Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coatings
2.3. Application of the Coatings on the Wood Substrate
2.4. Characterization of the Coatings
2.4.1. pH and Relative Density
2.4.2. Shear Viscosity
2.4.3. CIELAB Color Measurements
2.4.4. Adhesion Strength
2.4.5. Surface Morphology
2.4.6. Contact Angle and Water Absorption Measurements
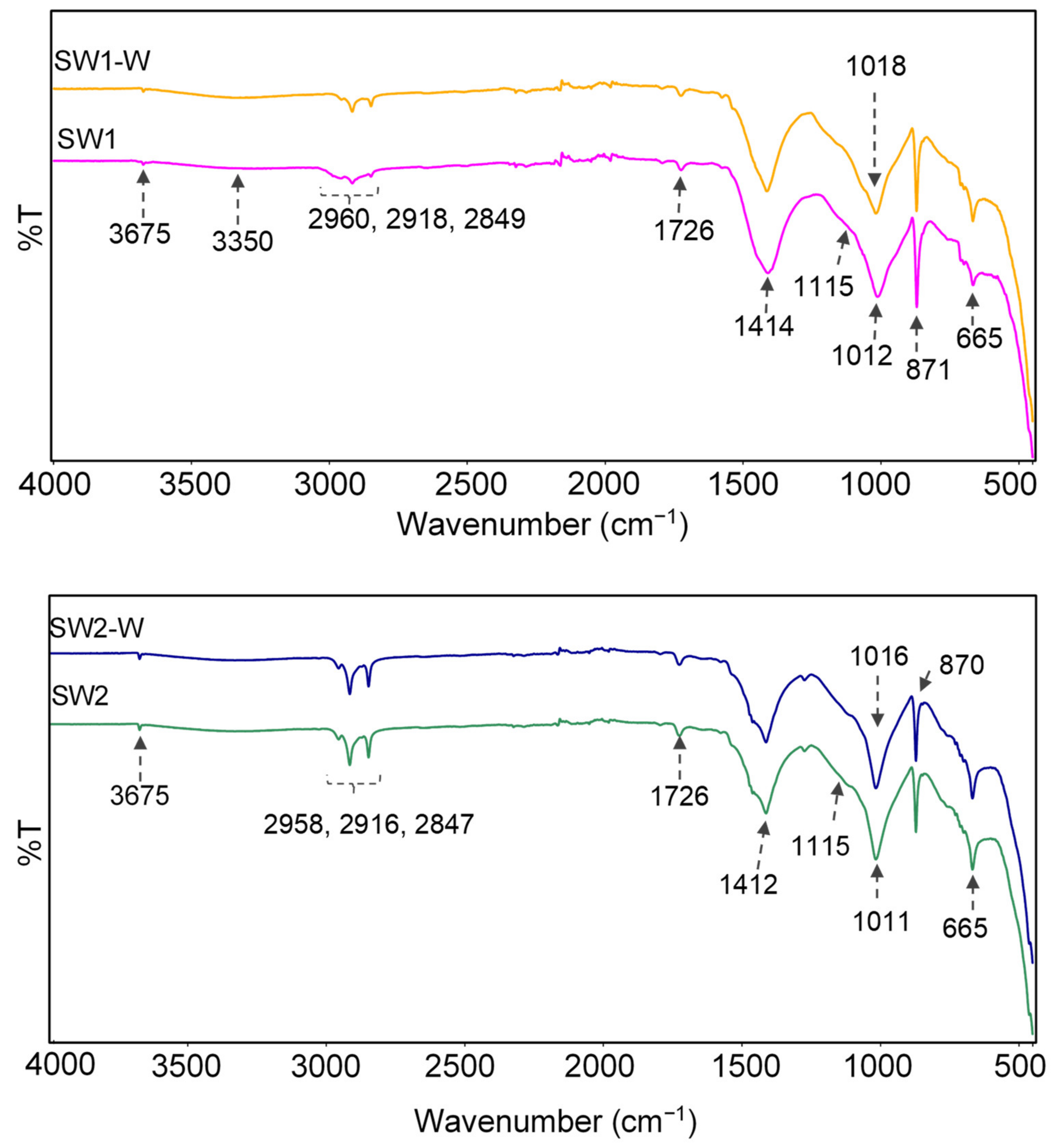
2.4.7. ATR-FTIR
3. Results and Discussion
3.1. Physicochemical Properties of the Fresh Coatings
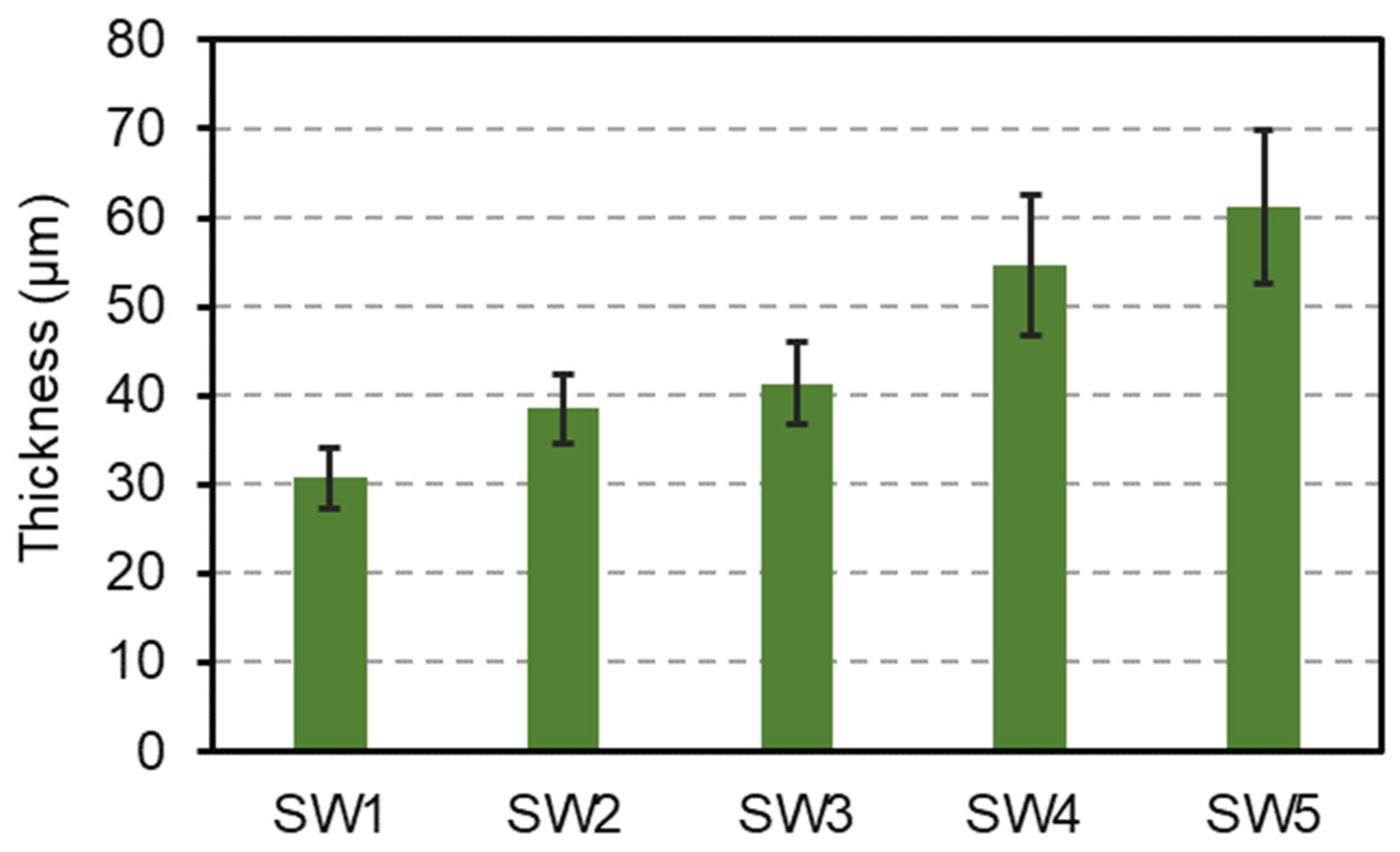
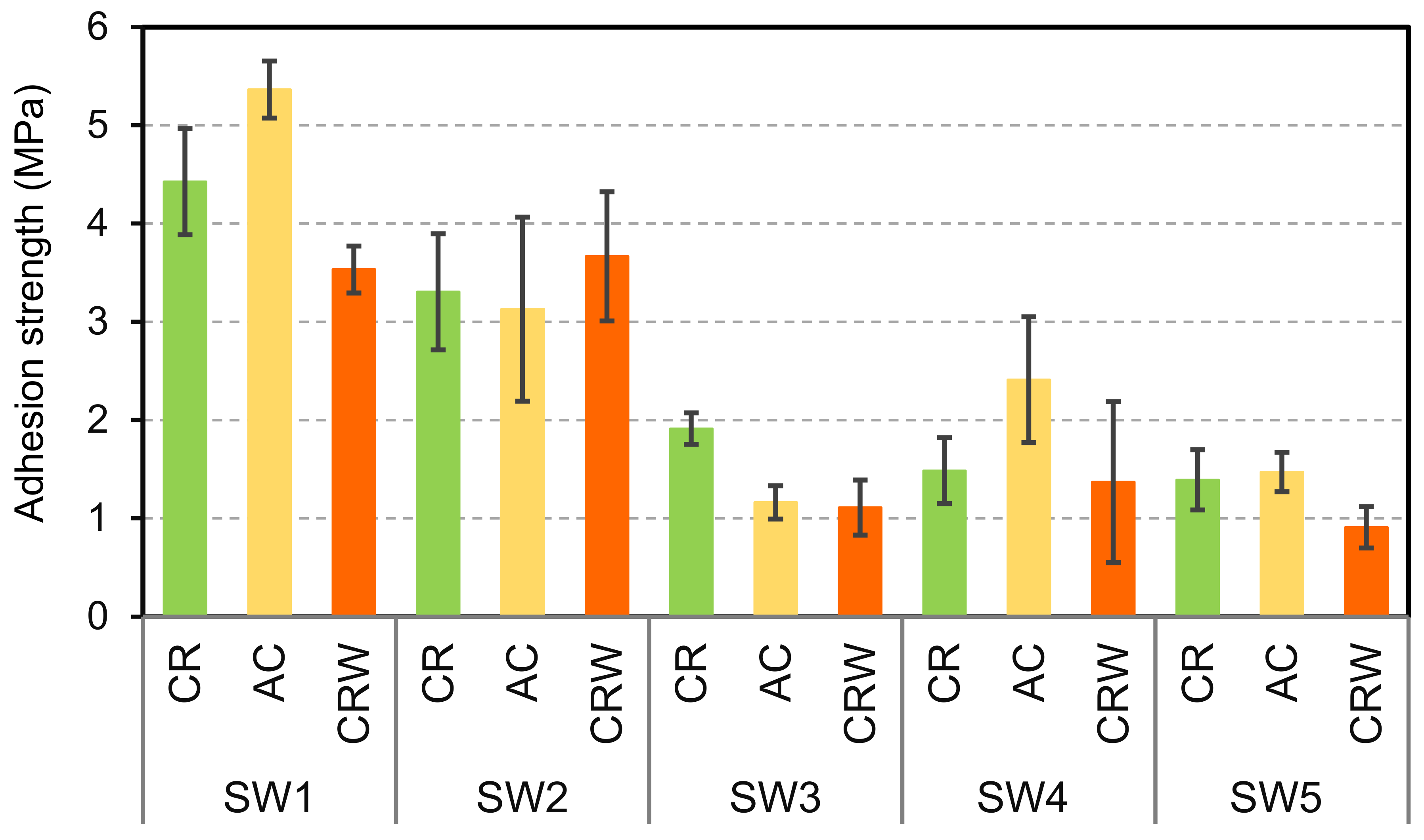
3.2. Surface Appearance, Thickness, and Adhesion of the Coatings on Wood
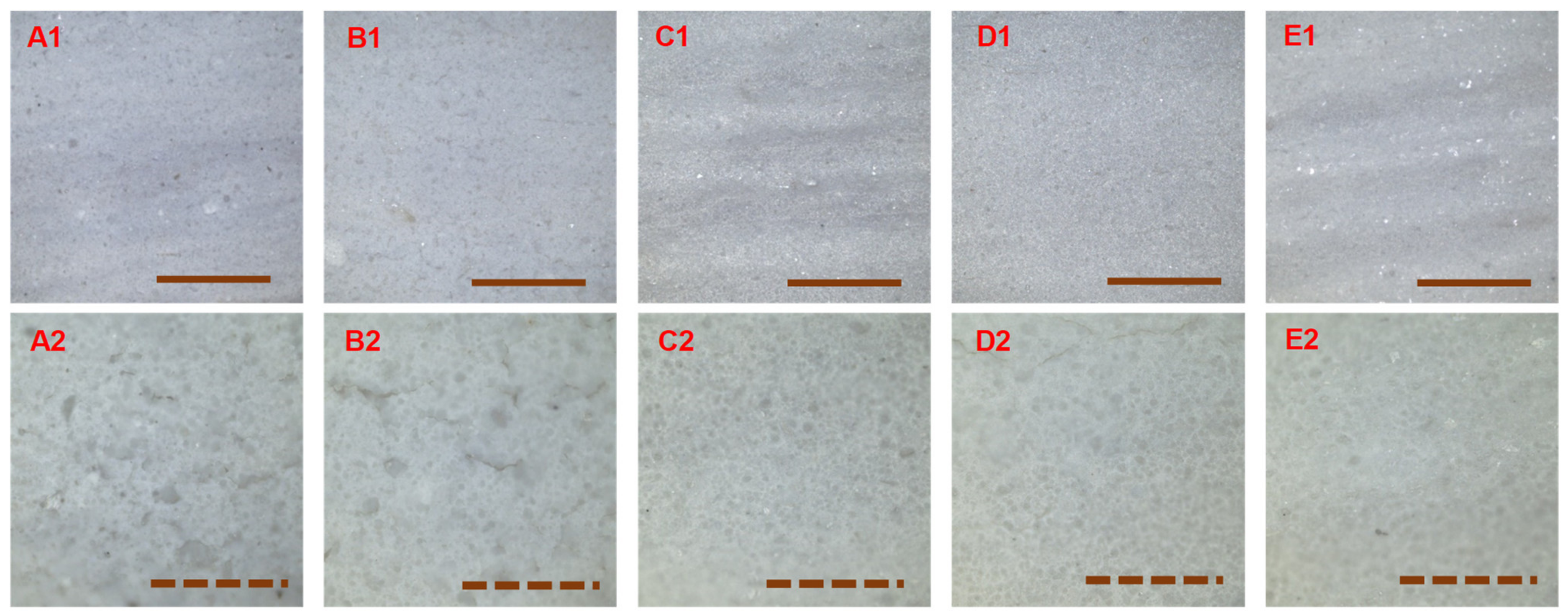
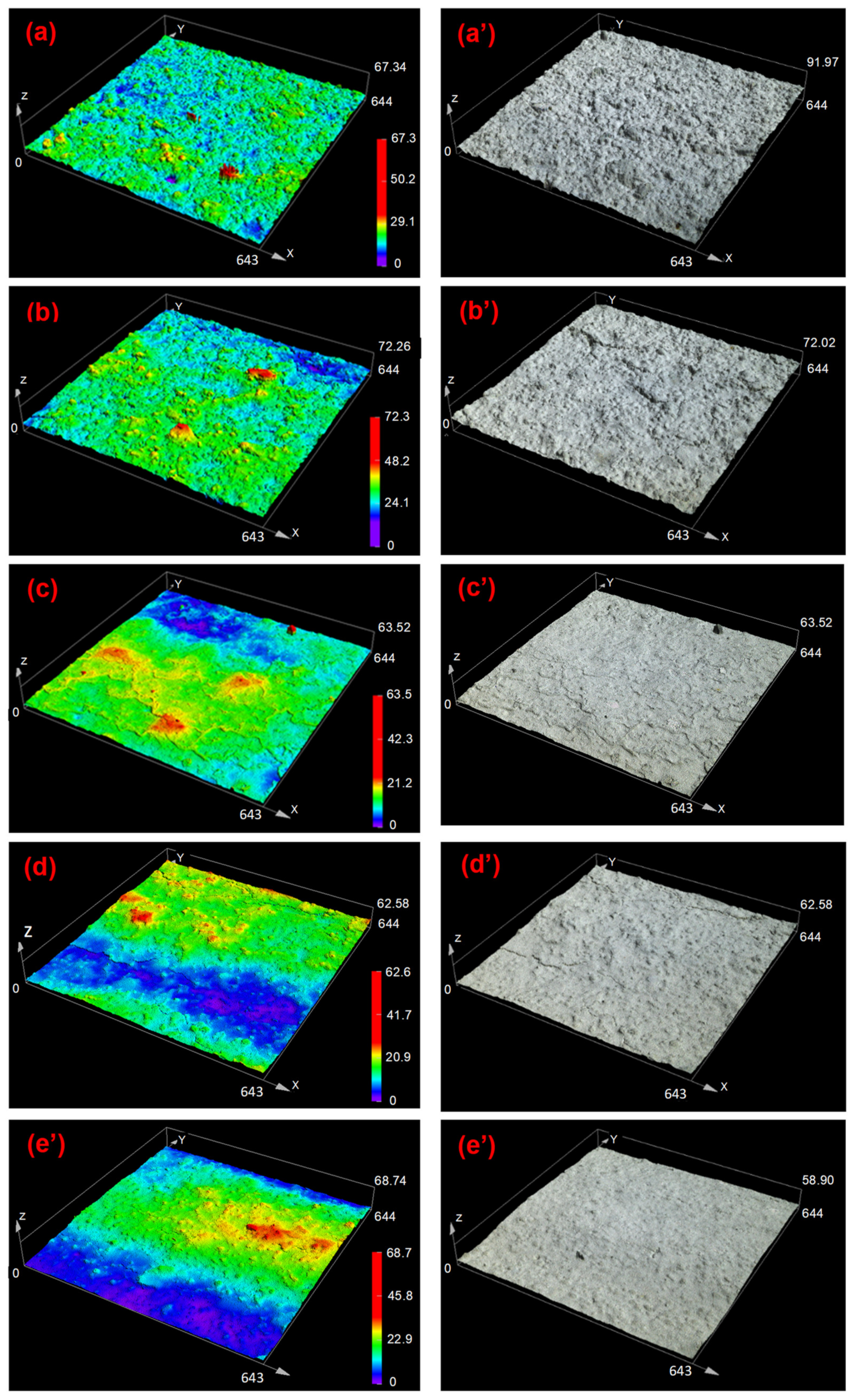
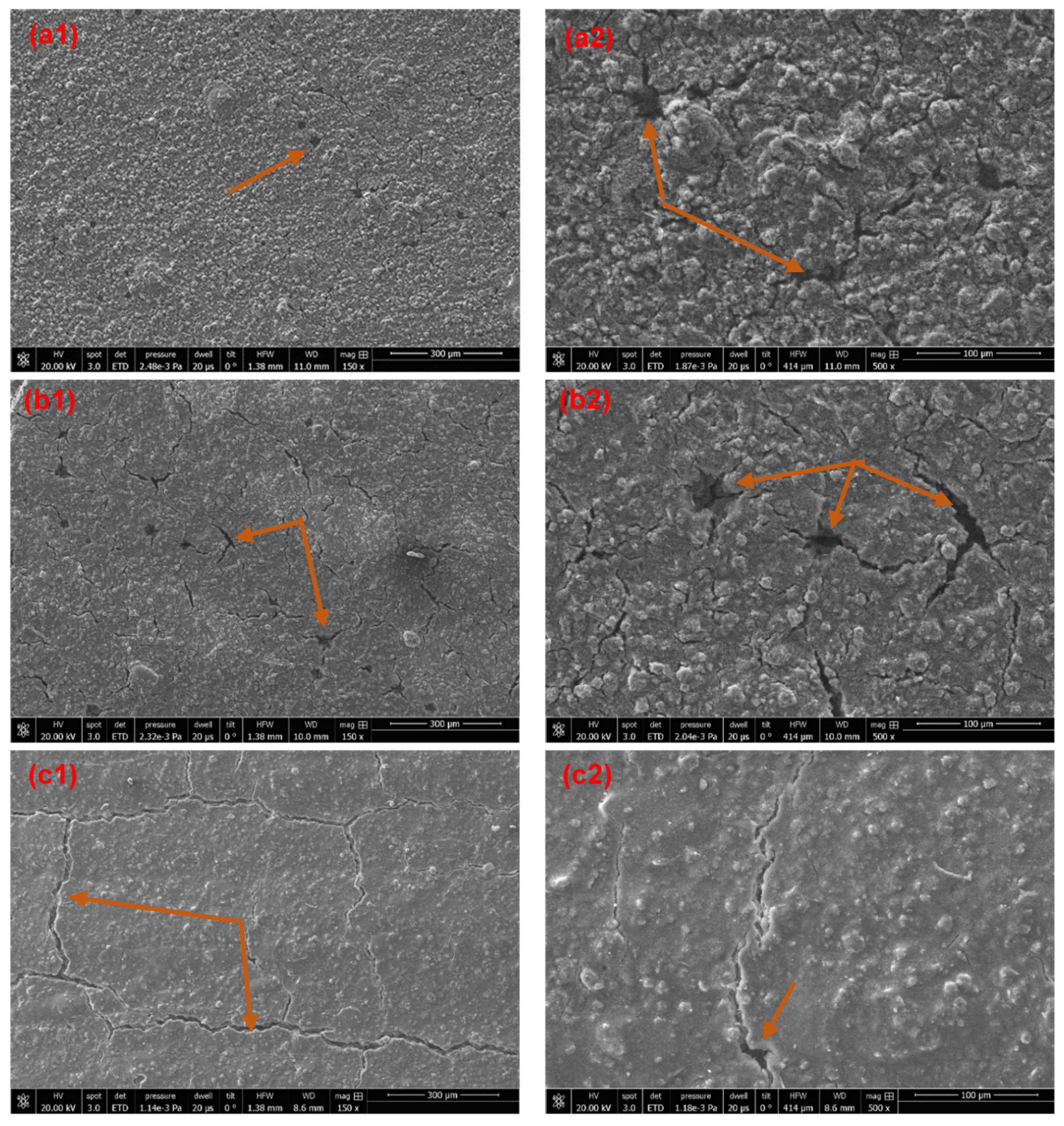
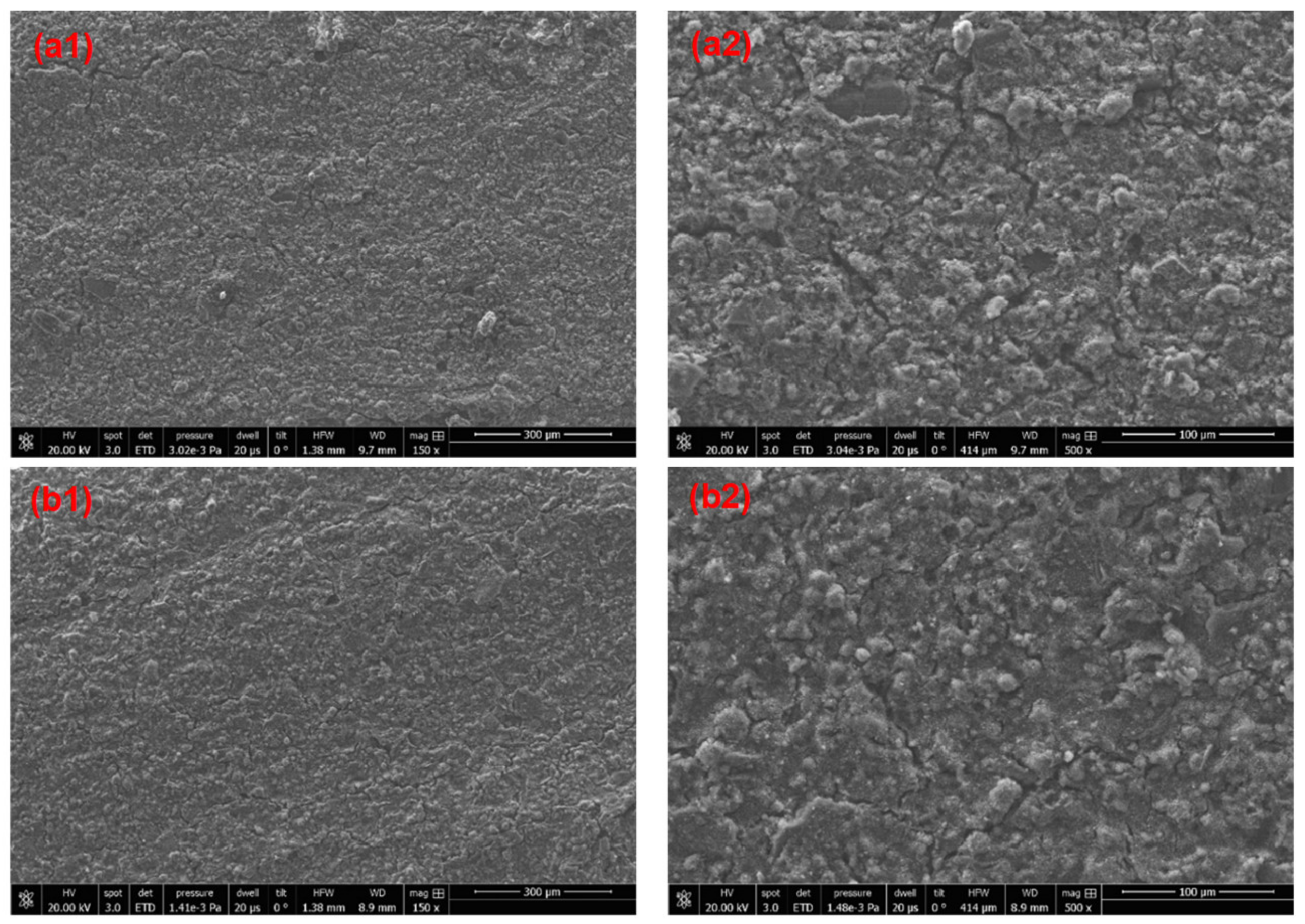
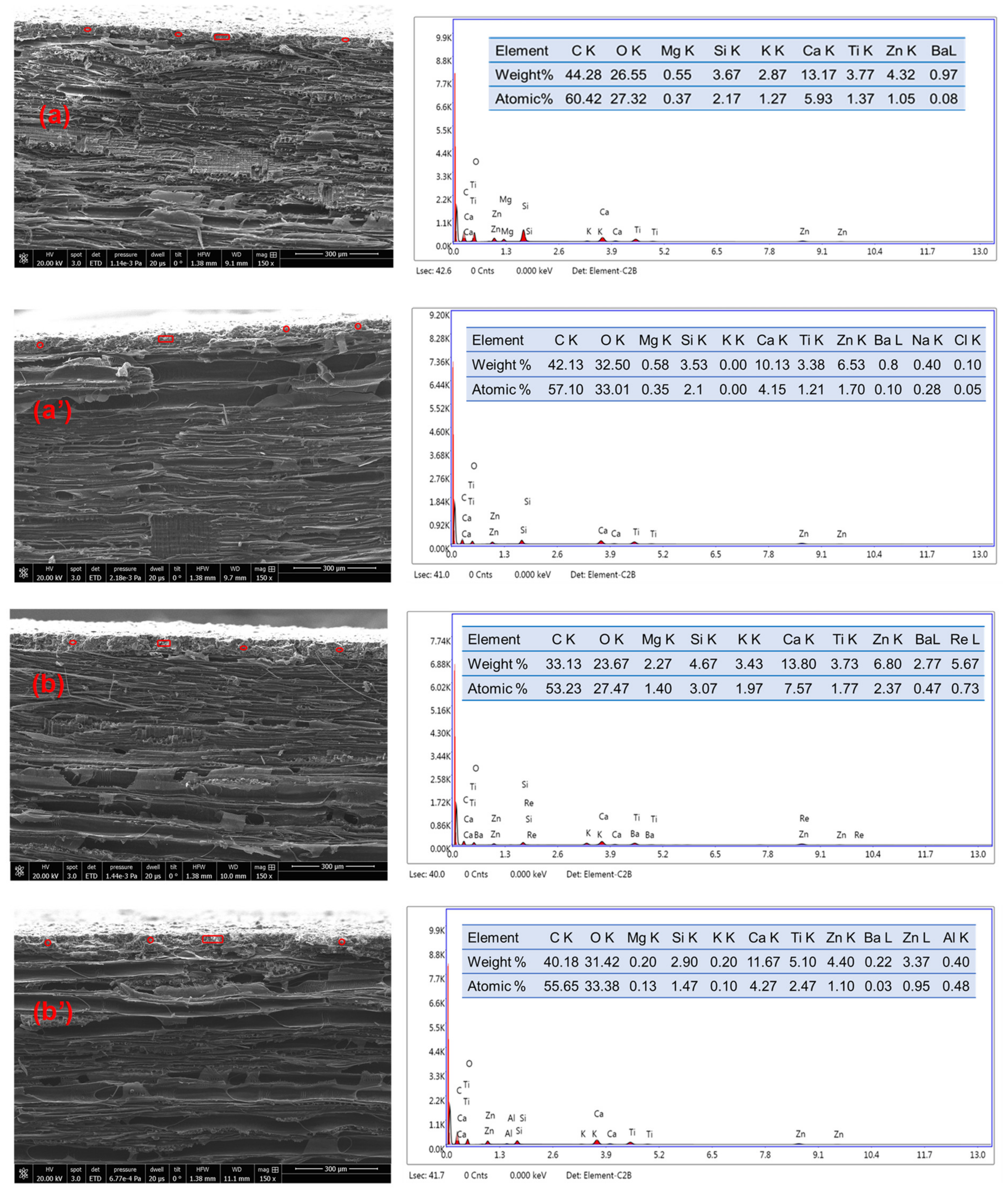
3.3. Surface Morphology of the Cured Coatings
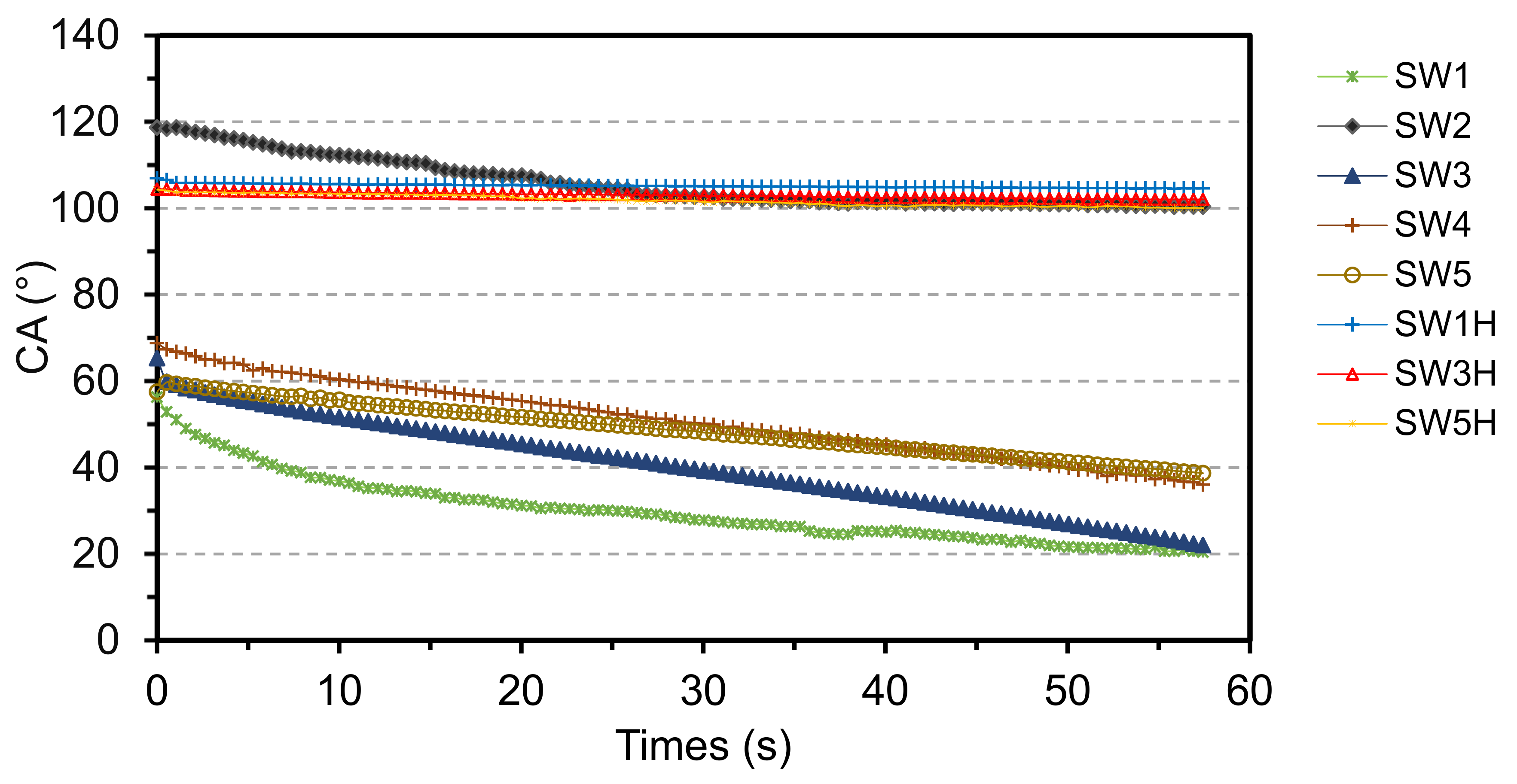
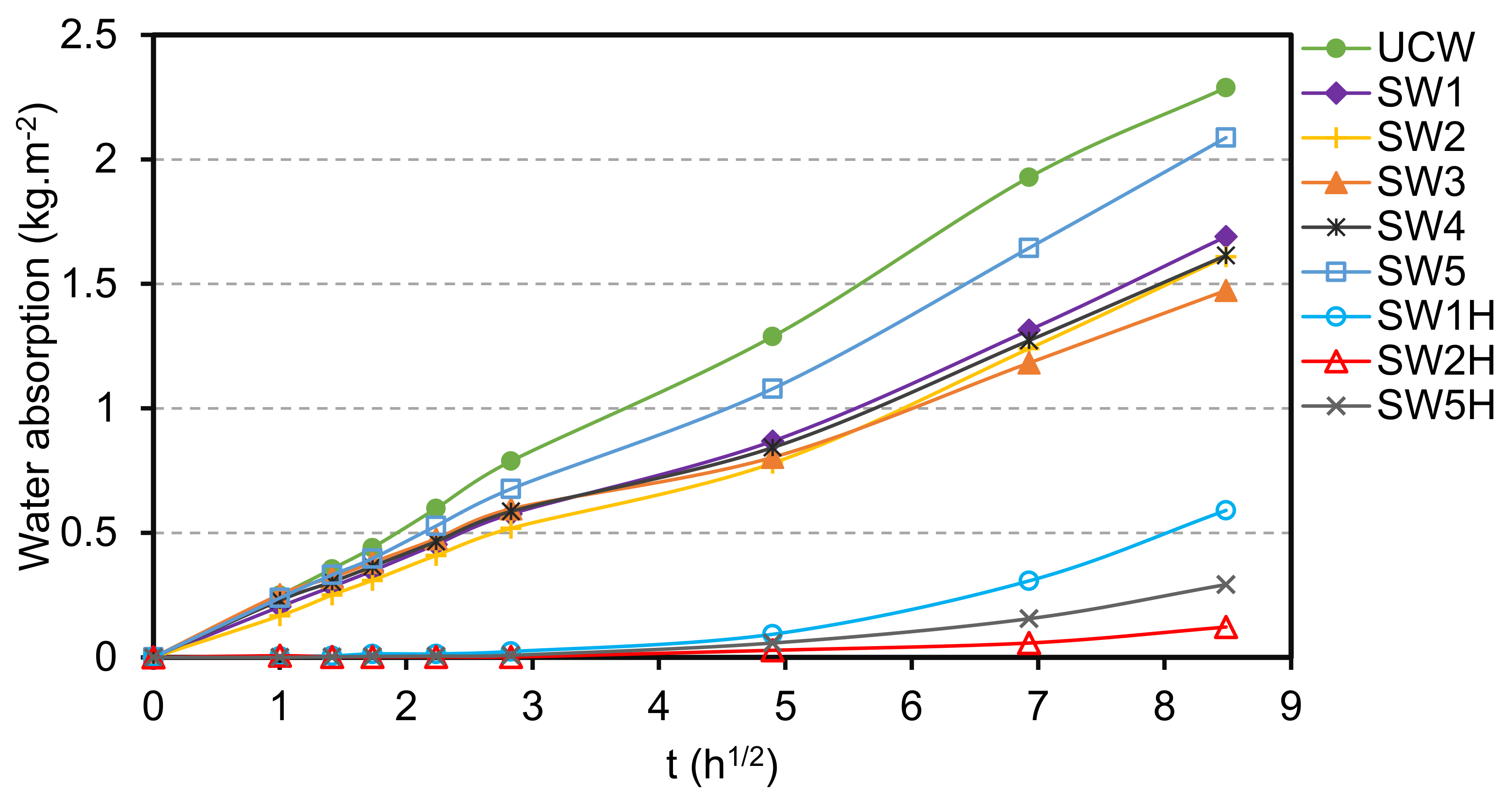
3.4. Contact Angle and Water Absorption
3.5. Improvement of Water Resistance and Adhesion
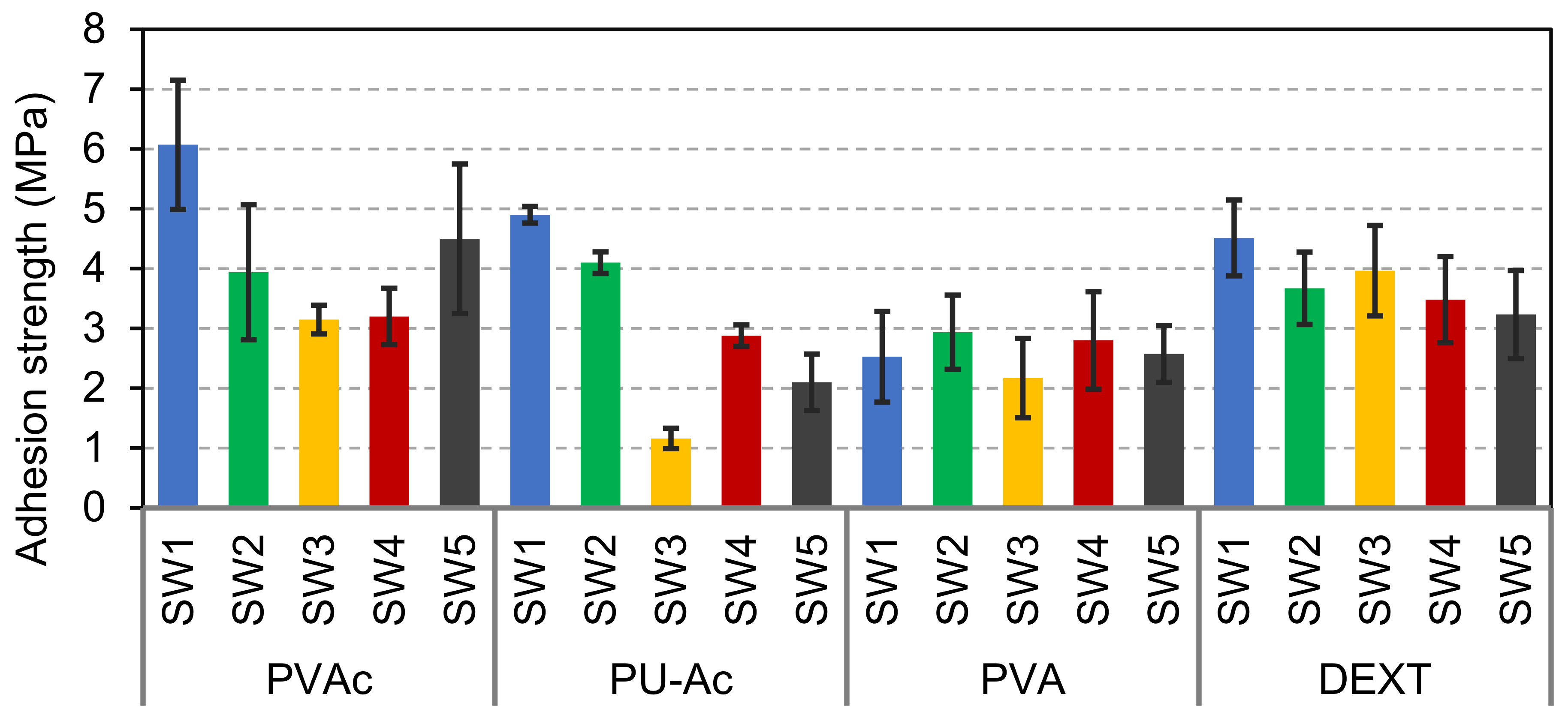
3.6. Improvement of the Adhesion of the Coatings
3.7. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraci, A.T. Waterborne Silicates in Coatings and Construction Chemicals. In Inorganic Zinc Coatings, History, Chemistry, Properties, Applications and Alternatives, 2nd ed.; The Australasian Corrosion Association Inc.: Victoria, Australia, 2013; pp. 227–258. [Google Scholar]
- Parashar, G.; Srivastava, D.; Kumar, P. Ethyl silicate binders for high performance coatings. Prog. Org. Coat. 2001, 42, 1–14. [Google Scholar] [CrossRef]
- Parashar, G.; Bajpayee, M.; Kamani, P.K. Water-borne non-toxic high-performance inorganic silicate coatings. Surf. Coat. Int. B Coat. Trans. 2003, 86, 169–246. [Google Scholar] [CrossRef]
- Stachowicz, M.; Granat, K.; Nowak, D.; Haimann, K. Effect of hardening methods of moulding sands with water glass on structure of bonding bridges. Arch. Foundry Eng. 2010, 10, 123–128. [Google Scholar]
- Zhang, X.L.; Wu, Y.Q.; Yang, S.L.; Liu, X.M. Effect of curing technology on bonding properties of silicate wood adhesive. Mater. Res. Innov. 2014, 18, 532–536. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Karevan, M. Effect of nano-silica on the mechanical properties of acrylic polyurethane coatings. Prog. Org. Coat. 2016, 101, 477–485. [Google Scholar] [CrossRef]
- Maganty, S.; Roma, M.P.; Meschter, S.J.; Starkey, D.; Gomez, M.; Edwards, D.G.; Ekin, A.; Elsken, K.; Cho, J. Enhanced mechanical properties of polyurethane composite coatings through nanosilica addition. Prog. Org. Coat. 2016, 90, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Afsharimani, N.; Durán, A.; Galusek, D.; Castro, Y. Hybrid sol–gel silica coatings containing graphene nanosheets for improving the corrosion protection of aa2024-t3. Nanomaterials 2020, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol–gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Figueira, R.B. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Lim, H.M.; Kim, S.M.; Kim, B.M.; Kim, D.S.; Lee, S.H. Effect of Colloidal Silica Contents in the Organic-Inorganic Hybrid Coatings on the Physical Properties of the Film on the Metal Surface. Solid State Phenom. 2007, 124–126, 655–658. [Google Scholar] [CrossRef]
- Chu, L.; Daniels, M.W.; Francis, L.F. Use of (Glycidoxypropyl)trimethoxysilane as a Binder in Colloidal Silica Coatings. Chem. Mater. 1997, 9, 2577–2582. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Facile Preparation of stable superhydrophobic coatings on wood surfaces using silica-polymer nanocomposites. BioResources 2015, 10, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Gettwert, G.; Rieber, W.; Bonarius, J. One-component silicate binder systems for coatings. Surf. Coat. Int. 1998, 12, 596–603. [Google Scholar] [CrossRef]
- Loganina, V.I.; Kislitsynab, S.N.; Mazhitov, Y.B. Development of sol-silicate composition for decoration of building walls. Case Stud. Constr. Mater. 2018, 9, e00173. [Google Scholar] [CrossRef]
- Loganina, V.I.; Mazhitov, Y.B.; Skachkov, Y.P. Durability of Coatings Based on Sol Silicate Paint. Defect Diffus. Forum 2019, 394, 1–4. [Google Scholar] [CrossRef]
- Hebao, T.; Chaocan, Z.; Peng, L.; Xi, L.; Lili, W.; Yanjun, C. combination of silica sol and potassium silicate via isothermal heat conduction microcalorimetry. Chin. J. Chem. 2011, 29, 356–362. [Google Scholar]
- Budunoglu, H.; Yildirimab, A.; Bayindir, M. Flexible and mechanically stable antireflective coatings from nanoporous organically modified silica colloids. J. Mater. Chem. 2012, 22, 9671. [Google Scholar] [CrossRef] [Green Version]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Nadargi, D.Y.; Latthe, S.S.; Hirashima, H.; Rao, A.V. Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater. 2009, 117, 617–626. [Google Scholar] [CrossRef]
- Parale, V.G.; Lee, K.-Y.; Park, H.-H. Flexible and transparent silica aerogels: An overview. J. Korean Ceram. Soc. 2017, 54, 184–199. [Google Scholar] [CrossRef] [Green Version]
- Kazmina, O.; Lebedeva, E.; Mitina, N.; Kuzmenko, A. Fire-proof silicate coatings with magnesium-containing fire retardant. J. Coat. Technol. Res. 2018, 15, 543–554. [Google Scholar] [CrossRef]
- Cheumani Yona, A.M.; Žigon, J.; Dahle, S.; Petrič, M. Study of the adhesion of silicate-based coating formulations on a wood substrate. Coatings 2021, 11, 61. [Google Scholar] [CrossRef]
- ISO 4624-2016 Paints and Varnishes—Pull-off Test for Adhesion; International Organization for Standardization: Geneva, Switzerland, 2016.
- EN 927-5:2006-Paints and Varnishes-Coating Materials and Coating Systems for Exterior Wood-Part 5: Assessment of the Liquid Water Permeability; Comite Europeen de Normalisation: Brussels, Belgium.
- T.A. Instruments. Rheological Characterization of Paints and Coatings. 2015. Available online: www.azom.com/article.aspx?ArticleID=12120 (accessed on 15 February 2021).
- Whittingstall, P. Paint Evaluation Using Rheology. RH-059. Available online: www.tainstruments.com/pdf/literature/RH059.pdf (accessed on 15 February 2021).
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Eccleston, G. The effect of cure temperature and humidity on the properties of solvent-borne zinc silicate coatings. J. Prot. Coat. Linings 1998, 15, 36–45. [Google Scholar]
- Jiang, J.; Cao, J.; Wang, W. Characteristics of wood-silica composites influenced by the pH value of silica sols. Holzforschung 2018, 72, 311–319. [Google Scholar] [CrossRef]
- Pries, M.; Mai, C. Fire resistance of wood treated with a cationic silica sol. Eur. J. Wood Wood Prod. 2013, 71, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Yong, Q.; Chang, J.; Liu, Q.; Jiang, F.; Wei, D.; Li, H. Matt polyurethane coatings: Correlation of surface roughness on measurement length and gloss. Polymers 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, X.; Li, F.; Lu, C. Visualization of materials using the confocal laser scanning microscopy technique. Chem. Soc. Rev. 2020, 49, 2408–2425. [Google Scholar] [CrossRef]
- Loganina, V.; Mazhitov, E.; Demyanova, V. Regularities in the Formation of the Structure and Properties of Coatings Based on Silicate Paint Sol. In 14th International Congress for Applied Mineralogy (ICAM2019), Proceedings of the ICAM 2019, Belgorod, Russia, 23–27 September 2019; Glagolev, S., Ed.; Springer Proceedings in Earth and Environmental Sciences; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H. Evaluation of the surface roughness of a standard abraded dental porcelain following different polishing techniques. J. Dent. Sci. 2012, 7, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.Y.; Patanen, M.; Kauppinen, S.; Kosonen, H.; Ristolainen, M.; Hall, S.A.; Liimatainen, H. Surface analysis of tissue paper using laser scanning confocal microscopy and micro-computed topography. Cellulose 2020, 27, 8989–9003. [Google Scholar] [CrossRef]
- Bentz, D.P. Early-age cracking review: Mechanisms, material properties, and mitigation strategies. In Current Understanding of the Parameters that Influence Hydraulic and Leaching Properties and Uncertainty Analysis of Cementitious Barriers; Langton, C., Ed.; DOE-CBP Report 901945; U.S. Department of Energy: Washington, DC, USA, 2009. [Google Scholar]
- Bentur, A.; Kovler, K. Evaluation of early age cracking characteristics in cementitious systems. Mater. Struct. 2003, 36, 183–190. [Google Scholar] [CrossRef]
- Safiuddin, M.; Amrul Kaish, A.B.M.; Woon, C.-O.; Raman, S.N. Early-age cracking in concrete: Causes, consequences, remedial measures, and recommendations. Appl. Sci. 2018, 8, 1730. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Qin, Y.; Lin, Z.; Tian, Z.; Cui, X. One-Part Plastic Formable inorganic coating obtain from alkali-activated slag/starch (cms) hybrid composites. Molecules 2020, 25, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications, a review article. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef] [Green Version]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and RSi(OR)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Bobrowski, A.; Stypuła, B.; Hutera, B.; Kmita, A.; Drożyński, D.; Starowicz, M. Ftir spectroscopy of water glass-the binder moulding modified by ZnO nanoparticles. Metalurgija 2012, 51, 477–480. [Google Scholar]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2016, 52, 507–540. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. Cryst. Eng. Comm. 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Gridchin, A.M.; Loganina, V.I.; Mazhitov, E.B.; Ryapukhin, A.N. Assessment of the durability of coatings based on sol silicate paint. In Innovations and Technologies in Construction. BUILDINTECH BIT 2020; Klyuev, S., Lesovik, V., Vatin, N., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2020; Volume 95. [Google Scholar] [CrossRef]
- Loganina, V.; Mazhitov, E. Building and architecture paints and coatings. Intechopen 2019. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Wang, Y.; Liu, Z.; Li, S. A study on the properties and working mechanism of a waterborne polyurethane-modified silicate-based coating. RSC Adv. 2019, 9, 26817–26824. [Google Scholar] [CrossRef] [Green Version]



| Binder | Sty-Acr | CaCO3 | ZnO | Talc | TiO2 | BD20 | BQ40 | BA11 | HEC | DeF | Water |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37.5 | 8 | 16 | 6 | 6 | 5 | 0.2 | 0.6 | 0.3 | 0.2 | 0.2 | 20 |
| Binder | Formulation (wt %) a and Properties | Coating Designation b | ||||
|---|---|---|---|---|---|---|
| KSi | MeKSi | Ludox® AS 40 | pH | Density (Kg m−3) | ||
| Binder 1 | 100 | / | / | 12.13 | 1.31 | SW1 |
| Binder 2 | 75 | 25 | / | 12.41 | 1.28 | SW2 |
| Binder 3 | 25 | / | 75 | 11.49 | 1.26 | SW3 |
| Binder 4 | / | 25 | 75 | 12.26 | 1.26 | SW4 |
| Binder 5 | / | 15 | 85 | 11.91 | 1.24 | SW5 |
| Coating | pH (1 h) | pH (24 h) | Density (kg m−3) * | Viscosity at Various Shear Rate (Pa·s) * | ||
|---|---|---|---|---|---|---|
| 0.1 s−1 | 1 s−1 | 1000 s−1 | ||||
| SW1 | 12.05 | 11.95 | 1.42 | 1119.5 | 90.5 | 0.16 |
| SW2 | 11.91 | 11.84 | 1.41 | 632.7 | 62.3 | 0.23 |
| SW3 | 11.42 | 11.26 | 1.41 | 1545.2 | 26.4 | 0.08 |
| SW4 | 11.40 | 11.27 | 1.40 | 1557.4 | 91.8 | 0.05 |
| SW5 | 11.07 | 10.91 | 1.41 | 850.9 | 10.6 | 0.16 |
| Sample | L* ‡ | a* ‡ | b* ‡ |
|---|---|---|---|
| Uncoated wood | 74.41(0.99) | 6.06(0.22) | 16.89(0.33) |
| SW1 | 88.68(0.88) | 0.23(0.13) | −1.03(0.25) |
| SW2 | 90.65(1.17) | −0.15(0.12) | −0.80(0.24) |
| SW3 | 92.85(0.15) | 0.11(0.07) | 0.36(0.05) |
| SW4 | 91.92(0.70) | 0.07(0.12) | 0.93(0.38) |
| SW5 | 92.50(0.40) | 0.07(0.09) | 1.00(0.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheumani Yona, A.M.; Žigon, J.; Ngueteu Kamlo, A.; Pavlič, M.; Dahle, S.; Petrič, M. Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood. Materials 2021, 14, 3559. https://doi.org/10.3390/ma14133559
Cheumani Yona AM, Žigon J, Ngueteu Kamlo A, Pavlič M, Dahle S, Petrič M. Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood. Materials. 2021; 14(13):3559. https://doi.org/10.3390/ma14133559
Chicago/Turabian StyleCheumani Yona, Arnaud Maxime, Jure Žigon, Alexis Ngueteu Kamlo, Matjaž Pavlič, Sebastian Dahle, and Marko Petrič. 2021. "Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood" Materials 14, no. 13: 3559. https://doi.org/10.3390/ma14133559
APA StyleCheumani Yona, A. M., Žigon, J., Ngueteu Kamlo, A., Pavlič, M., Dahle, S., & Petrič, M. (2021). Preparation, Surface Characterization, and Water Resistance of Silicate and Sol-Silicate Inorganic–Organic Hybrid Dispersion Coatings for Wood. Materials, 14(13), 3559. https://doi.org/10.3390/ma14133559









