Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections?
Abstract
1. Introduction
2. The Role of Touched Surfaces in Pathogen Spread
3. Establishing the Case for the Re-emergence of Copper Surfaces
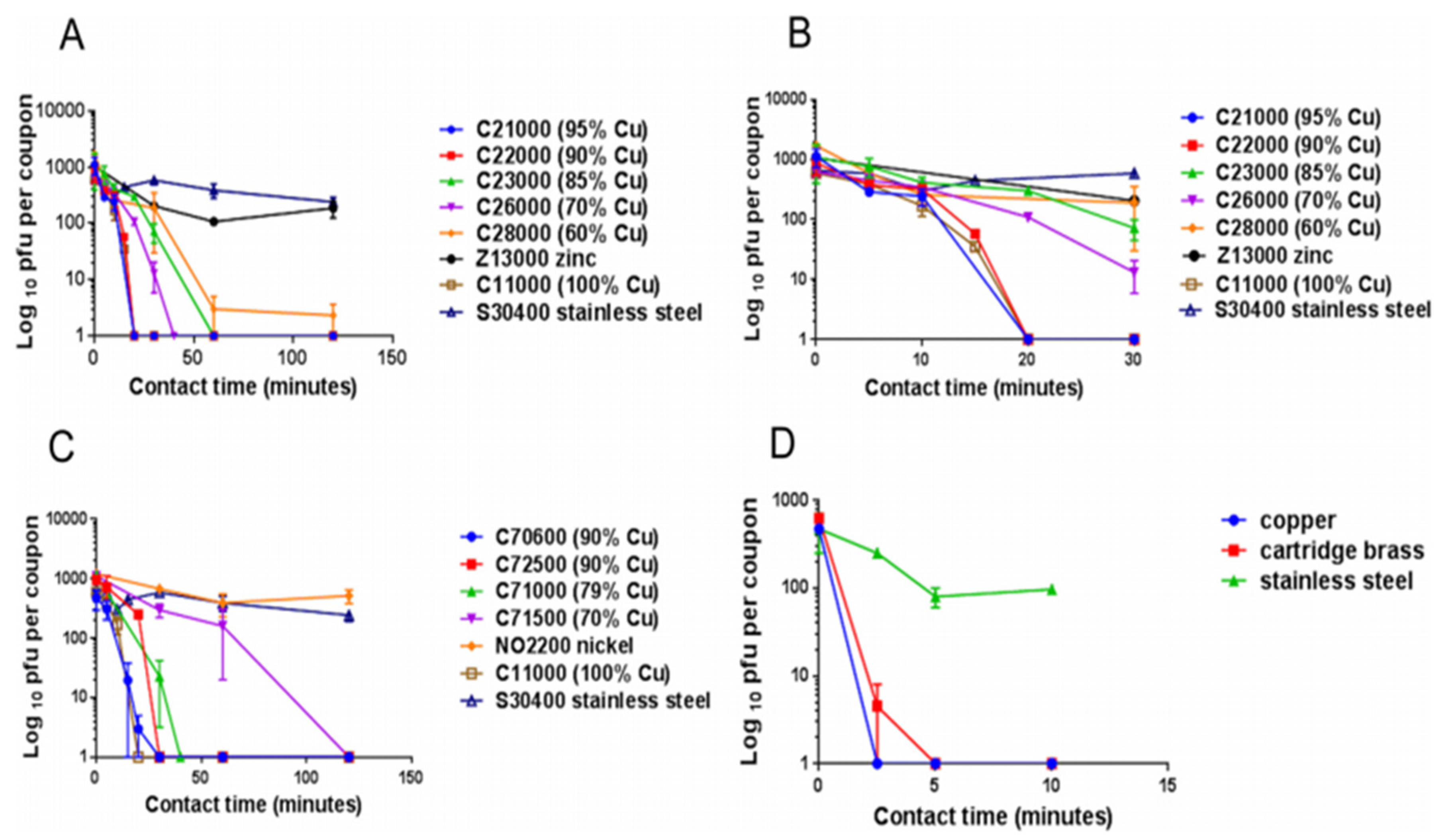
4. A Survey of the Antipathogenic Properties of Copper and Its Alloys
4.1. Copper as an Antibacterial Agent
4.2. Copper as an Antiviral Agent
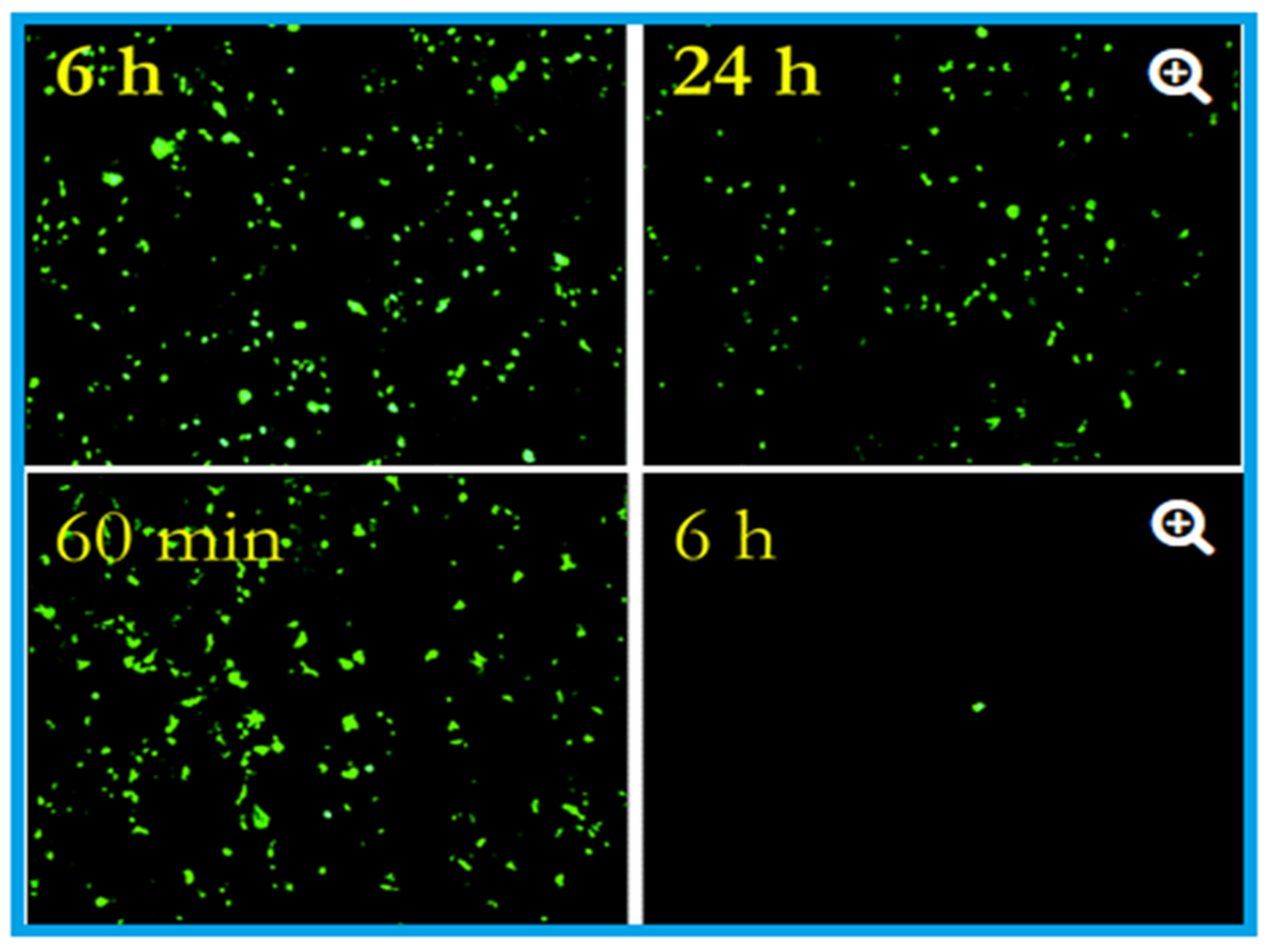
| Types of Virus(es) | Effect(s) | Reference(s) |
|---|---|---|
| SARS-CoV-2 | The virus was active only up to 4 h on the copper surface | [51] |
| SARS-CoV | The virus was active only up to 8 h on the copper surface | [51] |
| Influenza A virus | After incubation for six hours on copper 99% of the viral particles were inactivated | [92] |
| Influenza A virus | Solid-state copper oxide (Cu2O) inactivated the influenza A virus | [99] |
| Human coronavirus HuCoV-229E | Active only 20 min on copper surface | [93] |
| Hepatitis C virus (HCV) | Copper oxide-NPs significantly inhibit the infectivity of HCV, both at the entry and attachment stages | [100] |
| Murine norovirus-1 (MNV-1) | Copper alloy (65 to 99.9% Cu) dry surfaces inactivated the MNV-1 | [90] |
| Vesicular Stomatitis Virus Coxsackie Virus-B4 Respiratory Syncytial Virus | Curcumin-copper synthesised compound found to effective against these viruses and could be utilised for the development of vaginal microbicidal gel | [101] |
| Feline Calicivirus (FCV) | CuI-NPs reduced the infectivity of FCV by order of seven magnitude | [102] |
| H1N1 Influenza Virus 2009 Pandemic | CuI-NPs showed antiviral activity against influenza A virus of swine-origin | [10] |
| Human Immunodeficiency Virus-1 (HIV-1) | When exposed to copper oxide, the HIV-1 infectivity inhibited in a dose-dependant manner | [98] |
| Polio Virus | Copper sulphate (20 mg/L) completely inactivated the polio virus in the presence of hydrogen peroxide | [103] |
| Herpes Simplex Virus (HSV) | Reducing agents such as ascorbic acid, hydrogen peroxide and cysteine enhanced the antiviral property of copper | [104] |
4.3. Copper as an Antifungal Agent
| Species | Application Method (Wet (W)/Dry (D)) | Time to No Viable Forms Detected | Reference(s) |
|---|---|---|---|
| SARS-CoV-2 | D, 105.25 50% (TCID50) per mm | 4 h | [51] |
| SARS-CoV | D, 106.75–7.00 TCID50/mm | 8 h | [51] |
| Human coronavirus—HCoV-229E | W, 103 PFU | 20 min | [93] |
| Influenza A virus (H1N1) | W, 5 × 105 viruses h | 5 h | [92] |
| Penicillium crysogenum | W, (2–300) × 105 spores c | 24 h | [9] |
| Fusarium solani | W, (2–300) × 105 spores c | 24 h | [9] |
| Fusarium oxysporum | W, (2–300) × 105 spores c | 24 h | [9] |
| Fusarium culmonium | W, (2–300) × 105 spores c | 24 h | [9] |
| Aspergillus niger | W, (2–300) × 105 spores c | >576 h | [9] |
| Aspergillus fumigatus | W, (2–300) × 105 spores c | >120 h | [9] |
| Aspergillus flavus | W, (2–300) × 105 spores c | 120 h | [9] |
| Candida albicans | W, 105 CFU f | 1 h | [59] |
| Saccharomyces cerevisiae | D, 106 CFU k | 30 s | [117] |
| Candida albicans | D, 106 CFU k | 5 min | [117] |
| Candida albicans | W, (2–300) × 105 spores c | 24 h | [118] |
| MRSA d | W, 107 CFU f | 3 h | [59] |
| MRSA NCTC 10442 | W, 2 × 107 CFU | 75 min | [75] |
| EMRSA-16 e (NCTC13143) | W, (1–1.9) × 105 CFU c | 90 min | [74] |
| EMRSA-1 e (NCTC11939) | W, (1–1.9) × 107 CFU c | 1 h | [74] |
| MRSA d (NCTC10442) | W, (1–1.9) × 107 CFU c | 45 min | [74] |
| Acinetobacter baumannii | W, 107 CFU f | 3 h | [59] |
| Pseudomonas aeruginosa | W, 107 CFU f | 3 h | [59] |
| Klebsiella pneumoniae | W, 107 CFU f | 1 h | [59] |
| Mycobacterium tuberculosis | W, 2.5 × 107 CFU f | 5–15 days | [59] |
| C. difficile (ATCC 9689) vc&spores | W, 2.2 × 105 CFU c | 24–48 h | [73] |
| Pseudomonas aeruginosa PAO1 | W, 2.2 × 107 CFU j | 2 h | [74] |
| Escherichia coli O157 | W, 2.7 × 107 CFU c | 75 min | [75] |
| Listeria monocytogenes Scott A | W, 107 CFU c | 1 h | [77] |
| Escherichia coli O157 | W, (3–4) × 107 CFU c | 65 min | [78] |
| Brucella melitensis NCTC 10094 | D, 106 CFU k | <5 min | [80] |
| Burkholderia mallei NCTC 3709 | D, 106 CFU k | <5 min | [80] |
| Burkholderia pseudomallei NCTC 0816-03 | D, 106 CFU k | <5 min | [80] |
| Francisella tularensis FSC 237 | D, 106 CFU k | <5 min | [80] |
| Yersinia pestis NCTC 2028 | D, 106 CFU k | <5 min | [80] |
| C. difficile germinating spores | W, 8 × 106 CFU i | 3 h | [82] |
| C. difficile dormant spores | W, 8 × 106 CFU i | ua-3 h | [82] |
| C. difficile NCTC11204/R20291 vc | W, (1–5) × 106 CFU i | 30 min | [82] |
| Different Enterococcus spp. | W, 106 CFU f | 1 h | [96] |
| Enterococcus hirae ATCC 9790 | W, 107 CFU c | 90 min | [96] |
| Escherichia coli W3110 | D, 109 CFU k | 1 min | [119] |
| Brachybacterium conglomeratum DSM10241 | D, 109 CFU k | A few min | [119] |
| Staphylococcus warneri DSM 20316 | D, 109 CFU k | A few min | [119] |
| Pseudomonas oleovorans DSM1045 | D, 109 CFU k | 1 min | [119] |
| Pantoea stewartii DSM30176 | D, 109 CFU k | 1 min | [119] |
| Acinetobacter johnsoni SM6963 | D, 109 CFU k | 1 min | [119] |
| Campylobacter jejuni | W, 4.5 × 106 CFU b | 8 h | [120] |
| Salmonella enterica | W, 4.5 × 106 CFU b | 4 h | [120] |
5. Application of Copper Nanoparticles (Cu-NPs)
6. The Various Applications of Copper in the Built Environment
7. Possible Environmental Impacts Arising from the Use of Copper and Copper Alloys
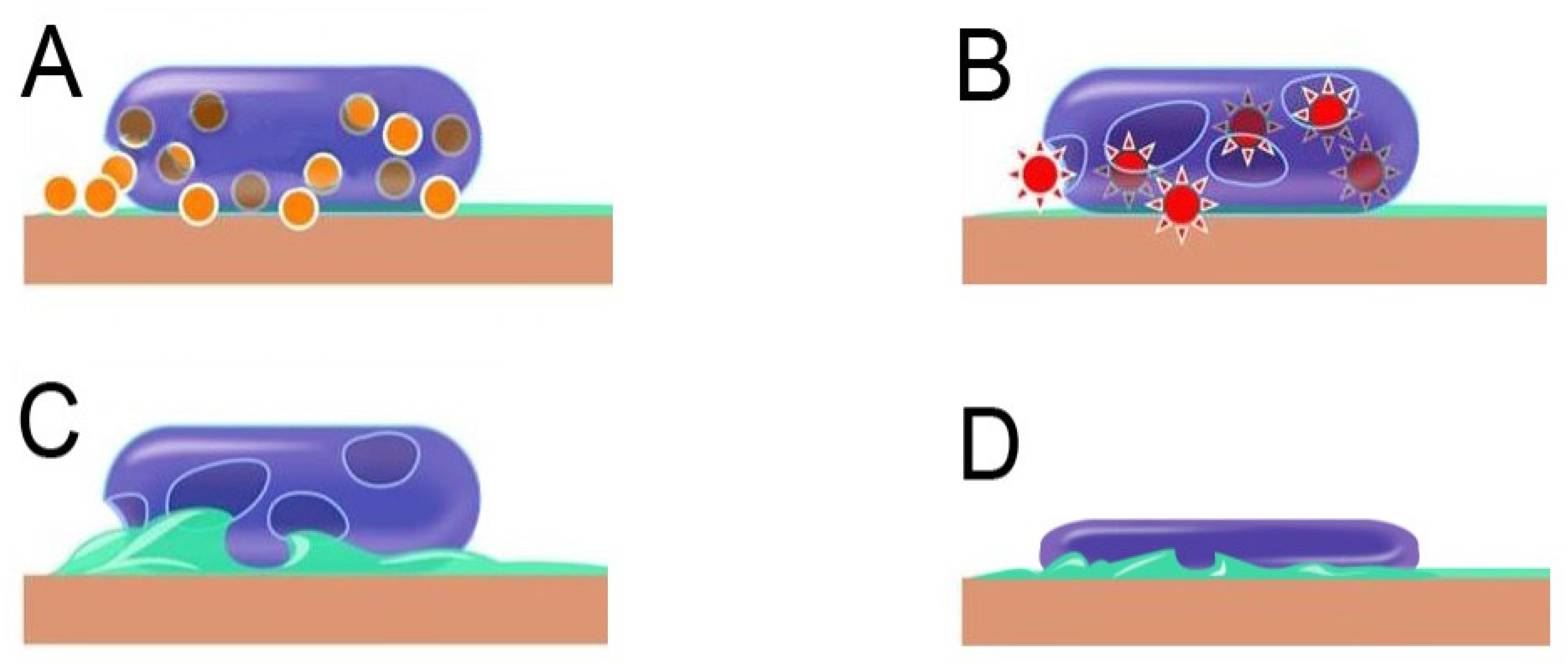
8. Antimicrobial Mechanism of Copper
8.1. Membrane Depolarisation
8.2. Reactive Oxygen Species Generation
9. The Economics of Copper Installation and Advantages?
- (i)
- It is easy and safe to install and maintain and is visually attractive;
- (ii)
- Whist the initial installation expenditure appears to be high, it gives excellent return when compared to otherwise required treatment expenditure;
- (iii)
- Once installed, copper products and surfaces continue to provide non-diminishing infection control;
- (iv)
- There is no requirement for energy input, apart from regular cleaning, needed for maintaining the antimicrobial properties;
- (v)
- Copper products and surfaces do not introduce any harmful side effects to the health when installed in public places; and
- (vi)
- It is a convenient and effective way to control superbugs such as MRSA and VRE.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borkow, G.; Gabbay, J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2019, 3, 272–278. [Google Scholar] [CrossRef]
- Chaturvedi, K.S.; Henderson, J.P. Pathogenic adaptations to host-derived antibacterial copper. Front. Cellu. Infect. Micobiol. 2014, 4, 3. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Dollwet, H.; Sorenson, J.R.J. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Carson, K.C.; Bartlett, J.G.; Tan, T.J.; Riley, T.V. In vitro susceptibility of methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus to a new antimicrobial, copper silicate. Antimicrob. Agents Chemother. 2007, 51, 4505–4507. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agent 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Stout, J.E.; Murder, R.R.; Mietzner, S.; Wagner, M.M.; Perri, M.B.; DeRoss, K.; Goodrich, D.; Arnold, W.; Theresa Williamson, T.; Ruark, O.; et al. Role of environmental surveillance in determining the risk of hospital-acquired legionellosis: A national surveillance study with clinical correlations. Infect. Contr. Hosp. Epidemiol. 2007, 28, 818–824. [Google Scholar] [CrossRef]
- Weaver, L.; Michels, H.T.; Keevil, C.W. Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Lett. Appl. Microbiol. 2010, 50, 18–23. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791S–796S. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Barceloux, D. Copper. J. Toxicol Clini. Toxicol 1999, 37, 217–230. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper in medicine. Curr. Opin. Chem. Biol. 2003, 7, 207–212. [Google Scholar] [CrossRef]
- O’Brien, P.A.; Kulier, R.; Helmerhorst, F.M.; Usher-Patel, M.; d’Arcangues, C. Copper-containing, framed intrauterine devices for contraception: A systematic review of randomized controlled trials. Contraception 2008, 77, 318–327. [Google Scholar] [CrossRef]
- Michels, H.T.; Anderson, D.G. Antimicrobial regulatory efficacy testing of solid copper alloy surfaces in the USA. In Met Ions Biology and Medicine; Collery, P., Marymard, I., Theophanides, T., Khassanova, L., Collery, T., Eds.; Copper Development Association Inc.: McLean, VA, USA, 2008; Volume 10, pp. 185–190. Available online: www.copperalloystewardship.com (accessed on 20 December 2020).
- Schmidt, M.G.; Attaway, H.H.; Sharpe, P.A.; John, J., Jr.; Sepkowitz, K.A.; Morgan, A.; Fairey, S.E.; Singh, S.; Steed, L.L.; Cantey, J.R.; et al. Sustained reduction of microbial burden on common hospital surfaces through the introduction of copper. J. Clin. Microbiol. 2012, 50, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Howard, J.; Mohr, D.; Craig, S. Self-disinfecting copper beds sustain terminal cleaning and disinfection effects throughout patient care. Appl. Environ. Microbiol. 2019, 86, e01886-19. [Google Scholar] [CrossRef]
- Lazary, A.; Weinberg, I.; Vatine, J.-J.; Jefidoff, A.; Bardenstein, R.; Borkow, G.; Ohana, N. Reduction of healthcare-associated infections in a long-term care brain injury ward by replacing regular linens with biocidal copper oxide impregnated linens. Int. J. Infect. Dis. 2014, 24, 23–29. [Google Scholar] [CrossRef]
- Niiyama, N.; Sasahara, T.; Mase, H.; Abe, M.; Saito, H.; Katsuoka, K. Use of copper alloy for preventing transmission of methicillin-resistant Staphylococcus aureus contamination in the dermatology ward. Acta Derm. Venereol. 2013, 93, 294–300. [Google Scholar] [CrossRef]
- Selvamani, V.; Zareeri, A.; Elkashiff, A.; MAruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical micro/mesoporous copper structure with enhanced antimicrobial property via laser surface texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Hinsa-Leasure, S.M.; Nartey, Q.; Vaverka, J.; Schmidt, M.G. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am. J. Infect. Control 2016, 44, e195–e203. [Google Scholar] [CrossRef] [PubMed]
- Dietz, L.; Horve, P.F.; Coil, D.A.; Fretz, M.; Eisen, J.A.; Van Den Wymelenberg, K. 2019 novel coronavirus (COVID-19) pandemic: Built environment considerations to reduce transmission. Msystems 2020, 5, e00245-20. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A. Survival of microorganisms on animate and inanimate surfaces and their disinfection. In Proceedings of the Disinfection, sterilization and antisepsis: Principles and practices in healthcare facilities, Minneapolis, MN, USA, 22–23 June 2000; Association for Professionals in Infection Control and Epidemiology, Inc.: Washington DC, USA, 2001; pp. 195–205. [Google Scholar]
- Reynolds, K.A.; Watt, P.M.; Boone, S.A.; Gerba, C.P. Occurrence of bacteria and biochemical markers on public surfaces. Int. J. Environ. Health Res. 2005, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightyngale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliot, T.S.J. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections–an overview. Infect. Drug Resist. 2018, 11, 2321. [Google Scholar] [CrossRef]
- Michels, H.T.; Keevil, C.W.; Salgado, C.D.; Schmidt, M.G. From laboratory research to a clinical trial: Copper alloy surfaces kill bacteria and reduce hospital-acquired infections. HERD: Health Environ. Resear. Design J. 2015, 9, 64–79. [Google Scholar] [CrossRef]
- CDC. Data Portal, Healthcare Associated Infections (HAI), Centre for Disease Control (CDC), USA. 2018. Available online: https://www.cdc.gov/hai/data/portal/index.html (accessed on 12 October 2020).
- WHO. Patient Safety. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organisation: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf;jsessionid=9680B80D81E0C4E7C2348B5769668CD0?sequence=1 (accessed on 10 October 2020).
- ECDPC. Healthcare-Associated Infections—A Threat to Patient Safety in Europe. European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/infographic-healthcare-associated-infections-threat-patient-safety-europe2018 (accessed on 17 October 2020).
- OECD. Healthcare-associated infections. In Health at a Glance: Europe 2018: State of Health in the EU Cycle, Organisation of Economic Cooperation and Development; OECD Publishing: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect. Control Hosp. Epidemiol. 2013, 34, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Keating, T.; Could, D.; Wigglesworth, N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ 2020, 10, e033367. [Google Scholar] [CrossRef] [PubMed]
- HAI. Healthcare-Associated Infections, Hospital-Acquired Complication-3, Australian Commission on Safety and Quality in Health Care. Report. Available online: https://www.safetyandquality.gov.au/sites/default/files/migrated/Healthcare-associated-infection-detailed-fact-sheet.pdf (accessed on 11 November 2020).
- Mitchell, B.G.; Shaban, R.Z.; MacBeth, D.; Wood, C.J.; Russo, P.L. The burden of healthcare-associated infection in Australian hospitals: A systematic review of the literature. Infect. Dis. Health 2017, 22, 117–128. [Google Scholar] [CrossRef]
- Allen, J.; Rey-Conde, T.; North, J.B.; Kruger, P.; Babidge, W.J.; Wysocki, A.P.; Ware, R.S.; Veerman, J.L.; Maddern, G.J. Processes of care in surgical patients who died with hospital-acquired infections in Australian hospitals. J. Hosp. Infect. 2018, 99, 17–23. [Google Scholar] [CrossRef]
- WHO. Food Safety. World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 21 January 2021).
- CDC. Estimates of foodborne illness in the United States. Centres for Disease Control and Prevention. Available online: https://www.cdc.gov/foodborneburden/index.html (accessed on 10 November 2020).
- Hoffmann, S.; Maculloch, B.; Batz, M. Economic Burden of Major Foodborne Illness Acquired in the United States. Economic Research Services, United States Department of Agriculture, Economic Information Bulletin Number 140. Available online: https://www.ers.usda.gov/webdocs/publications/43984/52807_eib140.pdf (accessed on 12 November 2020).
- World Bank. The Safe Food Imperative: Accelerating Progress in Low and Middle-Income countries. World Bank. Available online: https://www.worldbank.org/en/topic/agriculture/publication/the-safe-food-imperative-accelerating-progress-in-low-and-middle-income-countries (accessed on 17 December 2020).
- Boone, S.A.; Gerba, C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Yezli, S.; Khan, A. COVID-19 social distancing in the Kingdom of Saudi Arabia: Bold measures in the face of political, economic, social and religious challenges. Trav. Med. Infect. Dis. 2020, 37, 101692. [Google Scholar] [CrossRef] [PubMed]
- Wildman, W.J.; Bulbulia, J.; Sosis, R.; Schjoedt, U. Religion and COVID-19 pandemic. Relig. Brain Behav. 2020, 10, 115–117. [Google Scholar] [CrossRef]
- Rawlinson, S.; Ciric, L.; Cloutman-Green, E. COVID-19 pandemic—Let’s not forget surfaces. J. Hosp. Infect. 2020, 105, 790–791. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Nicoloff, H.; Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Microbiol. 2019, 17, 479–496. [Google Scholar] [CrossRef]
- Majno, G. The Healing Hand: Man and Wound in the Ancient World; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar] [CrossRef]
- Rogers, J.U.L.I.E.; Keevil, C.W. Immunogold and fluorescein immunolabelling of Legionella pneumophila within an aquatic biofilm visualized by using episcopic differential interference contrast microscopy. Appl. Environ. Microbiol. 1992, 58, 2326–2330. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Bassett, P.; Sultan, A.H. The use of copper as an antimicrobial agent in health care, including obstetrics and gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. NEJM 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Michels, H.T.; Michels, C.A. Copper alloys-The new ‘old’ weapon in the fight against infectious disease. Microbiology 2016, 10, 23–45. [Google Scholar]
- Ibrahim, Z.; Petrusan, A.J.; Hooke, P.; Hinsa-Leasure, S.M. Reduction of bacterial burden by copper alloys on high-touch athletic center surfaces. Am. J. Infect. Control 2018, 46, 197–201. [Google Scholar] [CrossRef]
- Wagenvoort, J.H.T.; Penders, R.J.R. Long-term in-vitro survival of an epidemic MRSA phage-group III-29 strain. J. Hosp. Infect. 1997, 35, 322–325. [Google Scholar] [CrossRef]
- Hammett, E. How long does Coronavirus survive on different surfaces? BDJ Team 2020, 7, 14–15. [Google Scholar] [CrossRef]
- Kuehnert, M.J.; Hill, H.A.; Kupronis, B.A.; Tokars, J.I.; Solomon, S.L.; Jernigan, D.B. Methicillin-resistant–Staphylococcus aureus hospitalizations, United States. Emerg. Infect. Dis. 2005, 11, 468. [Google Scholar] [CrossRef]
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Steed, L.L.; Michels, H.T.; Salgado, C.D. Copper continuously limits the concentration of bacteria resident on bed rails within the ICU. Infect. Control Hosp. Epidemiol. 2013, 34, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Majka, G.; Bulanda, M. Antimicrobial effect of copper alloys on Acinetobacter species isolated from infections and hospital environment. Antimicrob. Resist. Infect. Control 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehtar, S.; Wiid, I.; Todorov, S.D. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef]
- Weaver, L.; Noyce, J.O.; Michels, H.T.; Keevil, C.W. Potential action of copper surfaces on meticillin—Resistant Staphylococcus aureus. J. Appl. Microbiol. 2010, 109, 2200–2205. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Casey, A.L.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Elliot, T.S.J. The antimicrobial efficacy of copper alloy furnishing in the clinical environment: A crossover study. Infect. Control Hosp. Epidemiol. 2012, 33, 3–9. [Google Scholar] [CrossRef] [PubMed]
- ECI. Copper—A New Weapon to Fight the Influenza a Virus- New Research Finds Copper Effective at Inactivating H1N1 Virus. European Copper Institute. Available online: https://pr.euractiv.com/pr/copper-new-weapon-fight-influenza-virus-new-research-finds-copper-effective-inactivating-h1n1 (accessed on 12 January 2021).
- Barker, J.; Vipond, I.B.; Bloomfield, S.F. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2014, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.J. Doorknobs: A source of nosocomial infection. Diagn. Med. 1983, 6, 62–63. [Google Scholar]
- Colin, M.; Klingelschmitt, F.; Charpentier, E.; Josse, J.; Kanagaratnam, L.; Champs, C.D.; Gangloff, S.C. Copper alloy touch surfaces in healthcare facilities: An effective solution to prevent bacterial spreading. Materials 2018, 11, 2479. [Google Scholar] [CrossRef]
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, P. The Role of Antimicrobial Copper Surfaces in Reducing Healthcare-Associated Infections. Eur. Infect. Dis. 2011, 5, 125–128. Available online: http://www.anamedis.com/demo/wp-content/uploads/2017/07/03.efstathiou.pdf (accessed on 17 October 2020).
- O’gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- von Dessauer, B.; Navarrete, M.S.; Benadof, D.; Benavente, C.; Schmidt, M.G. Potential effectiveness of copper surfaces in reducing health care–associated infection rates in a pediatric intensive and intermediate care unit: A nonrandomized controlled trial. Am. J. Infect. Control 2016, 44, e133–e139. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.G.; Dessauer, B.v.; Benavente, C.; Benadof, D.; Cifuentes, P.; Elgueta, A.; Duran, C.; Navaratte, M.S. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a paediatric intensive care unit. Am. J. Infec. Control 2016, 44, 203–209. [Google Scholar] [CrossRef]
- Zhu, L.; Elguindi, J.; Rensing, C.; Ravishankar, S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012, 30, 303–310. [Google Scholar] [CrossRef]
- Souli, M.; Antoniadou, A.; Katsarolis, I.; Mavrou, I.; Paramythiotou, E.; Papadomichelakis, E.; Drogari-Apiranthitou, M.; Panagea, T.; Giamarellou, H.; Petrikkos, G.; et al. Reduction of environmental contamination with multidrug-resistant bacteria by copper-alloy coating of surfaces in a highly endemic setting. Infect. Control Hosp. Epidemiol. 2017, 38, 765–771. [Google Scholar] [CrossRef]
- Weaver, L.; Michels, H.T.; Keevil, C.W. Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J. Hosp. Infect. 2008, 68, 145–151. [Google Scholar] [CrossRef]
- Elguindi, J.; Wagner, J.; Rensing, C. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 2009, 106, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin—Resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Appl. Microbiol. 2009, 49, 191–195. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.T.; Keevil, C.W. Survival of Listeria monocytogenes Scott A on metal surfaces: Implications for cross-contamination. Int. J. Food Microbiol. 2006, 111, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bleichert, P.; Santo, C.E.; Hanczaruk, M.; Meyer, H.; Grass, G. Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. Biometals 2014, 27, 1179–1189. [Google Scholar] [CrossRef]
- Inkinen, J.; Mäkinen, R.; Keinänen-Toivola, M.M.; Nordström, K.; Ahonen, M. Copper as an antibacterial material in different facilities. Lett. Appl. Microbiol. 2017, 64, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, L.J.; Worthington, T.; Lambert, P.A.; Hilton, A.C.; Lowden, C.J.; Elliott, T.S.J. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: The germination theory. J. Antimicrob. Chemother. 2008, 62, 522–525. [Google Scholar] [CrossRef]
- Goudarzi, M.; Saviz, S.; Ghoranneviss, M.; Salar Elahi, A. Medical equipment antiseptic processes using the atmospheric plasma sprayed copper coatings. J. X-ray Sci. Technol. 2017, 25, 479–485. [Google Scholar] [CrossRef]
- Silk, B.J.; Mahon, B.E.; Griffin, P.M.; Gould, L.H.; Tauxe, R.V.; Crim, S.M.; Jackson, K.A. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR. Morb. Mortal. Wkly. Rep. 2013, 62, 448. [Google Scholar] [PubMed]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Listeria monocytogenes in fresh produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef]
- Wei, P.; Bao, R.; Fan, Y. Brainstem Encephalitis Caused by Listeria monocytogenes. Pathogens 2020, 9, 715. [Google Scholar] [CrossRef]
- Aisha, A. Antimicrobial Effects of Copper and Brass Ions on the Growth of Listeria Monocytogenes at Different Temperatures, pH and Nutrients. Louisiana State University in Shreveport, USA, 2005. Available online: http://www.openthesis.org/documents/Antimicrobial-Effects-Copper-Brass-Ions-526730.html (accessed on 10 February 2021).
- Brennan, S.; McDonald, S.; McKenzie, J.; Cheng, A.; Green, S.; Allen, K. Systematic review of antimicrobial surfaces to reduce infection rates in hospitalized populations. Cochrane Aust. 2017, 1–21. [Google Scholar]
- Bauer, D.J. The chemotherapeutic activity of compounds of copper, rhodium and certain other metals in mice infected with neurovaccinia and ectromelia viruses. Br. J. Exp. Pathol. 1958, 39, 480. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2082242/?page=1 (accessed on 12 February 2021).
- Warnes, S.L.; Keevil, C.W. Inactivation of norovirus on dry copper alloy surfaces. PLoS ONE 2013, 8, e75017. [Google Scholar] [CrossRef]
- Science Daily. Copper Could Prevent the Spread of Flu Infections (14 February 2006). Available online: https://www.sciencedaily.com/releases/2006/02/060214080834.htm (accessed on 29 December 2020).
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza a virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- Cordis. Copper Could Stop Spread of Flu. Available online: https://cordis.europa.eu/article/id/25151-copper-could-stop-spread-of-flu (accessed on 12 January 2021).
- Santo, C.S.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal efficacy of copper alloys against pathogenic enterococci involves the degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401. [Google Scholar] [CrossRef] [PubMed]
- Michels, H.; Moran, W.; Michel, J. Antimicrobial properties of copper alloy surfaces, with a focus on hospital-acquired infections. Int. J. Met. 2008, 2, 47–56. [Google Scholar] [CrossRef]
- Borkow, G.; Lara, H.H.; Covington, C.Y.; Nyamathi, A.; Gabbay, J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 2008, 52, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Chauhan, G.; Rath, G.; Goyal, A.K. In-vitroanti-viral screening and cytotoxicity evaluation of copper-curcumin complex. Artif. Cells Nanomed. Biotech. 2013, 41, 276–281. [Google Scholar] [CrossRef]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioengin. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- ICA. The International Copper Association. Available online: http://www.copperinfo.com (accessed on 11 February 2021).
- Sagripanti, J.L.; Routson, L.B.; Bonifacino, A.C.; Lytle, C.D. Mechanism of copper-mediated inactivation of herpes simplex virus. Antimicrob. Agents Chemother. 1997, 41, 812–817. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Cha, J.S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef]
- Cooksey, D.A. copper uptake and resistance in bacteria. Mol. Microbiol. 1993, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, A.S.; Padhye, S.B.; Saraf, A.P.; Mahajan, H.B.; Chopade, B.A.; West, D.X. Novel broad-spectrum metal-based antifungal agents. Biol. Met. 1991, 4, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Mumcuoglu, K.Y.; Gabbay, J.; Borkow, G. Copper oxide-impregnated fabrics for the control of house dust mites. Int. J. Pest Manag. 2008, 54, 235–240. [Google Scholar] [CrossRef]
- Bellí, N.; Marín, S.; Sanchis, V.; Ramos, A.J. Impact of fungicides on Aspergillus carbonarius growth and ochratoxin A production on synthetic grape-like medium and on grapes. Food Addit. Contam. 2006, 23, 1021–1029. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Ballo, M.K.; Rtimi, S.; Kiwi, J.; Pulgarin, C.; Entenza, J.M.; Bizzini, A. Fungicidal activity of copper-sputtered flexible surfaces under dark and actinic light against azole-resistant Candida albicans and Candida glabrata. J. Photochem. Photobiol. B Biol. 2017, 174, 229–234. [Google Scholar] [CrossRef]
- Creaven, B.S.; Duff, B.; Egan, D.A.; Kavanagh, K.; Rosair, G.; Thangella, V.R.; Waslh, M. Anticancer and antifungal activity of copper (II) complexes of quinolin-2 (1H)-one-derived Schiff bases. Inorg. Chim. Acta 2010, 363, 4048–4058. [Google Scholar] [CrossRef]
- Ghasemian, E.; Naghoni, A.; Tabaraie, B.; Tabaraie, T. In vitro susceptibility of filamentous fungi to copper nanoparticles assessed by rapid XTT colorimetry and agar dilution method. J. Mycol. Médicale 2012, 22, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Quaranta, D.; Krans, T.; Santo, C.S.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef]
- Molteni, C.; Abicht, H.K.; Solioz, M. The killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Remyadevi, J.; Jeyasubramanian, K.; MArikani, A.; Rajkumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Balela, M.D.L.; Amores, K.L.S. Electroless deposition of copper nanoparticles for antimicrobial coating. Mater. Chem. Phys. 2019, 225, 393–398. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Investigations into the antibacterial behaviour of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Anita, S.; Ramachandran, T.; Rajendran, R.; Koushik, C.V.; Mahalakshmi, M.A. Study of the antimicrobial property of encapsulated copper oxide nanoparticles on cotton fabric. Text. Res. J. 2011, 81, 1081–1088. [Google Scholar] [CrossRef]
- Harikumar, P.S.; Aravind, A. Antebacterial activity of copper nanoparticles and nanocomposites against Escherichia coli bacteria. Int. J. Sci. 2016, 5, 83–90. [Google Scholar] [CrossRef]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents. 2018, 51, 912–917. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Giannousi, K.; Lafazanis, K.; Arvanitidis, J.; Pantazaki, A.; Dendrinou-Samara, C. Hydrothermal synthesis of copper-based nanoparticles: Antimicrobial screening and interaction with DNA. J. Inorg. Biochem. 2014, 133, 24–32. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharma. Bull. 2015, 5, 19. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Černík, M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparti-cles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2009, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.A.; Xing, G.M.; Chen, C.Y.; Zhao, Y.L.; Jia, G.A.; Wang, T.C.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Edwards, M.; Sprague, N. Organic matter and copper corrosion by-product 455 release: A mechanistic study. Corros. Sci. 2001, 43, 1–18. [Google Scholar] [CrossRef]
- Sabrià, M.; Garcia-Nunez, M.; Pedro-Botet, M.L.; Sopena, N.; Gimeno, J.M.; Reynaga, E.; Morera, J.; Rey-Joly, C. Presence and chromosomal subtyping of Legionella species in potable water systems in 20 hospitals of Catalonia, Spain. Infect. Control Hosp. Epidemiol. 2001, 22, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Sabria, M.; Victor, L.Y. Hospital-acquired legionellosis: Solutions for a preventable infection. Lancet Infect. Dis. 2002, 2, 368–373. [Google Scholar] [CrossRef]
- Stout, J.E.; Victor, L.Y. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: Implications for the evaluation of other disinfection modalities. Infect. Control Hosp. Epidemiol. 2003, 24, 563–568. [Google Scholar] [CrossRef]
- Cachafeiro, S.P.; Naveira, I.M.; García, I.G. Is copper–silver ionisation safe and effective in controlling legionella? J. Hosp. Infect. 2007, 67, 209–216. [Google Scholar] [CrossRef]
- Casari, E.; Ferrario, A.; Montanelli, A. Prolonged effect of two combined methods for Legionella disinfection in a hospital water system. Ann. Ig. Med. Prev. Comunità 2007, 19, 525–532. [Google Scholar] [PubMed]
- Chen, Y.S.; Lin, Y.E.; Liu, Y.-C.; Hunag, W.K.; Shih, H.Y.; Wann, S.R.; Lee, S.S.; Tsai, H.C.; Li, C.H.; Chao, H.L.; et al. Efficacy of point-of-entry copper–silver ionisation system in eradicating Legionella pneumophila in a tropical tertiary care hospital: Implications for hospitals contaminated with Legionella in both hot and cold water. J. Hosp. Infect. 2008, 68, 152–158. [Google Scholar] [CrossRef]
- Thneibat, A.; Cochiran, M.A.; Gonzalez-Cabezas, C.; Moore, B.K.; Matis, B.A.; Lund, M.R. Anticariogenic and antibacterial properties of a copper varnish using an in vitro microbial caries model. Oper. Dent. 2008, 33, 142–148. [Google Scholar] [CrossRef]
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. The effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003, 24, 1797–1807. [Google Scholar] [CrossRef]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977–986. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef]
- Guldiren, D.; Aydın, S. Antimicrobial property of silver, silver-zinc and silver-copper incorporated soda lime glass prepared by ion exchange. Mater. Sci. Eng. C 2017, 78, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef]
- Sudha, V.P.; Ganesan, S.; Pazhani, G.P.; Ramamurthy, T.; Nair, G.B.; Venkatasubramanian, P. Storing drinking-water in copper pots kills contaminating diarrhoeagenic bacteria. J. Health Popul. Nutr. 2012, 30, 17. [Google Scholar] [CrossRef]
- Sudha, V.B.P.; Singh, K.O.; Prasad, S.R.; Venkatasubramanian, P. Killing of enteric bacteria in drinking water by a copper device for use in the home: Laboratory evidence. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 819–822. [Google Scholar] [CrossRef]
- Sharan, R.; Chhibber, S.; Attri, S.; Reed, R.H. Inactivation and sub-lethal injury of Escherichia coli in a copper water storage vessel: Effect of inorganic and organic constituents. Antonie Van Leeuwenhoek 2010, 98, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper- products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef]
- Gabbay, J.; Borkow, G.; Mishal, J.; Magen, E.; Zatcoff, R.; Shemer-Avni, Y. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323–335. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pinheiro, J.P.; Cancio, I.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Effects of copper nanoparticles exposure in the musselmytilus galloprovincialis. Environ. Sci. Technol. 2011, 45, 9356–9362. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.; Saho, L.; Sanjib, P. Excess copper induced oxidative stress and the response of antioxidants in rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Zhou, H.; Adeleye, A.; Wang, H.; Ortiz, C.; Mazer, S.; Keller, A. GC-TOF-MS based metabolomics and ICP-MS based metallomics of cucumber (cucumis sativus) fruits reveal alteration of metabolites profile and biological pathway disruption induced by nano copper. Environ. Sci. Nano 2016, 3, 1114–11121. [Google Scholar] [CrossRef]
- Zhao, L.; Ortiz, C.; Adeleye, A.; Hu, Q.; Zhou, H.; Huang, Y.; Keller, A. Metabolomics to detect response of lettuce (lactuca sativa) to Cu(OH)2 nanopesticides: Oxidative stress response and detoxification mechanism. Environ. Sci. Technol. 2016, 50, 9697–9707. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, K.; Sadrieh, N.; Savage, N.; Adair, P.; Bronaugh, R. Research strategies for safety evaluation of nanomaterials, part VII: Evaluating consumer exposure to nanoscale materials. Toxicol. Sci. 2006, 91, 14–19. [Google Scholar] [CrossRef]
- Chibber, S.; Shanker, R. Can CuO nanoparticles lead to epigenetic regulation of antioxydent enzyme system? J. Appl. Toxicol. 2017, 37, 84–91. [Google Scholar] [CrossRef]
- Sufian, M.M.; Khattak, J.Z.K.; Yousaf, S.; Rana, M.S. Safety issues associatewith the use of nanoparticles in human body. Photodiag. Photodynam. Ther. 2017, 19, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Khandan, A. Safety, regulatory issues, long-term biotoxicity, and the processing environment. In Nanobiomaterials Science, Development and Evaluation; Razavi, M., Thakor, A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 261–279. [Google Scholar] [CrossRef]
- Madl, A.; Pinkerton, K. Health effects of inhaled engineered and incidental nanoparticles. Crit. Rev. Toxicol. 2009, 39, 629–658. [Google Scholar] [CrossRef]
- Lee, I.; Park, S.; Lim, J.; Shin, I.; Moon, C.; Kim, S.; Hero, J.; Kim, J. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomedicine 2016, 11, 2883–2900. [Google Scholar] [PubMed]
- Pra, D.; Franke, S.I.R.; Giulian, R.; Yoneama, M.L.; Dias, J.F.; Erdtmann, B.; Henriques, J.A.P. Genotoxicity and mutagenicity of iron and copper in mice. Biometals 2008, 21, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Nawaz, M.; Manzl, C.; Lacher, V.; Krumschnabel, G. Copperinduced stimulation of extracellular signal-regulated kinase in trout hepatocytes: The role of reactive oxygen species, Ca2+, and cell energetics and the impact of extracellular signalregulated kinase signaling on apoptosis and necrosis. Toxicol. Sci. 2006, 92, 464–475. [Google Scholar] [CrossRef]
- Pandit, A.; Bhave, S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am. J. Clin. Nutr. 1996, 63, 830S–835S. [Google Scholar] [CrossRef]
- Dietrich, A.M.; Glindemann, D.; Pizarro, F.; Gidi, V.; Olivares, M.; Araya, M.; Edwards, M. Health and aesthetic impacts of copper corrosion on drinking water. Water Sci. Technol. 2004, 49, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wen, X.; Hu, Y.; Zhang, X.; Wang, D.; Yin, S. Copper nanoparticles induced oxidation stress, cell apoptosis and immune response in the liver of juvenile Takifugu fasciatus. Fish Shellfish Immunol. 2019, 84, 648–655. [Google Scholar] [CrossRef]
- Cheng, T.C.; Guida, V.G.; Butler, M.S.; Howland, K.H. Use of copper compounds in shellfish depuration and disease control in mariculture. Incra Proj. 2001, 262B, 31. [Google Scholar]
- Chandra, S.; Raizada, S.; Tyagi, M.; Gautam, A. Synthesis, spectroscopic, and antimicrobial studies on bivalent nickel and copper complexes of bis (thiosemicrbazone). Bioinorg. Chem. Appl. 2007, 51483. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N. Nanoparticles: Environmental problems or problem solvers? Bioscience 2018, 68, 241–246. [Google Scholar] [CrossRef]
- Lewis, A.; Keevil, C.W. Antibacterial Properties of Alloys and Its Alloys in HVAC&R Systems; International Copper Association: New York, NY, USA, 2004. [Google Scholar]
- Thurman, R.; Gerba, C.A. Small sample of research and articles supporting the efficacy of silver as an antimicrobial agent follow: The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. CRC Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Rosenberg, M.; Vija, H.; Kahru, A.; Keevil, C.W.; Ivask, A. Rapid in situ assessment of Cu-ion mediated effects and antibacterial efficacy of copper surfaces. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of copper surface toxicity in Escherichia coli O157: H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram—Positive bacteria. Environ. Microbiol. 2012, 14, 1730–1743. [Google Scholar] [CrossRef]
- Li, M.; Ma, Z.; Zhu, Y.; Yao, M.; Chu, X.; Wang, X.; Yang, K.; Yang, M.; Zhang, Y.; Mao, C. Toward a molecular understanding of the antibacterial mechanism of copper—Bearing titanium alloys against staphylococcus aureus. Adv. Healthc. Mater. 2016, 5, 557–566. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 2012, 1, 46–52. [Google Scholar] [CrossRef]
- Langley, S.; Beveridge, T.J. Effect of O-side-chain-lipopolysaccharide chemistry on metal binding. Appl. Environ. Microbiol. 1999, 65, 489–498. [Google Scholar] [CrossRef]
- Fang, L.; Cai, P.; Chen, W.; Liang, W.; Hong, Z.; Huang, Q. Impact of cell wall structure on the behavior of bacterial cells in the binding of copper and cadmium. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 50–55. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Peters, K.; Pazos, M.; Edoo, Z.; Hugonnet, J.; MArtorana, A.M.; Polissi, A.; VanNieuwenhze, M.S.; Arthur, M.; Vollmer, W. Copper inhibits peptidoglycan LD-transpeptidases suppressing β-lactam resistance due to bypass of penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10786–10791. [Google Scholar] [CrossRef]
- Warnes, S.L.; Keevil, C.W. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Kuehl, R.; Brunetto, P.S.; Woisching, A.-K.; Varisco, M.; Rajacic, Z.; Vosbeck, J.; Terraciano, L.; Fromm, K.M.; Khanna, N. Preventing implant-associated infections by silver coating. Antimicrob. Agent Chemother. 2016, 60, 2467–2475. [Google Scholar] [CrossRef]
- Gibbard, J. Public health aspects of the treatment of water and beverages with silver. Am. J. Public Health Nations Health 1937, 27, 112–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Hein, C.; Mücklich, F.; Solioz, M. Killing of bacteria by copper, cadmium, and silver surfaces reveals relevant physicochemical parameters. Biointerphases 2017, 12, 020301. [Google Scholar] [CrossRef]
- Knobloch, J.K.; Tofem, S.; Kunz, W.; Schutze, S.; Riecke, M.; Solbach, W.; Wuske, T. “Life-like” assessment of antimicrobial surfaces by a new touch transfer assay displays strong superiority of a copper alloy compared to silver containing surfaces. PLoS ONE 2017, 12, e0187442. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact-killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Champagne, V.K.; Helfritch, D.J.A. Demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.D. The Direct Medical Cost of Healthcare-Associatedinfections in U.S Hospitals and the Benefits of Prevention; Division of Healthcare Quality Promotion, National Centre for reparedness Detection and Control of Infectious Diseases, Centre for Disease Control and Prevention: Atlanta, GA, USA, 2009; No CS200891-A.
| Sl. No. | Country | No of Cases Per Annum | No of Mortalities Per Annum | Expenditure Per Annum (USD) | Reference(s) |
|---|---|---|---|---|---|
| 1 | USA | 687,000 | 72,000 | 35 to 45 billion | [28] |
| 2 | Europe | 3.8 million | 90,000 | 8.3 billion | [31] |
| 3 | England | 834,000 | 28,500 | 3.71 billion | [33] |
| 4 | Australia | 165,000 | Unknown | 4.7 billion | [34,35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraham, J.; Dowling, K.; Florentine, S. Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections? Materials 2021, 14, 3444. https://doi.org/10.3390/ma14133444
Abraham J, Dowling K, Florentine S. Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections? Materials. 2021; 14(13):3444. https://doi.org/10.3390/ma14133444
Chicago/Turabian StyleAbraham, Joji, Kim Dowling, and Singarayer Florentine. 2021. "Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections?" Materials 14, no. 13: 3444. https://doi.org/10.3390/ma14133444
APA StyleAbraham, J., Dowling, K., & Florentine, S. (2021). Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections? Materials, 14(13), 3444. https://doi.org/10.3390/ma14133444







