Manufacturing of Al2O3/Ni/Ti Composites Enhanced by Intermetallic Phases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Base Powders
2.2. Slurries
2.3. Composites
3. Results and discussion
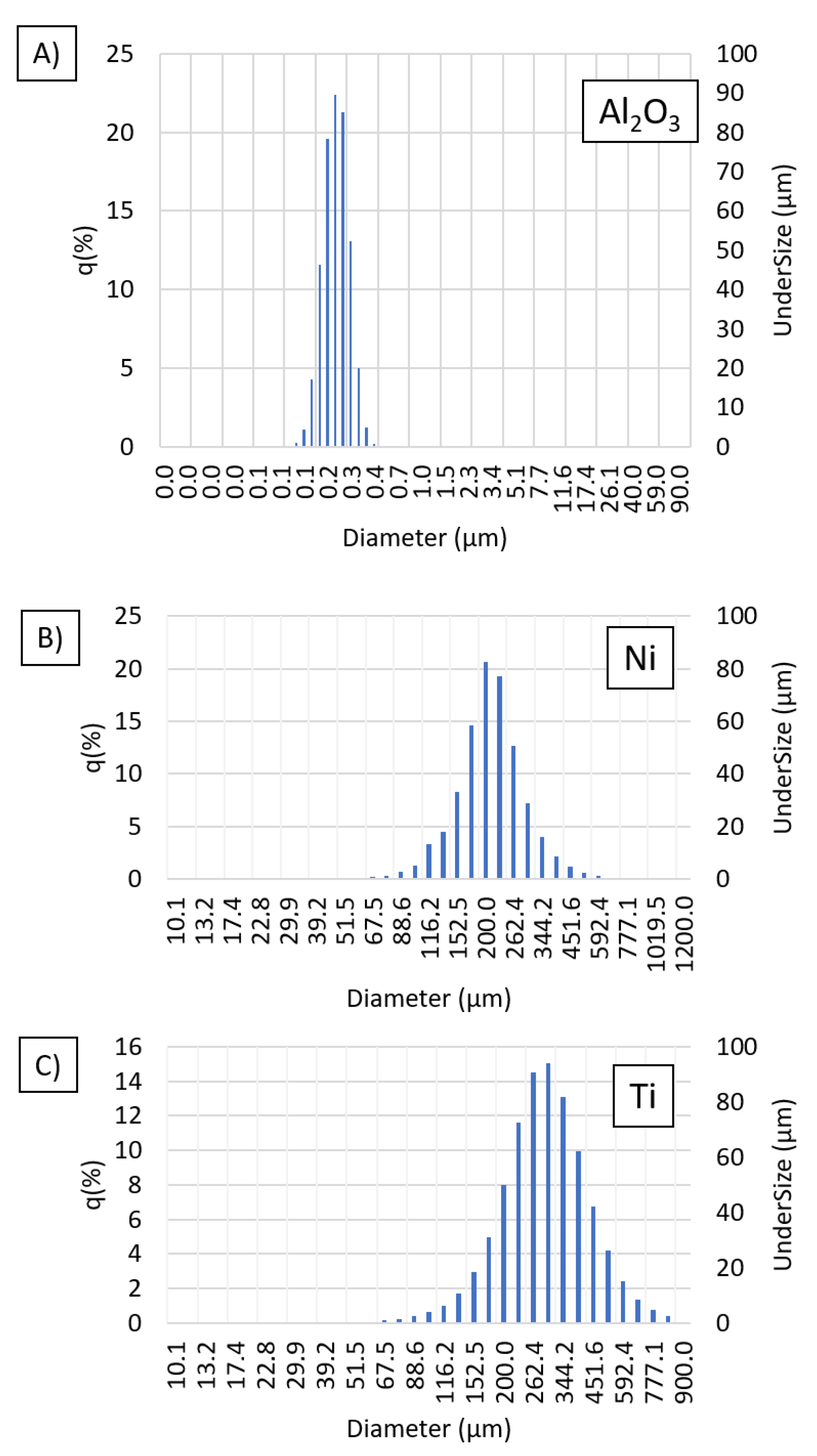
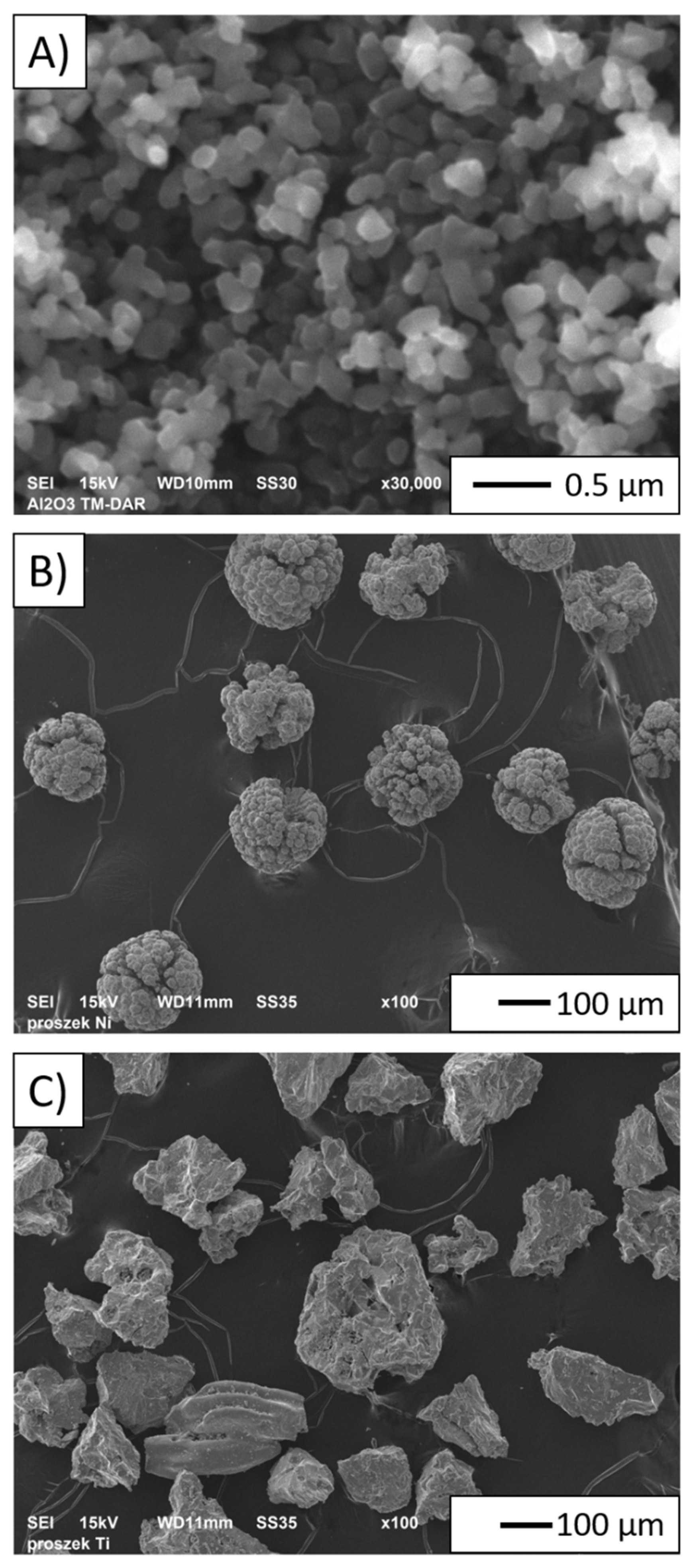
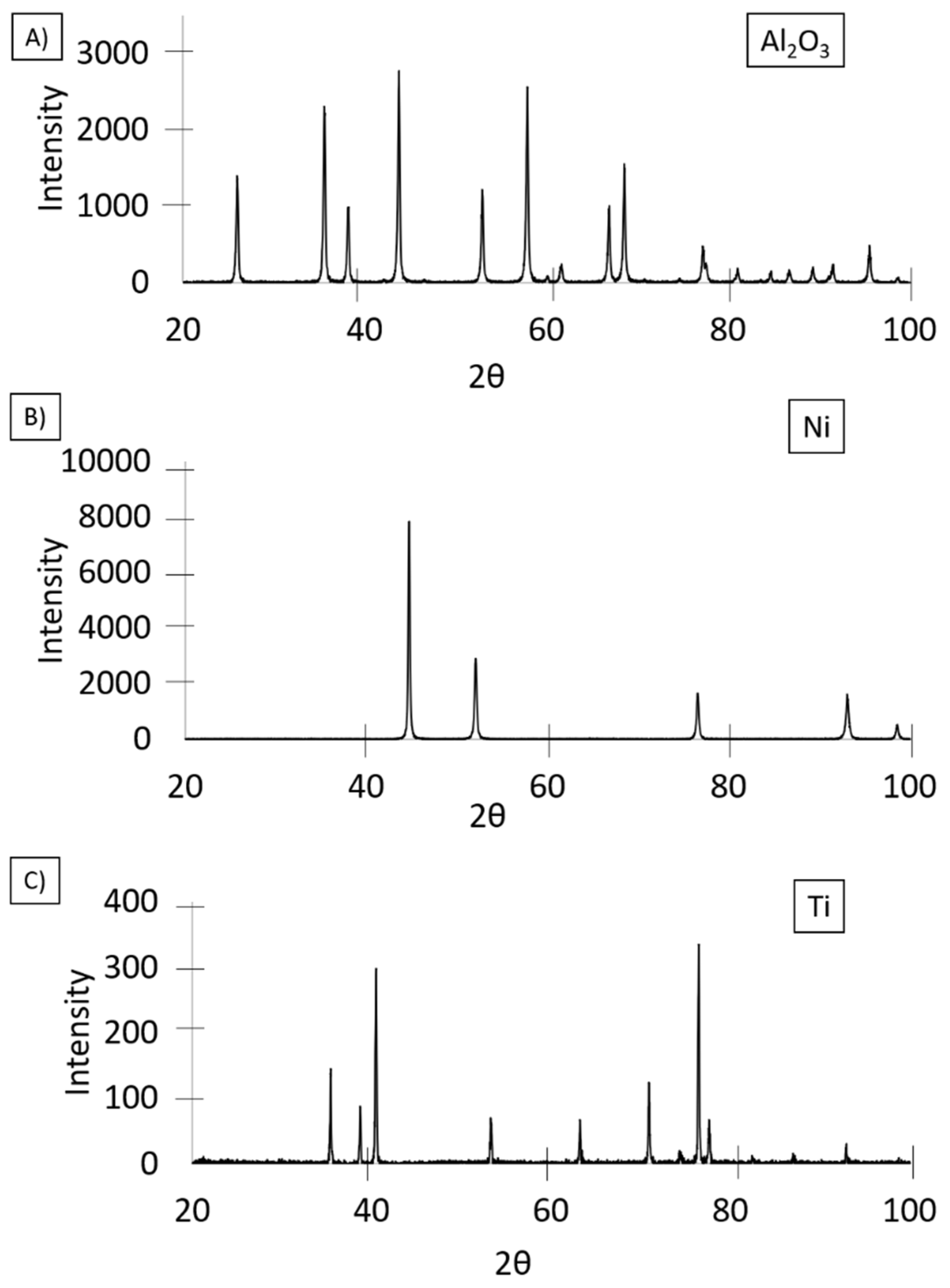
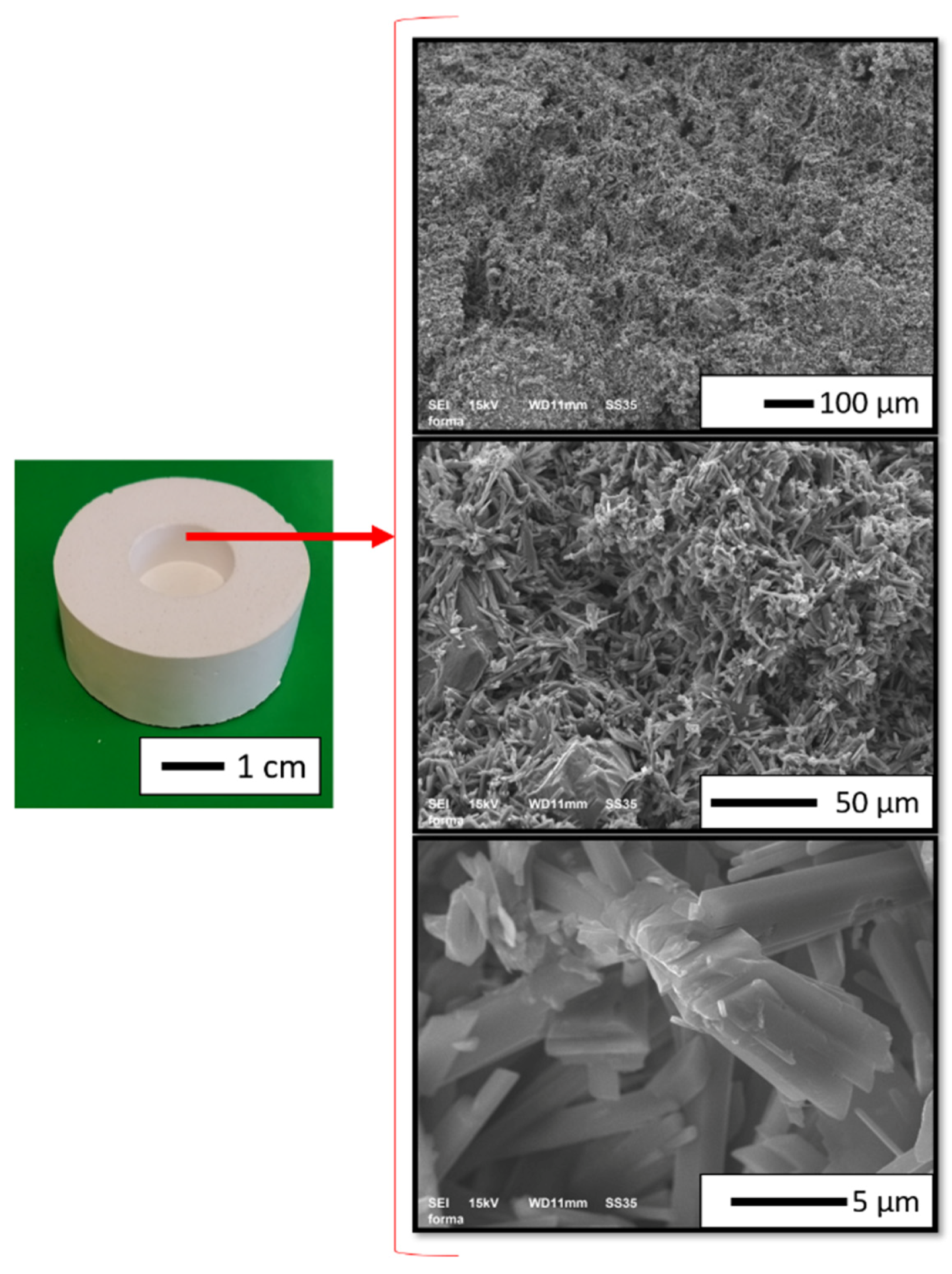
3.1. Base Powders
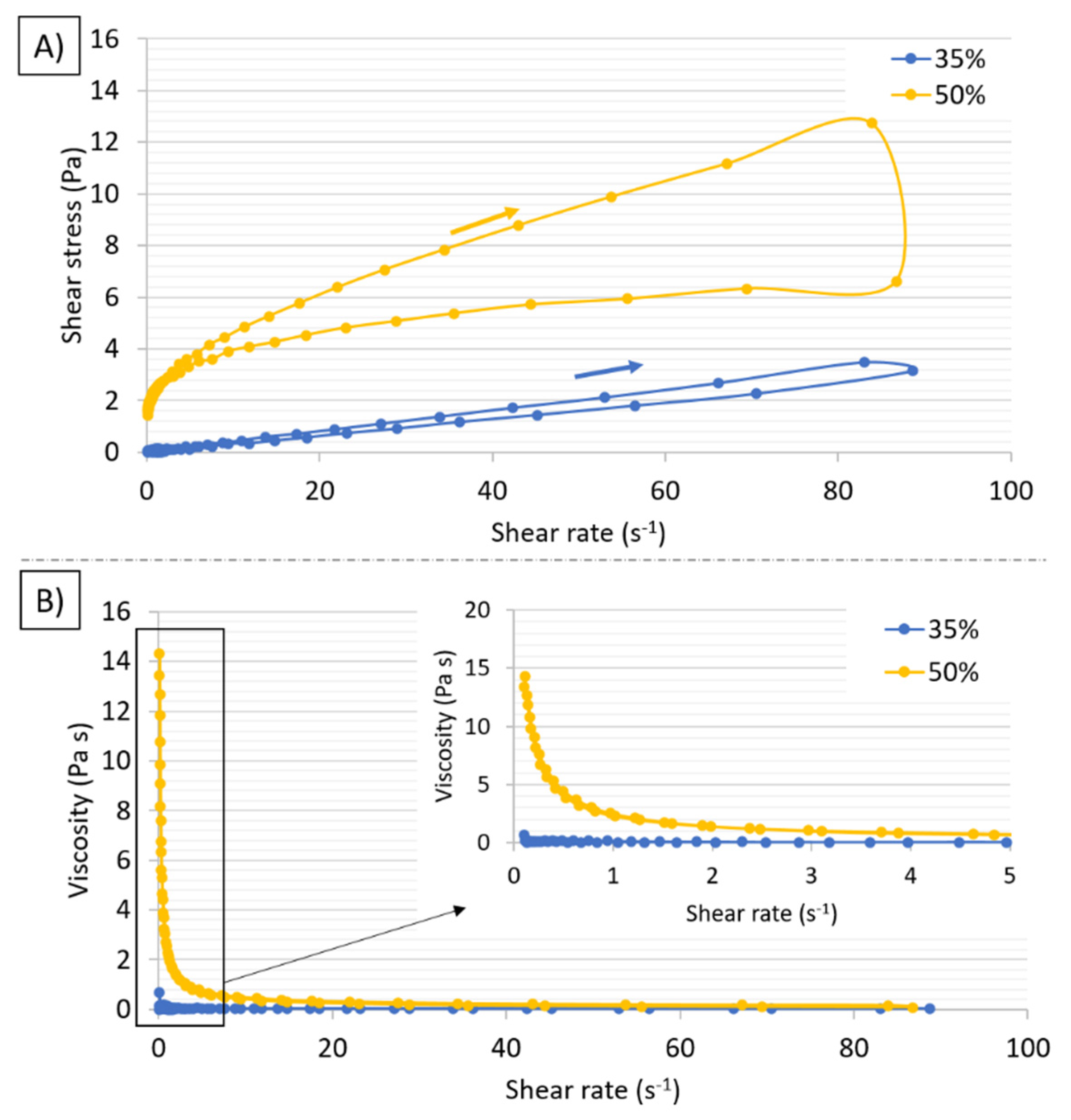
3.2. Slurries
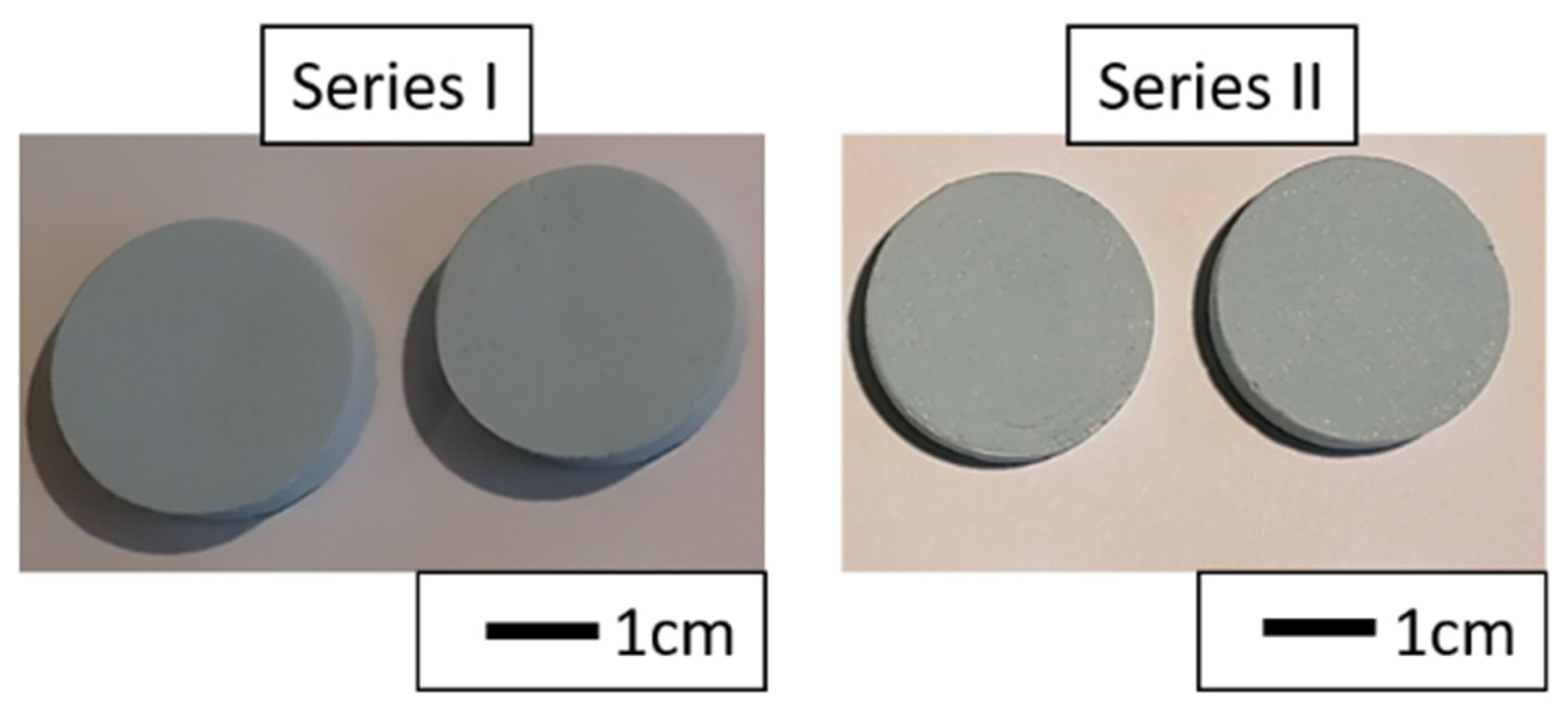
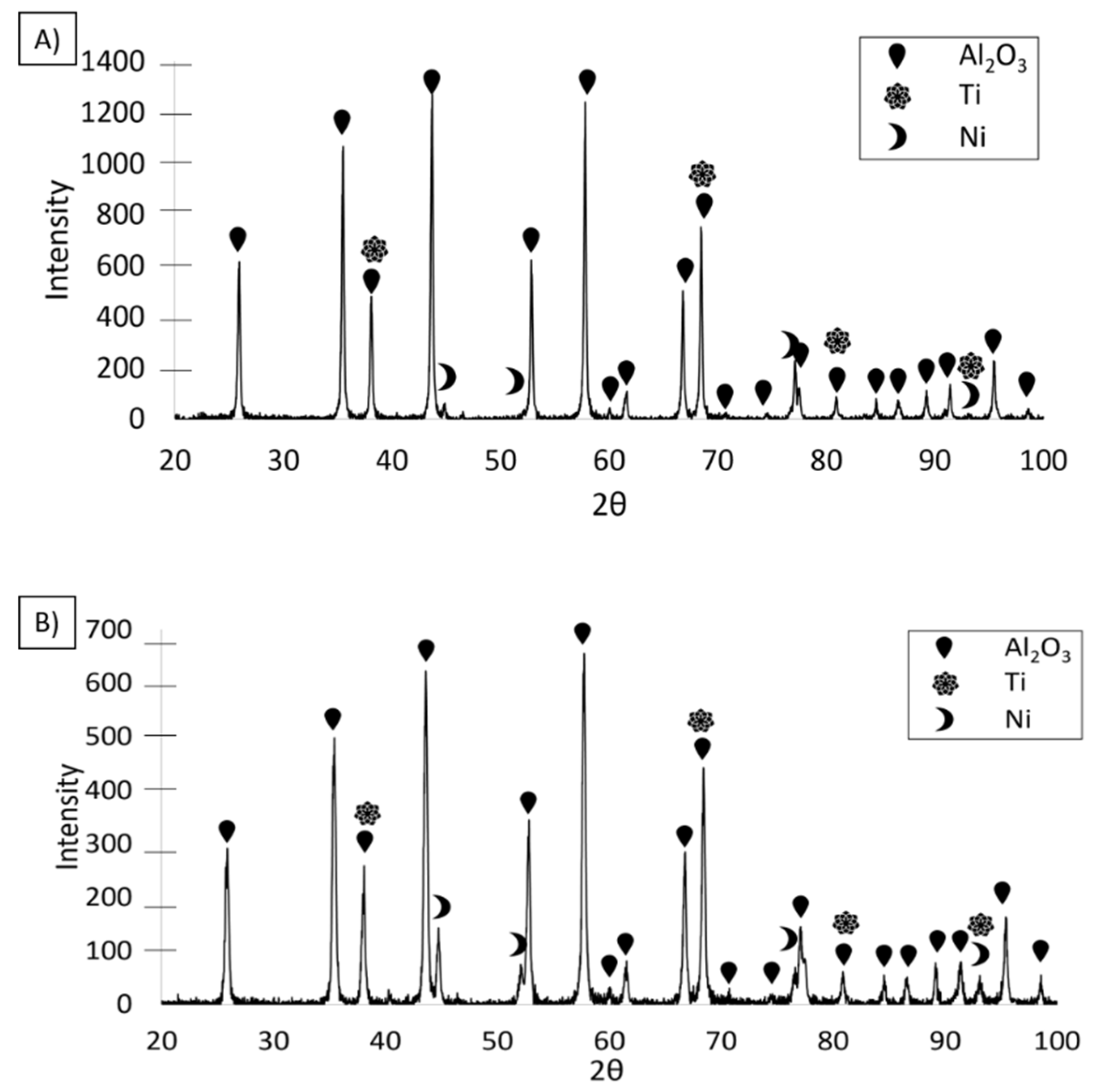
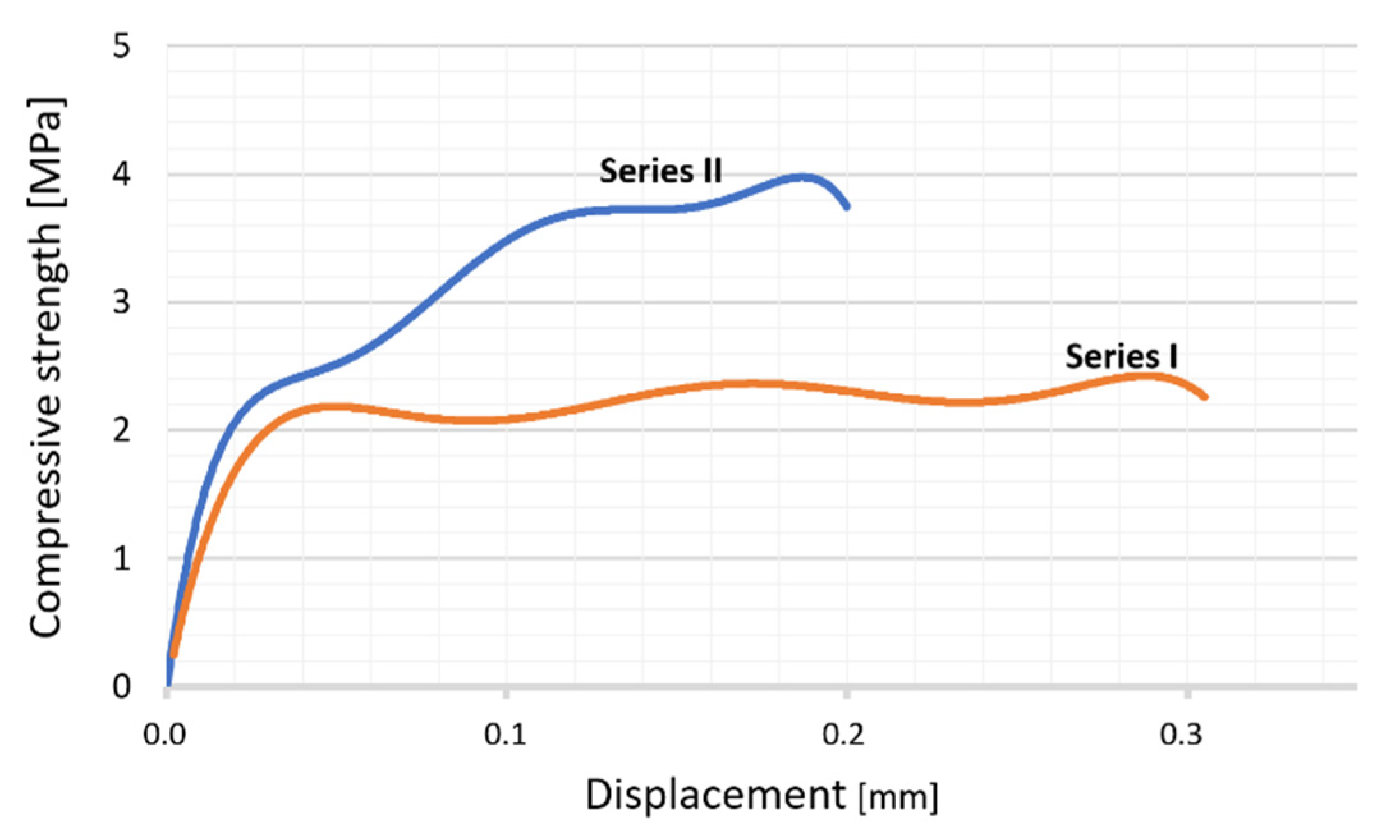
3.3. Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Cong, W. A review on laser deposition additive manufacturing of ceramics and ceramic reinforced metal matrix composites. Ceram. Int. 2018, 44, 20599–20612. [Google Scholar] [CrossRef]
- Yeh, C.L.; Ke, C.Y. Intermetallic/Ceramic Composites Synthesized from Al–Ni–Ti Combustion with B4C Addition. Metals 2020, 10, 873. [Google Scholar] [CrossRef]
- Travitzky, N. Processing of ceramic-Metal composites. Adv. Appl. Ceram. 2012, 111, 286–300. [Google Scholar] [CrossRef]
- Evans, A.G. The mechanical properties of reinforced ceramic, metal and intermetallic matrix composites. Mater. Sci. Eng. A 1991, 143, 63–76. [Google Scholar] [CrossRef]
- Haušild, P.; Karlík, M.; Cech, J.; Průša, F.; Nová, K.; Novák, P.; Minárik, P.; Kopeček, J. Preparation of Fe-Al-Si Intermetallic Compound by Mechanical Alloying and Spark Plasma Sintering. Acta Phys. Pol. A 2018, 134, 724–728. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Stark, A.; Esikov, M.A.; Paul, J.; Bataev, I.A.; Kashimbetova, A.A.; Mali, V.I.; Lorenz, U.; Pyczak, F. Ceramic-Reinforced γ-TiAl-Based Composites: Synthesis, Structure, and Properties. Materials 2019, 12, 629. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Zou, G.; Ren, J.; Ren, W.; Li, S. Heat-resistant joints of Si3N4 ceramics with intermetallic compounds formed in situ. J. Mater. Sci. 2001, 36, 2673–2678. [Google Scholar] [CrossRef]
- Kaliński, D.; Chmielewski, M.; Pietrzak, K. An influence of mechanical mixing and hot-pressing on the properties of NiAl/Al2O3 composites. Arch. Metall. Mater. 2012, 57, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Danforth, S.C. Processing of ceramic-ceramic composites. In Processing of the Ceramic and Metal Matrix Composites; Mostaghaci, H., Ed.; Pergamon: Oxford, UK, 1989; pp. 107–119. [Google Scholar] [CrossRef]
- Dittmer, R.; Schaefer, C.M.; Fischer, J.-F.; Hausch, U.; Troetzschel, J.; Specht, H. Advanced CerMet ceramic composites for medical applications. J. Mech. Behav. Biomed. Mater. 2017, 75, 206–211. [Google Scholar] [CrossRef]
- Zhou, W.; Ai, S.; Chen, M.; Zhang, R.; He, R.; Pei, Y.; Fang, D. Preparation and thermodynamic analysis of the porous ZrO2/(ZrO2 + Ni) functionally graded bolted joint. Compos. Part B Eng. 2015, 82, 13–22. [Google Scholar] [CrossRef]
- Amosov, A.; Amosov, E.; Latukhin, E.; Kichaev, P.; Umerov, E. Producing TiC-Al Cermet by Combustion Synthesis of TiC Porous Skeleton with Spontaneous Infiltration by Aluminum Melt. In Proceedings of the 7th International Congress on Energy Fluxes and Radiation Effects (EFRE), Tomsk, Russia, 14–26 September 2020; pp. 1057–1062. [Google Scholar] [CrossRef]
- Lengauer, W.; Scagnetto, F. Ti(C,N)-Based Cermets: Critical Review of Achievements and Recent Developments. Solid State Phenom. 2018, 274, 53. [Google Scholar] [CrossRef]
- Huang, S.; Nie, H.B.; Guo, X.Y.; Vleugels, J.; Huang, J.H.; Mohrbacher, H.; Rajendhran, N.; Sukumaran, J.; Debaets, P.; Cannizza, E.; et al. Microstructural investigation and machining performance of NbC-Ti(C0.5N0.5) matrix cermets. Int. J. Refract. Met. Hard Mater. 2019, 84, 105038. [Google Scholar] [CrossRef]
- Almeida, M.; Vieira, J.M. Processing of Ceramics by Direct Coagulation Casting; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Adams, F.E. Slip-Cast Ceramics. In High Temperature Oxides Part IV Refractory Glasses, Glass-Ceramics, and Ceramics; Alper, A.M., Ed.; Academic Press: New York, NY, USA; London, UK, 1971; pp. 145–183. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Ren, B.; Wang, C.; Rong, Y.; Gan, K.; Xu, J.; Yang, J. Room-Temperature Gelcasting of Alumina with Tartaric Acid and Glutaraldehyde. Ceram. Int. 2020, 46, 11432–11435. [Google Scholar] [CrossRef]
- Huisman, W.; Graule, T.; Gauckler, L.J. Centrifugal Slip Casting of Zirconia (TZP). J. Eur. Ceram. Soc. 1994, 13, 33–39. [Google Scholar] [CrossRef]
- Wachowski, M.; Kosturek, R.; Winkler, H.; Miazga, A.; Lada, P.; Kaszuwara, W.; Konopka, K.; Zygmuntowicz, J. Manufacturing of ZrO2-Ni graded composites via centrifugal casting in the magnetic field. Bull. Pol. Acad. Sci. Tech. 2020, 68, 539–545. [Google Scholar] [CrossRef]
- Lada, P.; Falkowski, P.; Miazga, A.; Konopka, K.; Szafran, M. Fabrication of ZrO2-Ti Composites by Slip Casting Method. Arch. Metall. Mater. 2016, 61, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Gizowska, M.; Konopka, K.; Szafran, M. Properties of Water-Based Slurries for Fabrication of Ceramic-Metal Composites by Slip Casting Method. Arch. Metall. Mater. 2011, 56, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Zygmuntowicz, J.; Żurowski, R.; Tomaszewska, J.; Wachowski, M.; Torzewski, J.; Piotrkiewicz, P.; Gloc, M.; Konopka, K. Al2O3/ZrO2 Materials as an Environmentally Friendly Solution for Linear Infrastructure Applications. Materials 2021, 14, 2375. [Google Scholar] [CrossRef]
- Zygmuntowicz, J.; Łoś, J.; Kurowski, B.; Piotrkiewicz, P.; Kaszuwara, W. Investigation of microstructure and selected properties of Al2O3-Cu and Al2O3-Cu-Mo composites. Adv. Compos. Hybrid Mater. 2021, 4, 212–222. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Mali, V.I.; Jorge, A.M.; Stark, A.; Pyczak, F.; Ogneva, T.S.; Maliutina, I.N. Synthesis of metal-intermetallic laminate (MIL) composites with modified Al3Ti structure and in situ synchrotron X-ray diffraction analysis of sintering process. Mater. Des. 2018, 151, 8–16. [Google Scholar] [CrossRef]
- Kosturek, R.; Wachowski, M.; Śnieżek, L.; Kruk, A.; Torzewski, J.; Grzelak, K.; Mierzyński, J. Research on the microstructure of a Ti6Al4V-AA1050 explosive-welded bimetallic joint. Mater. Tehnol. 2019, 53, 109–113. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, B.; Ya, B.; Jing, D.; Jiang, Y.; Zhang, D.; Zhang, X. Microstructures and Properties of AlMgTi-Based Metal-Intermetallic Laminate Composites by Dual-Step Vacuum Hot Pressing. Materials 2020, 13, 3932. [Google Scholar] [CrossRef]
- Wachowski, M.; Kosturek, R.; Śnieżek, L.; Mróz, S.; Stefanik, A.; Szota, P. The Effect of Post-Weld Hot-Rolling on the Properties of Explosively Welded Mg/Al/Ti Multilayer Composite. Materials 2020, 13, 1930. [Google Scholar] [CrossRef] [Green Version]
- Klassen, T.; Günther, R.; Dickau, B.; Gärtner, F.; Bartels, A.; Bormann, R.; Mecking, H. Processing and Properties of Intermetallic/Ceramic Composites with Interpenetrating Microstructure. J. Am. Ceram. Soc. 1998, 81, 2504–2506. [Google Scholar] [CrossRef]
- Mokgalaka, M.; Pityana, S.; Popoola, P.; Mathebula, T. NiTi Intermetallic Surface Coatings by Laser Metal Deposition for Improving Wear Properties of Ti-6Al-4V Substrates. Adv. Mater. Sci. Eng. 2014, 2014, 363917. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tang, S.; Gao, Y.; Ma, S.; Zheng, Q.; Cheng, Y. Mechanical and thermodynamic properties of intermetallic compounds in the Ni–Ti system. Int. J. Mod. Phys. B 2017, 31, 1750161. [Google Scholar] [CrossRef]
- Li, B.; Rong, L.; Li, Y.; Gjunter, V. Fabrication of cellular NiTi intermetallic compounds. J. Mater. Res. 2000, 15, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Ergin, N.; Ozdemir, O. An investigation on TiNi intermetallics produced by electric current activated sintering. Acta Phys. Pol. A 2013, 123, 248–249. [Google Scholar] [CrossRef]
- Sadrnezhaad, S.K.; Selahi, A.R. Effect of Mechanical Alloying and Sintering on Ni–Ti Powders. Mater. Manuf. Process 2004, 19, 475–486. [Google Scholar] [CrossRef]
- Fu, Y.; Moochhala, S.; Shearwood, C. Spark plasma sintering of TiNi nanopowders. BioMEMS Nanotechnol. 2004, 5275, 9–17. [Google Scholar] [CrossRef]
- Yener, T.; Siddique, S.; Walther, F.; Zeytin, S. Effect of electric current on the production of NiTi intermetallics via electric-current-activated sintering. Mater. Tehnol. 2015, 49, 721–724. [Google Scholar] [CrossRef]
- Zygmuntowicz, J.; Wachowski, M.; Miazga, A.; Konopka, K.; Kaszuwara, W. Characterization of Al2O3/Ni composites manufactured via CSC technique in magnetic field. Compos. Part B Eng. 2019, 156, 113–120. [Google Scholar] [CrossRef]


| Series No. | Chemical Composition | Metallic Phase Content [vol.%] | Solid Content [vol.%] | Titanium/Nickel Ratio [%] |
|---|---|---|---|---|
| 1. | Al2O3/Ti/Ni | 15 | 35 | 50/50 |
| 2. | 50 |
| Parameter | α-Al2O3 (Taimei Chemicals) | Ti (Alfa Aesar) | Ni (Alfa Aesar) |
|---|---|---|---|
| Average particle size [µm] | 0.100 ± 0.025 | 149–250 | 149–297 |
| Average particle size from PSD analysis (Particle Size Distribution) [µm] | 0.218 ± 0.05 | 271.39 ± 115.10 | 206.78 ± 67.71 |
| Density [g/cm3] | 3.980 | 4.506 | 8.900 |
| Density was measured with a pycnometer [g/cm3] | 3.896 | 4.431 | 8.733 |
| Purity [%] | 99.9 | 99.5 | 99.7 |
| Series No. | Solid Content [Vol. %] | Maximum Compression Load F [N] | Compression Strength K [MPa] |
|---|---|---|---|
| 1. | 35 | 378 | 3.78 |
| 2. | 50 | 802 | 4.37 |
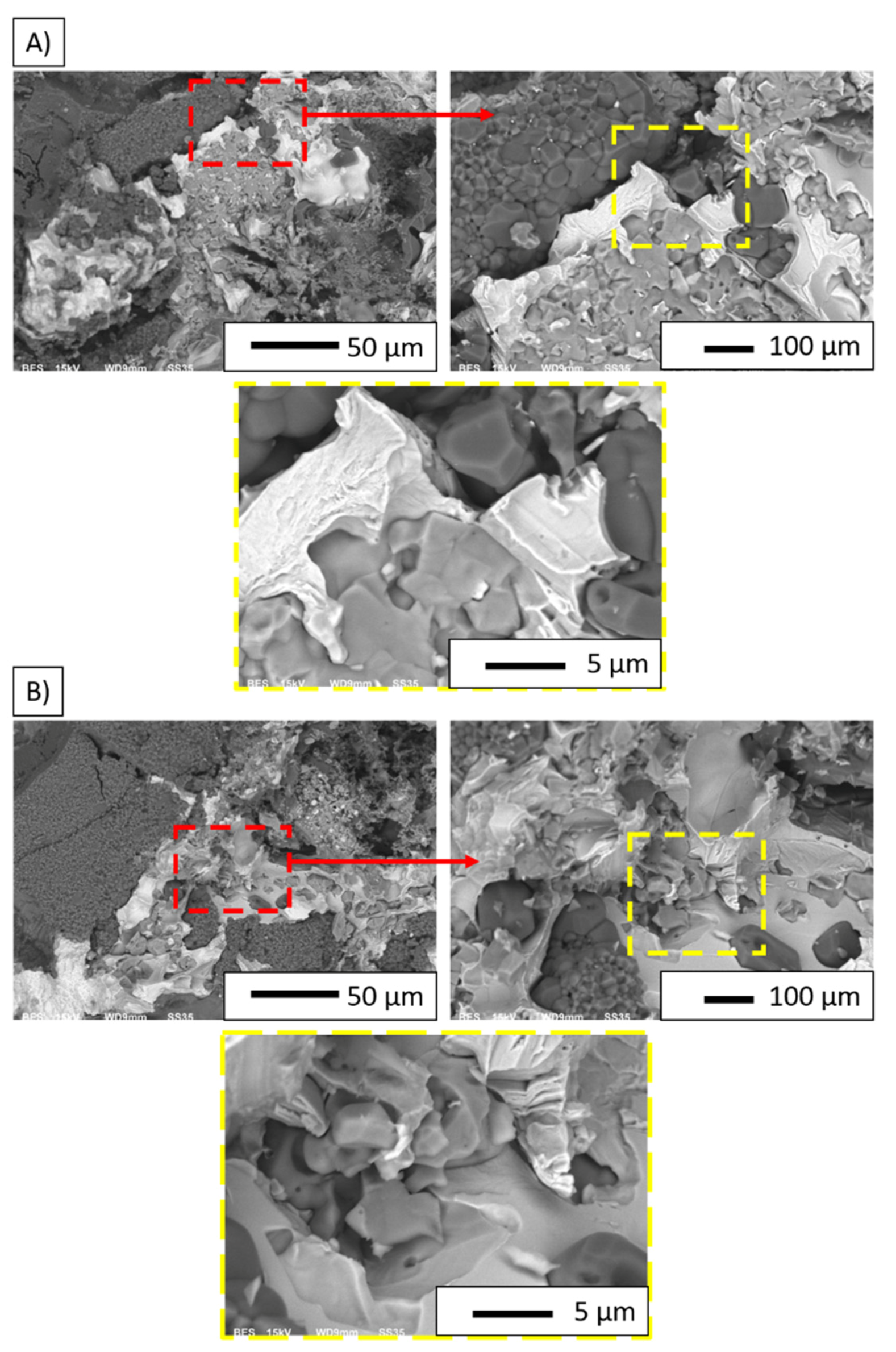
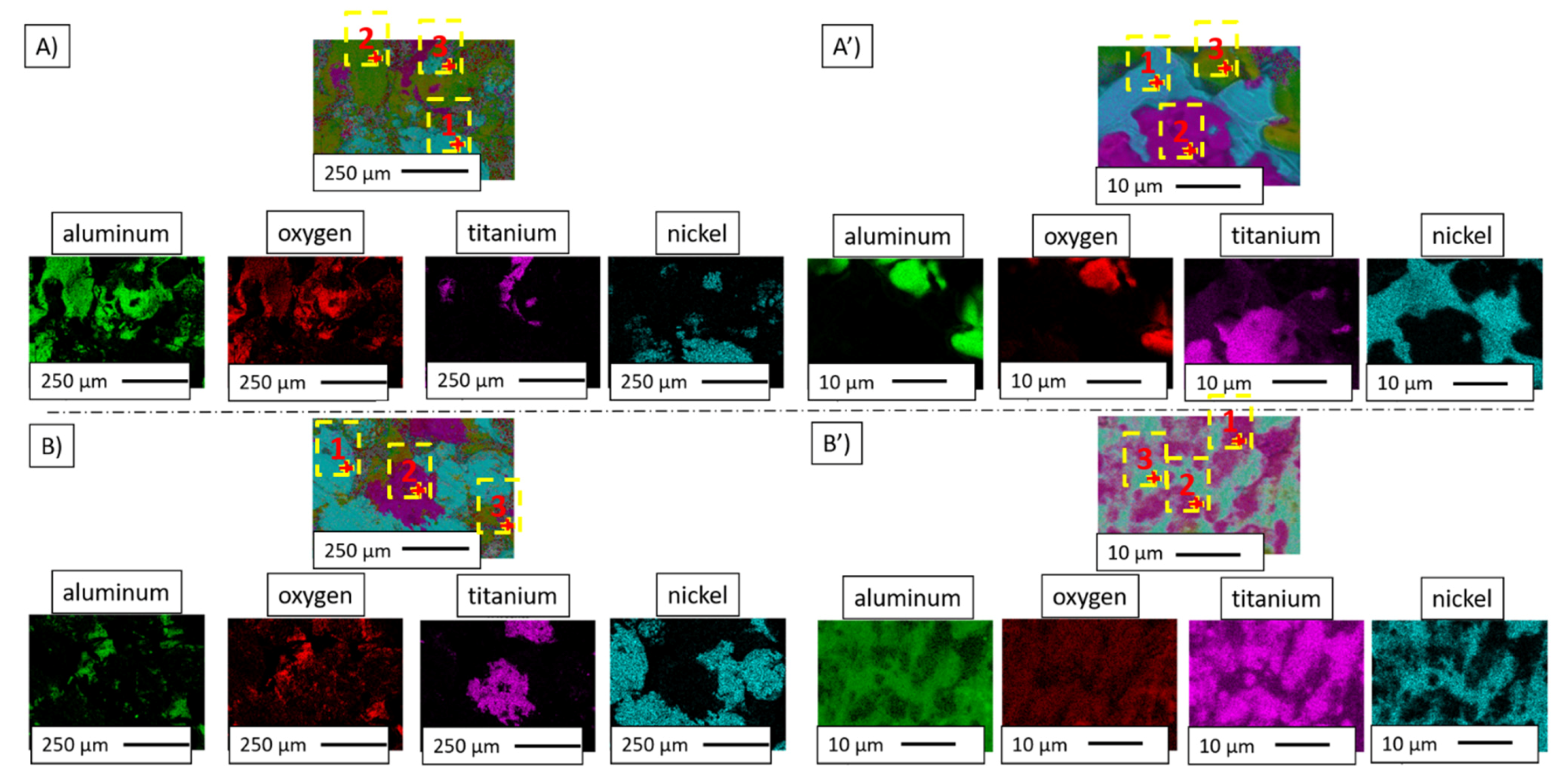
| Series | Image | Point | Chemical Composition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Ti | Ni | |||||||
| %wt. | %at. | %wt. | %at. | %wt. | %at. | %wt. | %at. | |||
| I | A | 1 | 2.10 | 7.24 | 0.91 | 1.85 | --- | --- | 96.99 | 90.91 |
| 2 | 37.62 | 50.93 | 59.95 | 48.12 | 0.66 | 0.30 | 1.77 | 0.65 | ||
| 3 | 1.66 | 5.69 | 2.05 | 4.18 | --- | --- | 96.29 | 90.13 | ||
| A’ | 1 | 1.72 | 5.74 | 1.87 | 3.71 | 13.60 | 15.17 | 82.81 | 75.37 | |
| 2 | 18.20 | 40.00 | 0.46 | 0.60 | 79.16 | 58.10 | 2.18 | 1.30 | ||
| 3 | 46.73 | 60.60 | 49.00 | 37.68 | 2.63 | 1.14 | 1.64 | 0.58 | ||
| II | B | 1 | 1.26 | 4.44 | 0.77 | 1.61 | --- | --- | 97.97 | 93.95 |
| 2 | 33.54 | 59.28 | 3.64 | 3.82 | 61.07 | 36.06 | 1.75 | 0.84 | ||
| 3 | 35.77 | 52.61 | 45.27 | 39.48 | 3.44 | 1.69 | 15.51 | 6.22 | ||
| B’ | 1 | 20.32 | 43.29 | 2.99 | 3.77 | 64.11 | 45.63 | 12.59 | 7.31 | |
| 2 | --- | --- | 13.56 | 24.20 | 26.43 | 26.57 | 60.02 | 49.23 | ||
| 3 | 21.58 | 45.83 | 3.20 | 3.94 | 60.07 | 41.66 | 15.14 | 8.57 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachowski, M.; Zygmuntowicz, J.; Kosturek, R.; Konopka, K.; Kaszuwara, W. Manufacturing of Al2O3/Ni/Ti Composites Enhanced by Intermetallic Phases. Materials 2021, 14, 3510. https://doi.org/10.3390/ma14133510
Wachowski M, Zygmuntowicz J, Kosturek R, Konopka K, Kaszuwara W. Manufacturing of Al2O3/Ni/Ti Composites Enhanced by Intermetallic Phases. Materials. 2021; 14(13):3510. https://doi.org/10.3390/ma14133510
Chicago/Turabian StyleWachowski, Marcin, Justyna Zygmuntowicz, Robert Kosturek, Katarzyna Konopka, and Waldemar Kaszuwara. 2021. "Manufacturing of Al2O3/Ni/Ti Composites Enhanced by Intermetallic Phases" Materials 14, no. 13: 3510. https://doi.org/10.3390/ma14133510
APA StyleWachowski, M., Zygmuntowicz, J., Kosturek, R., Konopka, K., & Kaszuwara, W. (2021). Manufacturing of Al2O3/Ni/Ti Composites Enhanced by Intermetallic Phases. Materials, 14(13), 3510. https://doi.org/10.3390/ma14133510









