Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Preparation of Calcium Carbonate (CaCO3) Particles
2.2. Characterization of Crystal State of CaCO3 Particles by Powder X-ray Diffraction
2.3. Morphological Studies Using Electron Microscopy
2.4. Inductively-Coupled Plasma Optical Emission Spectroscopy
3. Results
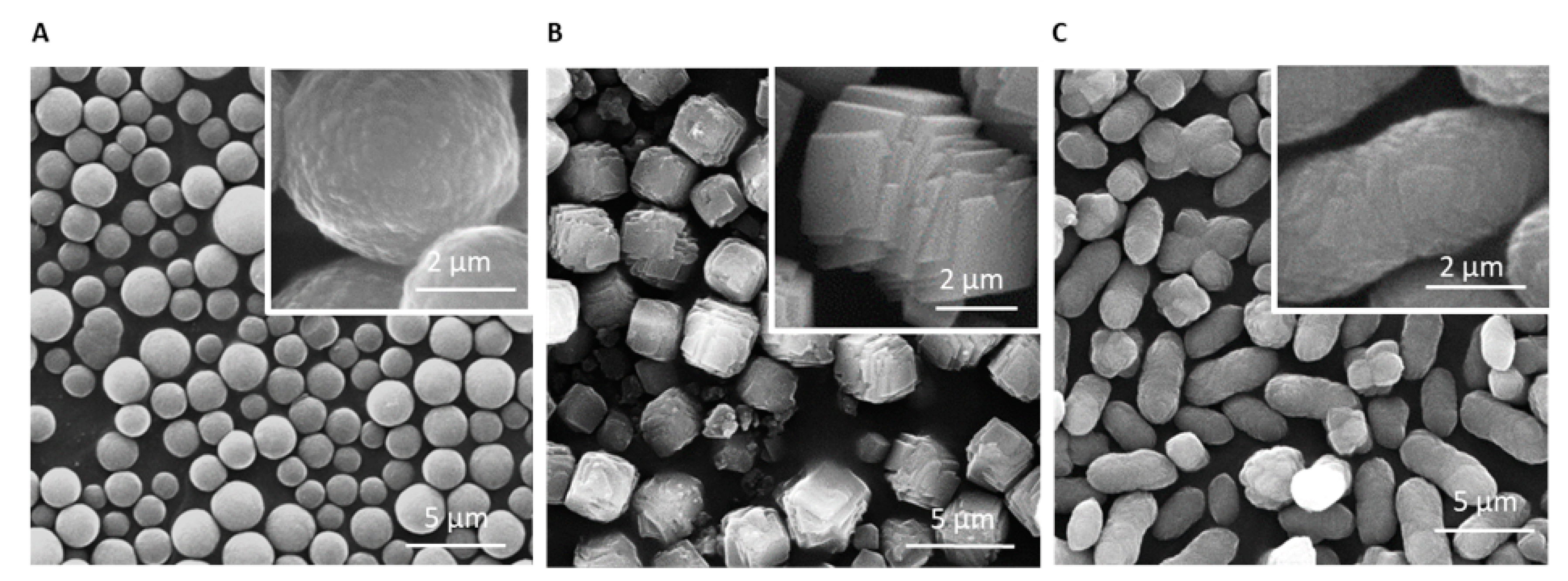
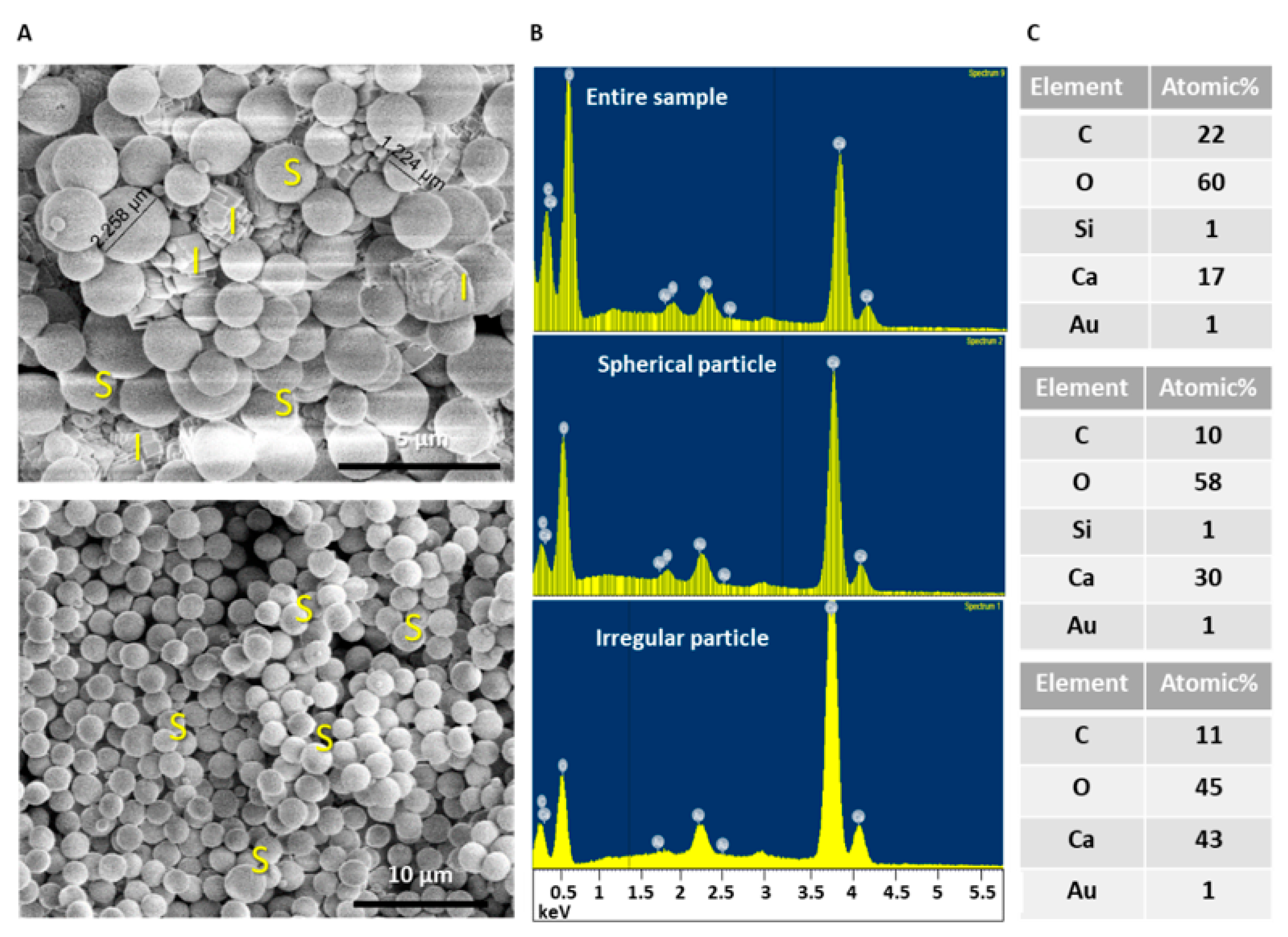
3.1. Analyzing Morphology and Composition of Calcium Carbonate Samples
3.2. Addition of Silicon-Containing Additives and their Effect on Calcium Carbonate Particles
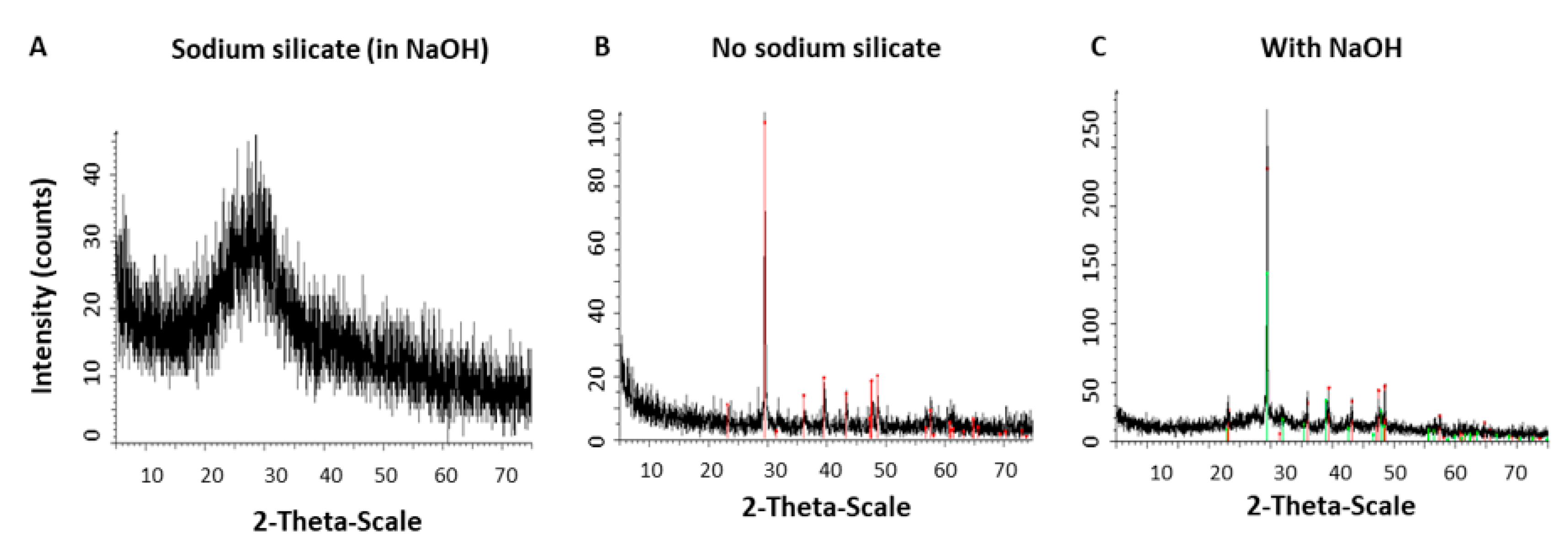
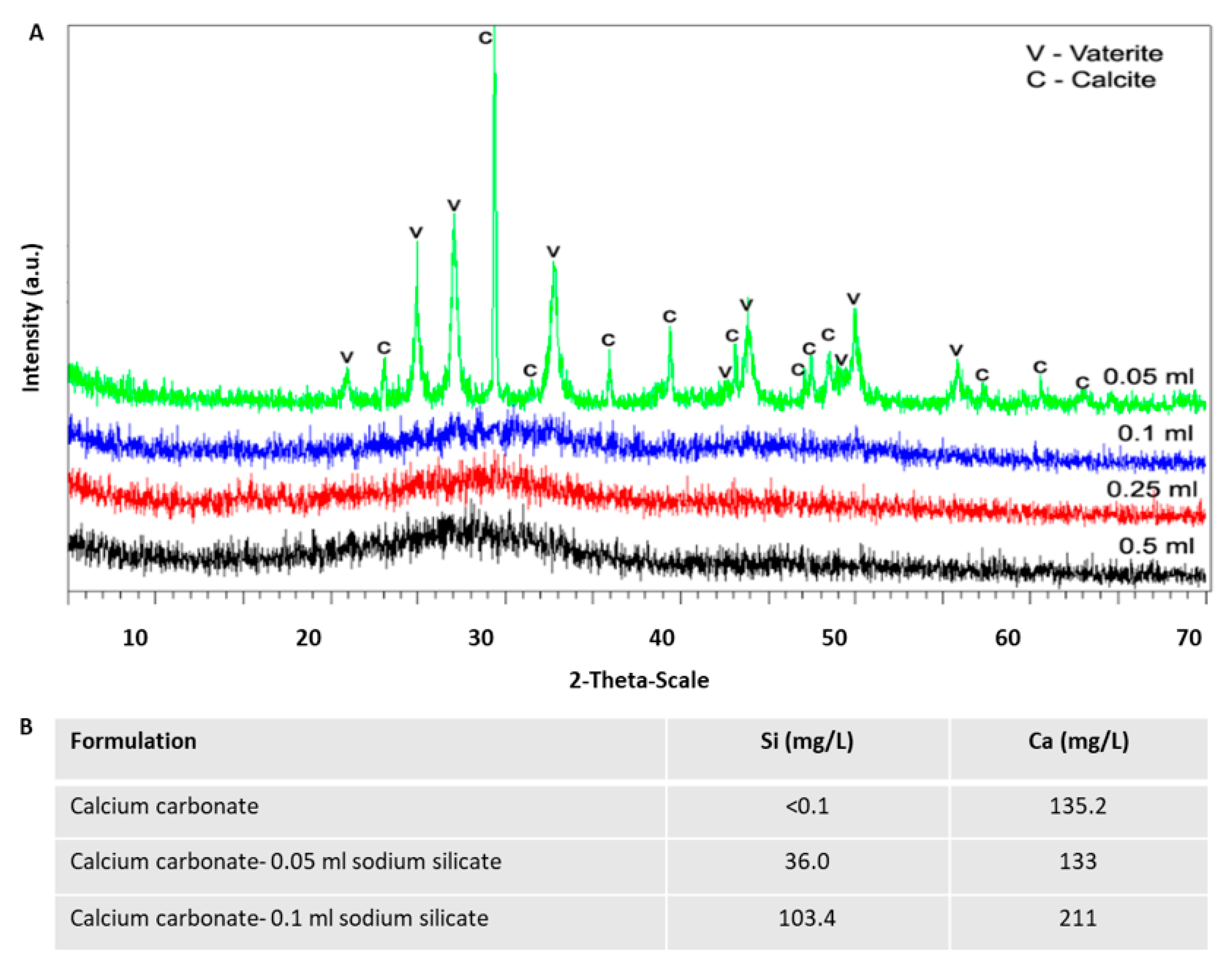
3.3. Crystalline State of Calcium Carbonate with Various Content of Silicate
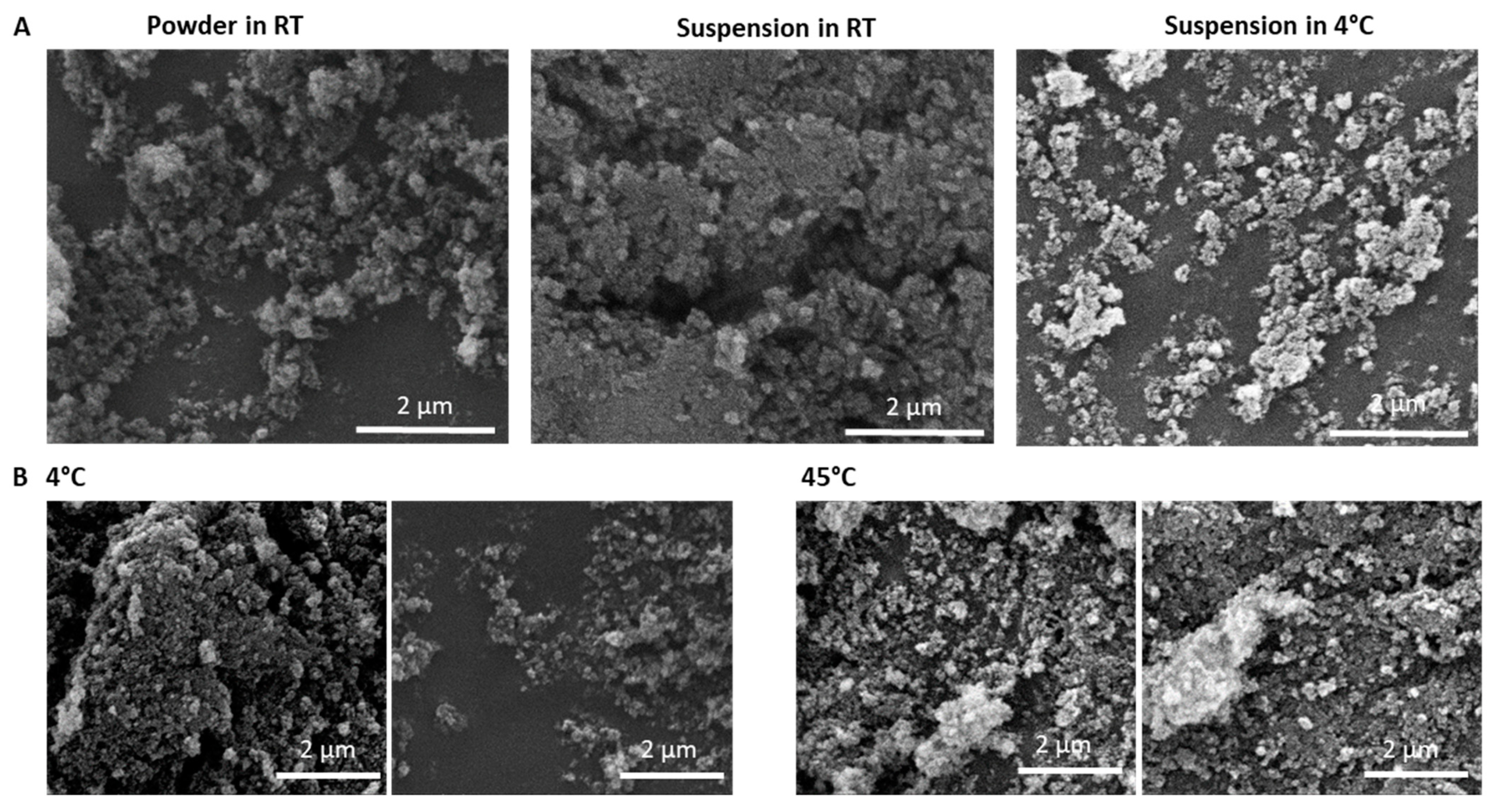
3.4. The Effect of CMC and Product Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of Micro and Nano-Sized Calcium Carbonate Particles and Their Applications. J. Mater. Chem. A 2014, 7, 14270–14288. [Google Scholar] [CrossRef]
- Chan, K.Y.; Heenan, D.P. Effect of Lime (CaCO3) Application on Soil Structural Stability of a Red Earth. Aust. J. Soil Res. 1998, 36, 73–86. [Google Scholar] [CrossRef]
- Sang, H.C.; Jin, K.P.; Seung, K.L.; Sung, M.J.; Im, H.K.; Ji, W.A.; Hwan, K. Synthesis of Precipitated Calcium Carbonate Using a Limestone and Its Application in Paper Filler and Coating Color. Mater. Sci. Forum 2007, 10, 881–884. [Google Scholar]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The Role of Calcium Carbonate in Cement Hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Nicar, M.J.; Pak, C.Y.C. Calcium Bioavailability from Calcium Carbonate Andcalcium Citrate. J. Clin. Endocrinol. Metab. 1985, 61, 391–393. [Google Scholar] [CrossRef]
- Mortensen, L.; Charles, P. Bioavailability of Calcium Supplements and the Effect of Vitamin D: Comparisons between Milk, Calcium Carbonate, and Calcium Carbonate plus Vitamin D. Am. J. Clin. Nutr. 1996, 63, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Decktor, D.L.; Robinson, M.; Maton, P.N.; Lanza, F.L.; Gottlieb, S. Effects of Aluminum/Magnesium Hydroxide and Calcium Carbonate on Esophageal and Gastric pH in Subjects with Heartburn. Am. J. Ther. 1995, 2, 546–552. [Google Scholar] [CrossRef]
- Peck, G.E.; Soh, J.L.P.; Morris, K.R.; Augsburger, L.L.; Hoag, S.W.; Lachman, L.; Lieberman, H.A.; Schwartz, J.B. Pharmaceutical Dosage Forms; Informa Healthcare: Boca Raton, FL, USA, 2008; Volume 3. [Google Scholar] [CrossRef]
- De Villiers, M.M.; Otto, D.P.; Lvov, Y.M. Application of Electrostatic Layer-by-Layer Nanocoating in Drug Delivery. Am. Pharm. Rev. 2012, 3, 2304. [Google Scholar]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.H.; Zhao, Y. A Preloaded Amorphous Calcium Carbonate/doxorubicin@silica Nanoreactor for pH-Responsive Delivery of an Anticancer Drug. Angew. Chem. Int. Ed. 2015, 54, 919–922. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Volodkin, D.V.; Günther, A.M.; Petrov, A.I.; Shenoy, D.B.; Möhwald, H. Porous Calcium Carbonate Microparticles as Templates for Encapsulation of Bioactive Compounds. J. Mater. Chem. 2004, 2073–2081. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Z.; Gao, C.; Shen, J. Assembly of Multilayer Microcapsules on CaCO3 particles from Biocompatible Polysaccharides. J. Biomater. Sci. Polym. Ed. 2006, 17, 997–1014. [Google Scholar] [CrossRef] [Green Version]
- Abebe, M.; Hedin, N.; Bacsik, Z. Spherical and Porous Particles of Calcium Carbonate Synthesized with Food Friendly Polymer Additives. Cryst. Growth Des. 2015, 15, 3609–3616. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 3358–3393. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, P.; Wang, Y.; Du, J.; Zhou, Q.; Zhu, Z.; Yang, X.; Yuan, J. Biocompatibility of Porous Spherical Calcium Carbonate Microparticles on Hela Cells. World J. Nano Sci. Eng. 2012, 2, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Kamba, A.S.; Ismail, M.; Azmi Tengku Ibrahim, T.; Zakaria, Z.A.B. Biocompatibility of Bio Based Calcium Carbonate Nanocrystals Aragonite Polymorph on Nih 3T3 Fibroblast Cell Line. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 31–38. [Google Scholar] [CrossRef]
- Kuusisto, J.E.; Maloney, T.C. The Effect of Carbonation Conditions on the Properties of Carbohydrate-Calcium Carbonate Hybrid Pigments. BioResources 2015, 10, 3277–3292. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Lei, M.; Cheng, B.; Zhao, X. Effects of PAA Additive and Temperature on Morphology of Calcium Carbonate Particles. J. Solid State Chem. 2004, 177, 681–689. [Google Scholar] [CrossRef]
- Westin, K.J.; Rasmuson, A.C. Precipitation of Calcium Carbonate in the Presence of Citrate and EDTA. Desalination 2003, 159, 107–118. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, T.; Brannon-Peppas, L. Micro-and Nanofabrication Methods in Nanotechnological Medical and Pharmaceutical Devices. Int. J. Nanomed. 2006, 483–495. [Google Scholar] [CrossRef]
- Yadav, K.S.; Chuttani, K.; Mishra, A.K.; Sawant, K.K. Effect of Size on the Biodistribution and Blood Clearance of Etoposide-Loaded PLGA Nanoparticles. PDA J. Pharm. Sci. Technol. 2011, 65, 131–139. [Google Scholar] [PubMed]
- Jennings, L.; Ivashchenko, O.; Marsman, I.J.C.; Laan, A.C.; Denkova, A.G.; Waton, G.; Beekman, F.J.; Schosseler, F.; Mendes, E. In Vivo Biodistribution of Stable Spherical and Filamentous Micelles Probed by High-Sensitivity SPECT. Biomater. Sci. 2016, 4, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Sha, X.; Guo, J.; Chen, Y.; Fang, X. Effect of Phospholipid Composition on Pharmacokinetics and Biodistribution of Epirubicin Liposomes. J. Liposome Res. 2012, 22, 80–88. [Google Scholar] [CrossRef]
- Aizenberg, J.; Lambert, G.; Weiner, S.; Addadi, L. Factors Involved in the Formation of Amorphous and Crystalline Calcium Carbonate: A Study of an Ascidian Skeleton. J. Am. Chem. Soc. 2002. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Lababidi, H.M.S.; Al-Adwani, H.; Gleason, K.K. A Review of Heterogeneous Nucleation of Calcium Carbonate and Control Strategies for Scale Formation in Multi-Stage Flash (MSF) Desalination Plants. Desalination 2018, 75–88. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minkowicz, L.; Dagan, A.; Uvarov, V.; Benny, O. Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate. Materials 2021, 14, 3525. https://doi.org/10.3390/ma14133525
Minkowicz L, Dagan A, Uvarov V, Benny O. Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate. Materials. 2021; 14(13):3525. https://doi.org/10.3390/ma14133525
Chicago/Turabian StyleMinkowicz, Lior, Arie Dagan, Vladimir Uvarov, and Ofra Benny. 2021. "Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate" Materials 14, no. 13: 3525. https://doi.org/10.3390/ma14133525
APA StyleMinkowicz, L., Dagan, A., Uvarov, V., & Benny, O. (2021). Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate. Materials, 14(13), 3525. https://doi.org/10.3390/ma14133525




