Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Powders and Preparing the Composite Powder
2.2. Pulse Plasma Sintering (PPS) Process
2.3. Experimental Techniques
3. Results and Discussion
3.1. Initial Powder Characterization
3.2. NiAl–Al2O3 Composite Powder Characterization
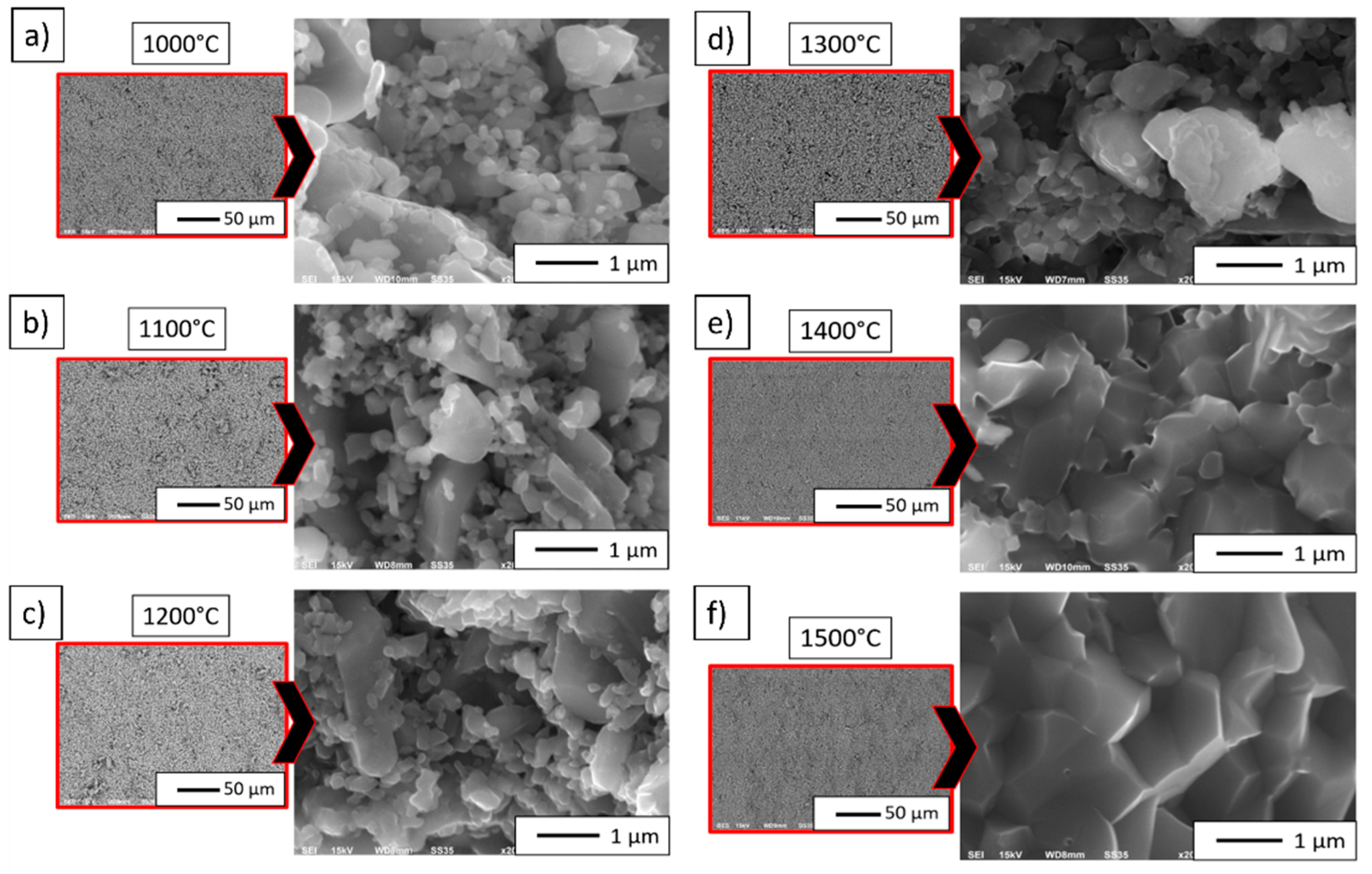
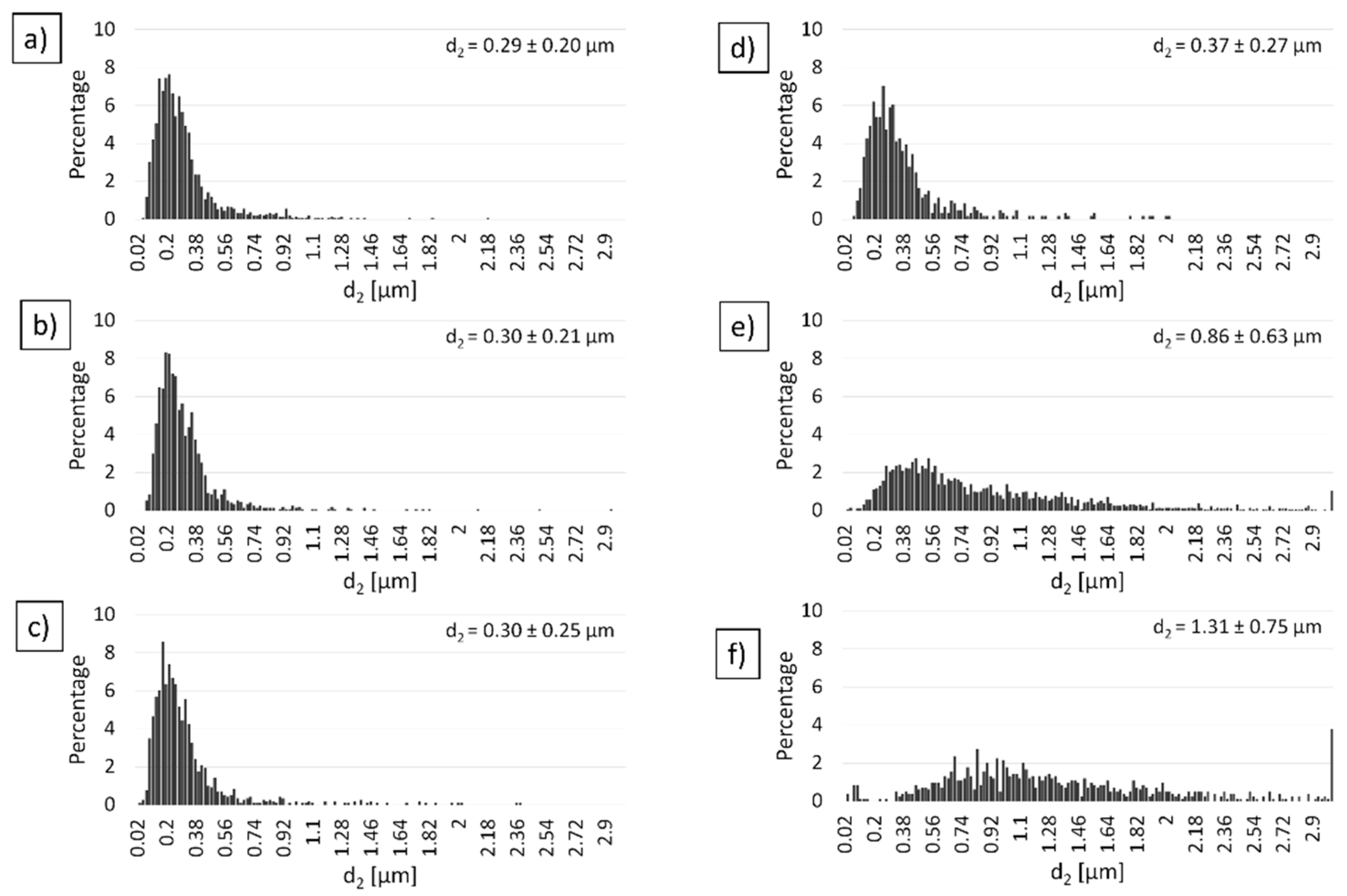
3.3. Characterization of Al2O3 Powder Compacted by PPS
| Author | Equation | Type of Crack System |
|---|---|---|
| Niihara [52] | Radial—median | |
| Anstis [53] | Radial—median | |
| Lankford [54] | Any kind |
| Sintering Temperature | Results of the Helium Pycnometer Measurements and Archimedes Measurements | ||||
|---|---|---|---|---|---|
| Density Determined by Helium Pycnometer [g/cm3] | Apparent Density [g/cm3] | Relative Density [%] | Open Porosity [%] | Water Absorptivity [%] | |
| 1000 °C | 3.93 | 2.6168 | 66.58 | 31.50 | 12.04 |
| 1100 °C | 3.93 | 2.7309 | 69.49 | 28.23 | 10.34 |
| 1200 °C | 3.93 | 2.9364 | 74.72 | 24.29 | 8.27 |
| 1300 °C | 3.93 | 3.2498 | 82.69 | 16.79 | 5.17 |
| 1400 °C | 3.93 | 3.8088 | 96.92 | 0.81 | 0.21 |
| 1500 °C | 3.93 | 3.9298 | 99.99 | - | - |
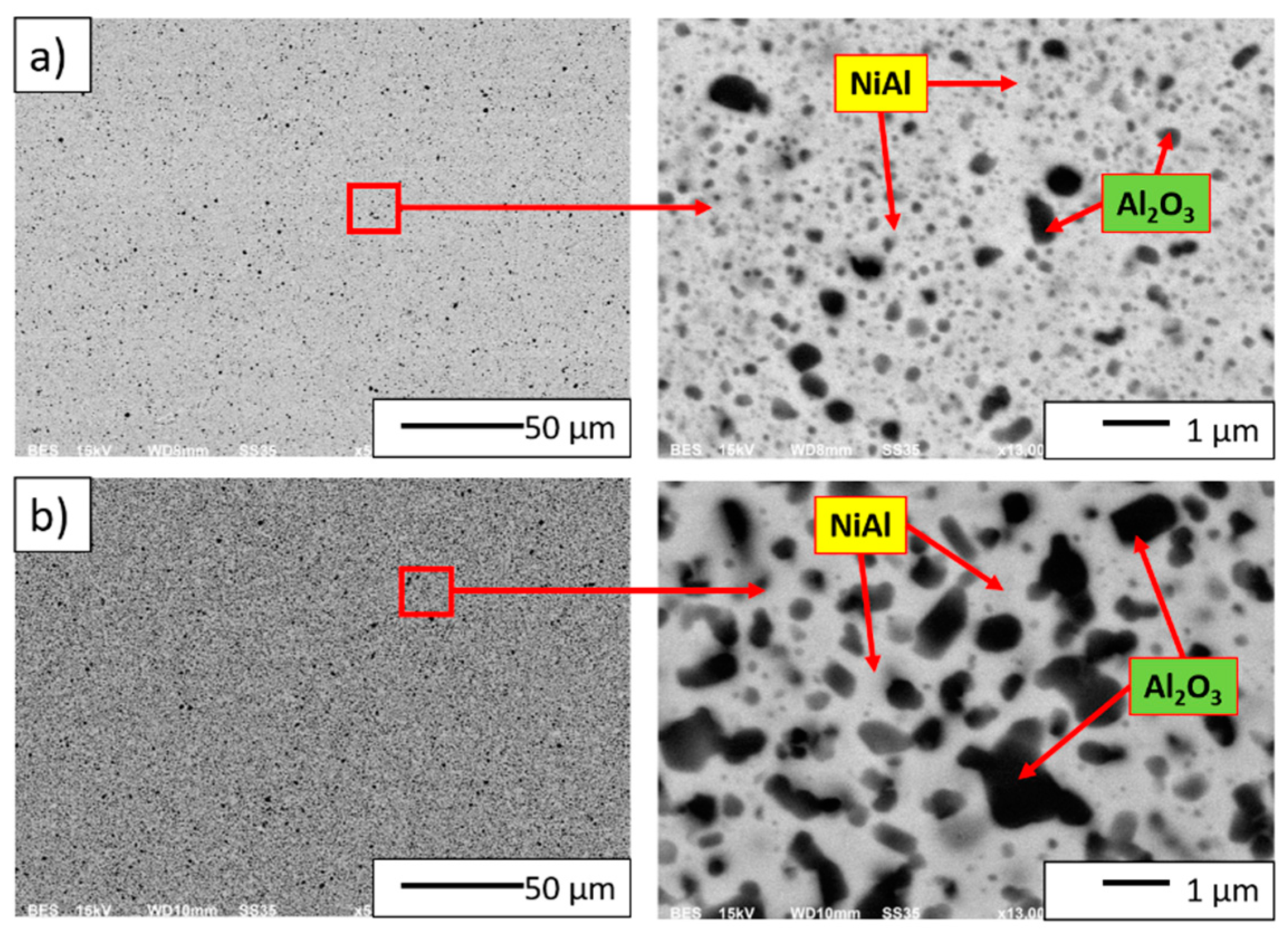
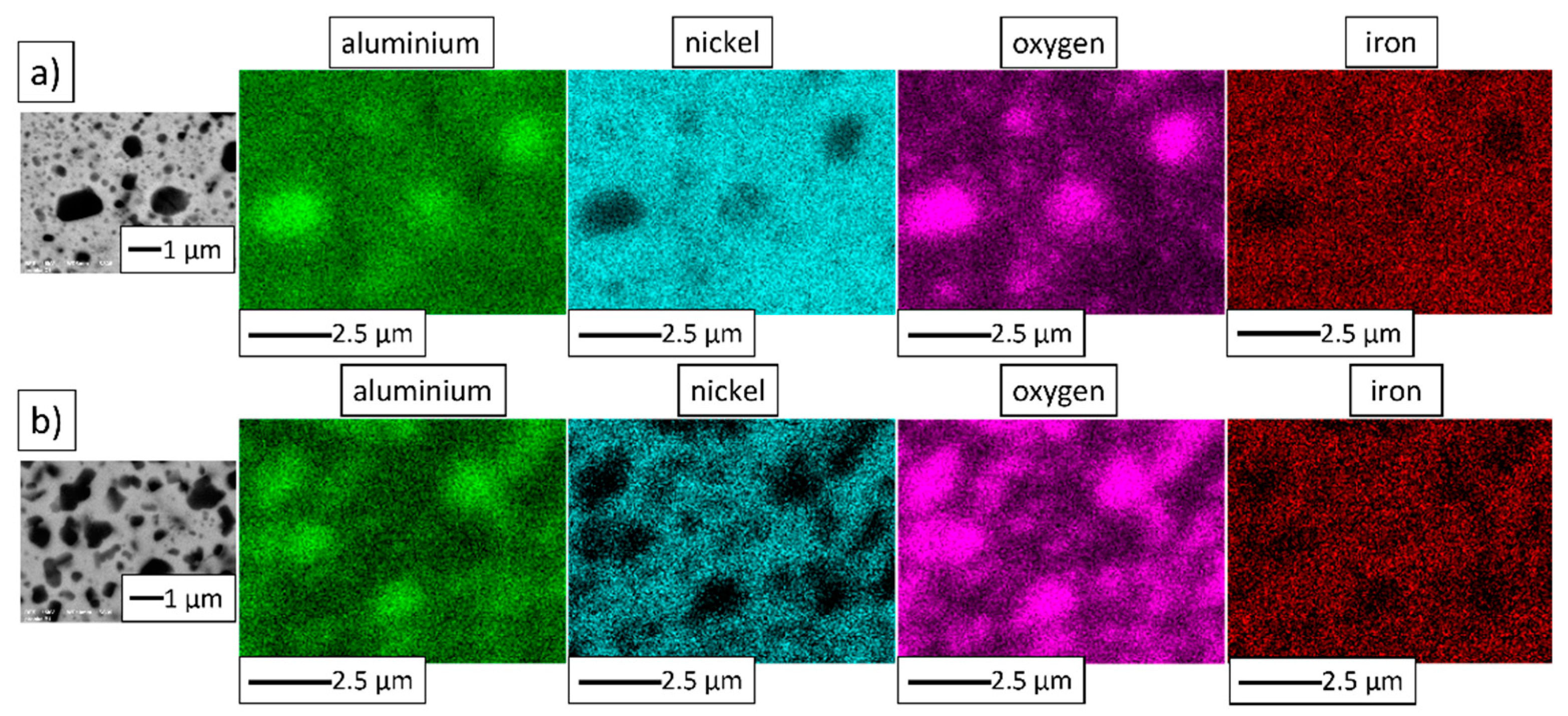
3.4. Characterization of NiAl–Al2O3 Powder Compacted by PPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akter, N.; Becerril-Gonzalez, J.; Karabiyik, M.; Alam, F.; Pala, N.; Oskam, G.; Arés-Muzio, O. FDTD modeling of sputtered Mo–Al2O3 nanocomposites. Sol. Energy Mater. Sol. Cells 2021, 225, 111027. [Google Scholar] [CrossRef]
- Okumus, S.C. Microstructural and mechanical characterization of plasma sprayed Al2O3–TiO2 composite ceramic coating on Mo/cast iron substrates. Mater. Lett. 2005, 59, 3214–3220. [Google Scholar] [CrossRef]
- Gong, F.; Zhao, J.; Liu, G.; Ni, X. Design and fabrication of TiB2–TiC–Al2O3 gradient composite ceramic tool materials reinforced by VC/Cr3C2 additives. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Cui, E.; Sun, Z.; Yu, H. Grain growth kinetics and grain refinement mechanism in Al2O3/WC/TiC/graphene ceramic composite. J. Eur. Ceram. Soc. 2021, 41, 1391–1398. [Google Scholar] [CrossRef]
- Fenga, T.; Zhenga, W.; Chena, W.; Shia, Y.; Fu, Y.Q. Enhanced interfacial wettability and mechanical properties of Ni2Al2O3/Cu ceramic matrix composites using spark plasma sintering of Ni coated Al2O3 powders. Vacuum 2021, 184, 109938. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, G.; Li, Z.; Chen, L.; Han, W.; Du, Z. A novel approach to fabricate ceramic/metal interpenetrating phase composites by ultrasonic-assisted spontaneous infiltration. Ceram. Int. 2021, 47, 2903–2907. [Google Scholar] [CrossRef]
- Das, S.; Das, S. Properties for Polymer, Metal and Ceramic Based Composite Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Konopka, K.; Bucki, J.; Gierlotka, S.; Zielinski, W.; Kurzydłowski, K. Characterization of the metal particles fraction in ceramic matrix composites fabricated under high pressure. Mater. Charact. 2006, 56, 394–398. [Google Scholar] [CrossRef]
- Rebillat, F. Ceramic Matrix Fiber Composites. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 317–339. [Google Scholar]
- Gunasekaran, T.; Vijayan, S.; Prakash, P.; Satishkumar, P. Mechanical properties and characterization of Al7075 aluminum alloy based ZrO2 particle reinforced metal-matrix composites. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Balokhonov, R.; Romanova, V.; Kulkov, A. Microstructure-based analysis of deformation and fracture in metal-matrix composite materials. Eng. Fail. Anal. 2020, 110, 104412. [Google Scholar] [CrossRef]
- Akçamlı, N.; Şenyurt, B. Fabrication and characterization of in-situ Al3Ni intermetallic and CeO2 particulate-reinforced aluminum matrix composites. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Fan, X.; Huang, W.; Zhou, X.; Zou, B. Preparation and characterization of NiAl–TiC–TiB2 intermetallic matrix composite coatings by atmospheric plasma spraying of SHS powders. Ceram. Int. 2020, 46, 10512–10520. [Google Scholar] [CrossRef]
- Lu, Y.; Watanabe, M.; Miyata, R.; Nakamura, J.; Yamada, J.; Kato, H.; Yoshimi, K. Microstructures and mechanical properties of TiC-particulate-reinforced Ti–Mo–Al intermetallic matrix composites. Mater. Sci. Eng. A 2020, 790, 139523. [Google Scholar] [CrossRef]
- Shi, S.; Cho, S.; Goto, T.; Sekino, T. The effects of sintering temperature on mechanical and electrical properties of Al2O3/Ti composites. Mater. Today Commun. 2020, 25, 101522. [Google Scholar] [CrossRef]
- Li, H.; Motamedi, P.; Hogan, J. Characterization and mechanical testing on novel (γ + α2)—TiAl/Ti3Al/Al2O3 cermet. Mater. Sci. Eng. A 2019, 750, 152–163. [Google Scholar] [CrossRef]
- Taotao, A. Microstructure and Mechanical Properties of In-situ Synthesized Al2O3/TiAl Composites. Chin. J. Aeronaut. 2008, 21, 559–564. [Google Scholar] [CrossRef]
- Lu, X.; Li, J.; Chen, X.; Ran, C.; Wang, Y.; Liu, B.; Liu, Y.; Rashad, M.; Pan, F. Grinding mechanism and mechanical properties of the in-situ synthesized Al2O3/TiAl composites. Ceram. Int. 2019, 45, 12113–12121. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, C.; Chen, H.; He, J. Pinning effect of reactive elements on structural stability and adhesive strength of environmental sulfur segregation on Al2O3/NiAl interface. Scr. Mater. 2020, 188, 174–178. [Google Scholar] [CrossRef]
- Troncy, R.; Bonnet, G.; Pedraza, F. Microstructural characterization of NiAl–Al2O3 composite materials obtained by in situ aluminothermic reduction of NiO for potential coating applications. Mater. Chem. Phys. 2020, 251, 123124. [Google Scholar] [CrossRef]
- Beyhaghi, M.; Khaki, J.V.; Manawan, M.; Kiani-Rashid, A.; Kashefi, M.; Jonsson, S. In-situ synthesis and characterization of nano-structured NiAl-Al2O3 composite during high energy ball milling. Powder Technol. 2018, 329, 95–106. [Google Scholar] [CrossRef]
- Mehrizi, M.Z.; Mofrad, S.S. Synthesis of NiAl/TiC–Al2O3 composite by mechanically activated combustion synthesis. Ceram. Int. 2021, 47, 9258–9263. [Google Scholar] [CrossRef]
- Beyhaghi, M.; Kiani-Rashid, A.R.; Khaki, J.V.; Kashefi, M.; Jonsson, S. Influences of mechanical activation and heating rate on reaction processes in combustion synthesis of NiAl-Al2O3 composites. Powder Technol. 2019, 346, 237–247. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Yeh, C.-T.; Tuan, W.-H. Heat capacity of Al2O3-NiAl composites, a key parameter for thermal management. Ceram. Int. 2017, 43, S705–S709. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Gottstein, G. Long term stability and mechanical properties of Al2O3–NiAl composites reinforced with partially fragmented long fibers. Mater. Sci. Eng. A 2011, 528, 7790–7800. [Google Scholar] [CrossRef]
- Abe, O.; Ohwa, Y. Oxidation of NiAl/Al2O3 composites for controlled development of surface layers and toughening. Solid State lonics 2004, 172, 553–556. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Jimba, Y.; Kondo, S.; Yu, H.; Wang, H.; Okuno, Y.; Kasada, R. Effect of mechanically alloyed sintering aid on sinterability of TiB2. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Zuo, K.-S.; Xi, S.-Q.; Zhou, J.-E. Effect of sintering methods on properties of Cu-Based composite reinforced by mechanically alloyed Cu–Mo–C powder. Results Mater. 2021, 9, 100153. [Google Scholar] [CrossRef]
- Macía, E.; García-Junceda, A.; Serrano, M.; Hong, S.; Campos, M. Effect of mechanical alloying on the microstructural evolution of a ferritic ODS steel with (Y–Ti–Al–Zr) addition processed by Spark Plasma Sintering (SPS). Nucl. Eng. Technol. 2021. [Google Scholar] [CrossRef]
- Krasnowski, M.; Gierlotka, S.; Kulik, T. NiAl-B composites with nanocrystalline intermetallic matrix produced by mechanical alloying and consolidation. Adv. Powder Technol. 2019, 30, 2742–2750. [Google Scholar] [CrossRef]
- Boehlert, C.J. Powder Metallurgy Processed Ti-Based Intermetallic Alloys. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Han, Y.; Jiang, F.; Lin, C.; Yuan, D.; Huang, H.; Wang, E.; Wang, Z.; Guo, C. Microstructure and mechanical properties of continuous ceramic SiC and shape memory alloy NiTi hybrid fibers reinforced Ti-Al metal-intermetallic laminated composite. J. Alloys Compd. 2017, 729, 1145–1155. [Google Scholar] [CrossRef]
- Makino, A. Fundamental aspects of the heterogeneous flame in the self-propagating high-temperature synthesis (SHS) process. Prog. Energy Combust. Sci. 2001, 27, 1–74. [Google Scholar] [CrossRef]
- Shishkovsky, I.V. 11—Laser-controlled intermetallics synthesis during surface cladding. In Laser Surface Engineering; Lawrence, J., Waugh, D.G., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Sawston, UK, 2015; pp. 237–286. [Google Scholar]
- Wieczorek-Ciurowa, K. 9—Mechanochemical synthesis of metallic–ceramic composite powders. In High-Energy Ball Milling; Sopicka-Lizer, M., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 193–223. [Google Scholar]
- Zygmuntowicz, J.; Falkowski, P.; Wachowski, M.; Cymerman, K.; Piotrkiewicz, P.; Kaszuwara, W. Effect of the sintering temperature on microstructure and properties of Al2O3–Cu–Ni hybrid composites obtained by PPS. Int. J. Appl. Ceram. Technol. 2020, 17, 1731–1741. [Google Scholar] [CrossRef]
- Cymerman, K.; Oleszak, D.; Rosinski, M.; Michalski, A. Structure and mechanical properties of TiB2/TiC–Ni composites fabricated by pulse plasma sintering method. Adv. Powder Technol. 2018, 29, 1795–1803. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 18754:2013. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Density and Apparent Porosity; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Michalski, J.; Wejrzanowski, T.; Pielaszek, R.; Konopka, K.; Łojkowski, W.; Kurzydłowski, K.J. Application of image analysis for characterization of powders. Mater. Sci. Pol. 2005, 23, 79–86. [Google Scholar]
- Wejrzanowski, T.; Spychalski, W.; Rożniatowski, K.; Kurzydłowski, K. Image Based Analysis of Complex Microstructures of Engineering Materials. Int. J. Appl. Math. Comput. Sci. 2008, 18, 33–39. [Google Scholar] [CrossRef]
- Krasnowski, M.; Gierlotka, S.; Ciołek, S.; Kulik, T. Nanocrystalline NiAl intermetallic alloy with high hardness produced by mechanical alloying and hot-pressing consolidation. Adv. Powder Technol. 2019, 30, 1312–1318. [Google Scholar] [CrossRef]
- Suryanarajana, C.; Grant Norton, M. X-ray Diffraction. A Practical Approach; Springer Science+Business Media: New York, NY, USA, 1998. [Google Scholar]
- Maiti, K.; Sil, A. Relationship between fracture toughness characteristics and morphology of sintered Al2O3 ceramics. Ceram. Int. 2010, 36, 2337–2344. [Google Scholar] [CrossRef]
- Ouyang, Y.; Bai, L.; Sun, Z.; Ding, F.; Yuan, F. A new strategy for dense Al2O3 ceramics by spherical powders prepared via thermal plasma. Ceram. Int. 2019, 45, 2012–2019. [Google Scholar] [CrossRef]
- Xu, J.; Lang, J.; An, D.; Liu, J.; Hu, Z.; Xie, Z. A novel alternating current-assisted sintering method for rapid densification of Al2O3 ceramics with ultrahigh flexural strength. Ceram. Int. 2020, 46, 5484–5488. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, J.; Li, J.; Liu, J.; Zhao, K.; Liu, D.; An, L. Oscillatory pressure sintering of Al2O3 ceramics. Ceram. Int. 2020, 46, 15670–15673. [Google Scholar] [CrossRef]
- Žmak, I.; Ćorić, D.; Mandić, V.; Ćurković, L. Hardness and Indentation Fracture Toughness of Slip Cast Alumina and Alumina-Zirconia Ceramics. Materials 2019, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Bysakh, S.; Muraleedharan, K.; Rao, T.N.; Sundaresan, R. Spark Plasma Sintering of Magnesia-Doped Alumina with High Hardness and Fracture Toughness: Spark Plasma Sintering of Magnesia Doped Alumina. J. Am. Ceram. Soc. 2007, 91, 203–208. [Google Scholar] [CrossRef]
- Belmonte, M.; Nieto, M.I.; Osendi, M.I.; Miranzo, P. Influence of the SiC grain size on the wear behaviour of Al2O3/SiC composites. J. Eur. Ceram. Soc. 2006, 26, 1273–1279. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of KIc of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1982, 1, 13–16. [Google Scholar] [CrossRef]
- Anstis, G.; Chantikul, P.; Lawn, B.; Marshall, D. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Lankford, J. Indentation microfracture in the Palmqvist crack regime: Implications for fracture toughness evaluation by the indentation method. J. Mater. Sci. Lett. 1982, 1, 493–495. [Google Scholar] [CrossRef]
- Michalski, A.; Jaroszewicz, J.; Rosiński, M.; Siemiaszko, D. NiAl–Al2O3 composites produced by pulse plasma sintering with the participation of the SHS reaction. Intermetallics 2006, 14, 603–606. [Google Scholar] [CrossRef]
- Rahaman, M.N. Sintering of Ceramics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
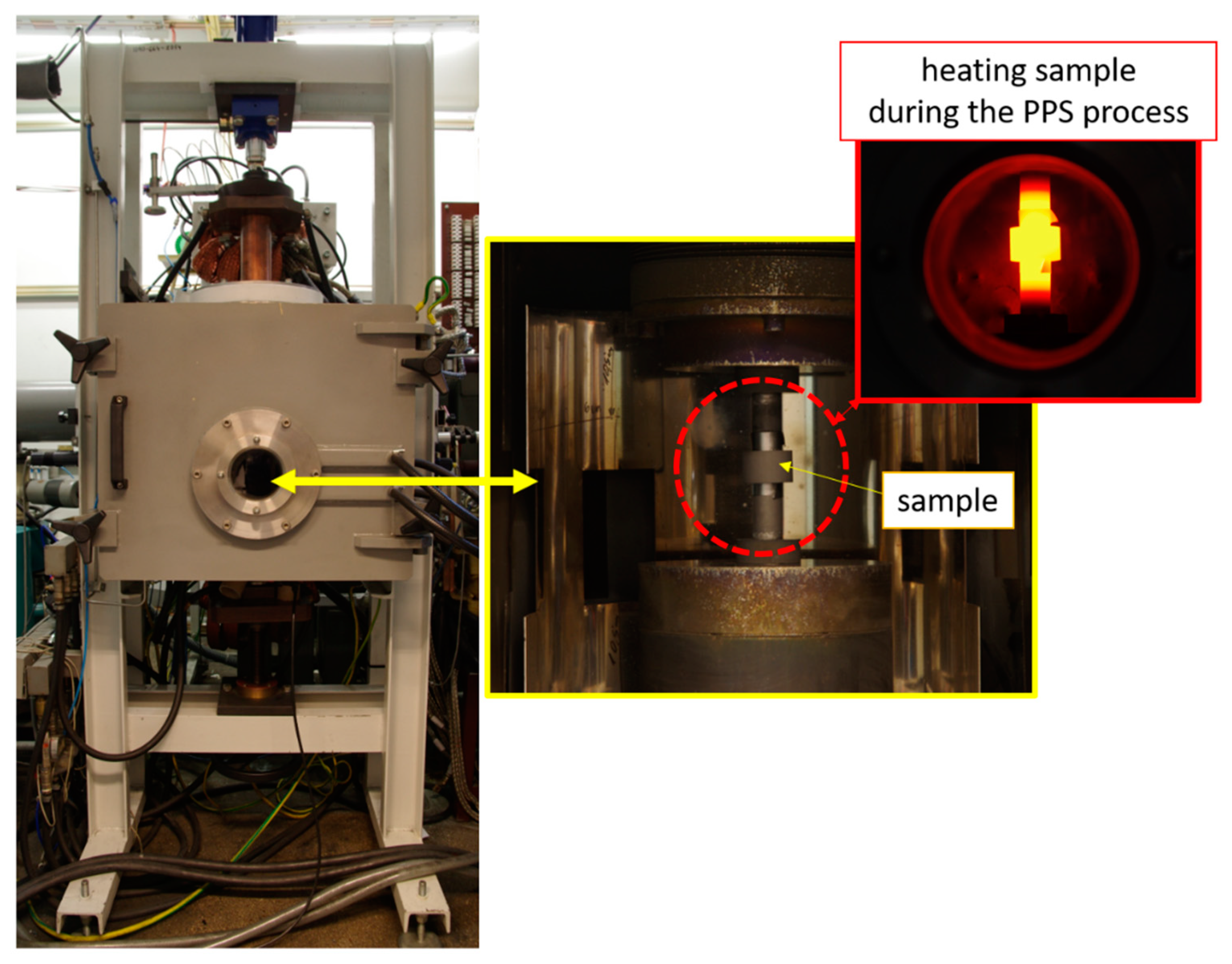
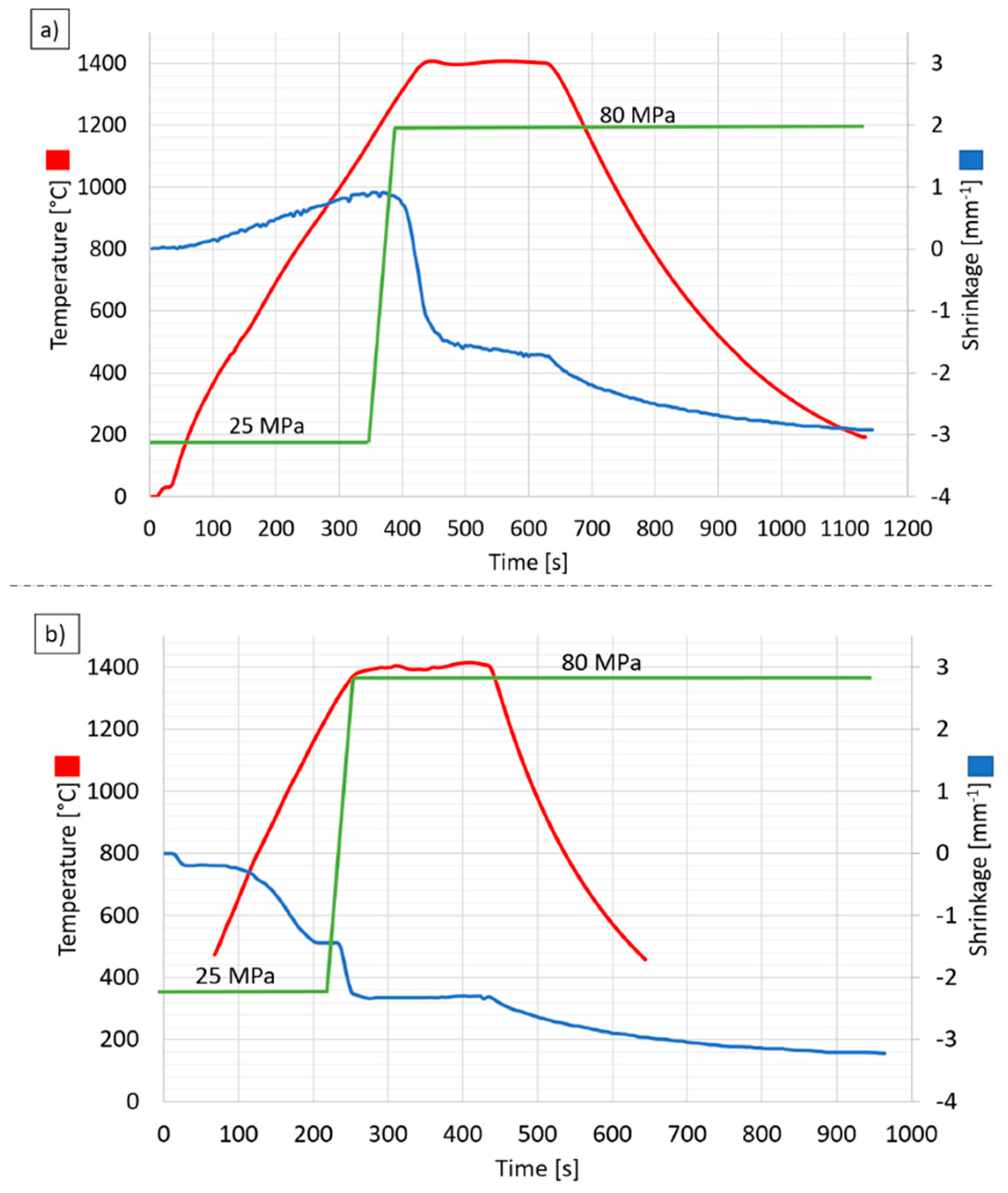
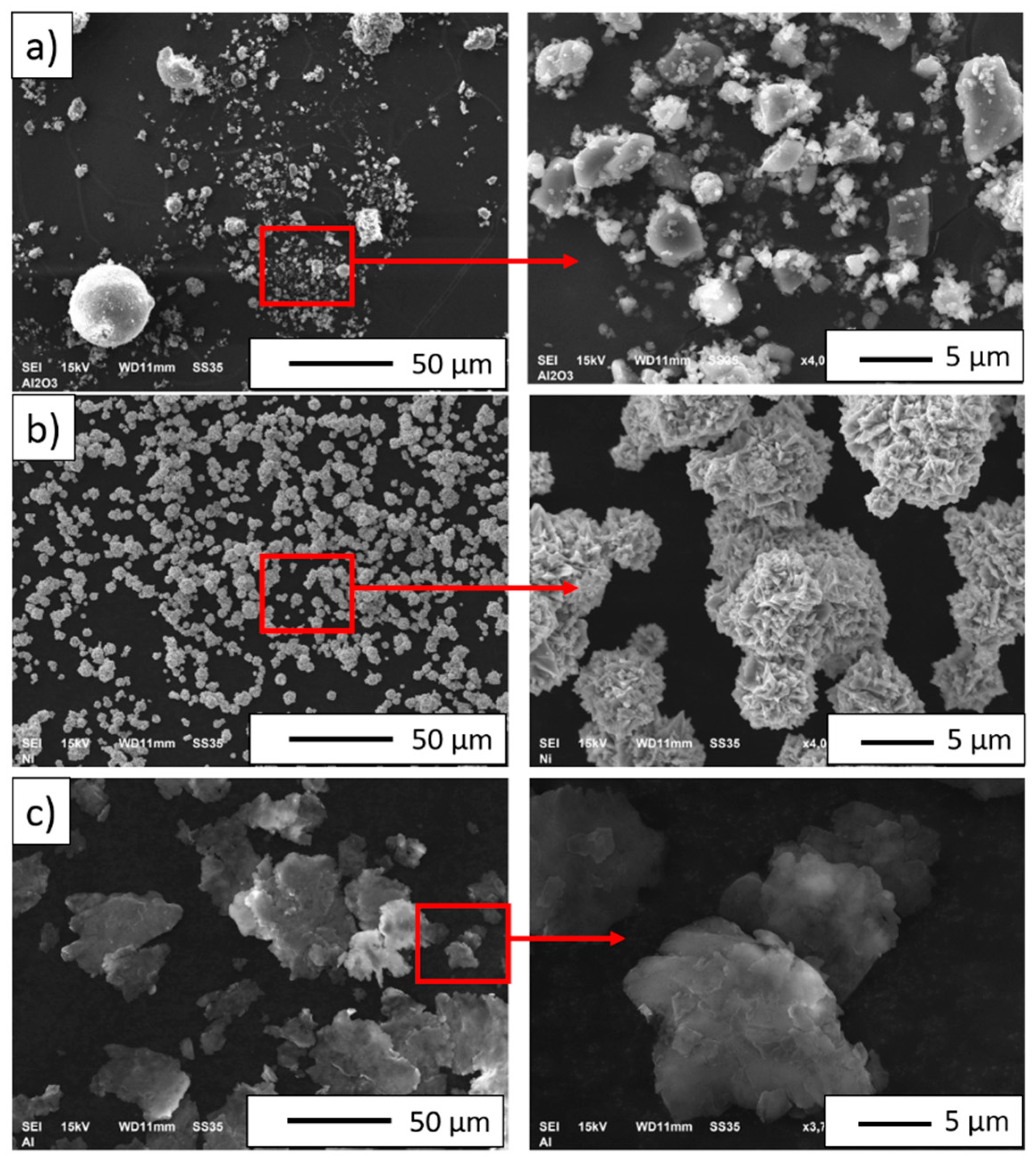
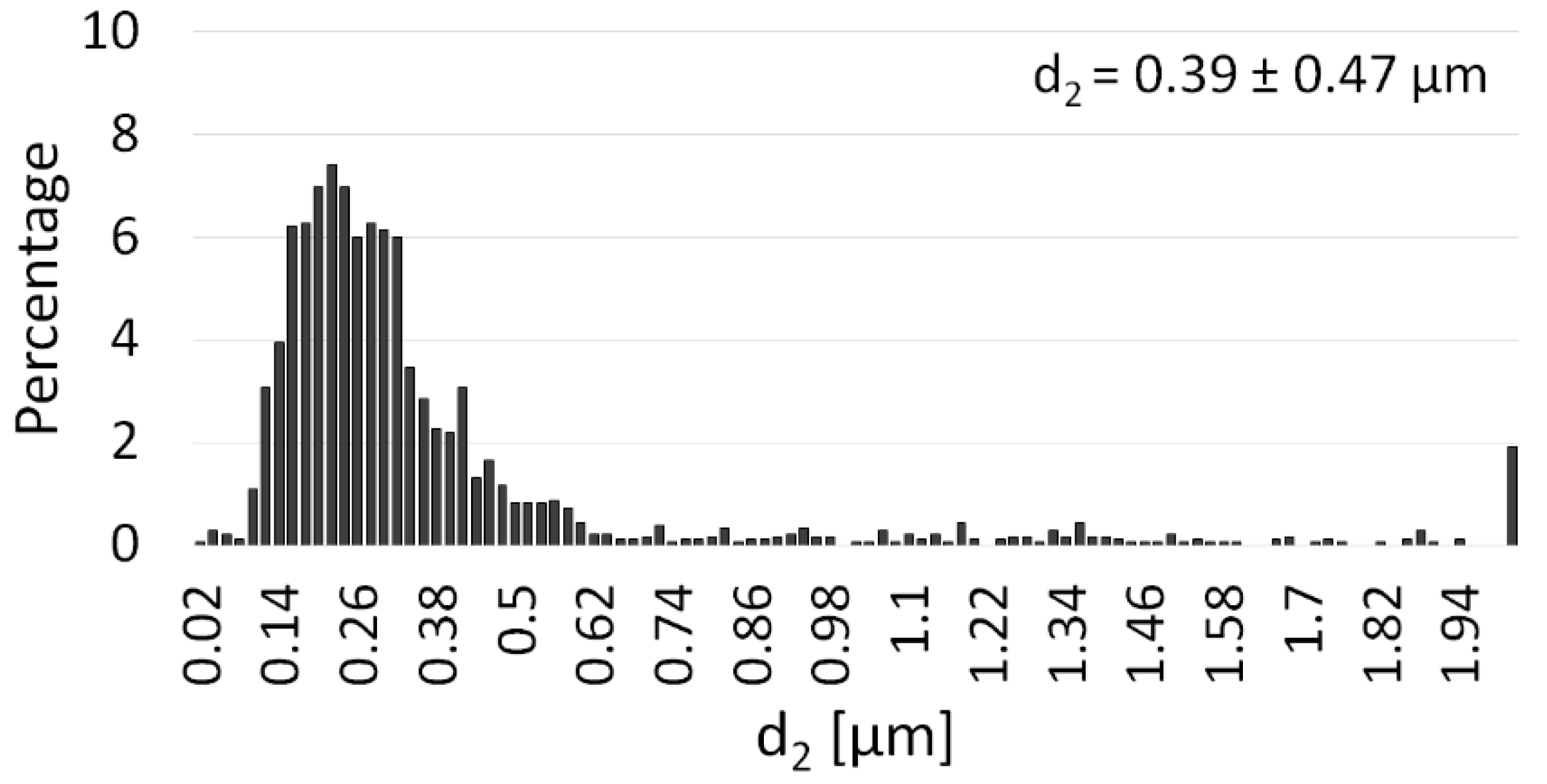
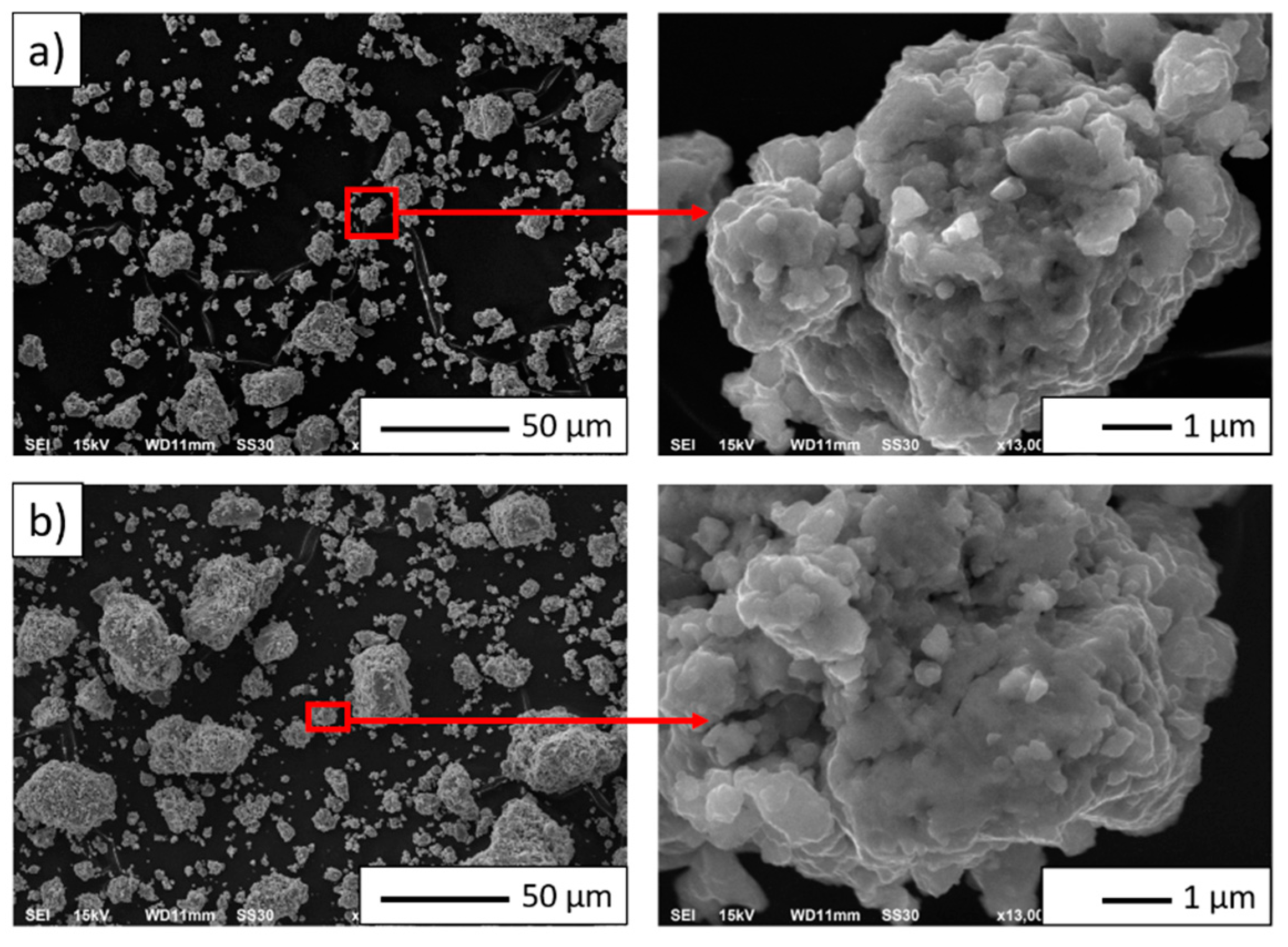
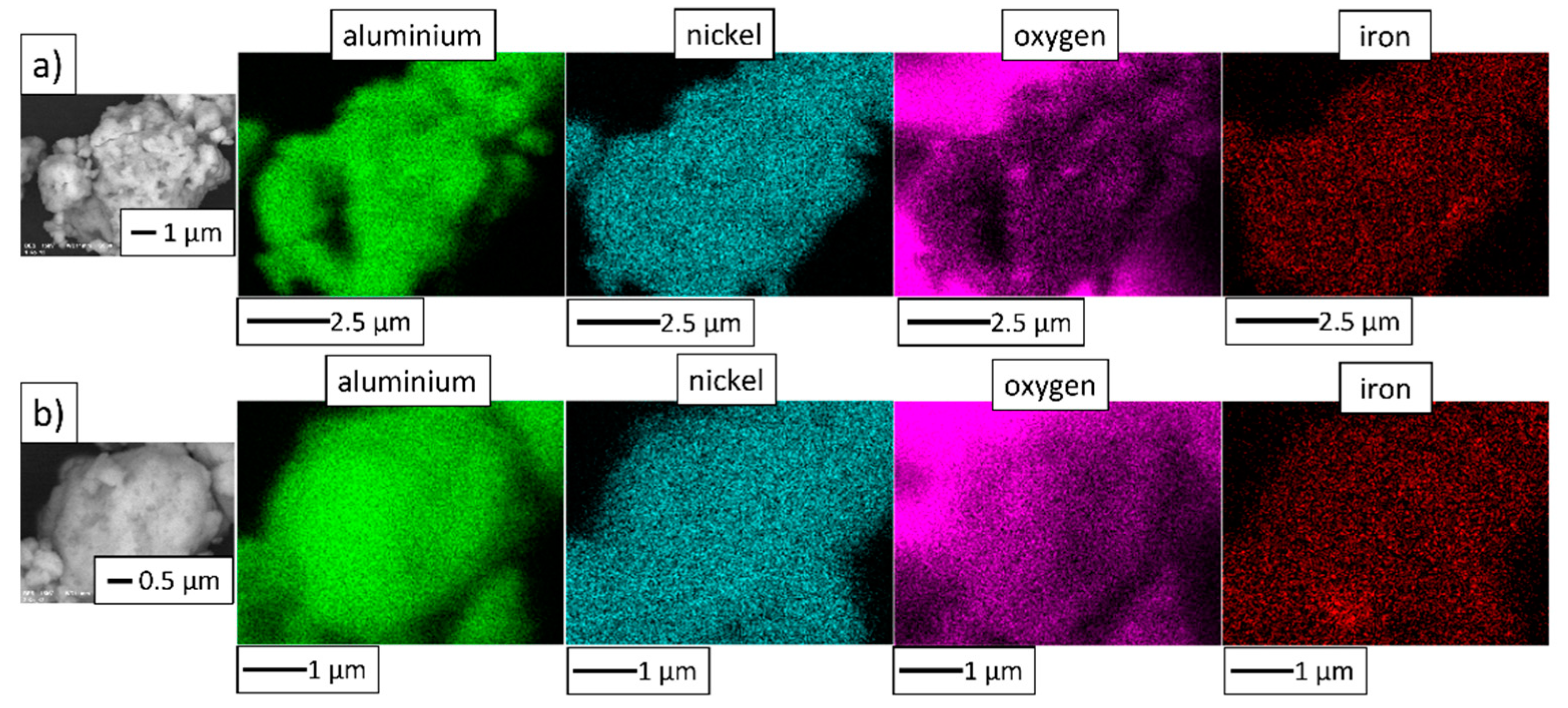
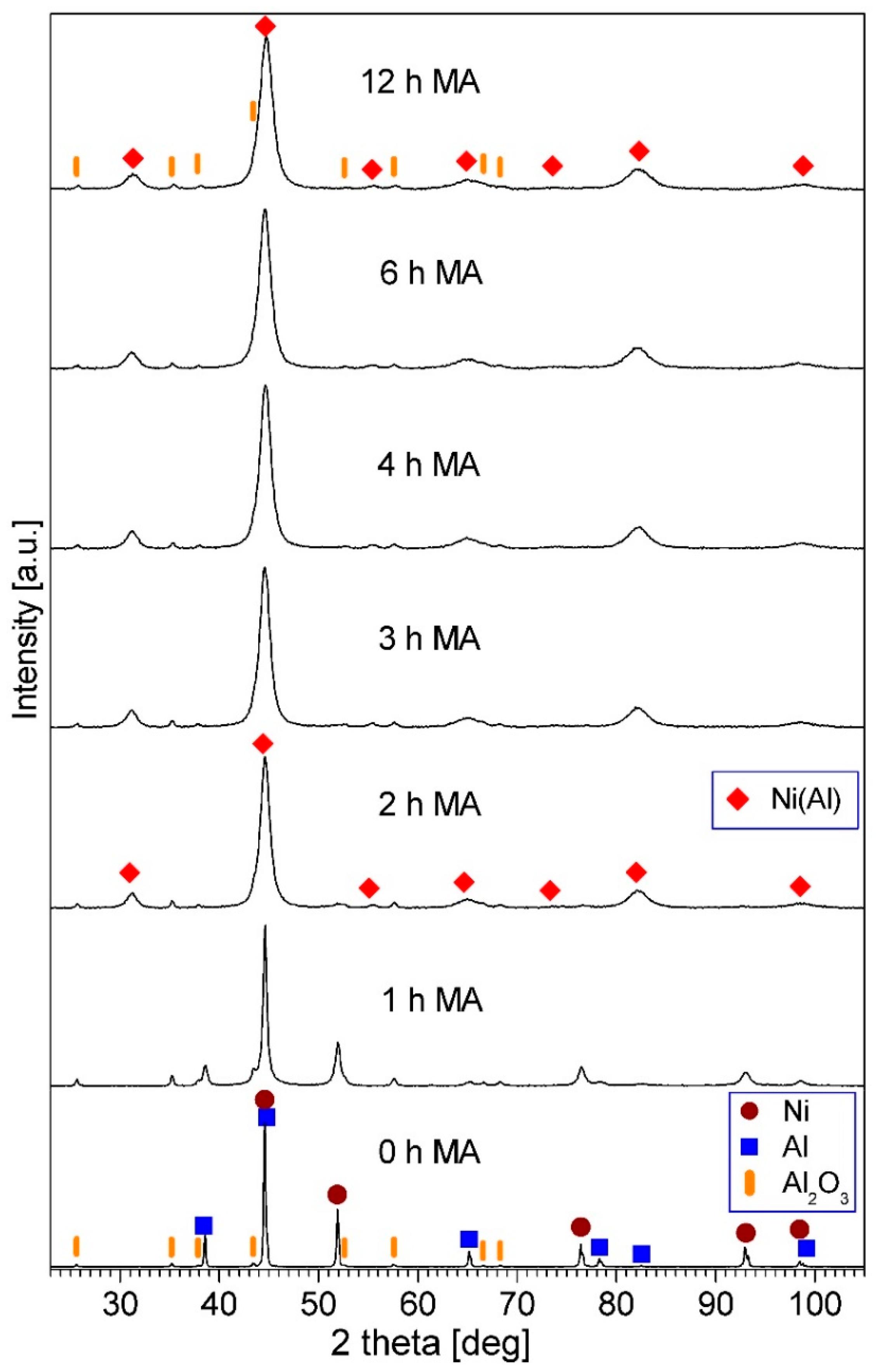
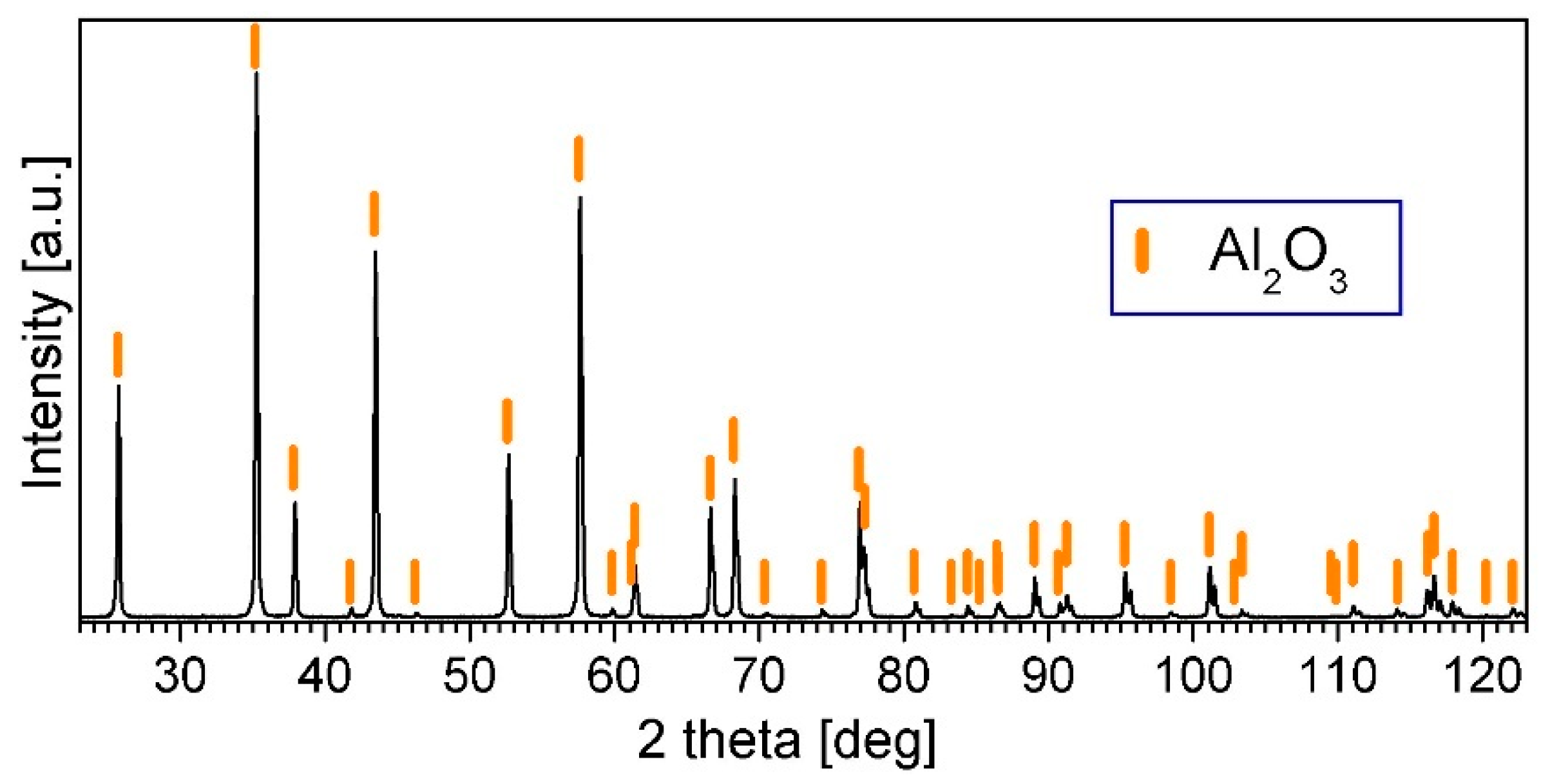

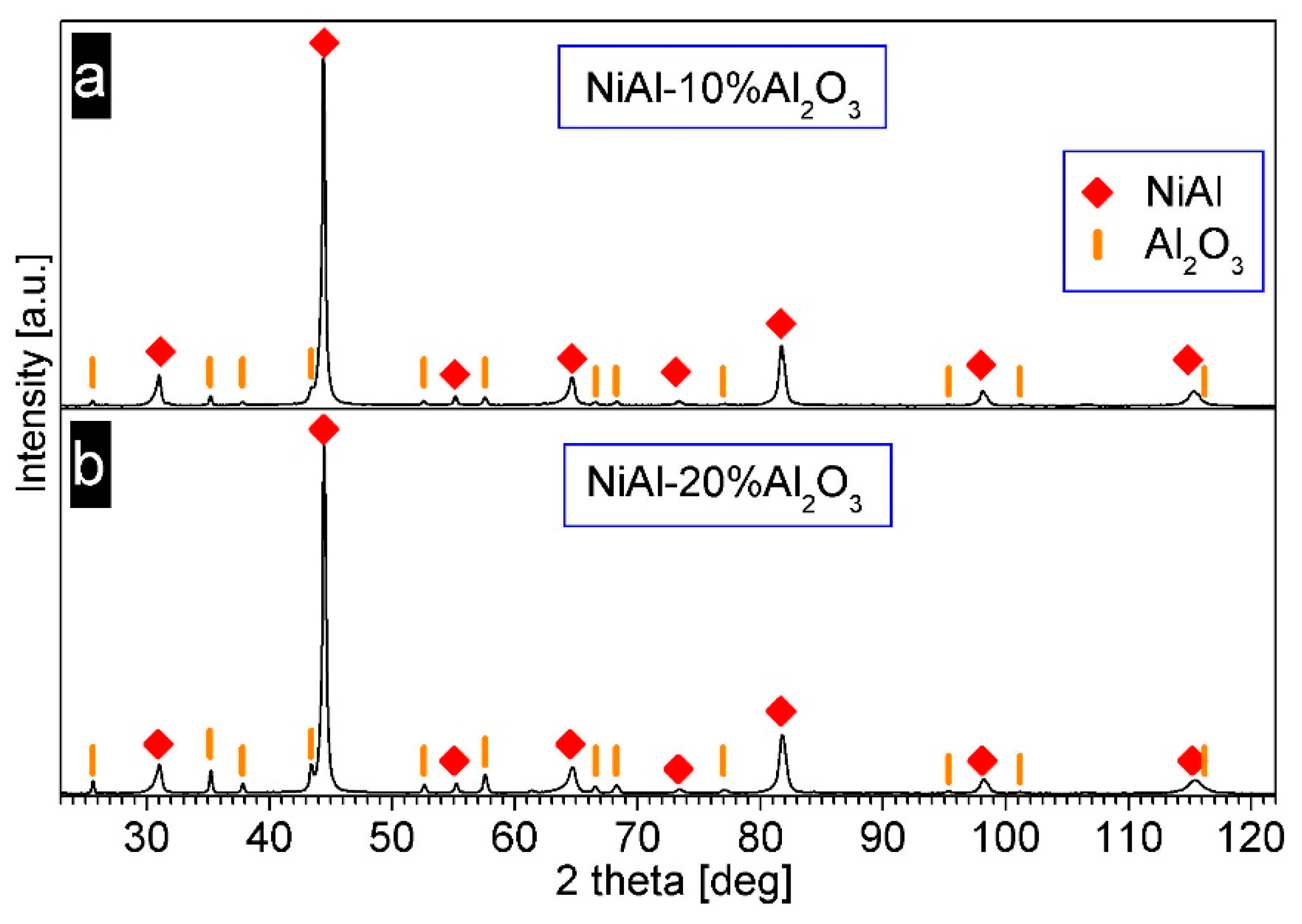
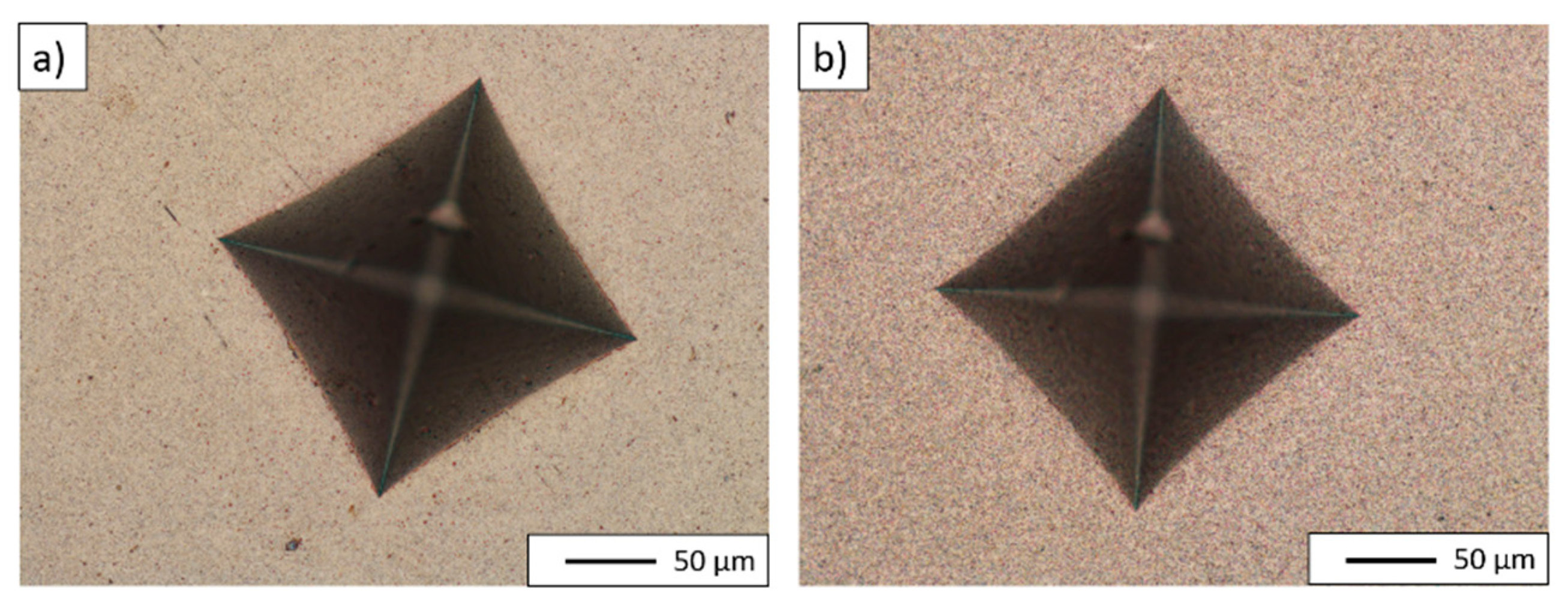
| PPS Process Parameter | Al2O3 Samples | NiAl–Al2O3 Composite Samples |
|---|---|---|
| Stored Energy | 4.06 ÷ 5.05 kJ | 2.77 kJ |
| Voltage | 5.2 ÷ 5.8 kV | 4.3 kV |
| Electro-pulse repetition | 1 ÷ 1.3 s | 1.3 s |
| Heating rate | 250 °C/min | 250 °C/min |
| Sintering temperaturę | 1000 °C, 1100 °C, 1200 °C, 1300 °C, 1400 °C, 1500 °C | 1400 °C |
| Load | 20–80 MPa | 20–80 MPa |
| Sintering time | 3 min | 3 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, K.; Krasnowski, M.; Zygmuntowicz, J.; Cymerman, K.; Wachowski, M.; Piotrkiewicz, P. Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering. Materials 2021, 14, 3398. https://doi.org/10.3390/ma14123398
Konopka K, Krasnowski M, Zygmuntowicz J, Cymerman K, Wachowski M, Piotrkiewicz P. Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering. Materials. 2021; 14(12):3398. https://doi.org/10.3390/ma14123398
Chicago/Turabian StyleKonopka, Katarzyna, Marek Krasnowski, Justyna Zygmuntowicz, Konrad Cymerman, Marcin Wachowski, and Paulina Piotrkiewicz. 2021. "Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering" Materials 14, no. 12: 3398. https://doi.org/10.3390/ma14123398
APA StyleKonopka, K., Krasnowski, M., Zygmuntowicz, J., Cymerman, K., Wachowski, M., & Piotrkiewicz, P. (2021). Characterization of Al2O3 Samples and NiAl–Al2O3 Composite Consolidated by Pulse Plasma Sintering. Materials, 14(12), 3398. https://doi.org/10.3390/ma14123398








