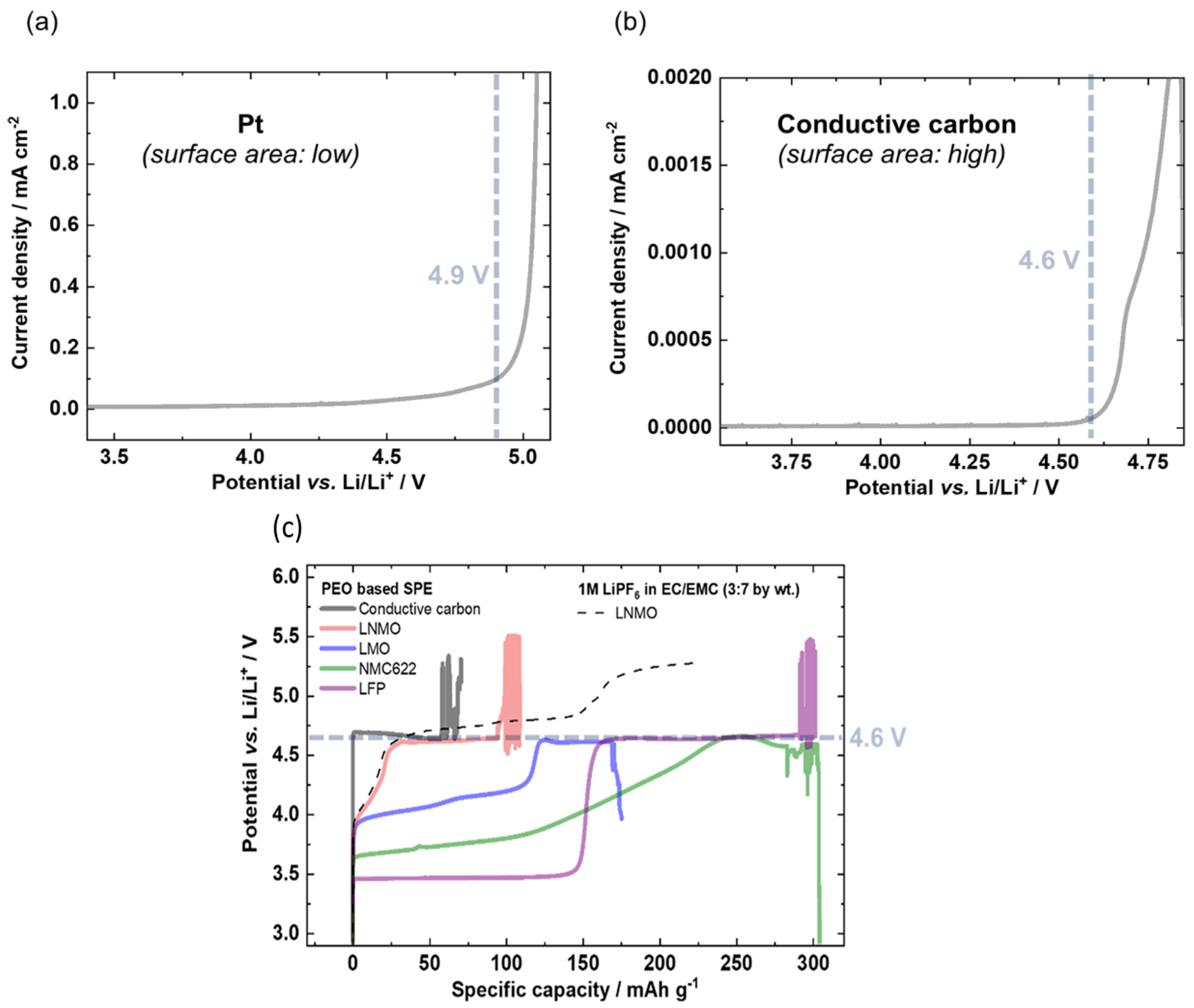
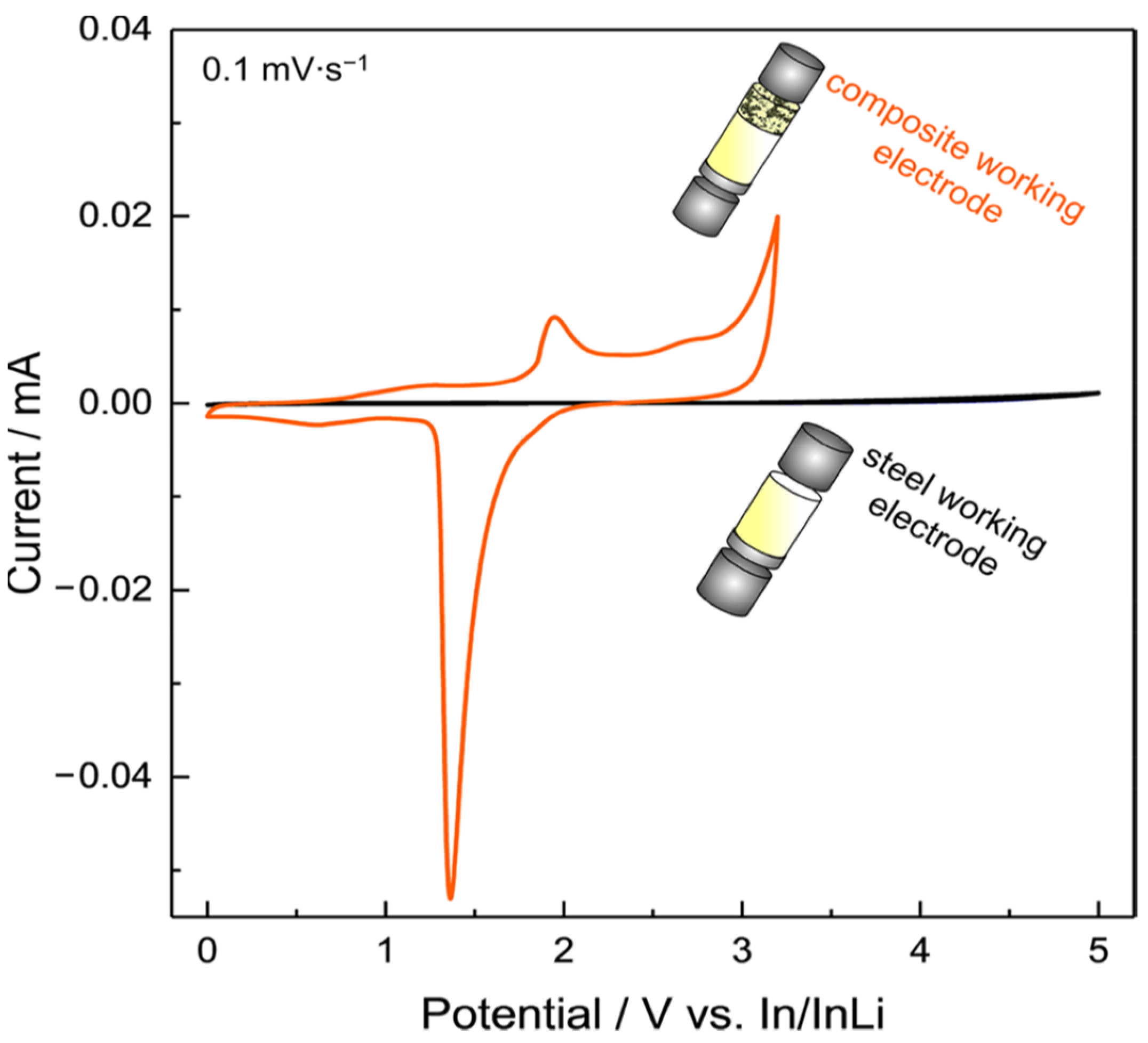
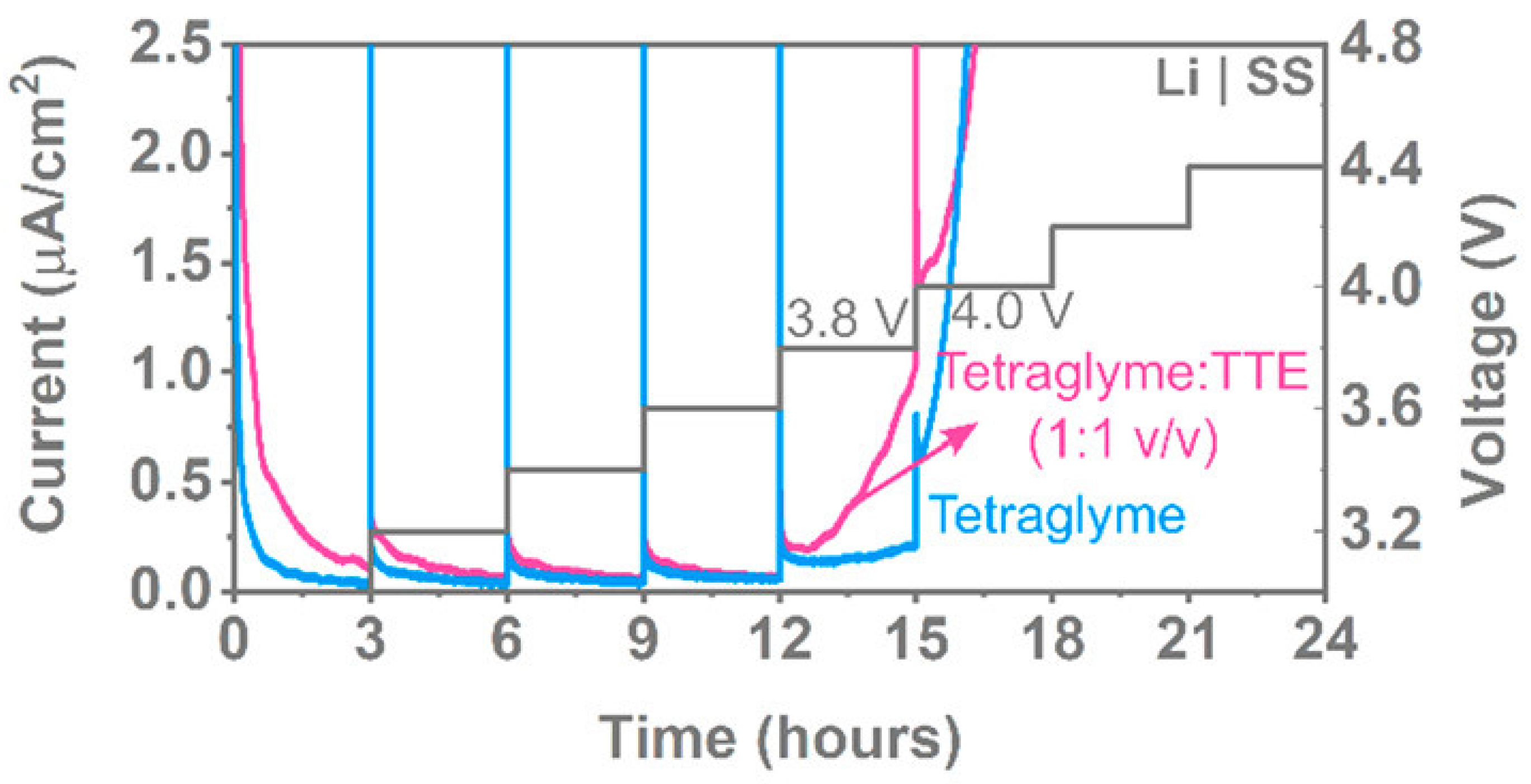
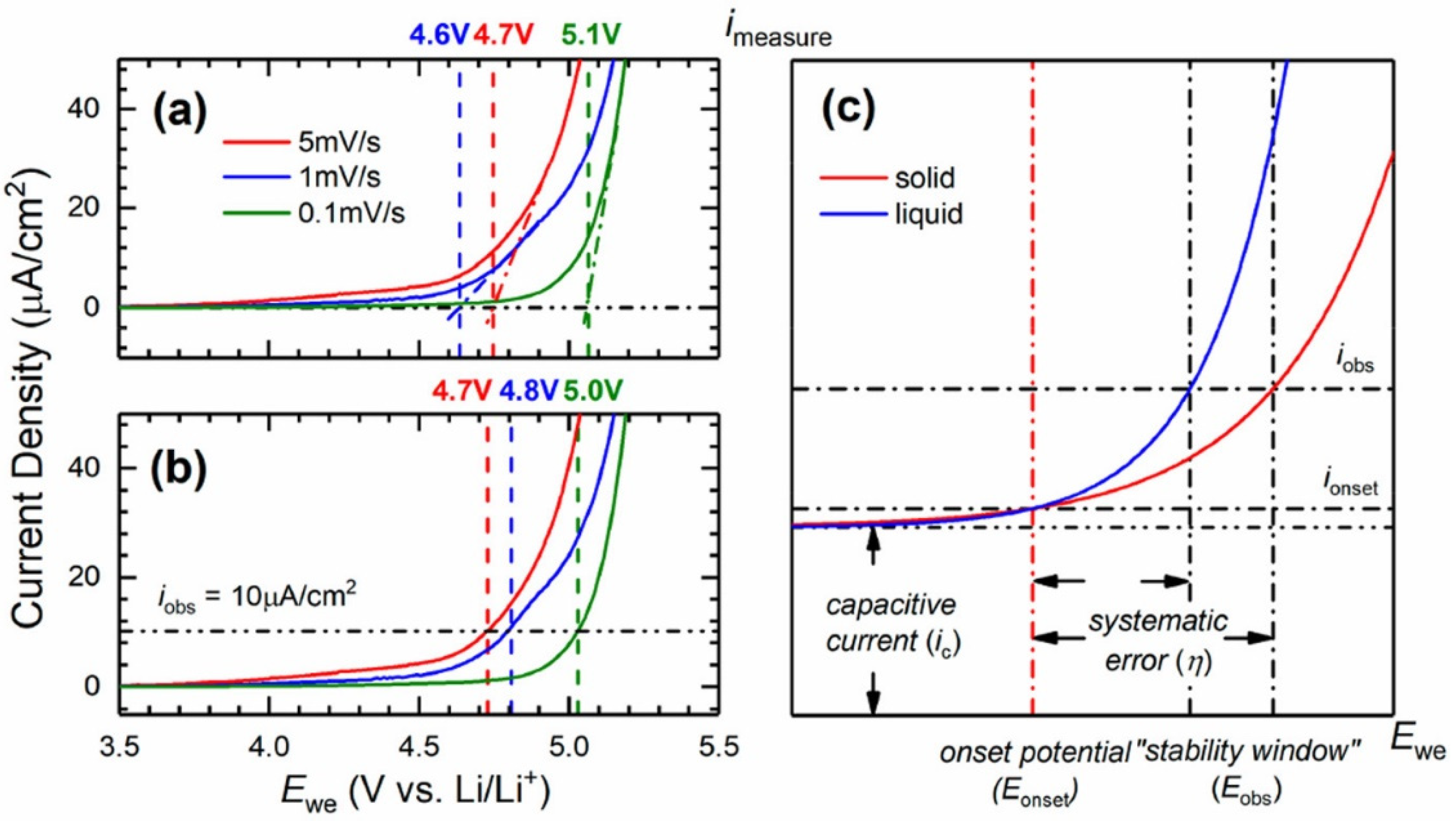
| Solid Electrolyte/Sample | Salt | Ionic Conductivity (S/cm) | Conductivity T (°C) | Eox (V vs. Li+/Li) | Scan Rate (mV/s) | ESW T (°C) | Method | References |
|---|---|---|---|---|---|---|---|---|
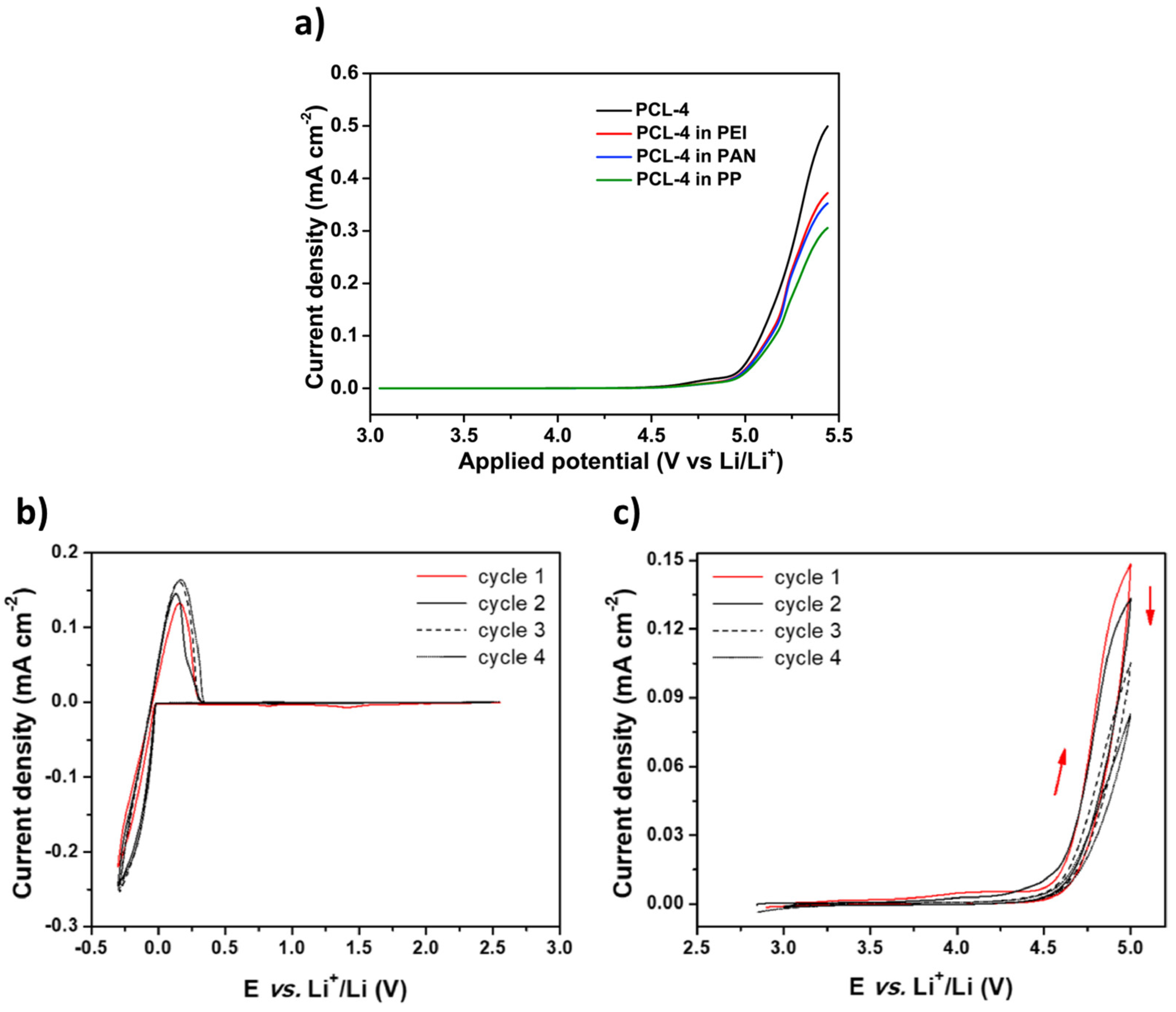
| PCL | LiTFSI | 2.5 × 10−5 | RT | 4.6 | 1 | 55 | LSV | [17] |
| PIL-SN-PCE | LiTFSI | 6.54 × 10−4 | RT | 5.4 | 1 | / | LSV | [18] |
| DAVA + ETTMP 1300 | LiPF6 | 7.65 × 10−4 | RT | 6 | 100 | / | LSV | [19] |
| PEO8–LiPCSI | LiPCSI | 7.33 × 10−5 | 60 | 5.53 | 0.2 | 60 | LSV | [20] |
| 3D ANF framework/PEO-LiTFSI | LiTFSI | 8.8 × 10−5 | RT | >4.5 | 1 | / | LSV | [21] |
| PEO–LiClO4–LLZTO | LiClO4 | / | 60 | 4 to 4.5 | 0.3 | / | LSV | [22] |
| PEO-LiTFSI-3%VSB-5 | LiTFSI | 4.83 × 10−5 | 30 | 4.13 | 1 | / | LSV | [23] |
| PVA/GA with 24 wt% of LiClO4 | LiClO4 | 1.6 × 10−4 | 25 | / | / | / | / | [24] |
| BCT (copolymer dblock) | LiTFSI | 9.1 × 10−6 | 30 | 5 | 1 | 60 | CV | [25] |
| 5PEG-SSH | LiTFSI | 7.28 × 10−6 | 30 | 5 | 10 | 60 | LSV | [26] |
| Li(FSI-ethyl cellulose)/PEO | LiTFSI | 0.5 × 10−4 | 70 | 4 | 10 | 70 | LSV | [27] |
| Li-HCFu-PH | LiPF6 | 6.4 × 10−3 | RT | 4.7 | 1 | RT | CV/ LSV | [28] |
| CPEG (copo EC/EO) | LiTFSI | 1.84 × 10−4 | 30 | 4.75 | 1 | 60 | LSV | [29] |
| (PEO)-based NASICON−LiZr2(PO4) | LiTFSI | 1.2 × 10−4 | 30 | 5 | / | / | LSV | [30] |
| Dual-Li SPEs | LiTFSI + LiPVFM | 5.7 × 10−4 | 25 | 4.5 | 5 | / | LSV | [31] |
| POSS-PEGDA/PEO/LiTFSI | LiTFSI | 3.83 × 10−4 | 60 | 5.3 | 10 | 60 | LSV | [32] |
| PVT-EMIMTFSI | EMIMTFSI | 1.26 × 10−4 | RT | 4.5 | 10 | 25 | LSV | [33] |
| PEO(LiTFSI)-LLZO+PEGDME | LiTFSI | 4.7 × 10−4 | 60 | 5.2 | 1 | 60 | LSV | [34] |
| PEO-SiO2 | LiClO4 | 1.1 × 10−4 | 30 | 5 | 1 | / | LSV | [35] |
| PEO/MnO2 | LiTFSI | 1.95 × 10−5 | 30 | 4.5 | 1 | 60 | LSV | [36] |
| SIGPE | LiSTFSI | 0.84 × 10−3 | RT | 5.2 | 1 | RT | LSV | [37] |
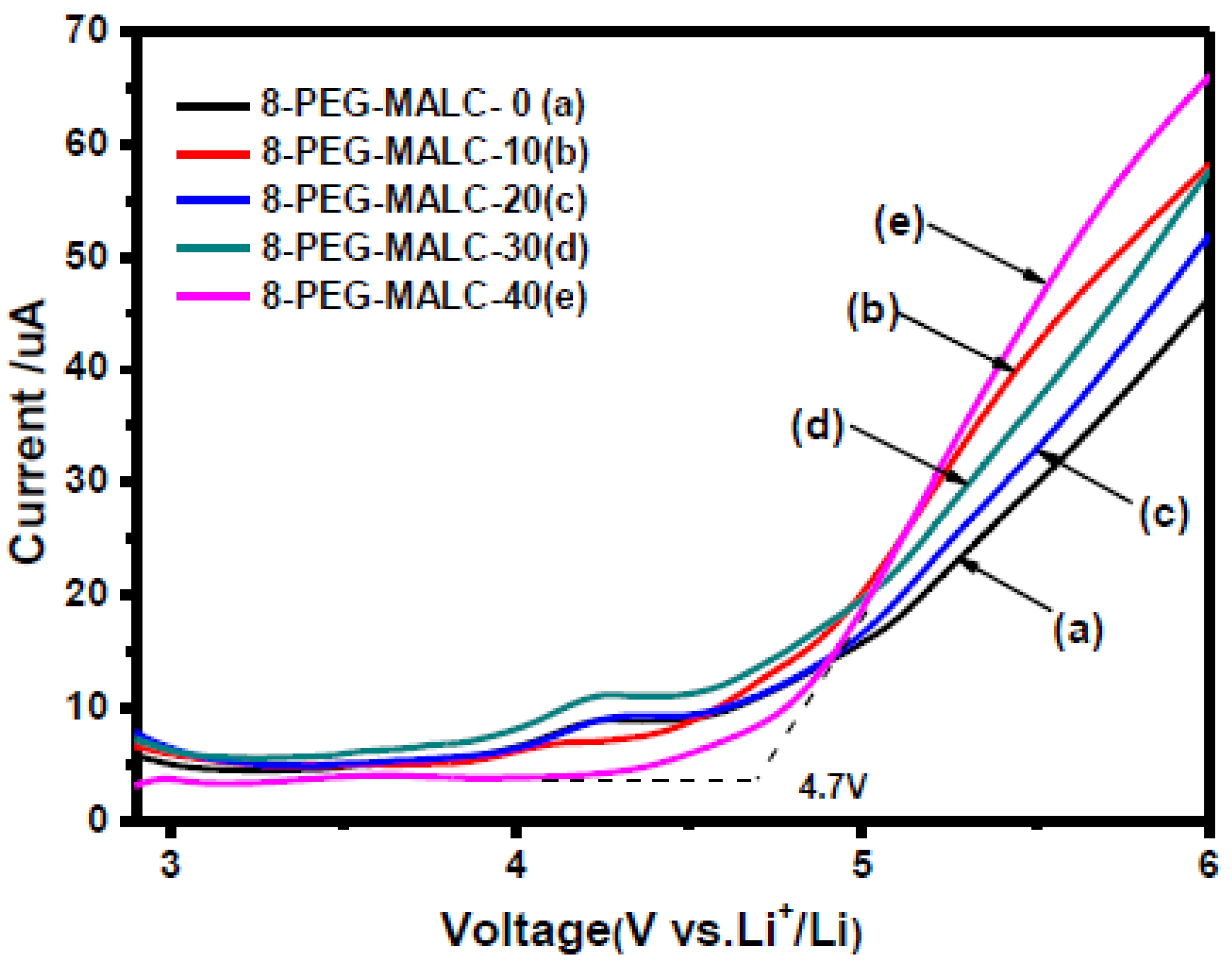
| 8-PEG-MALC, 8-PEG, PEGDA | LiTFSI | 6.2 × 10−5 | RT | 4.5 | 1 | RT | LSV | [38] |
| PEO/Al-MOF 5% | LiTFSI | 2.09 × 10−5 | 30 | 4.7 | 5 | 30 | LSV | [39] |
| PEO/MOF-UIO66 | LiTFSI | 1.47 × 10−4 | 30 | 5.2 | 0.5 | / | LSV | [40] |
| PEO/PVDF/LiClO4/TiO2/PC | LiClO4 | 10.2 × 10−6 | 27 | 3 | / | RT | LSV | [41] |
| polycarbonates/polyethers with linear and cyclic carbonates linkages | LiTFSI | 5.6 × 10−5 | 25 | 5.6 | 0.5 | 70 | CV | [42] |
| LiTFPFB/P(PO/EM) | LiTFPFB | 1.55 × 10−4 | 70 | 4.6 | 5 | 70 | LSV | [43] |
| CPE-(SiO2@PMMA) | LiTFSI | 8.54 × 10−5 | 60 | 4.7 | 0.5 | / | LSV | [44] |
| PEO/LDH (layer double hydroxide) | LiTFSI | 1.1 × 10−5 | 30 | 5 | 0.5 | / | LSV | [45] |
| CSE (PEO-LiClO4-PVDF/Al-LLZO) | LiClO4 | 1.73 × 10−4 | 70 | 5.25 | 2 | 70 | LSV | [46] |
| PVDF-HFP-LLZO | LiTFSI | 1.12 × 10−3 | 30 | 4.6 to 4.9 | 1 | / | LSV | [47] |
| LAGP–PEO LAGP-PEA LAGP-epoxy | LiTFSI and LiCLO4 | 1.25 × 10−4 7.4 × 10−4 8.4 × 10−4 | 25 25 25 | 4.5 | 0.1 | / | CV | [48] |
| CS(chitosan)-LiTFSI-PEO | LiTFSI | 6.8 × 10−4 | RT | 5 | 5 | / | LSV | [49] |
| LLTO-PAN-SN(succinonitrile) | LiTFSI | 2.2 × 10−3 | 30 | 5.1 | 1 | 25 | LSV | [50] |
| LSZP-PVDF | LiTFSI | 5.76 × 10−5 | 25 | 4.73 | 0.2 | RT | LSV | [51] |
| P(EGDMA-DODT) | LiTFSI | 2.7 × 10−5 | RT | 4.3 | / | 25 | CV | [52] |
| PVDF/PEO/LiClO4 | LiClO4 | 2.01 × 10−5 | 27 | 3 | 50 | RT | LSV | [53] |
| poly(ethylene oxide carbonate) | LiTFSI | 1.2 × 10−4 | 70 | 4.9 | 0.5 | 70 | LSV | [54] |
| HCPE (derived PEG SPE) | LiTFSI | 5.62 × 10−5 | RT | 5.28 | 10 | 25 | LSV | [55] |
| poly (PEGDA-PEMP-PDMS) | LiTFSI | 1.08 × 10−5 | 25 | 5 | 1 | 60 | LSV | [56] |
| Cross-linked nanoparticle-polymer composites (CNPCs) OH-PEO-SiO2 | LiTFSI | 3 × 10−3 | 25 | 5 | 1 | RT | LSV/CV | [57] |
| PEO-LATP | LiTFSI | 1.15 × 10−5 | 30 | 5 | / | 30 | CV/LSV | [58] |
| PVAc in P(VdF-HFP)-LiTFSI-EC | LiTFSI | 1.1 × 10−3 | RT | 4.7 | 0.5 | RT | LSV | [59] |
| poly(PEGDGE-PEMP-PDMS) | LiTFSI | 1.5 × 10−6 | RT | 5.3 | 0.1 | 60 | CV | [60] |
| PEG250-POSS-4PEG2k | LiTFSI | 3 × 10−4 | RT | 4 | / | 90 | CV | [61] |
| PEO/LAGP | LiTFSI | 1.6 × 10−5 | 20 | 4.5 | 0.1 | 60 | CV | [62] |
| LLTO(NF)/PEO | LiClO4 | 4.01 × 10−4 | 60 | 5.1 | 1 | 60 | LSV | [63] |
| PEO34-PC 10 wt% MA | LiTFSI | 1.3 × 10−3 | 70 | 4.9 | 0.5 | 70 | CV | [64] |
| P(SSPSILi-alt-MA)/PEO | SSPSILi | 3.08 × 10−4 | 25 | 5 | 10 | 80 | LSV | [65] |
| HSPE(polysiloxane/polyetherdiamine) | LiClO4 | 5.8 × 10−4 | 80 | 4.8 | 1 | / | LSV | [66] |
| Grafted polyrotaxane | LiTFSI | 1 × 10−4 | RT | 4.7 | 0.05 | 60 | LSV | [67] |
| NOE/PEO and LSA/PEO | LiTFSI | 5.08 × 10−5 | RT | 4.2 | 10 | / | CV | [68] |
| PEO-CuO fillers | LiTFSI | 1 × 10−4 | 30 | 4.8 | 1 | 25 | LSV | [69] |
| bis(2-methoxyethyl) ether (diglyme) | LiNO3 | / | / | 4.5 | 10 | RT | LSV | [70] |
| PEO-BaTiO3 | LiTFSI | 1.8 × 10−5 1.6 × 10−3 | 25 80 | 4.7 | 0.5 | 80 | LSV | [71] |
| PEGDGE | LiTFSI LiBF4 | 0.11 × 10−3 | RT | 5.5 | 0.1 | 60 | LSV/CV | [72] |
| PEO/TDI/PEG | LiTFSI | 0.17 × 10−3 | 60 | 5 | 0.5 | 60 | LSV/CV | [73] |
| PIL-LiTFSI-LATP | LiTFSI | 7.78 × 10−5 | 30 | 4.5 | 5 | 60 | LSV | [74] |
| Anion-regulated PEGPEA-SiO2 | LiTFSI | 2.16 × 10−5 | RT | 4.8 | 0.1 | 55 | CV | [75] |
| PEO@GF | LiTFSI | 1.9 × 10−4 | 60 | 4.9 | 0.1 | / | LSV | [76] |
| UV-PCCE | LiTFSI | 0.91 × 10−3 | RT | 4.78 | 0.1 | 25 | CV/LSV | [77] |
| (PTHF)-based SPE | LiClO4 | 2.3 × 10−4 | 60 | 4.5 | 1 | 60 | CV | [78] |
| HGO(holey graphene oxide)-PEO | LiTFSI | 6.05 × 10−4 | 60 | 5.2 | 5 | / | LSV | [79] |
| PEO:LiTFSI:SN(15%):LAO(10%) | LiTFSI | 1.36 × 10−5 | 30 | 5.2 | 10 | 60 | LSV | [80] |
| PEO-LLZTO-MMT | LiTFSI | 4.7 × 10−3 | 70 | 4.6 | 10 | / | LSV | [81] |
| cross-linked-PEO-TEGDME-TEGDMA | LiTFSI | 2.7 × 10−4 | 24 | 5.38 | 0.1 | 25 | CV/LSV | [82] |
| PEO-BaTiO3 | LiTFSI | 2.2 × 10−5 1.9 × 10−3 | 25 80 | 5 | 0.5 | 80 | LSV | [83] |
| PVA, PMP-TFSI | LiTFSI | 3 × 10−3 | 60 | 4.6 | 0.5 | / | LSV | [84] |
| NH2-PEG-NH2 | LiClO4, LiTFSI, LiBF4 | 1.9 × 10−4 | RT | 5 | 0.1 | / | LSV | [85] |
| PEO grafted polyimide (PI-g-PEO) | LiTFSI | 1 × 10−4 | 40 | 5 | 0.1 | 60 | CV/LSV | [86] |
| LLZN NWs filled PMMA-LiClO4 | LiClO4 | 2.2 × 10−5 | RT | 4.7 | 1 | 60 | LSV | [87] |
| Halloysite nanotubes (HNTs)-PEO | LiTFSI | 9.23 × 10−5 | 25 | 5.14 | 10 | 25 | LSV | [88] |
| PVDF-HFP/PEO/LAGP | LiFSI | 3.27 × 10−3 | RT | 4.9 | 1 | / | LSV | [89] |
| POSS−PEG−PIL | LiTFSI | 1.86 × 10−5 2.07 × 10−4 | 25 60 | 4.7 | 1 | 90 | LSV | [90] |
| vertically aligned LAGP- PEO | LiTFSI | 1.67 × 10−4 | RT | 4.5 | 5 | 60 | CV | [91] |
| PVDF-HFP/LiTFSI/LLZO | LiTFSI | 9.5 × 10−4 | RT | 5.2 | 0.1 | RT | LSV | [92] |
| PIL-PEO | LiTFSI | 6.12 × 10−4 | 55 | 5.44 | 1 | 55 | LSV | [93] |
| LLZO-PVDF | LiClO4 | 2.6 × 10−4 | 25 | 4.8 | 1 | 25 | LSV | [94] |
| PVA-Upy-PEG750 | LiClO4 | 1.51 × 10−4 | 60 | 5 | 0.1 | RT | LSV | [95] |
| PS-PEG-PS | LiTFSI | 1.1 × 10−3 | 70 | 4.5 | 0.1 | 70 | CV/LSV | [96] |
| (PEO) K-SPE750-Li | LiClO4 | 2.82 × 10−5 | 20 | 5.4 5.3 | 0.1 | 60 25 | LSV LSV | [97] |
| LiFPFSI/PEO | LiFPFSI | 6.2 × 10−4 | 80 | 5.6 | 0.5 | 80 | LSV | [16] |
| sPS-LiTFSI/PEGDA/succinonitrile | LiTFSI | 0.43 × 10−3 | RT | 5–5.3 | 0.5 | / | LSV/CV | [98] |
| PGO | LiClO4 | 2.08 × 10−5 | 50 | 4.4 | 1 | RT | LSV | [99] |
| PPC-PEO 10 W [5:5]-1%wt LAGP | LiTFSI | 8.39 × 10−4 | 60 | 4.5 | 0.5 | 60 | LSV | [100] |
| PVDF/PVAC-LLZTO | LiClO4 | 4.8 × 10−4 | RT | 4.85 | 0.1 | / | LSV | [101] |
| PEO-N1222FSI-LiFSI | LiFSI | 2.14 × 10−4 | 50 | 5 | 1 | 50 | LSV | [102] |
| Li-Nafion/LLZAO | / | 2.26 × 10−4 | 30 | 4.8 | 0.1 | 30 | CV/LSV | [103] |
| PPO-PEO-PPO/HO-PEO-SiO2 | LiPF6/LiTFSI | 1.32 × 10−3 | 20 | 6.5 | 1 | 20 | CV | [104] |
| Sandwich-type PVDF-HFP-LLZTO | LiTFSI | 2.29 × 10−4 | 30 | 5.3 | 1 | 40 | LSV | [105] |
| LLZTO/PEO-LiTFSI | LiTFSI | 2.61 × 10−4 | 25 | 6 | 1 | / | LSV | [106] |
| PEO-ta-POSS | LiTFSI | 1.2 × 10−3 | 90 | 3.8 | 0.2 | / | CV | [107] |
| g-C3N4/PEO (CSPE) | LiTFSI | 1.7 × 10−5 | 30 | 4.7 | 5 | 60 | LSV | [108] |
| N1222FSI-PIL | LiTFSI | 2.08 × 10−4 | 25 | 5 | 1 | 40 | LSV | [109] |
| hbPPEGMAm-s-PSn | LiTFSI | 9.5 × 10−5 | 60 | 4.3 | 0.2 | / | LSV | [110] |
| PEO-cPTFBC | LiDFOB | 2.2 × 10−5 | 50 | 4.7 | 1 | 60 | LSV | [111] |
| PEO@SiO2 | LiClO4 | 1.1 × 10−4 | 30 | 4.8 | 10 | 90 | LSV | [112] |
| LLZTO/PEO | LiTFSI | 1.31 × 10−5 | 25 | 5.2 | 1 | RT | LSV | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méry, A.; Rousselot, S.; Lepage, D.; Dollé, M. A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes. Materials 2021, 14, 3840. https://doi.org/10.3390/ma14143840
Méry A, Rousselot S, Lepage D, Dollé M. A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes. Materials. 2021; 14(14):3840. https://doi.org/10.3390/ma14143840
Chicago/Turabian StyleMéry, Adrien, Steeve Rousselot, David Lepage, and Mickaël Dollé. 2021. "A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes" Materials 14, no. 14: 3840. https://doi.org/10.3390/ma14143840
APA StyleMéry, A., Rousselot, S., Lepage, D., & Dollé, M. (2021). A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes. Materials, 14(14), 3840. https://doi.org/10.3390/ma14143840







