Evaluation of the Anticancer Activity of Phytomolecules Conjugated Gold Nanoparticles Synthesized by Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.2.1. N. sativa
2.2.2. Z. officinale
2.3. Synthesis of Gold Nanoparticles
Gold with Z. officinale (GE) and N. sativa (NSE)
2.4. Characterization
2.5. Methodology
Cytotoxic Assessment of Z. officinale and N. sativa Doped AU-NPs
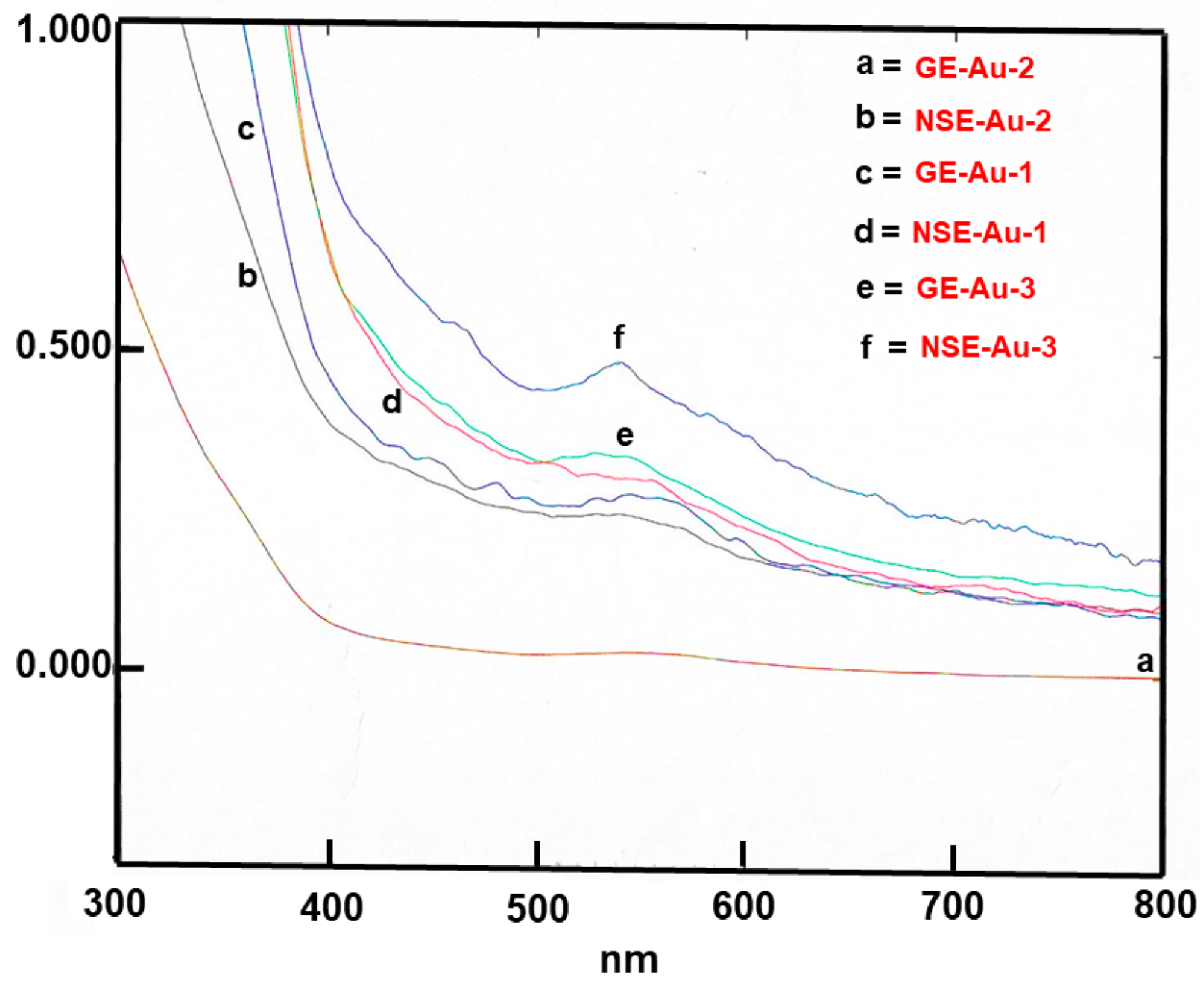
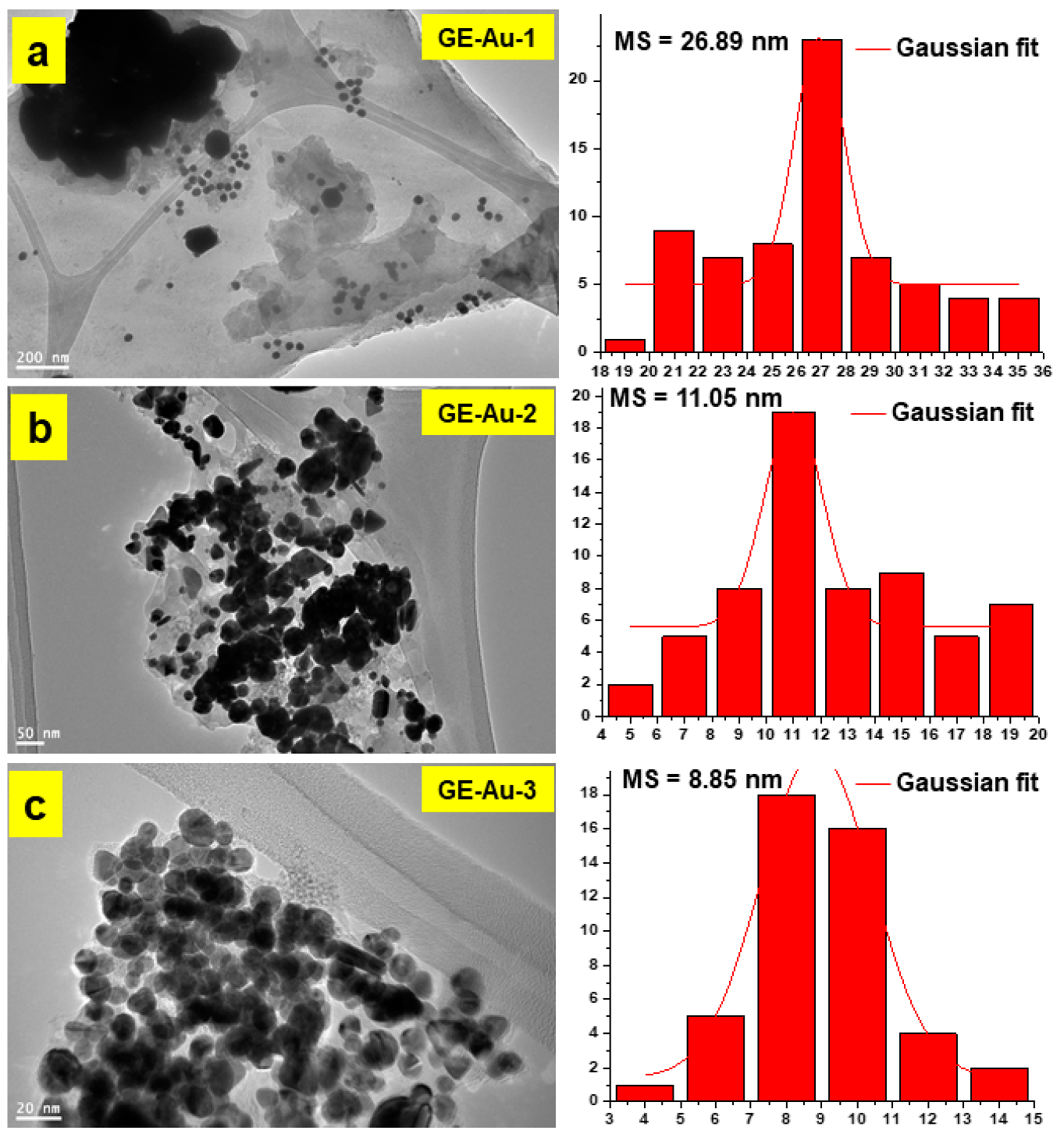
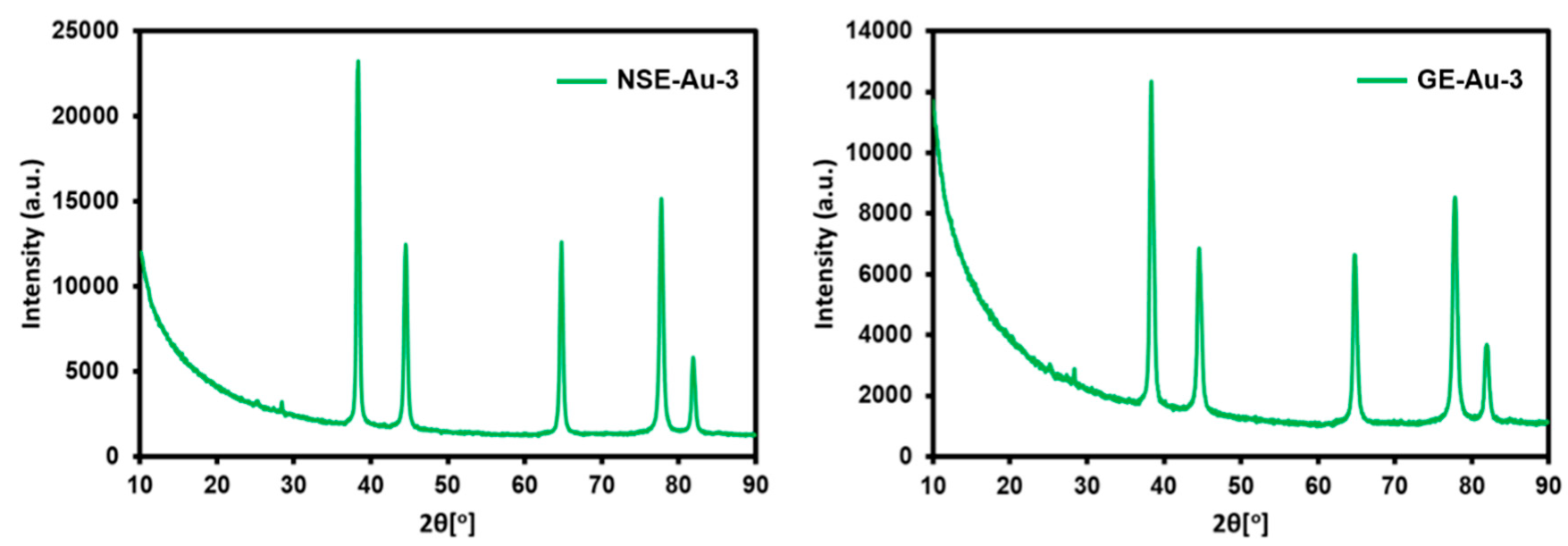
3. Results and Discussion
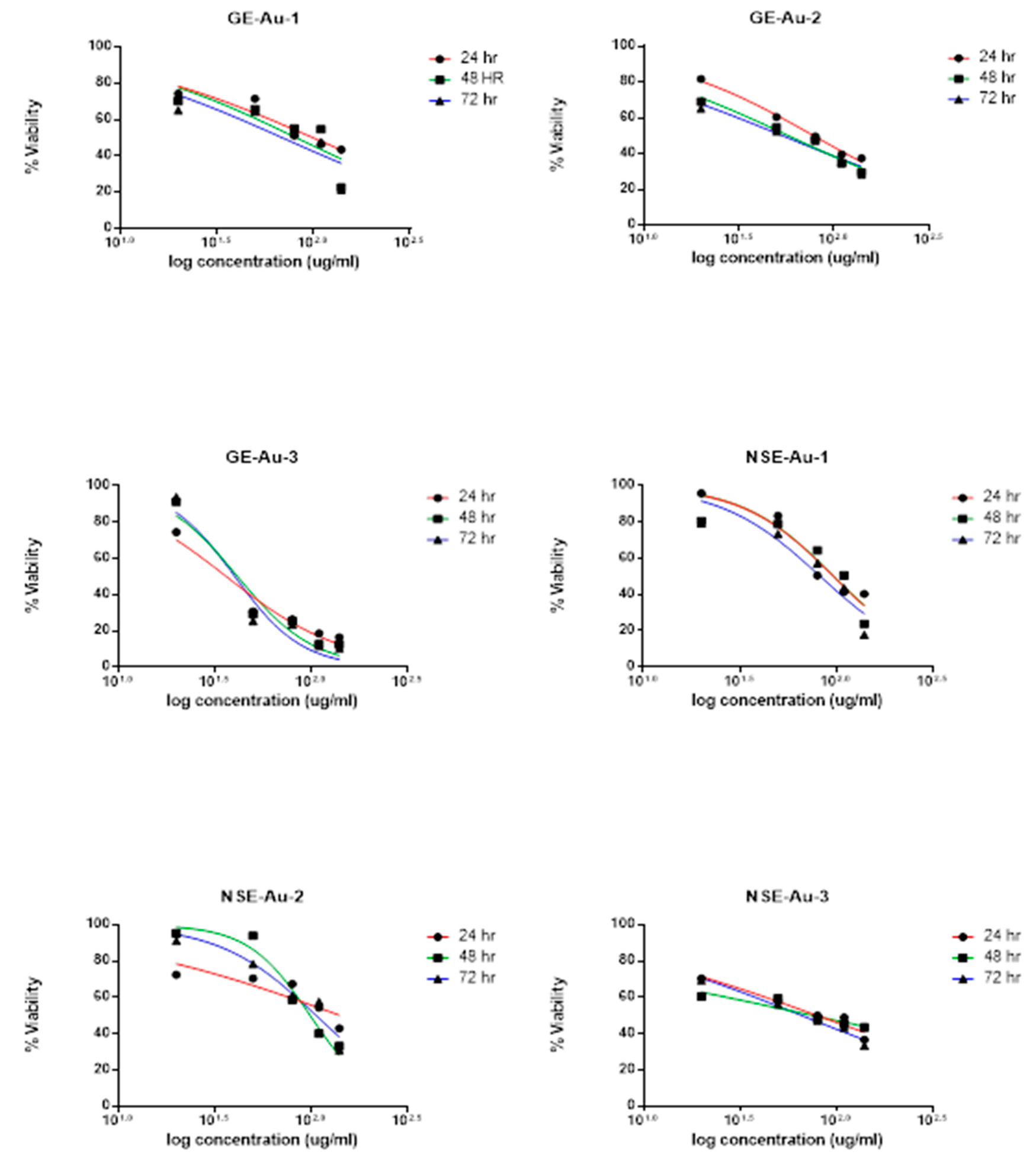
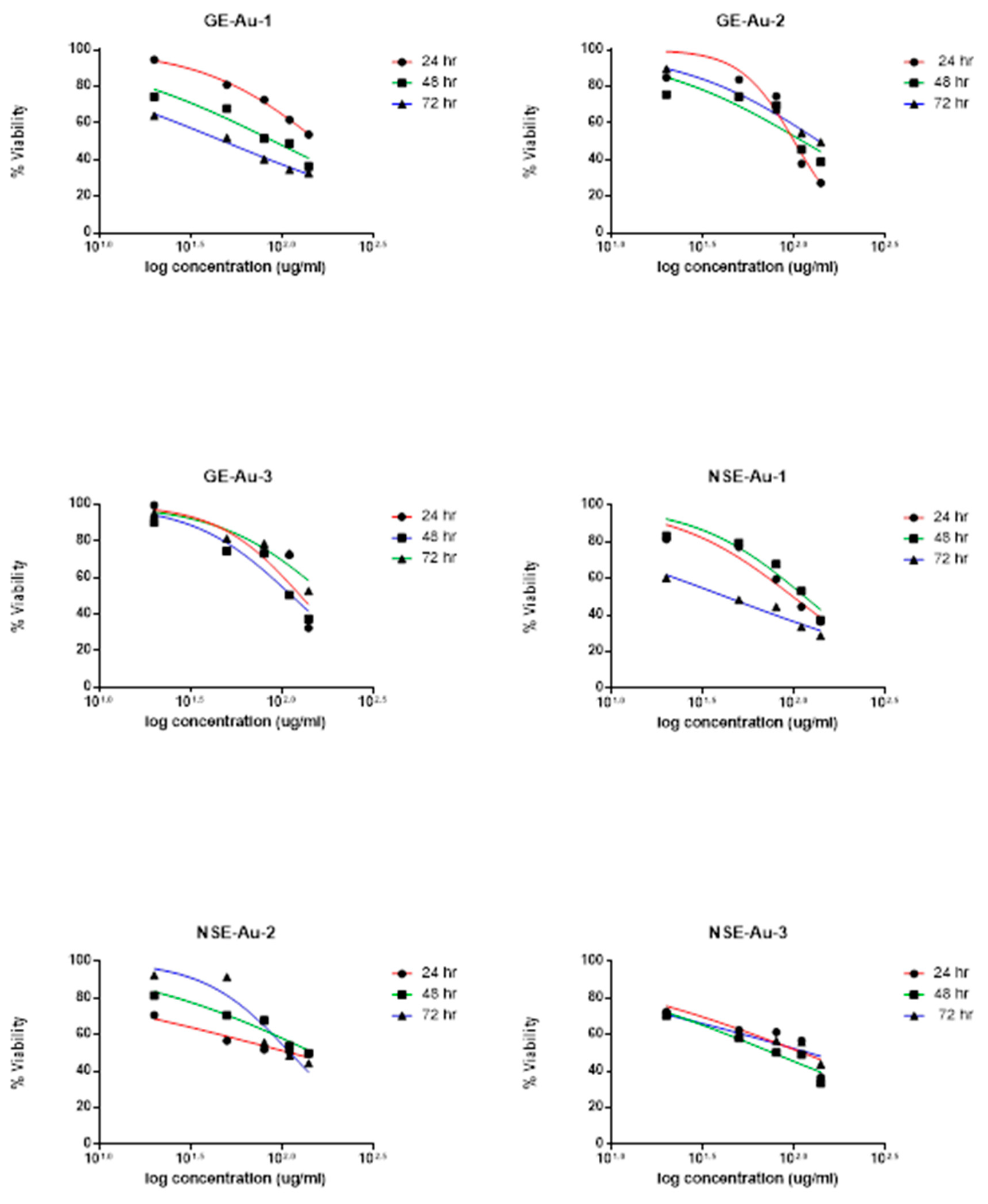
Cytotoxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H. Next-generation characterization of the cancer cell line encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Catt, S.; Starkings, R.; Shilling, V.; Fallowfield, L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: A systematic review. J. Cancer Surviv. 2017, 11, 211–232. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, C.M.; Bradbury, M.S.; Lanza, G.M.; Nel, A.E.; Rao, J.; Wang, A.Z.; Wiesner, U.B.; Yang, L.; Grodzinski, P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 2018, 12, 24–43. [Google Scholar] [CrossRef]
- Quinn, D.; Sandler, H.; Horvath, L.; Goldkorn, A.; Eastham, J. The evolution of chemotherapy for the treatment of prostate cancer. Ann. Oncol. 2017, 28, 2658–2669. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 12, 541340. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Miao, L.; Guo, S.; Lin, C.M.; Liu, Q.; Huang, L. Nanoformulations for combination or cascade anticancer therapy. Adv. Drug Del. Rev. 2017, 115, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef]
- Nakonieczna, S.; Grabarska, A.; Kukula-Koch, W. The Potential Anticancer Activity of Phytoconstituents against Gastric Cancer—A Review on In Vitro, In Vivo, and Clinical Studies. Int. J. Mol. Sci. 2020, 21, 8307. [Google Scholar] [CrossRef]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing delivery of plant based anticancer drugs. Front. Pharmacol. 2018, 8, 1002. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Yang, C.; Tanwar, R.S.; Saxena, R.; Gundala, S.R.; Zhang, Y.; Ghareeb, A.; Floyd, S.D.; Vangala, S.; Kuo, W.-W. Pharmacokinetic-pharmacodynamic correlations in the development of ginger extract as an anticancer agent. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; G Wasef, L.; Elewa, Y.H.; A Al-Sagan, A.; El-Hack, A.; Mohamed, E.; Taha, A.E.; M Abd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for cancer therapy based on chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.-K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Bora, V.; Patel, B.; Patel, M. Surface-engineered nanostructured lipid carrier systems for synergistic combination oncotherapy of non-small cell lung cancer. Drug Deliv. Transl. Res. 2020, 1–22. [Google Scholar] [CrossRef]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid. Med. Cell. Longev. 2016, 2016, 3685671. [Google Scholar] [CrossRef]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: A biocompatible anticancer therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef]
- Skóra, B.; Szychowski, K.A.; Gmiński, J. A concise review of metallic nanoparticles encapsulation methods and their potential use in anticancer therapy and medicine. Eur. J. Pharm. Biopharm. 2020, 154, 153–165. [Google Scholar] [CrossRef]
- Rixe, O.; Fojo, T. Is cell death a critical end point for anticancer therapies or is cytostasis sufficient? Clin. Cancer Res. 2007, 13, 7280–7287. [Google Scholar] [CrossRef]
- Hrabeta, J.; Adam, V.; Eckschlager, T.; Frei, E.; Stiborova, M.; Kizek, R. Metal containing cytostatics and their interaction with cellular thiol compounds causing chemoresistance. Anticancer Agents Med. Chem. (Former. Curr. Med. Chem. Anticancer Agents) 2016, 16, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.S.; Jousseaume, Y.; Piedade, M.F.M.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; de Brito, M.J.V.; Garcia, M.H. Important cytotoxic and cytostatic effects of new copper (i)–phosphane compounds with N, N, N, O and N, S bidentate ligands. Dalton Trans. 2018, 47, 7819–7829. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anticancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Mohamed, T.; Matou-Nasri, S.; Farooq, A.; Whitehead, D.; Azzawi, M. Polyvinylpyrrolidone-coated gold nanoparticles inhibit endothelial cell viability, proliferation, and ERK1/2 phosphorylation and reduce the magnitude of endothelial-independent dilator responses in isolated aortic vessels. Int. J. Nanomed. 2017, 12, 8813. [Google Scholar] [CrossRef]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J. Liposome supported metal oxide nanoparticles: Interaction mechanism, light controlled content release, and intracellular delivery. Small 2014, 10, 3927–3931. [Google Scholar] [CrossRef]
- Alphandéry, E. Natural metallic nanoparticles for application in nano-oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, X. Recent advances in amphiphilic polymers as the stabilizers of colloidal gold nanoparticles. Macromol. Mater. Eng. 2018, 303, 1800105. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. 2013, 4, 91–98. [Google Scholar] [CrossRef]
- Govindaraju, K.; Vasantharaja, R.; Suganya, K.U.; Anbarasu, S.; Revathy, K.; Pugazhendhi, A.; Karthickeyan, D.; Singaravelu, G. Unveiling the anticancer and antimycobacterial potentials of bioengineered gold nanoparticles. Process Biochem. 2020, 96, 213–219. [Google Scholar] [CrossRef]
- Mescola, A.; Canale, C.; Fragouli, D.; Athanassiou, A. Controlled formation of gold nanostructures on biopolymer films upon electromagnetic radiation. Nanotechnology 2017, 28, 415601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Kalla, D.; Uppuluri, K.B.; Anbazhagan, V. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydr. Polym. 2014, 112, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; de Oliveira Vercik, L.C.; Menezes, T.A.V.; Vercik, A. A simple and green method for synthesis of Ag and Au nanoparticles using biopolymers and sugars as reducing agent. MRS Online Proc. Libr. 2012, 1386, 1–7. [Google Scholar] [CrossRef]
- Khan, M.; Al-Hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Mondeshki, M. Synthesis of Au, Ag, and Au–Ag Bimetallic Nanoparticles Using Pulicaria undulata Extract and Their Catalytic Activity for the Reduction of 4-Nitrophenol. Nanomaterials 2020, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.; Lucas, J.M.; Morais, M.P.; Santos, S.A.; Silvestre, A.J.; Marques, P.A.; Freire, C.S. Demystifying the morphology and size control on the biosynthesis of gold nanoparticles using Eucalyptus globulus bark extract. Ind. Crop. Prod. 2017, 105, 83–92. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 1–21. [Google Scholar] [CrossRef]
- Anand, K.; Rajamanikandan, R.; Sharma, A.S.; Ilanchelian, M.; Khan, F.I.; Tiloke, C.; Katari, N.K.; Boomi, P.; Balakumar, C.; Saravanan, M. Human serum albumin interaction, in silico and anticancer evaluation of Pine-Gold nanoparticles. Process Biochem. 2020, 89, 98–109. [Google Scholar] [CrossRef]
- Alkhathlan, A.H.; AL-Abdulkarim, H.A.; Khan, M.; Khan, M.; AlDobiy, A.; Alkholief, M.; Alshamsan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H. Ecofriendly Synthesis of Silver Nanoparticles Using Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin) and Comparison of Their Antibacterial Potential. Sustainability 2020, 12, 10523. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W. Recent advances on the anticancer properties of Nigella sativa, a widely used food additive. J. Ayurveda Integr. Med. 2016, 7, 173–180. [Google Scholar] [CrossRef]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 650–653. [Google Scholar] [CrossRef]
- Suman, T.; Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Aromal, S.A.; Philip, D. Benincasa hispida seed mediated green synthesis of gold nanoparticles and its optical nonlinearity. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1329–1334. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anticancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- Loung, C.-Y.; Rasmussen, A.N.; Hoskin, D.W. The phenolic gingerols and gingerol-derived shogaols: Features and properties related to the prevention and treatment of cancer and chronic inflammation. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 395–405. [Google Scholar]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.-Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant properties of thymol, carvacrol, and thymoquinone and its efficiencies on the stabilization of refined and stripped corn oils. J. Food Meas. Charact. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Almomen, A.; Jarboe, E.A.; Dodson, M.K.; Peterson, C.M.; Owen, S.C.; Janát-Amsbury, M.M. Imiquimod induces apoptosis in human endometrial cancer cells in vitro and prevents tumor progression in vivo. Pharm. Res. 2016, 33, 2209–2217. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Agbaria, R.; Gabarin, A.; Dahan, A.; Ben-Shabat, S. Anticancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinone composition of the seed. Drug Des. Dev. Ther. 2015, 9, 3119. [Google Scholar]



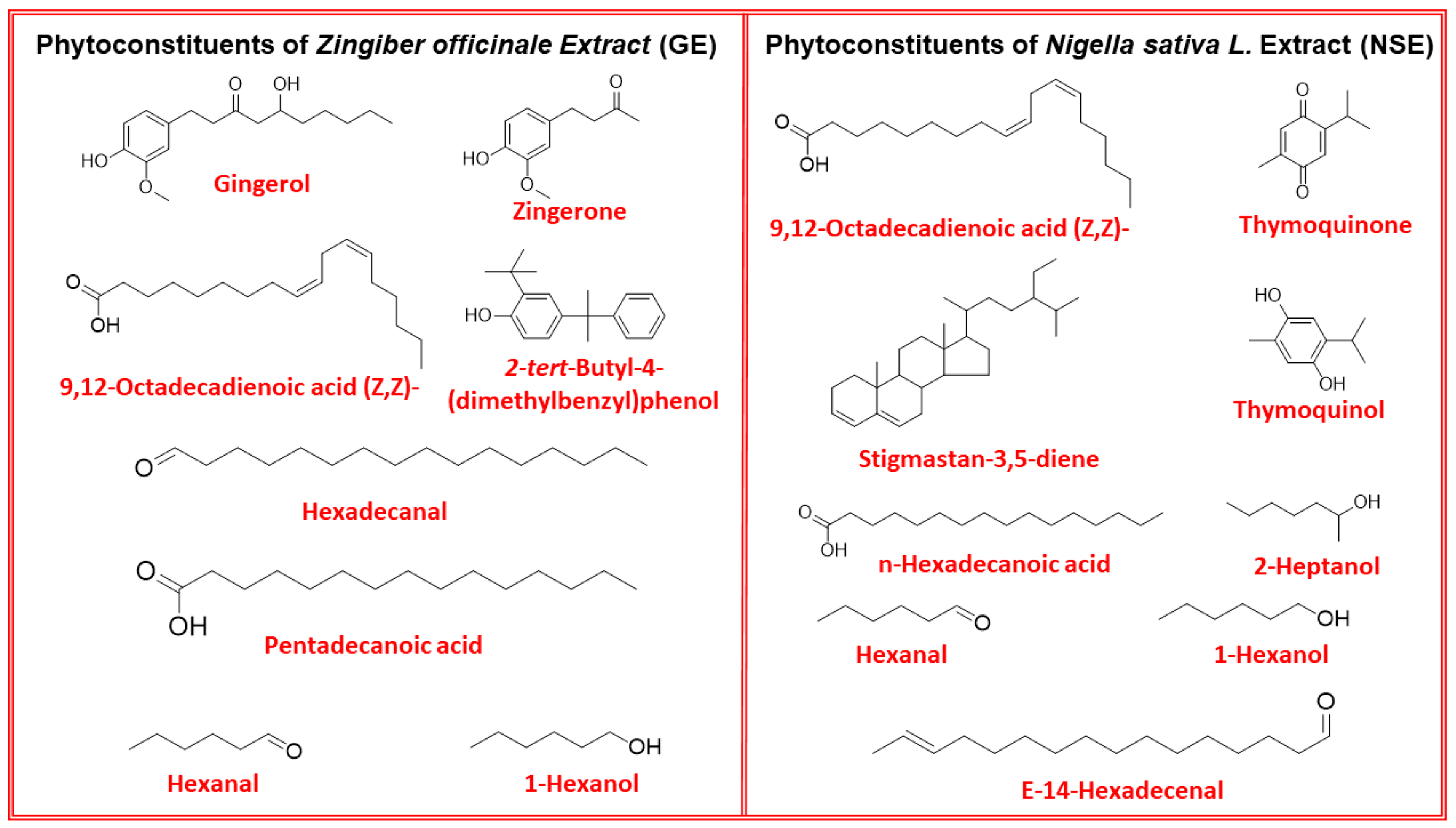
| Peak | Compounds | RT (Min.) | MF | MW | Peak Area (%) | |
|---|---|---|---|---|---|---|
| NS | ZO | |||||
| 1 | Hexanal | 6.28 | C6H12O | 100.16 | 0.4 | 4.8 |
| 2 | 1-Hexanol | 8.43 | C6H14O | 102.17 | 0.7 | 0.8 |
| 3 | 2-Heptanol | 9.09 | C7H16O | 116.2 | 0.3 | - |
| 4 | Dodecane | 20.80 | C12H26 | 170.33 | 0.5 | 0.8 |
| 5 | Tridecane | 24.24 | C13H28 | 184.36 | - | 0.6 |
| 6 | Tetradecane | 27.49 | C14H30 | 198.39 | 0.6 | 1.0 |
| 7 | Thymoquinone | 28.81 | C10H12O2 | 164.2 | 3.3 | - |
| 8 | Thymoquinol | 32.19 | C10H14O2 | 166.22 | 0.5 | - |
| 9 | Hexadecane | 33.45 | C16H34 | 226.44 | - | 1.0 |
| 10 | Zingerone | 34.87 | C11H14O3 | 194.22 | - | 3.0 |
| 11 | Hexadecanal | 39.35 | C16H32O | 240.42 | - | 1.1 |
| 12 | Pentadecanoic acid | 40.64 | C15H30O2 | 242.39 | - | 0.6 |
| 13 | n-Hexadecanoic acid | 42.84 | C16H32O2 | 256.42 | 3.1 | - |
| 14 | 2-tert-Butyl-4-(dimethylbenzyl)phenol | 46.74 | C19H24O | 268.4 | - | 1.0 |
| 15 | (Z,Z)-9,12-Octadecadienoic acid | 46.87 | C18H32O2 | 280.45 | 11.4 | 1.0 |
| 16 | 3-(6-Hydroxy-3,7-dimethyl-octa-2,7-dienyl)-4-methoxy-phenol | 50.56 | C17H24O3 | 276.00 | - | 7.0 |
| 17 | Gingerol | 52.32 | C17H26O4 | 294.38 | - | 11.3 |
| 18 | (E)-14-Hexadecenal | 52.35 | C16H30O | 238.41 | 0.7 | - |
| 19 | Pentacosane | 55.65 | C25H52 | 352.68 | - | 1.5 |
| 20 | n-Hexacosane | 58.04 | C26H54 | 366.70 | 0.8 | - |
| 21 | n-Heptacosane | 60.48 | C27H56 | 380.73 | 1.3 | - |
| 22 | Stigmastan-3,5-diene | 61.40 | C29H48 | 396.69 | 16.4 | - |
| Au-NPs | 24 h | 48 h | 72 h |
|---|---|---|---|
| GE-Au-1 | 100.22 ± 0.38 | 82.26 ± 0.28 | 69.67 ± 0.25 |
| GE-Au-2 | 78.85 ± 0.27 | 58.23 ± 0.07 | 54 ± 0.13 |
| GE-Au-3 | 36.4 ± 0.12 | 41.76 ± 0.07 | 40.3 ± 0.1 |
| NSE-Au-1 | 125.4 ± 0.21 | 99.04 ± 0.43 | 106.7 ± 0.22 |
| NSE-Au-2 | 97.07 ± 0.11 | 97.76 ± 0.22 | 83 ± 0.3 |
| NSE-Au-3 | 80.28 ± 0.21 | 76.54 ± 1 | 66.67 ± 0.42 |
| Au-NPs | 24 h | 48 h | 72 h |
|---|---|---|---|
| GE-Au-1 | 126.8 ± 0.22 | 114.96 ± 1.16 | NA |
| GE-Au-2 | 100.6 ± 0.17 | 111.92 ± 0.309 | 137.26 ± 0.41 |
| GE-Au-3 | 116.52 ± 15.55 | 90.47 ± 0.34 | 47.56 ± 0.21 |
| NSE-Au-1 | 111.73 ± 0.6 | 76.81 ± 0.34 | 118.76 ± 0.86 |
| NSE-Au-2 | 112.13 ± 0.77 | NA | 110.53 ± 0.26 |
| NSE-Au-3 | 99.36 ± 0.1 | 115.53 ± 0.17 | 42.6 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhathlan, A.H.; Al-Abdulkarim, H.A.; Khan, M.; Khan, M.; Alkholief, M.; Alshamsan, A.; Almomen, A.; Albekairi, N.; Alkhathlan, H.Z.; Siddiqui, M.R.H. Evaluation of the Anticancer Activity of Phytomolecules Conjugated Gold Nanoparticles Synthesized by Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin). Materials 2021, 14, 3368. https://doi.org/10.3390/ma14123368
Alkhathlan AH, Al-Abdulkarim HA, Khan M, Khan M, Alkholief M, Alshamsan A, Almomen A, Albekairi N, Alkhathlan HZ, Siddiqui MRH. Evaluation of the Anticancer Activity of Phytomolecules Conjugated Gold Nanoparticles Synthesized by Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin). Materials. 2021; 14(12):3368. https://doi.org/10.3390/ma14123368
Chicago/Turabian StyleAlkhathlan, Alaa H., Hessah A. Al-Abdulkarim, Merajuddin Khan, Mujeeb Khan, Musaed Alkholief, Aws Alshamsan, Aliyah Almomen, Norah Albekairi, Hamad Z. Alkhathlan, and M. Rafiq H. Siddiqui. 2021. "Evaluation of the Anticancer Activity of Phytomolecules Conjugated Gold Nanoparticles Synthesized by Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin)" Materials 14, no. 12: 3368. https://doi.org/10.3390/ma14123368
APA StyleAlkhathlan, A. H., Al-Abdulkarim, H. A., Khan, M., Khan, M., Alkholief, M., Alshamsan, A., Almomen, A., Albekairi, N., Alkhathlan, H. Z., & Siddiqui, M. R. H. (2021). Evaluation of the Anticancer Activity of Phytomolecules Conjugated Gold Nanoparticles Synthesized by Aqueous Extracts of Zingiber officinale (Ginger) and Nigella sativa L. Seeds (Black Cumin). Materials, 14(12), 3368. https://doi.org/10.3390/ma14123368









