Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection
Abstract
:1. Introduction
2. Materials and Methods
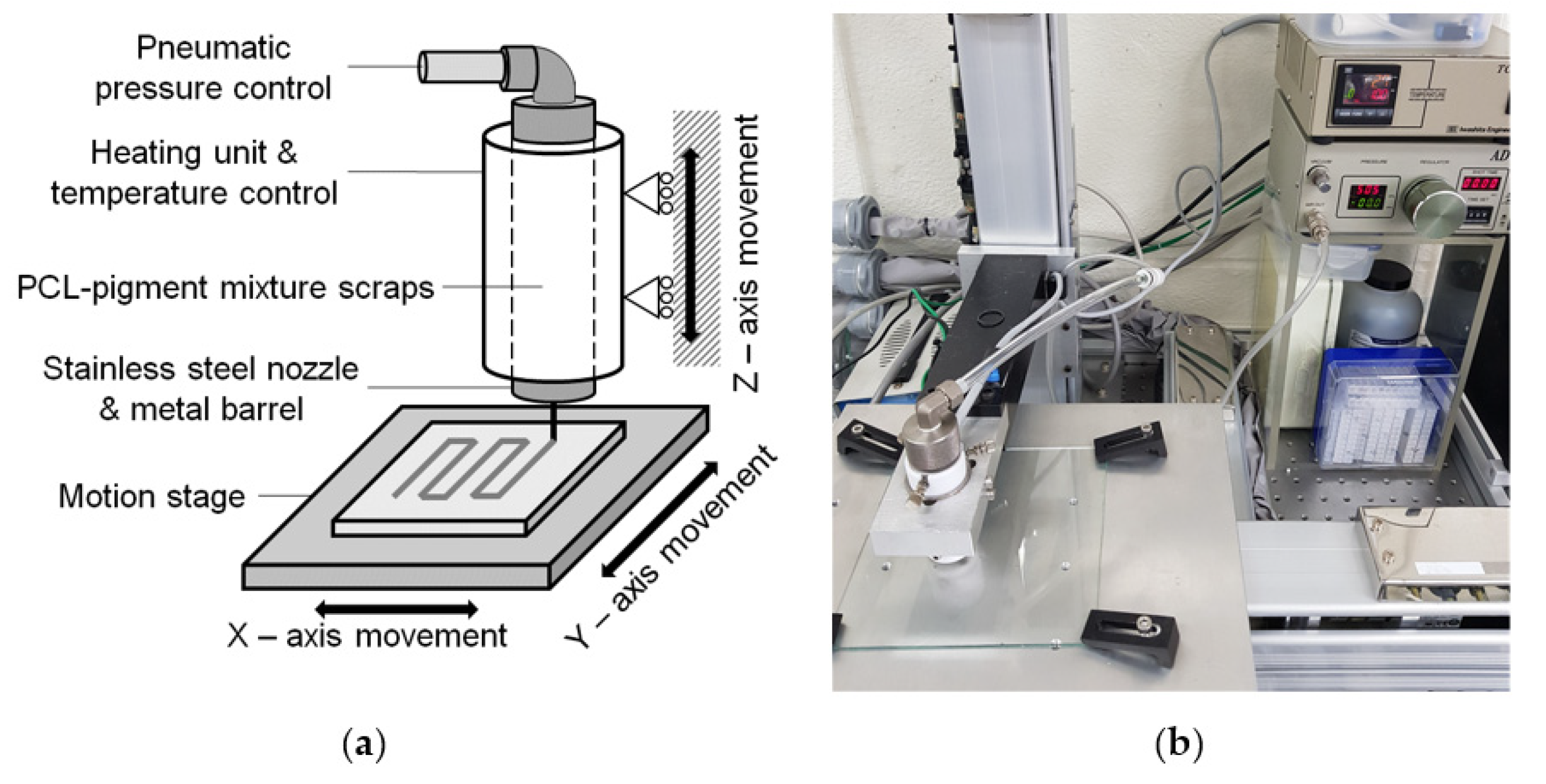
2.1. Materials and Experimental Equipment
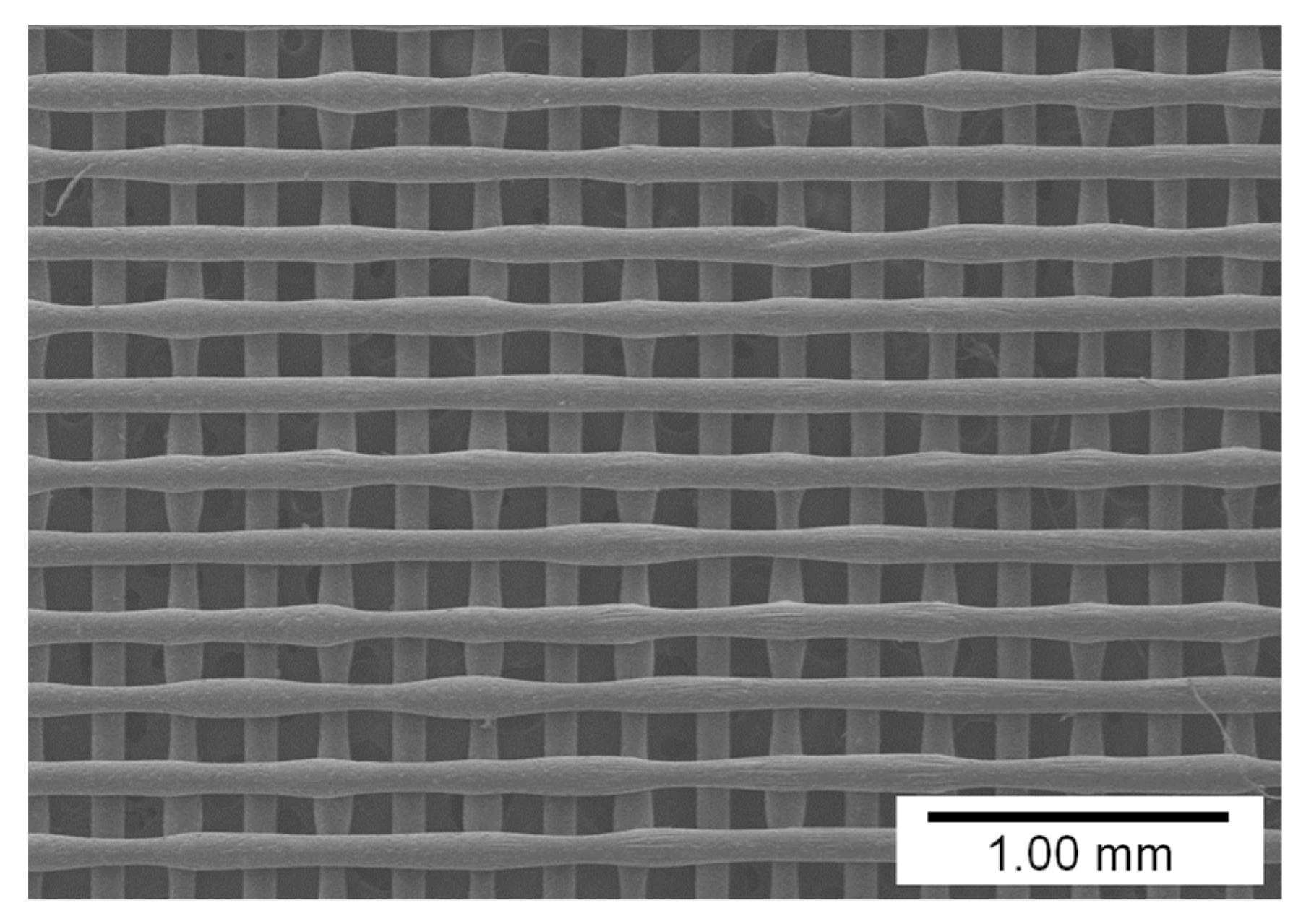
2.2. Fabrication of Lattice-Shaped Structure
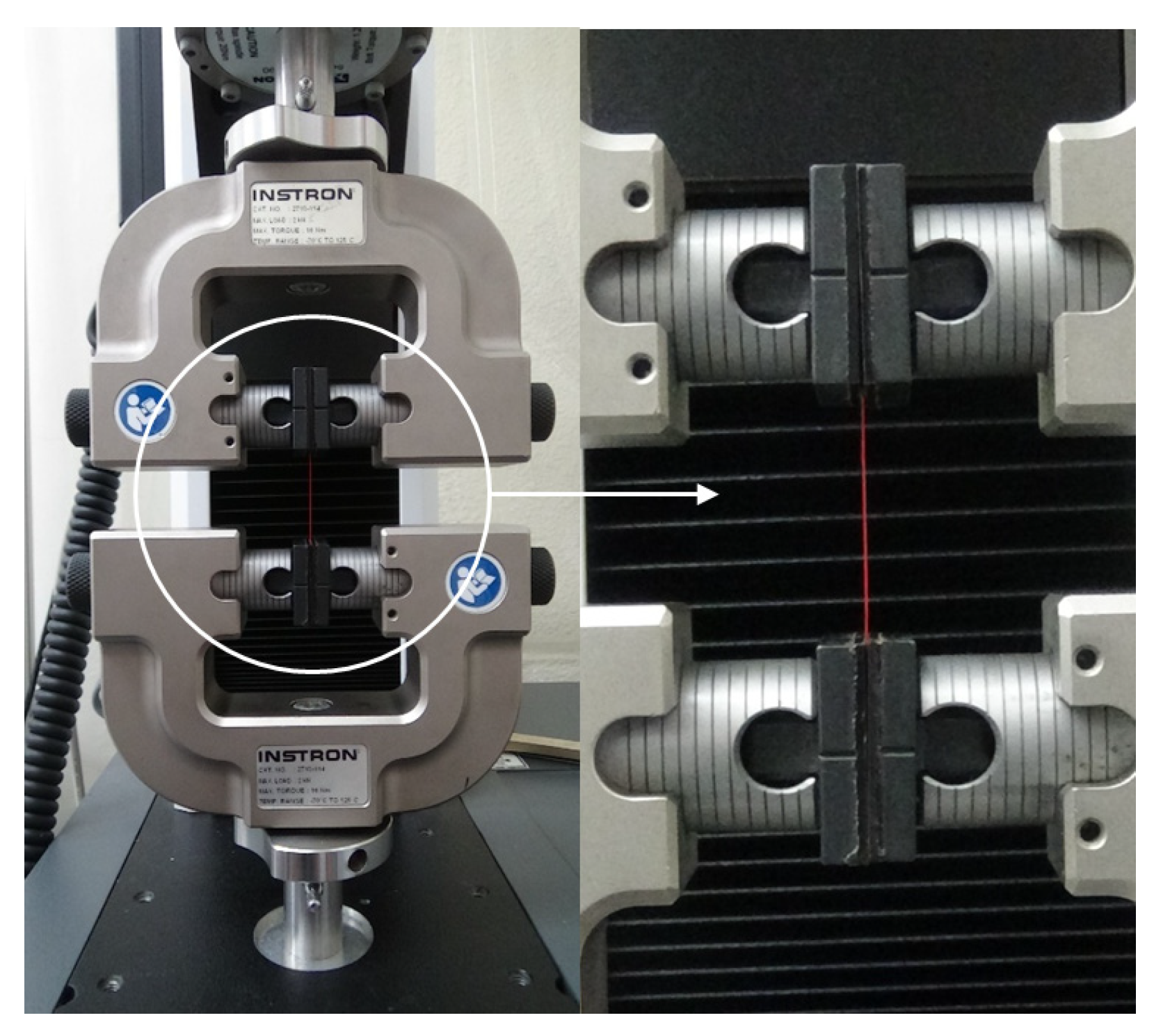
2.3. Morphology Observation and Tensile Tests
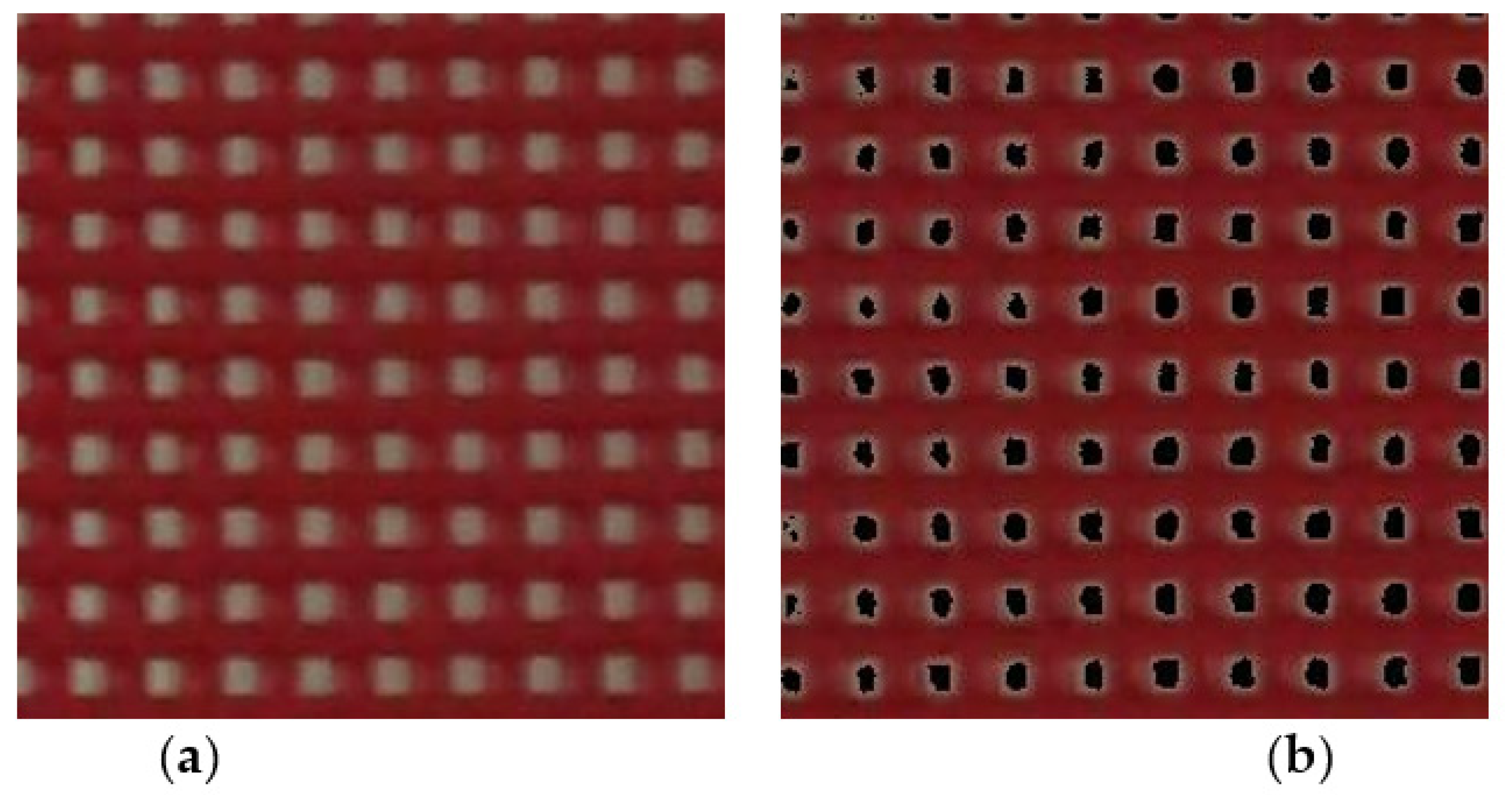
2.4. Color Changing at Transition Temperature and Image Processing
3. Results and Discussion
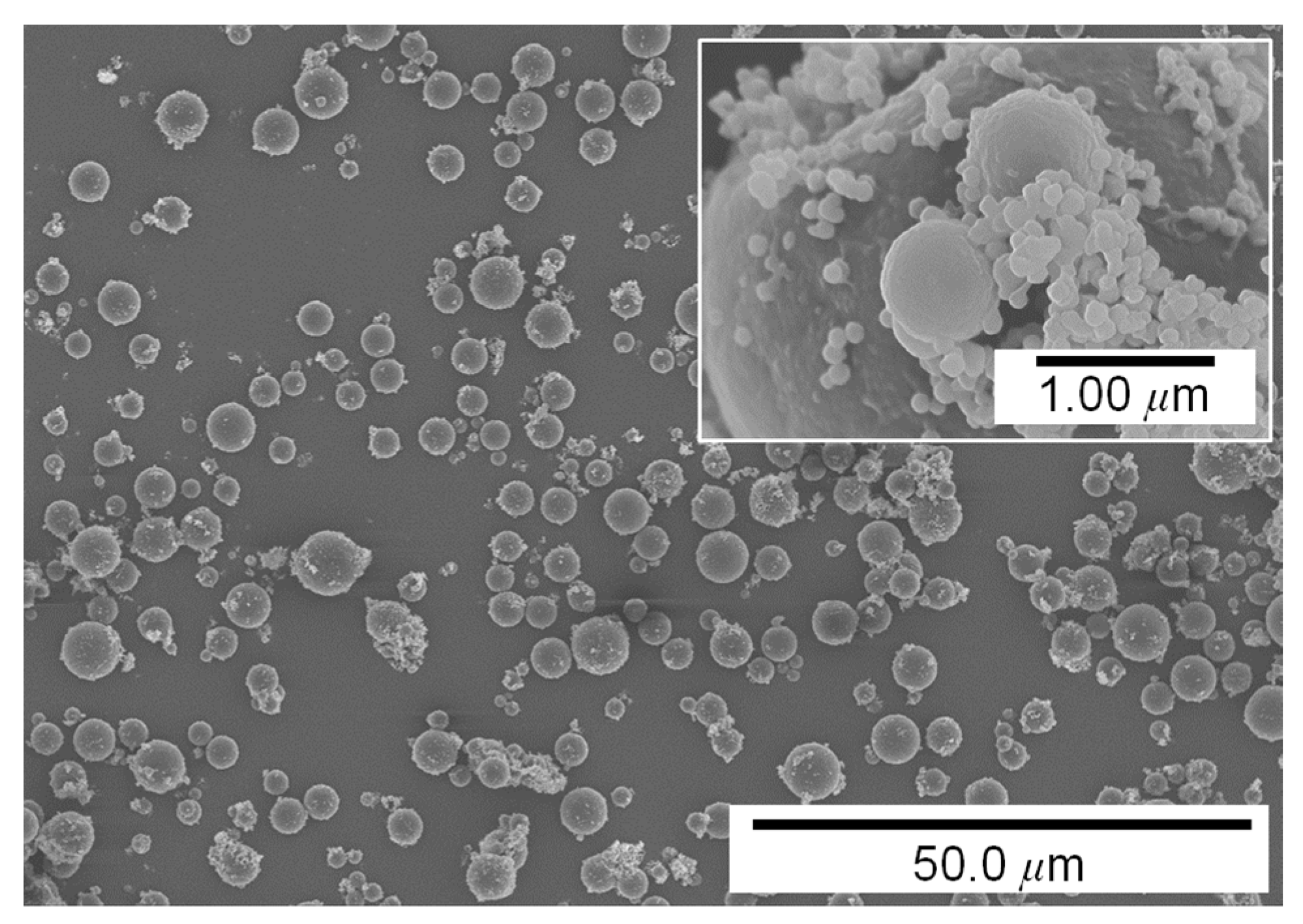
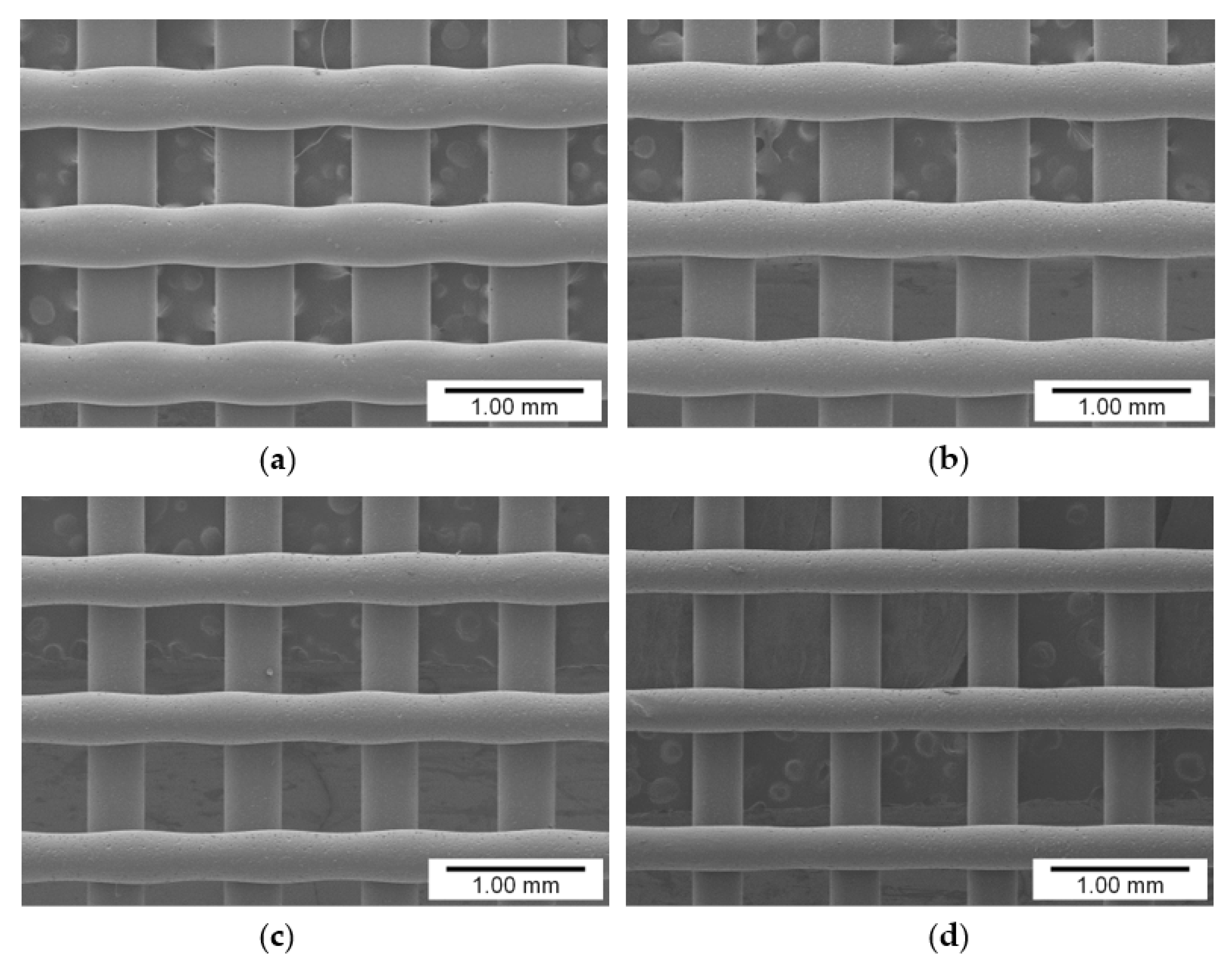
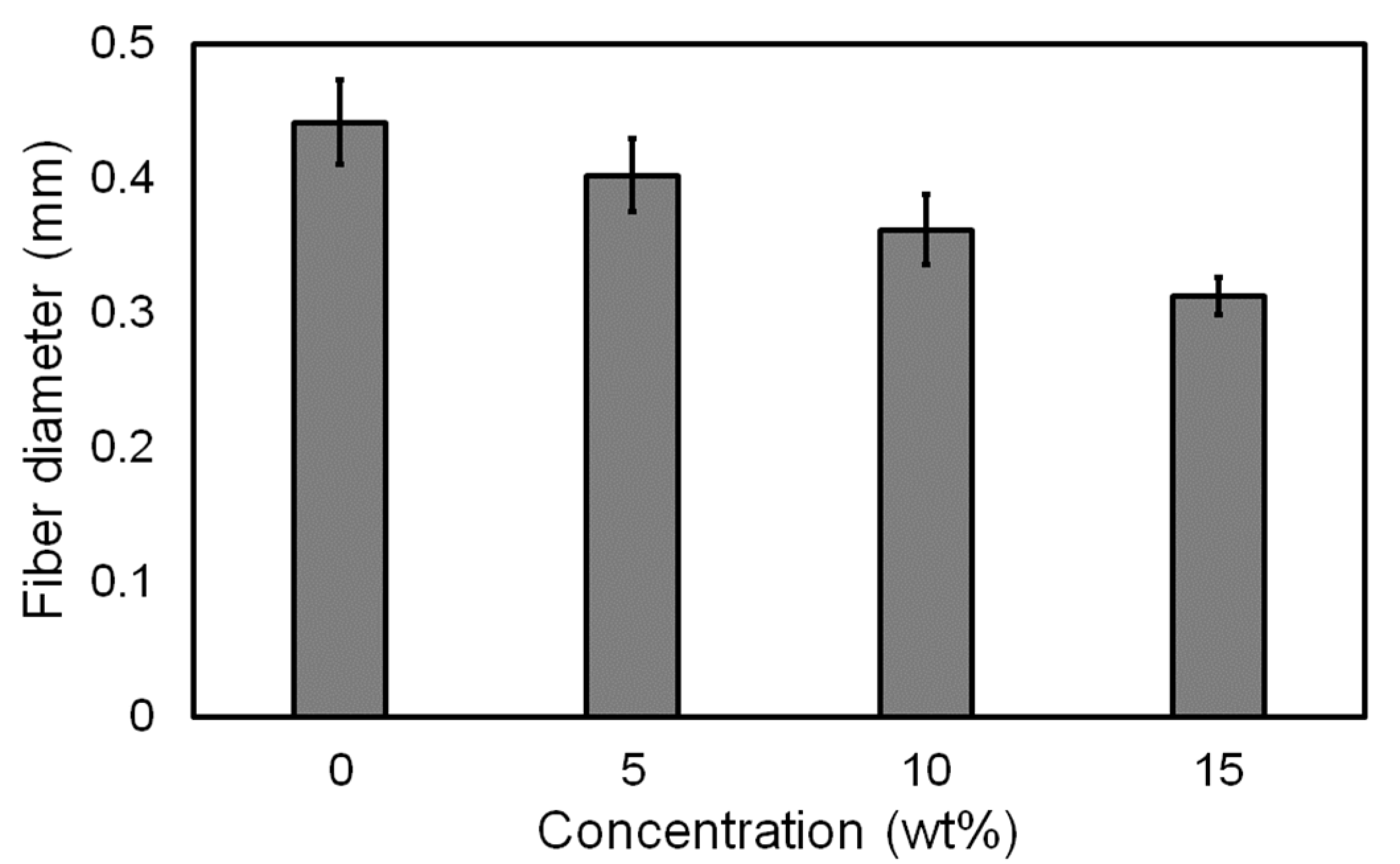
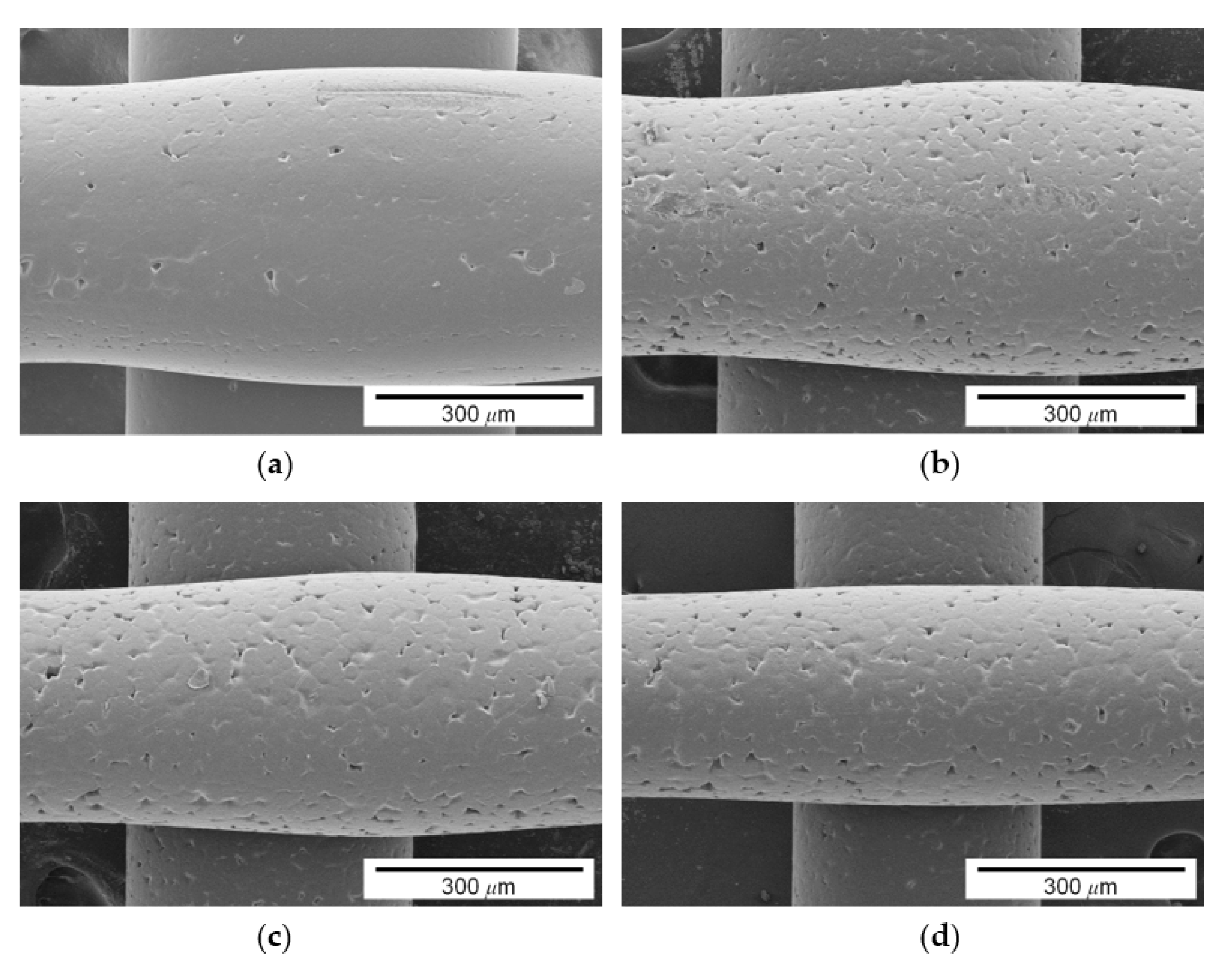
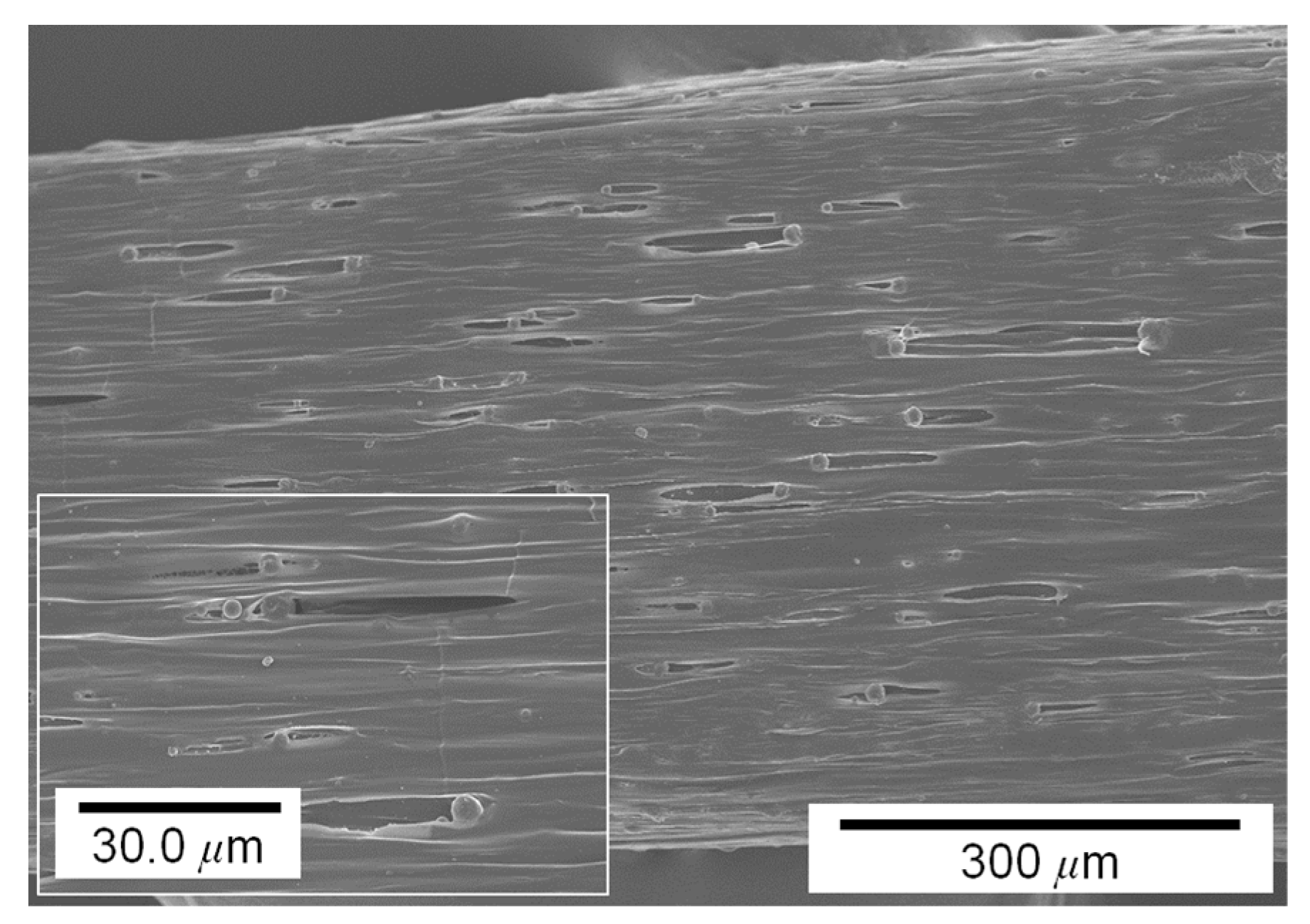
3.1. Influence of Pigment Concentration on Filament Diameter and Surface Quality
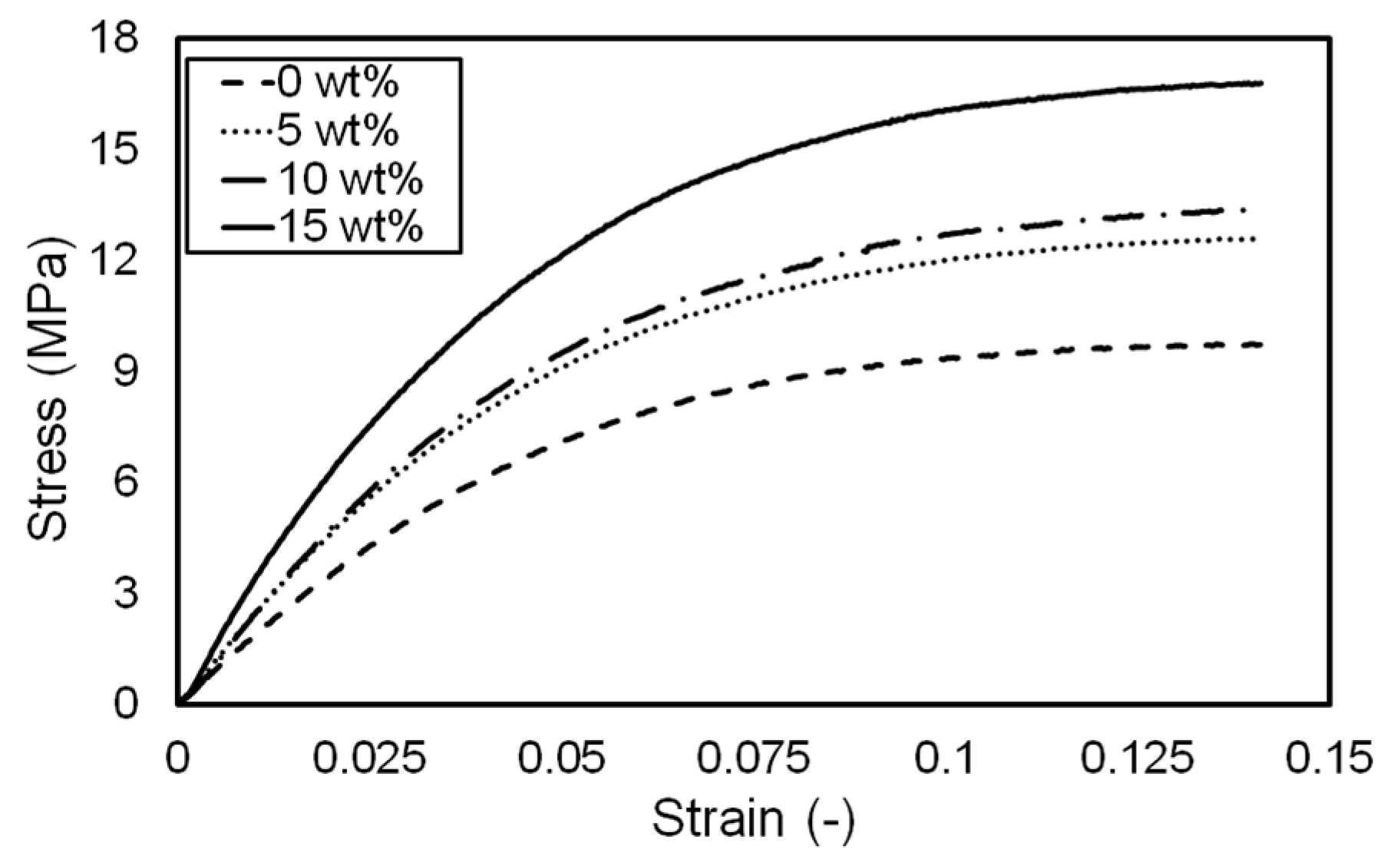
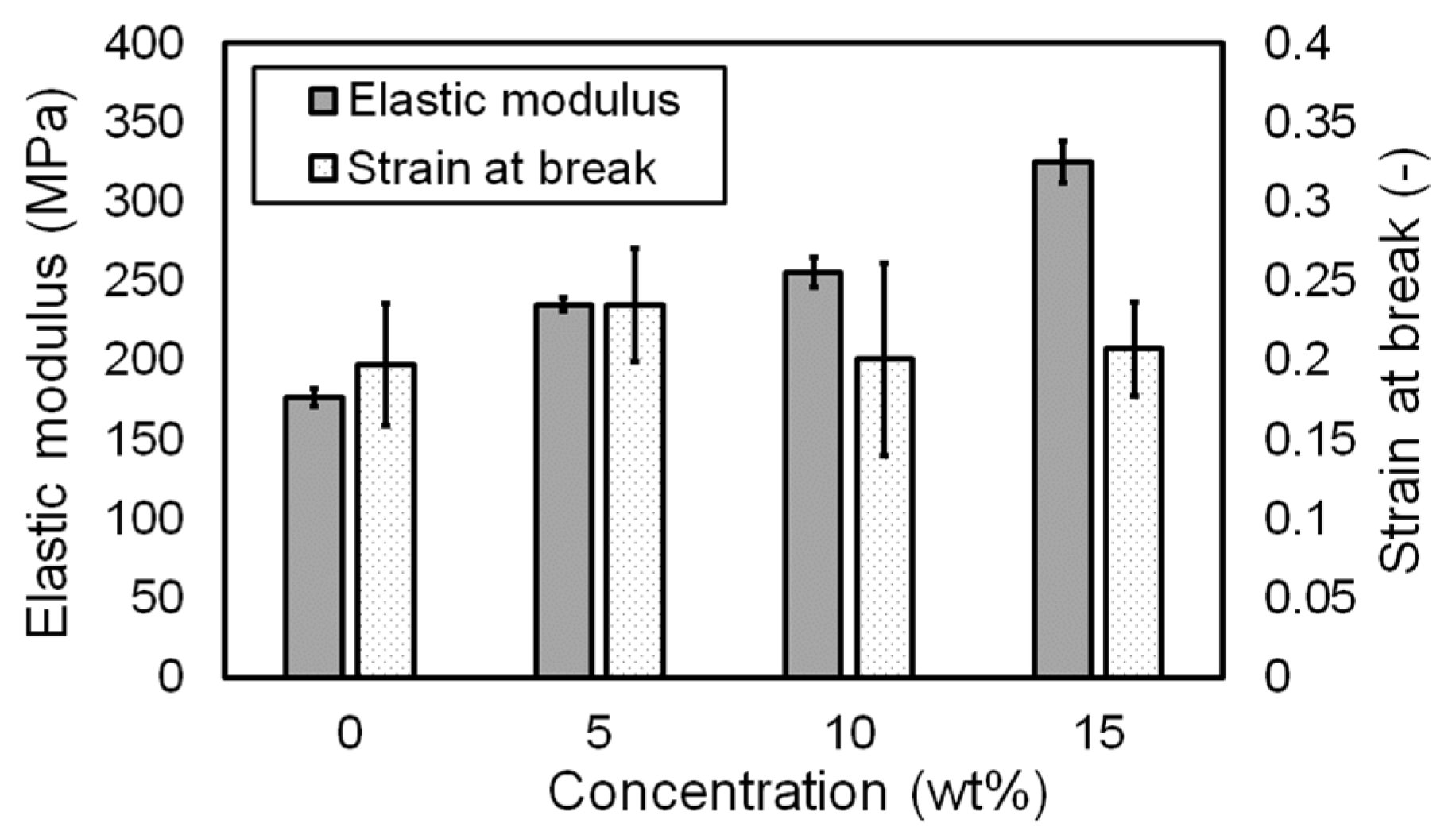
3.2. Influence of Pigment Concentration on Mechanical Properties
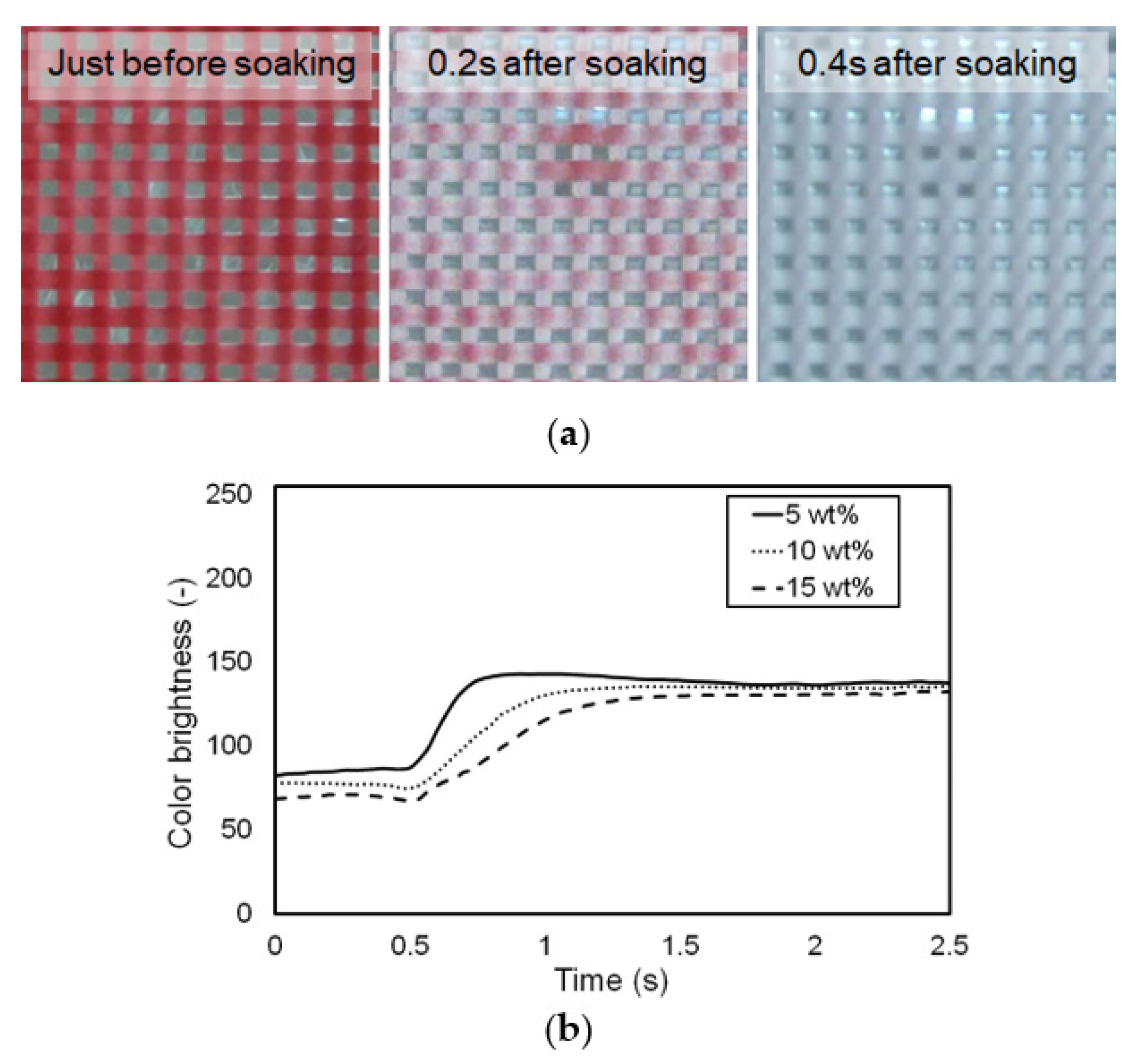
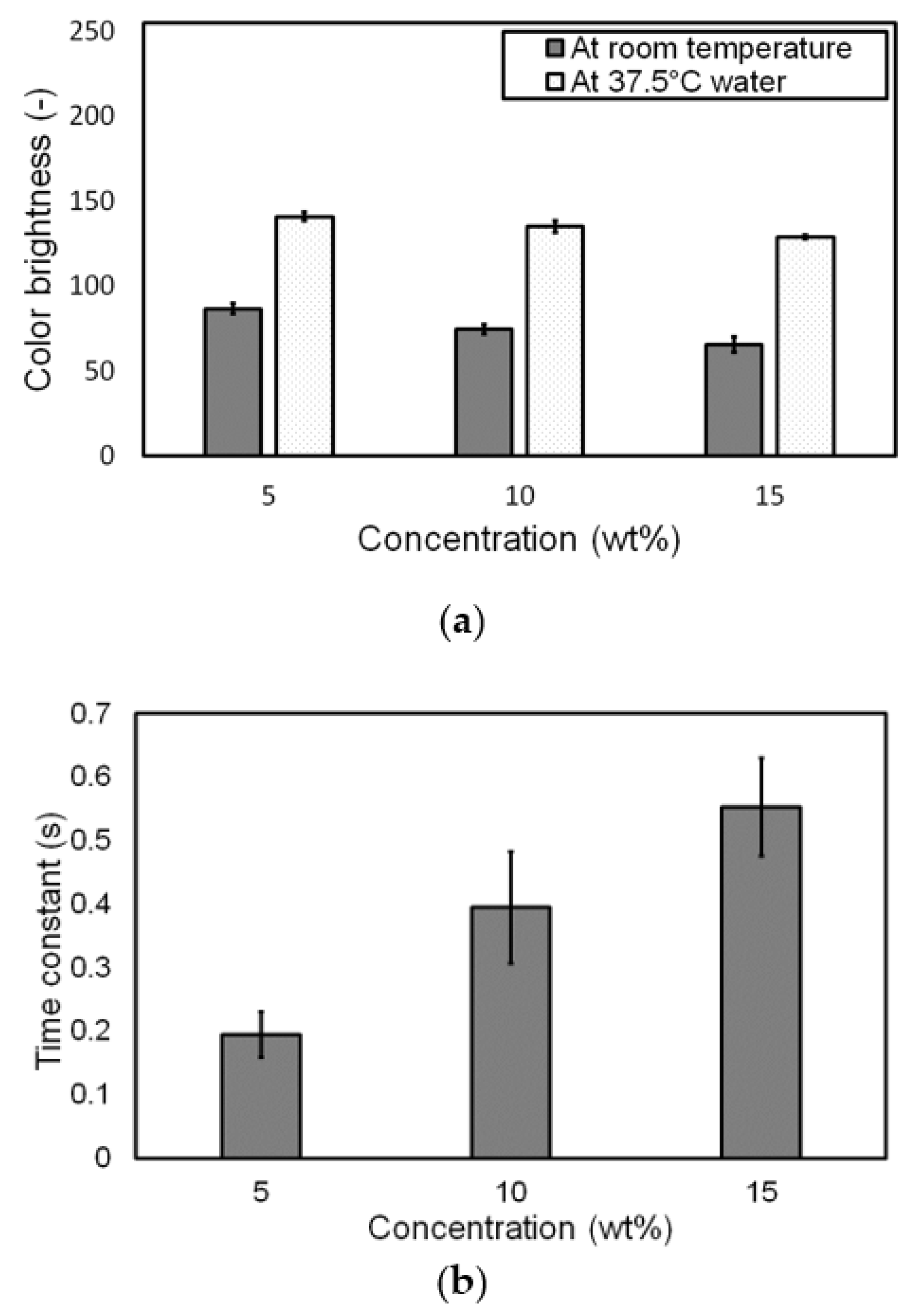
3.3. Influence of Pigment Concentration on Change in Color at Target Temperature
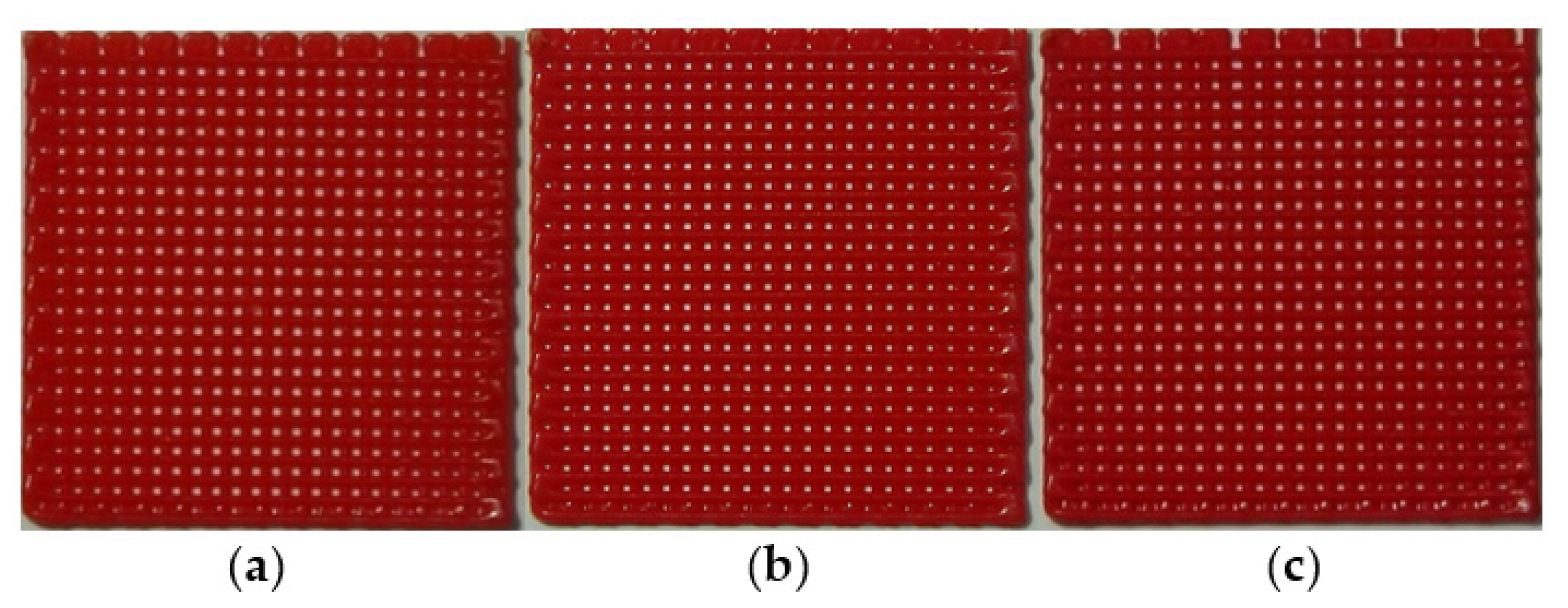
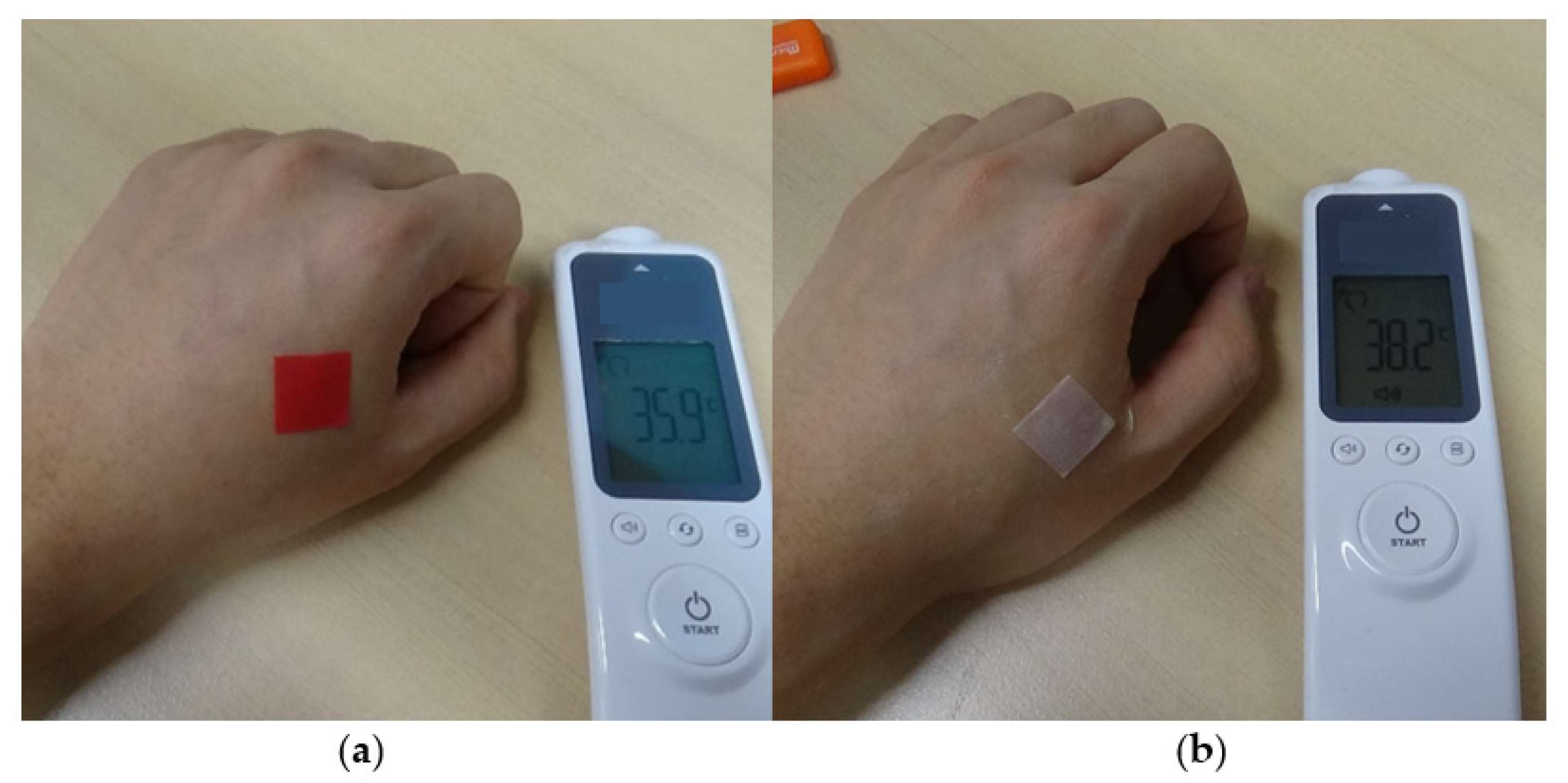
3.4. Fabrication of Thermochromic Patch for Fever Detecting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooper, V.D.; Andrews, J.O. Accuracy of Noninvasive Core Temperature Measurement in Acutely Ill Adults: The State of the Science. Biol. Res. Nurs. 2006, 8, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Cunha, J.P.S. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajbhandary, P.L.; Nallathambi, G. Feasibility of Continuous Monitoring of Core Body Temperature Using Chest-Worn Patch Sensor. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 4652–4655. [Google Scholar]
- Crawford, D.C.; Hicks, B.; Thompson, M.J. Which thermometer? Factors influencing best choice for intermittent clinical temperature assessment. J. Med. Eng. Technol. 2006, 30, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Crossley, B. Troubleshoot it: Accuracy of various thermometer types is important to consider during the COVID-19 pandemic. Biomed. Instrum. Technol. 2020, 54, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, S.; Vardon, F.; Geeraerts, T. Brain Temperature: Physiology and Pathophysiology after Brain Injury. Anesthesiol. Res. Pr. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Lin, J.-W.; Lu, M.-H.; Lin, Y.-H. A Thermal Camera Based Continuous Body Temperature Measurement System. In Proceedings of the 2019 IEEE/CVF International Conference on Computer Vision Workshop (ICCVW), Seoul, Korea, 27–28 October 2019; pp. 1681–1687. [Google Scholar]
- Sun, G.; Matsui, T.; Watai, Y.; Kim, S.; Kirimoto, T.; Suzuki, S.; Hakozaki, Y. Vital-SCOPE: Design and Evaluation of a Smart Vital Sign Monitor for Simultaneous Measurement of Pulse Rate, Respiratory Rate, and Body Temperature for Patient Monitoring. J. Sensors 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yamasue, K.; Hagiwara, H.; Tochikubo, O.; Sugimoto, C.; Kohno, R. Measurement of core body temperature by an ingestible capsule sensor and evaluation of its wireless communication performance. Adv. Biomed. Eng. 2012, 1, 9–15. [Google Scholar] [CrossRef] [Green Version]
- González-Montero, J.; Valenzuela, G.; Ahumada, M.; Barajas, O.; Villanueva, L. Management of cancer patients during COVID-19 pandemic at developing countries. World J. Clin. Cases 2020, 8, 3390–3404. [Google Scholar] [CrossRef]
- Seeboth, A.; Kriwanek, J.; Vetter, R. The first example of thermochromism of dyes embedded in transparent polymer gel networks. J. Mater. Chem. 1999, 9, 2277–2278. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R. First Example of a Non-Toxic Thermochromic Polymer Material—Based on a Novel Mechanism. J. Mater. Chem. C 2013, 1, 2811–2816. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Chang, J.Y. Reversible Thermochromic Polymer Film Embedded with Fluorescent Organogel Nanofibers. Langmuir 2014, 30, 13673–13679. [Google Scholar] [CrossRef]
- Kulčar, R.; Friškovec, M.; Hauptman, N.; Vesel, A.; Gunde, M.K. Colorimetric properties of reversible thermochromic printing inks. Dye. Pigment. 2010, 86, 271–277. [Google Scholar] [CrossRef]
- Muthyala, R. Chemistry and Applications of Leuco Dyes, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 159–205. [Google Scholar]
- Croisier, F.; Duwez, A.-S.; Jérôme, C.; Léonard, A.; van der Werf, K.; Dijkstra, P.; Bennink, M. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D.D.; Fockenberg, D.A.; Lemmens, K.P.H.; Ham, J.J.; Midden, C.J.H. Brightness differences influence the evaluation of affective pictures. Cogn. Emot. 2013, 27, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Kitano, T.; Sasahara, M.; Nishijima, K. Viscosity of particle filled polymer melts. Rheol. Acta 1978, 17, 149–155. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Szekely, V.; Rencz, M. Thermal dynamics and the time constant domain. IEEE Trans. Compon. Packag. Technol. 2000, 23, 587–594. [Google Scholar] [CrossRef]
| Parameter (Unit) | Value | |||
|---|---|---|---|---|
| Concentration (wt.%) | 0 | 5 | 10 | 15 |
| Barrel temperature (°C) | 100 | |||
| Ambient temperature (°C) | 20 | |||
| Pressure (kPa) | 550 | |||
| Nozzle inner diameter (mm) | 0.310 | |||
| Extrusion speed (mL/h) | 0.316 | 0.263 | 0.153 | 0.066 |
| Layer thickness (mm) | 0.280 | |||
| Printing speed (mm/s) | 0.800 | |||
| Concentration (wt.%) | Elastic Modulus (MPa) * | Strain at Break * |
|---|---|---|
| 0 | 176.778 ± 5.805 | 0.198 ± 0.038 |
| 5 | 235.308 ± 4.274 | 0.235 ± 0.036 |
| 10 | 255.618 ± 9.223 | 0.201 ± 0.061 |
| 15 | 325.335 ± 12.954 | 0.208 ± 0.030 |
| Concentration (wt.%) | Brightness * | Time Constant (s) * | |
|---|---|---|---|
| At Room Temp. | At 37.5 °C | ||
| 5 | 86.835 ± 3.231 | 141.027 ± 2.549 | 0.195 ± 0.036 |
| 10 | 74.647 ± 2.896 | 135.237 ± 3.310 | 0.395 ± 0.088 |
| 15 | 65.733 ± 4.435 | 128.955 ± 1.292 | 0.552 ± 0.077 |
| Parameter (Unit) | Value |
|---|---|
| Concentration (wt.%) | 5 |
| Barrel temperature (°C) | 100 |
| Ambient temperature (°C) | 20 |
| Pressure (kPa) | 600 |
| Nozzle inner diameter (mm) | 0.130 |
| Tip to collector distance (mm) | 0.120 |
| Collector feedrate (mm/s) | 0.500 |
| Pitch (mm) | 0.250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.S.; Kim, J.H.; Jun, M.B.G.; Jeong, Y.H. Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection. Materials 2021, 14, 3460. https://doi.org/10.3390/ma14133460
Jeon HS, Kim JH, Jun MBG, Jeong YH. Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection. Materials. 2021; 14(13):3460. https://doi.org/10.3390/ma14133460
Chicago/Turabian StyleJeon, Hyeon Seop, Jeong Hwa Kim, Martin B. G. Jun, and Young Hun Jeong. 2021. "Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection" Materials 14, no. 13: 3460. https://doi.org/10.3390/ma14133460
APA StyleJeon, H. S., Kim, J. H., Jun, M. B. G., & Jeong, Y. H. (2021). Fabrication of Thermochromic Membrane and Its Characteristics for Fever Detection. Materials, 14(13), 3460. https://doi.org/10.3390/ma14133460






