Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al,Si)C2 Materials
Abstract
1. Introduction
2. Experimental
3. Characterization
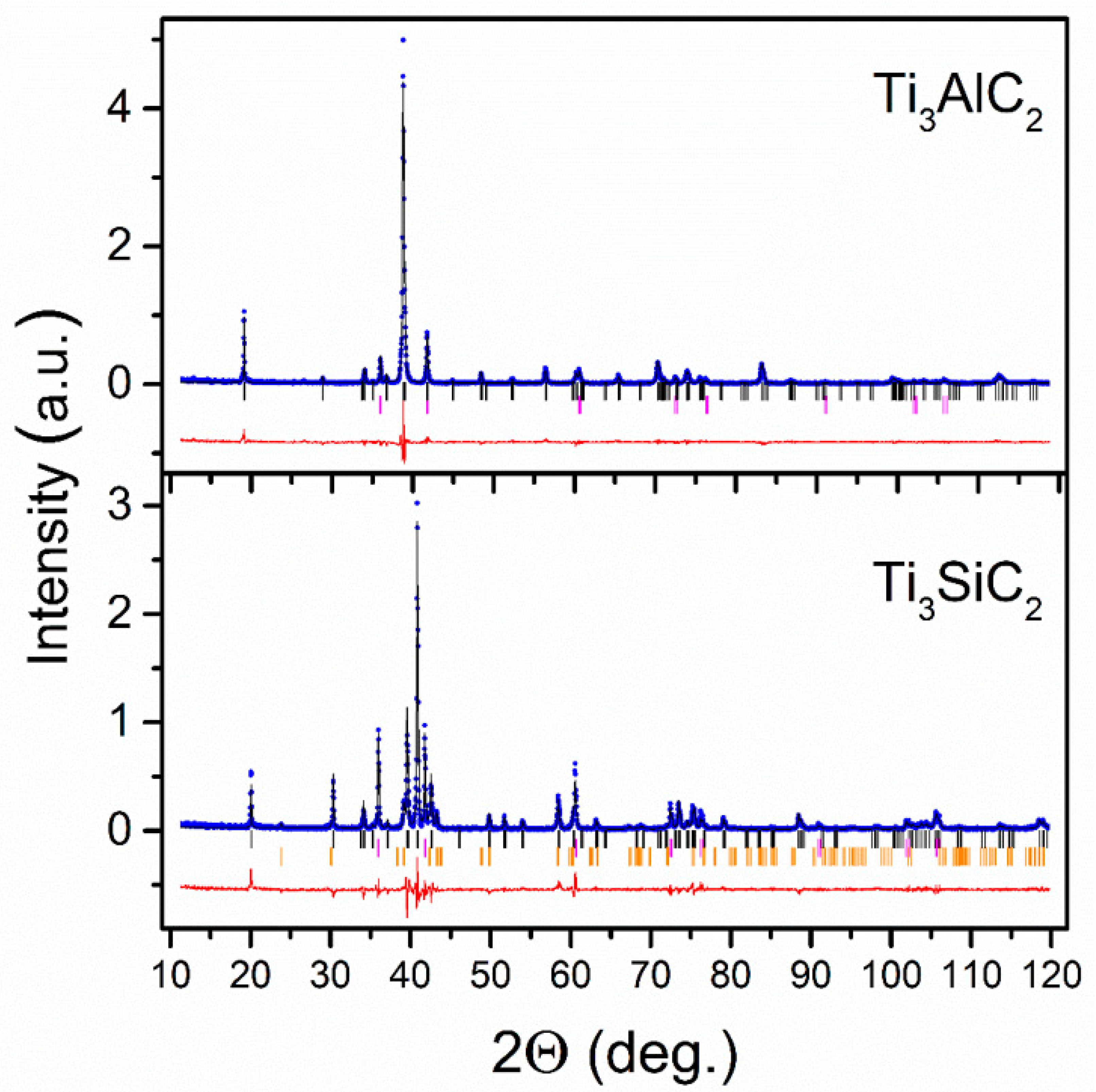
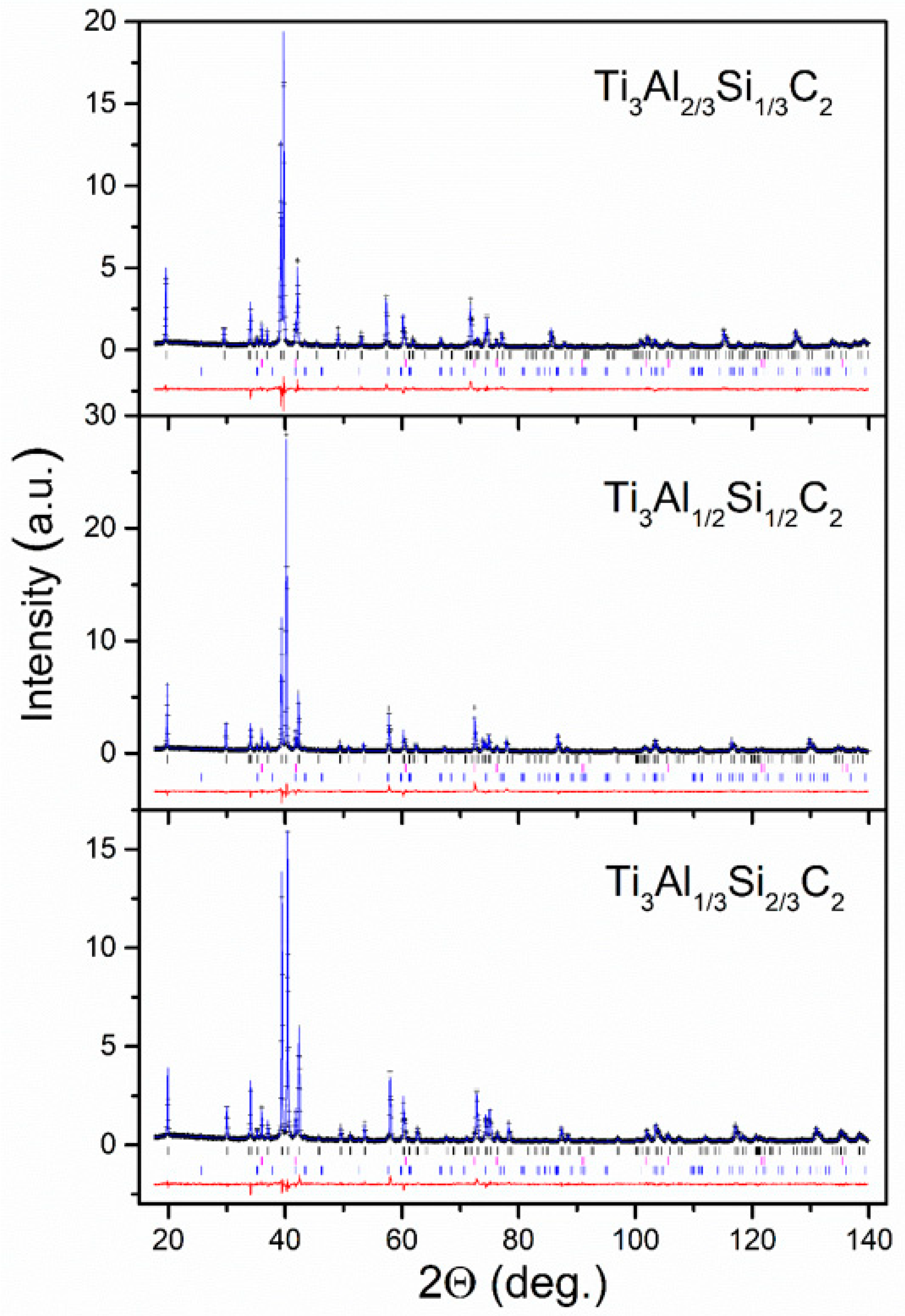
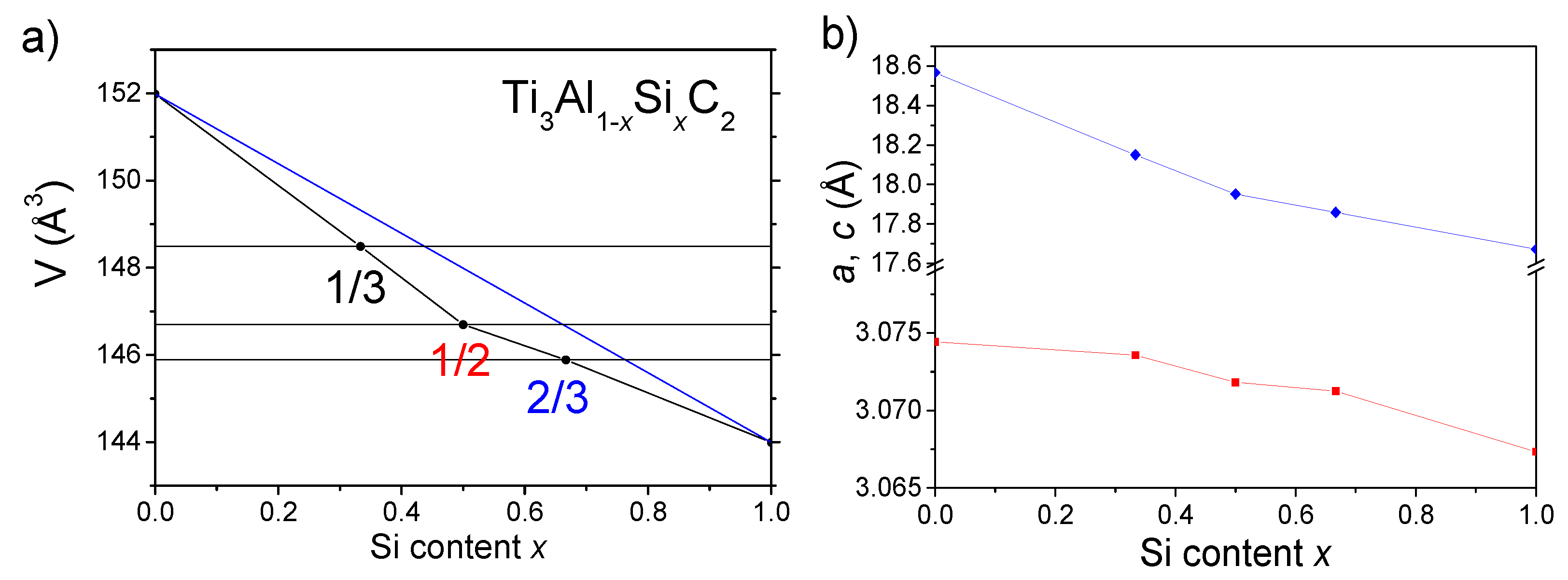
3.1. Characterization of the Crystallographic Structure
3.2. Microstructure Characterization
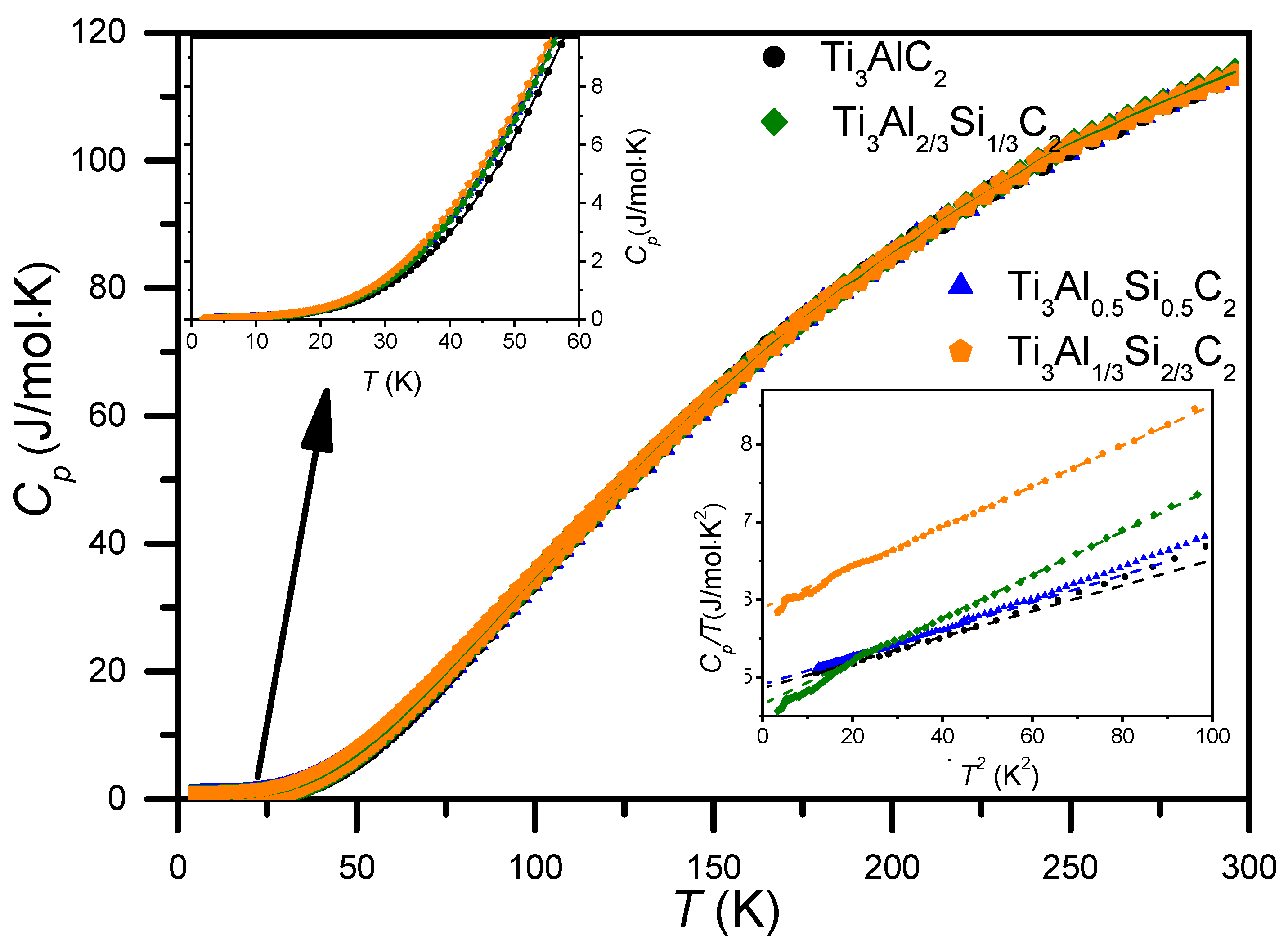
3.3. Heat Capacity
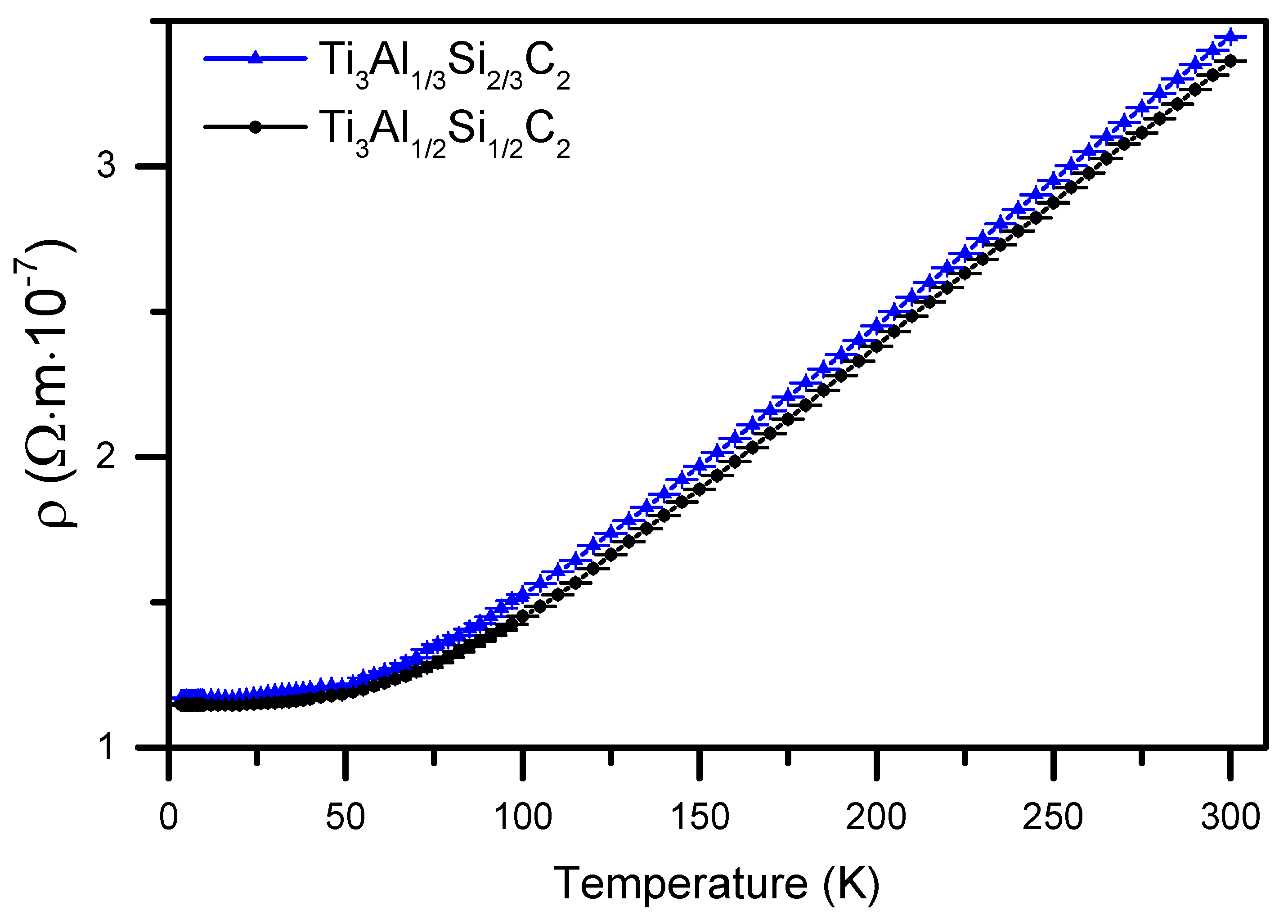
3.4. Electrical Resistivity and Magnetoresistance
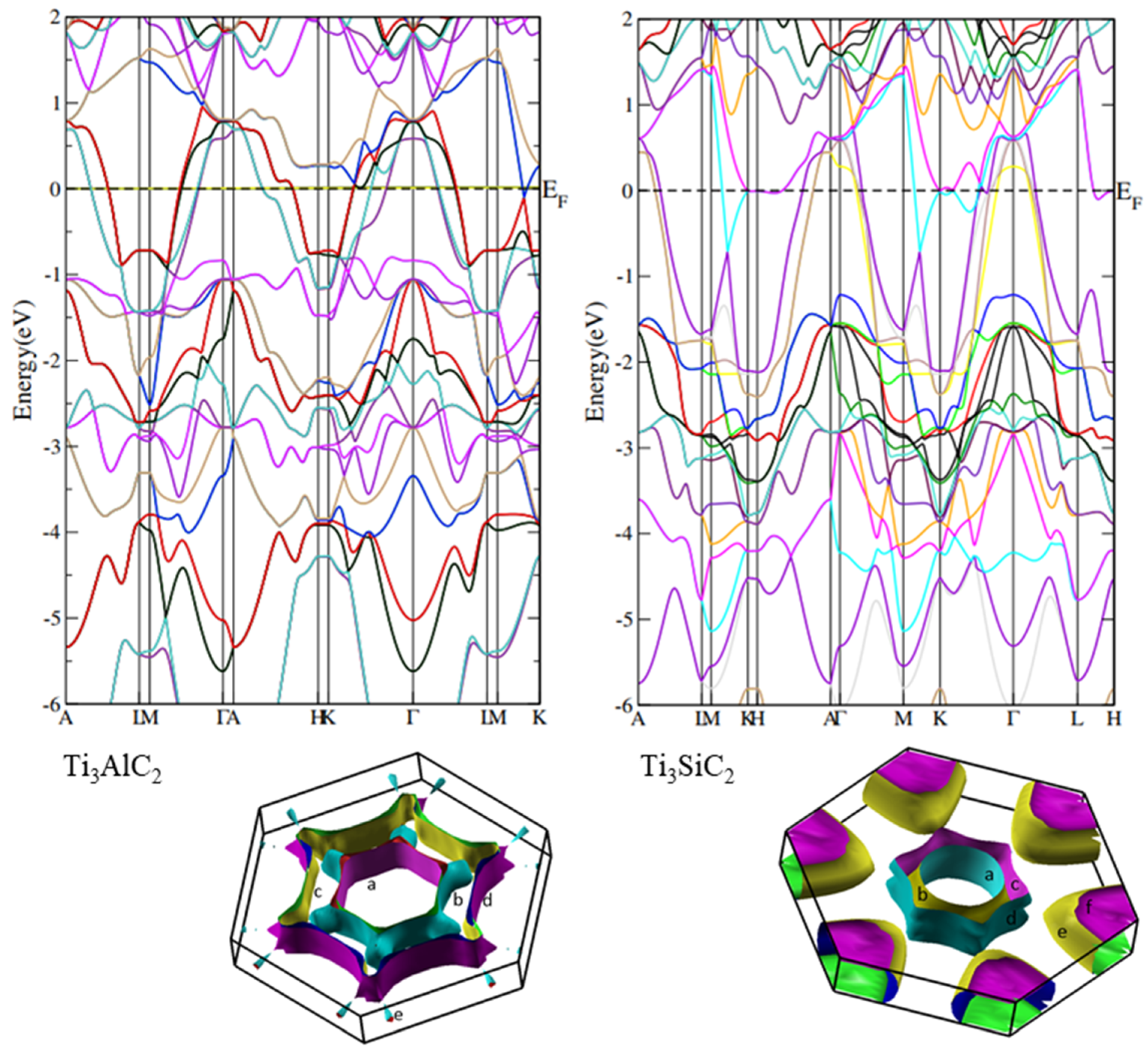
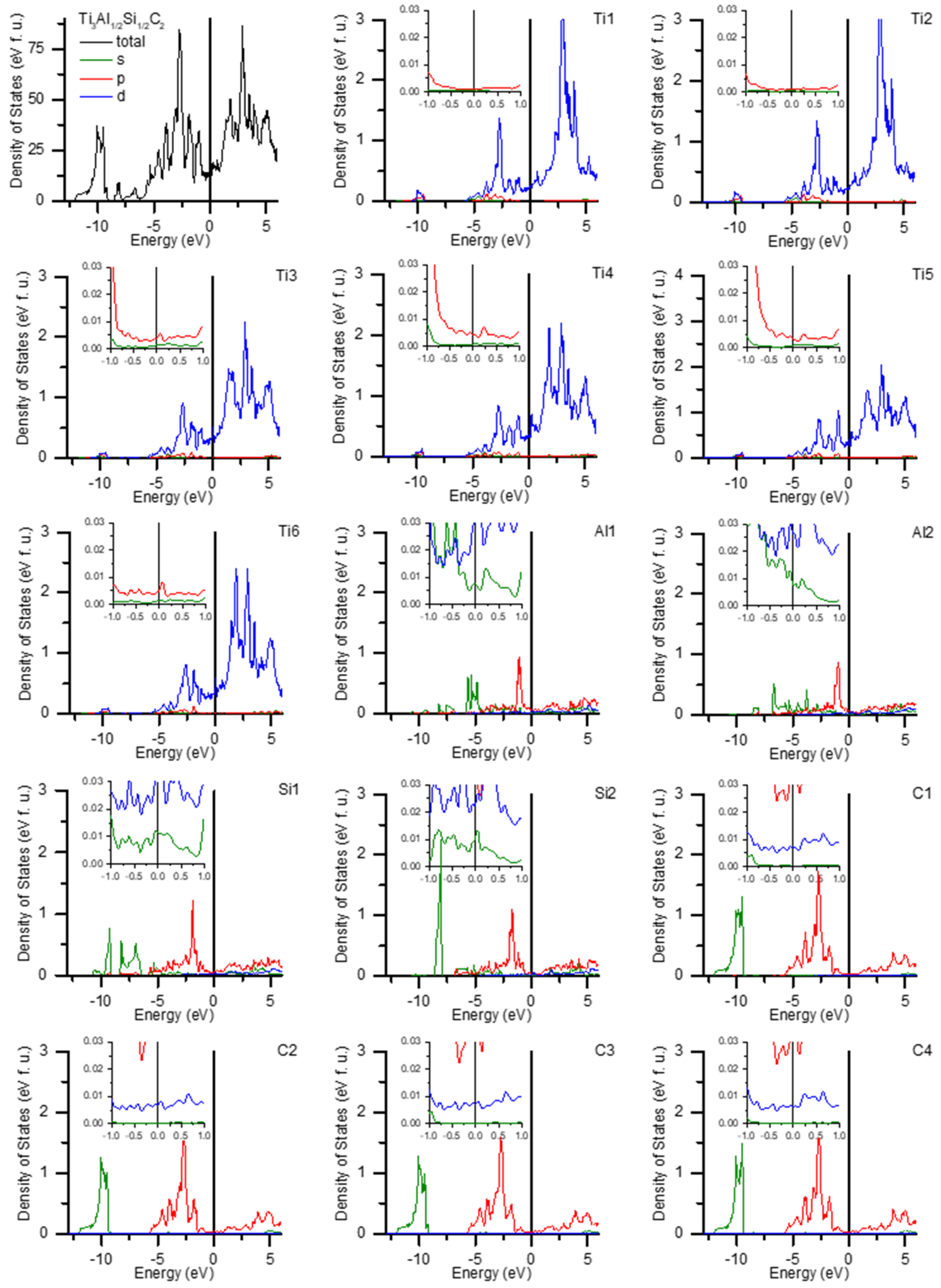
3.5. Density of States Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsoum, M.W. The MN+1AXN Phases: A New Class of Solids: Thermodynamically Stable Nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn+1AXn Phases: Materials Science and Thin-Film Processing. Thin Solid Films 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Low, I.-M. An Overview of Parameters Controlling the Decomposition and Degradation of Ti-Based Mn+1AXn Phases. Materials 2019, 12, 473. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Kohlenstoffhaltige ternäre Verbindungen (H-Phase). Mon. Für Chem. Verwandte Teile Anderer Wiss. 1963, 94, 672–676. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Barsoum, M.W.; Radovic, M. MAX Phases: Bridging the Gap between Metals and Ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Barsoum, M.W.; El-Raghy, T.; Ali, M. Processing and Characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5. Metall. Mater. Trans. A 2000, 31, 1857–1865. [Google Scholar] [CrossRef]
- Tzenov, N.V.; Barsoum, M.W. Synthesis and Characterization of Ti3AlC2. J. Am. Ceram. Soc. 2000, 83, 825–832. [Google Scholar] [CrossRef]
- Ying, G.; He, X.; Li, M.; Li, Y.; Du, S. Synthesis and Mechanical Properties of Nano-Layered Composite. J. Alloys Compd. 2010, 506, 734–738. [Google Scholar] [CrossRef]
- Chen, W.; Tang, J.; Shi, X.; Ye, N.; Yue, Z.; Lin, X. Synthesis and Formation Mechanism of High-Purity Ti3AlC2 Powders by Microwave Sintering. Int. J. Appl. Ceram. Technol. 2020, 17, 778–789. [Google Scholar] [CrossRef]
- Sobolev, K.V.; Kolincio, K.K.; Emelyanov, A.; Mielewczyk-Gryń, A.; Gazda, M.; Roman, M.; Pazniak, A.; Rodionova, V. Evolution of Magnetic and Transport Properties in (Cr1−xMnx)2AlC MAX-Phase Synthesized by Arc Melting Technique. J. Magn. Magn. Mater. 2020, 493, 165642. [Google Scholar] [CrossRef]
- Berger, O. The Correlation between Structure, Multifunctional Properties and Application of PVD MAX Phase Coatings. Part III. Multifunctional Applications. Surf. Eng. 2020, 36, 303–325. [Google Scholar] [CrossRef]
- Lane, N.J.; Naguib, M.; Lu, J.; Hultman, L.; Barsoum, M.W. Structure of a New Bulk Ti5Al2C3MAX Phase Produced by the Topotactic Transformation of Ti2AlC. J. Eur. Ceram. Soc. 2012, 32, 3485–3491. [Google Scholar] [CrossRef]
- Zhou, W.; Mei, B.; Zhu, J. Effect of Tin on the Reaction Synthesis of Ternary Carbide Ti3AlC2. J. Wuhan Univ. Technol. Mater Sci Ed. 2009, 24, 283–286. [Google Scholar] [CrossRef]
- Zhou, W.; Mei, B.; Zhu, J.; Hong, X. Synthesis of High-Purity Ti3SiC2 and Ti3AlC2 by Spark Plasma Sintering (SPS) Technique. J. Mater. Sci. 2005, 40, 2099–2100. [Google Scholar] [CrossRef]
- Yang, C.; Jin, S.Z.; Liang, B.Y.; Jia, S.S. Low-Temperature Synthesis of High-Purity Ti3AlC2 by MA-SPS Technique. J. Eur. Ceram. Soc. 2009, 29, 181–185. [Google Scholar] [CrossRef]
- Garbiec, D.; Leshchynsky, V.; Colella, A.; Matteazzi, P.; Siwak, P. Structure and Deformation Behavior of Ti-SiC Composites Made by Mechanical Alloying and Spark Plasma Sintering. Materials 2019, 12, 1276. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Kapusta, C.; Zientara, D.; Chabior, K.; Chachlowska, P. Phase Evolution Phenomenon During Hot Pressing of the SHS Obtained Ti3AlC2 Precursors Powders. In Additive Manufacturing and Strategic Technologies in Advanced Ceramics; Shimamura, K., Kirihara, S., Akedo, J., Ohji, T., Naito, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 219–228. ISBN 978-1-119-23601-6. [Google Scholar]
- Goc, K.; Prendota, W.; Chlubny, L.; Strączek, T.; Tokarz, W.; Chachlowska, P.B.; Chabior, K.W.; Bućko, M.M.; Przewoźnik, J.; Lis, J. Structure, Morphology and Electrical Transport Properties of the Ti3AlC2 Materials. Ceram. Int. 2018, 44, 18322–18328. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Layered Machinable and Electrically Conductive Influence of Precursors Stoichiometry on SHS Synthesis of Ti2AlC Powders and Influence of Precursors Stoichiometry on SHS Synthesis of Ti3AlC2 Powders Ceramics: A Review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yang, W.J. Formation of MAX Solid Solutions (Ti,V)2AlC and (Cr,V)2AlC with Al2O3 Addition by SHS Involving Aluminothermic Reduction. Ceram. Int. 2013, 39, 7537–7544. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; BuÐko, M.M. Densification of SHS Obtained Ti2AlC Active Precursor Powder by Hot Pressing Method. In Processing and Properties of Advanced Ceramics and Composites VII; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 203–212. ISBN 978-1-119-18386-0. [Google Scholar]
- Chlubny, L.; Lis, J.; Chabior, K.; Chachlowska, P.; Kapusta, C. Processing And Properties of MAX Phases-Based Materials Using SHS Technique. Arch. Metall. Mater. 2015, 60. [Google Scholar] [CrossRef]
- Lis, J.; Chlubny, L.; Witulska, K.; Borowiak, P.; Kozak, K.; Misztal, A.; Czajkowska, O. SHS of Ti3SiC2-Based Materials in the Ti–Si–C System: Impact of Silicon Excess. Int. J. Self Propagating High Temp. Synth. 2019, 28, 262–265. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Bućko, M.M. Sintering and Hot-Pressing of Ti2AlC Obtained by SHS Process. Adv. Sci. Technol. 2010, 63, 282–286. [Google Scholar] [CrossRef]
- Biswas, A.; Sengupta, A.; Rajput, U.; Singh, S.K.; Antad, V.; Hossain, S.M.; Parmar, S.; Rout, D.; Deshpande, A.; Nair, S.; et al. Growth, Properties, and Applications of Pulsed Laser Deposited Nanolaminate Ti3AlC2 Thin Films. Phys. Rev. Appl. 2020, 13, 044075. [Google Scholar] [CrossRef]
- Yu, W.; Li, S.; Sloof, W.G. Microstructure and Mechanical Properties of a Cr2Al(Si)C Solid Solution. Mater. Sci. Eng. A 2010, 527, 5997–6001. [Google Scholar] [CrossRef]
- Bei, G.P.; Gauthier-Brunet, V.; Tromas, C.; Dubois, S. Synthesis, Characterization, and Intrinsic Hardness of Layered Nanolaminate Ti3AlC2 and Ti3Al0.8Sn0.2C2 Solid Solution. J. Am. Ceram. Soc. 2012, 95, 102–107. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W.; Ganguly, A.; Zhen, T.; Finkel, P.; Kalidindi, S.R.; Lara-Curzio, E. On the Elastic Properties and Mechanical Damping of Ti3SiC2, Ti3GeC2, Ti3Si0.5Al0.5C2 and Ti2AlC in the 300–1573K Temperature Range. Acta Mater. 2006, 54, 2757–2767. [Google Scholar] [CrossRef]
- Mockute, A.; Dahlqvist, M.; Emmerlich, J.; Hultman, L.; Schneider, J.M.; Persson, P.O.Å.; Rosen, J. Synthesis and Ab Initio Calculations of Nanolaminated (Cr,Mn)2AlC Compounds. Phys. Rev. B 2013, 87, 094113. [Google Scholar] [CrossRef]
- Gao, H.; Benitez, R.; Son, W.; Arroyave, R.; Radovic, M. Structural, Physical and Mechanical Properties of Ti3(Al1−xSix)C2 Solid Solution with X=0–1. Mater. Sci. Eng. A 2016, 676, 197–208. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Yin, G.; Wang, C.-Y.; Hou, Y.-Y.; Tang, J.; Dai, J.-X.; Ren, C.-L.; Zhang, W.; Huai, P. First-Principles Study of Vacancies in Ti3SiC2 and Ti3AlC2. Materials 2017, 10, 103. [Google Scholar] [CrossRef]
- Ng, W.H.K.; Gnanakumar, E.S.; Batyrev, E.; Sharma, S.K.; Pujari, P.K.; Greer, H.F.; Zhou, W.; Sakidja, R.; Rothenberg, G.; Barsoum, M.W.; et al. The Ti3AlC2 MAX Phase as an Efficient Catalyst for Oxidative Dehydrogenation of N-Butane. Angew. Chem. Int. Ed. 2018, 57, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Peter, B.; Karlheinz, S.; Madsen, G.K.H.; Dieter, K.; Joachim, L.; Robert, L.; Fabien, T.; Laurence, D.M. WIEN2k-An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties; Vienna University of Technology: Vienna, Austria, 2018; ISBN 3-9501031-1-2. [Google Scholar]
- Szytuła, A.; Kaczorowski, D.; Szewczyk, A.; Gutowska, M.; Piekarz, P. Multiple Magnetic Phase Transitions in Tb3Cu4Si4. J. Phys. Condens. Matter 2007, 19, 246225. [Google Scholar] [CrossRef][Green Version]
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Harcourt College Publisher: New York, NY, USA, 1976. [Google Scholar]
- Finkel, P.; Barsoum, M.W.; Hettinger, J.D.; Lofland, S.E.; Yoo, H.I. Low-Temperature Transport Properties of Nanolaminates Ti3AlC2 and Ti4AlN3. Phys. Rev. B 2003, 67, 235108. [Google Scholar] [CrossRef]
- Sondheimer, E.H.; A, B.; Wilson, A.H.; S, F.R. The Theory of the Magneto-Resistance Effects in Metals. Proc. R. Soc. Lond. A 1947, 190, 435–455. [Google Scholar] [CrossRef]
- Kokalj, A. Computer Graphics and Graphical User Interfaces as Tools in Simulations of Matter at the Atomic Scale. Comput. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Lubatsch, A.; Frank, R. Self-Consistent Quantum Field Theory for the Characterization of Complex Random Media by Short Laser Pulses. Phys. Rev. Res. 2020, 2, 013324. [Google Scholar] [CrossRef]
- Vollhardt, D.; Wölfle, P. Diagrammatic, Self-Consistent Treatment of the Anderson Localization Problem in $d\ensuremath{\le}2$ Dimensions. Phys. Rev. B 1980, 22, 4666–4679. [Google Scholar] [CrossRef]
- Vollhardt, D.; Wölfle, P. Anderson Localization in $d<~2$ Dimensions: A Self-Consistent Diagrammatic Theory. Phys. Rev. Lett. 1980, 45, 842–846. [Google Scholar] [CrossRef]





| x (Si at.%) | Ti3(Al1−xSix)C2 | TiC | Al2O3 | TiSi2 |
|---|---|---|---|---|
| 0 | 88(3) | 12.3(7) | 0 | - |
| 1/3 | 89(2) | 6(1) | 5(1) | 0 |
| 1/2 | 87(2) | 8(1) | 3(1) | 0 |
| 2/3 | 91(2) | 6(2) | 3(1) | 0 |
| 1 | 62(3) | 27(2) | - | 11(1) |
| x (Si at.) | a (Å) | c (Å) | ||
|---|---|---|---|---|
| 0 | 3.0746(2) | 18.5670(6) | 0.1273(3) | 0.5692(8) |
| 1/3 | 3.0736(1) | 18.1500(3) | 0.1310(2) | 0.5720(5) |
| 1/2 | 3.0718(1) | 17.9511(3) | 0.1326(2) | 0.5711(5) |
| 2/3 | 3.0713(1) | 17.8588(2) | 0.1334(1) | 0.5723(4) |
| 1 | 3.0674(2) | 17.6723(5) | 0.1353(2) | 0.5743(9) |
| Parameter | Ti3AlC2 | Ti3Al2/3Si1/3C2 | Ti3Al1/2Si1/2C2 | Ti3Al1/3Si2/3C2 |
|---|---|---|---|---|
| (K) | 484.2 ± 1.6 | 412.1 ± 0.4 | 472.0 ± 0.7 | 420.6 ± 0.5 |
| (mJ⋅mol−1⋅K−2) | 4.848 ± 0.006 | 4.653 ± 0.005 | 4.891 ± 0.003 | 5.893 ± 0.005 |
| Parameter | Ti3AlC2 | Ti3Al1/2Si1/2C2 |
|---|---|---|
| (K) | 396.2 ± 4.1 | 371.9 ± 2.2 |
| (K) | 299.6 ± 3.4 | 296.2 ± 2.1 |
| (K) | 459.2 ± 4.8 | 506.2 ± 6.2 |
| (K) | 652.9 ± 3.4 | 638.3 ± 4.3 |
| (K) | 1107 ± 22 | 1098 ± 20 |
| (10−5⋅K−1) | 9.1 ± 1.0 | 8.5 ± 0.5 |
| Compound | Lattice Parameters (Å) | Atoms | Wyckoff Positions | x | y | z |
|---|---|---|---|---|---|---|
| Ti3AlC2 | a = 3.0547 b = 3.0547 c = 18.453 | Ti-1 | 2a | 0 | 0 | 0 |
| Ti-2 | 4f | 1/3 | 2/3 | 0.128 | ||
| Al | 2b | 0 | 0 | 0.25 | ||
| C | 4f | 1/3 | 2/3 | 0.064 | ||
| Ti3Al3/4Si1/4C2 | a = 6.090 b = 6.090 c = 18.396 | Ti-1 | 6g | 1/2 | 1/2 | 1/2 |
| Ti-2 | 2a | 0 | 0 | 1/2 | ||
| Ti-3 | 12k | 0.331 | 0.166 | 0.129 | ||
| Ti-4 | 4f | 1/3 | 2/3 | 0.127 | ||
| Si/Al | 2b | 1/2 | 0 | 1/4 | ||
| Al/Si | 6h | 0.498 | 0.502 | 1/4 | ||
| C-1 | 12k | 0.167 | 0.834 | 0.070 | ||
| C-2 | 4f | 1/3 | 2/3 | 0.57 | ||
| Ti3Al1/2Si1/2C2 | a = 6.103 b = 6.103 c = 18.064 | Ti-1 | 6n | 0.5006 | 0.4999 | 0.246 |
| Ti-2 | 2g | 0 | 0 | 0.247 | ||
| Ti-3 | 6n | 0.167 | 0.833 | 0.117 | ||
| Ti-4 | 6n | 0.834 | 0.166 | 0.378 | ||
| Ti-5 | 2h | 1/3 | 2/3 | 0.375 | ||
| Ti-6 | 2i | 2/3 | 1/3 | 0.113 | ||
| Si-1 | 3j | 0.490 | 0.510 | 0 | ||
| Si-2 | 1b | 0 | 0 | 1/2 | ||
| Al.-1 | 1a | 0 | 0 | 0 | ||
| Al.-2 | 3k | 0.493 | 0.507 | 1/2 | ||
| C-1 | 6n | 0.166 | 0.834 | 0.317 | ||
| C-2 | 6n | 0.833 | 0.167 | 0.176 | ||
| C-3 | 2h | 1/3 | 2/3 | 0.177 | ||
| C-4 | 2i | 2/3 | 1/3 | 0.318 | ||
| Ti3Al1/4Si3/4C2 | a = 6.157 b = 6.157 c = 18.012 | Ti-1 | 6g | 1/2 | 1/2 | 1/2 |
| Ti-2 | 2a | 0 | 0 | 1/2 | ||
| Ti-3 | 12k | 0.335 | 0.168 | 0.132 | ||
| Ti-4 | 4f | 1/3 | 2/3 | 0.134 | ||
| Al | 2b | 0 | 0 | 1/4 | ||
| Si | 6h | 0.504 | 0.496 | 1/4 | ||
| C-1 | 12k | 0.167 | 0.833 | 0.071 | ||
| C-2 | 4f | 1/3 | 2/3 | 0.571 | ||
| Ti3SiC2 | a = 3.0422 b = 3.0422 c = 17.620 | Ti-1 | 2a | 0 | 0 | 0 |
| Ti-2 | 4f | 1/3 | 2/3 | 0.135 | ||
| Si | 2b | 0 | 0 | 0.25 | ||
| C | 4f | 1/3 | 2/3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goc, K.; Przewoźnik, J.; Witulska, K.; Chlubny, L.; Tokarz, W.; Strączek, T.; Michalik, J.M.; Jurczyk, J.; Utke, I.; Lis, J.; et al. Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al,Si)C2 Materials. Materials 2021, 14, 3222. https://doi.org/10.3390/ma14123222
Goc K, Przewoźnik J, Witulska K, Chlubny L, Tokarz W, Strączek T, Michalik JM, Jurczyk J, Utke I, Lis J, et al. Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al,Si)C2 Materials. Materials. 2021; 14(12):3222. https://doi.org/10.3390/ma14123222
Chicago/Turabian StyleGoc, Kamil, Janusz Przewoźnik, Katarzyna Witulska, Leszek Chlubny, Waldemar Tokarz, Tomasz Strączek, Jan Marek Michalik, Jakub Jurczyk, Ivo Utke, Jerzy Lis, and et al. 2021. "Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al,Si)C2 Materials" Materials 14, no. 12: 3222. https://doi.org/10.3390/ma14123222
APA StyleGoc, K., Przewoźnik, J., Witulska, K., Chlubny, L., Tokarz, W., Strączek, T., Michalik, J. M., Jurczyk, J., Utke, I., Lis, J., & Kapusta, C. (2021). Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al,Si)C2 Materials. Materials, 14(12), 3222. https://doi.org/10.3390/ma14123222







