Evaluation of Rhodamine B Photocatalytic Degradation over BaTiO3-MnO2 Ceramic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents and Solvents
2.2. Chemical and Physicochemical Characterization
2.3. Adsorption and Photocatalytic Performance Tests
3. Results
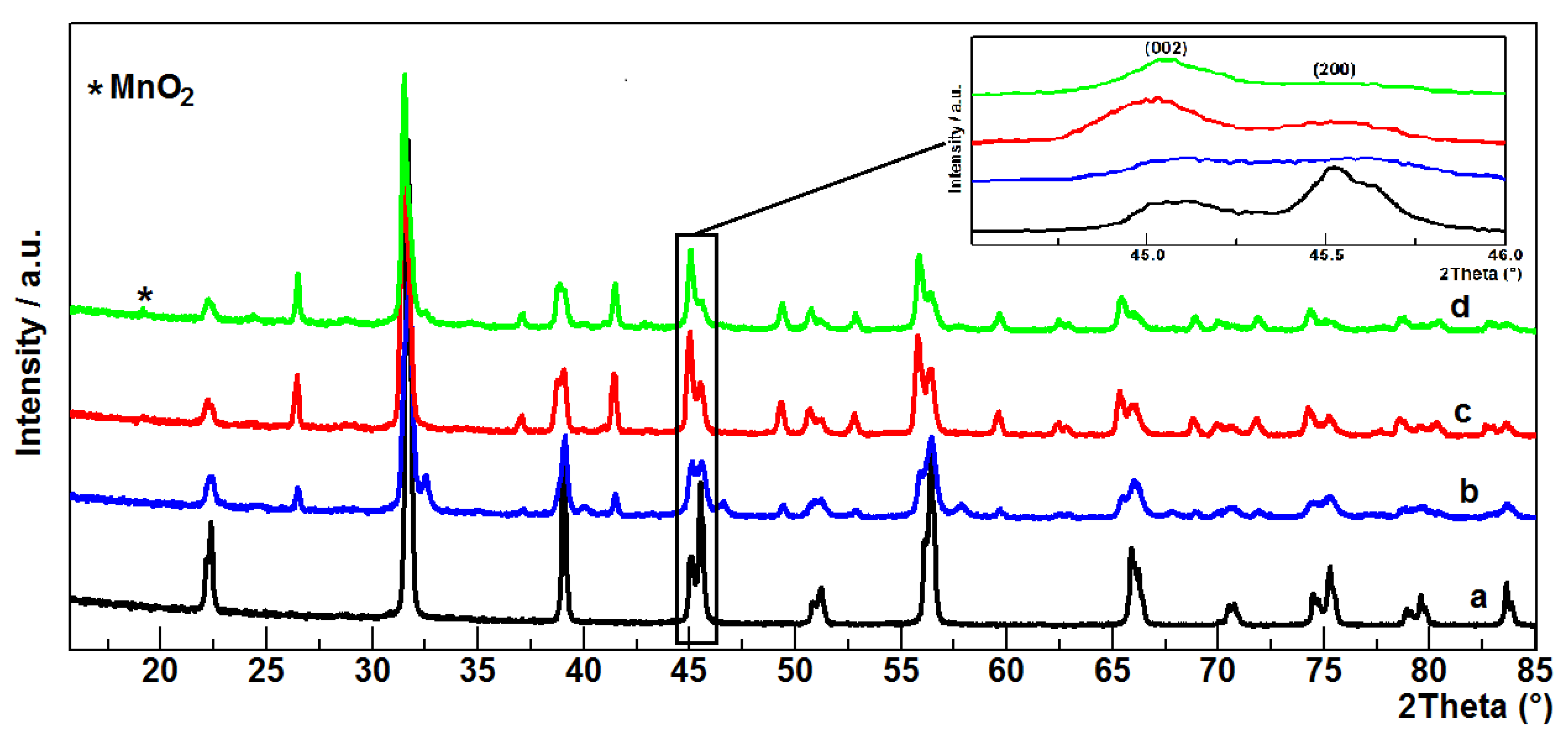
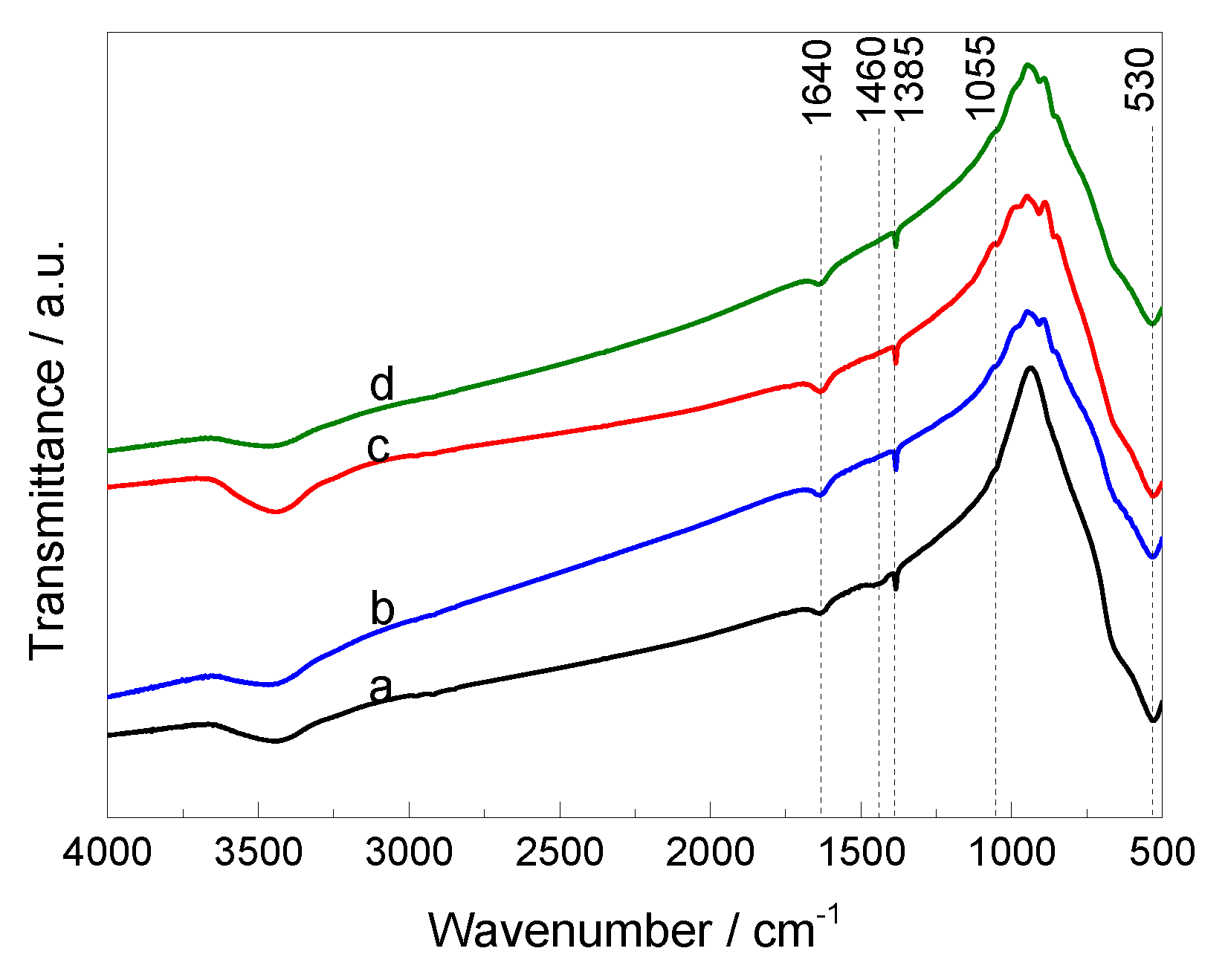
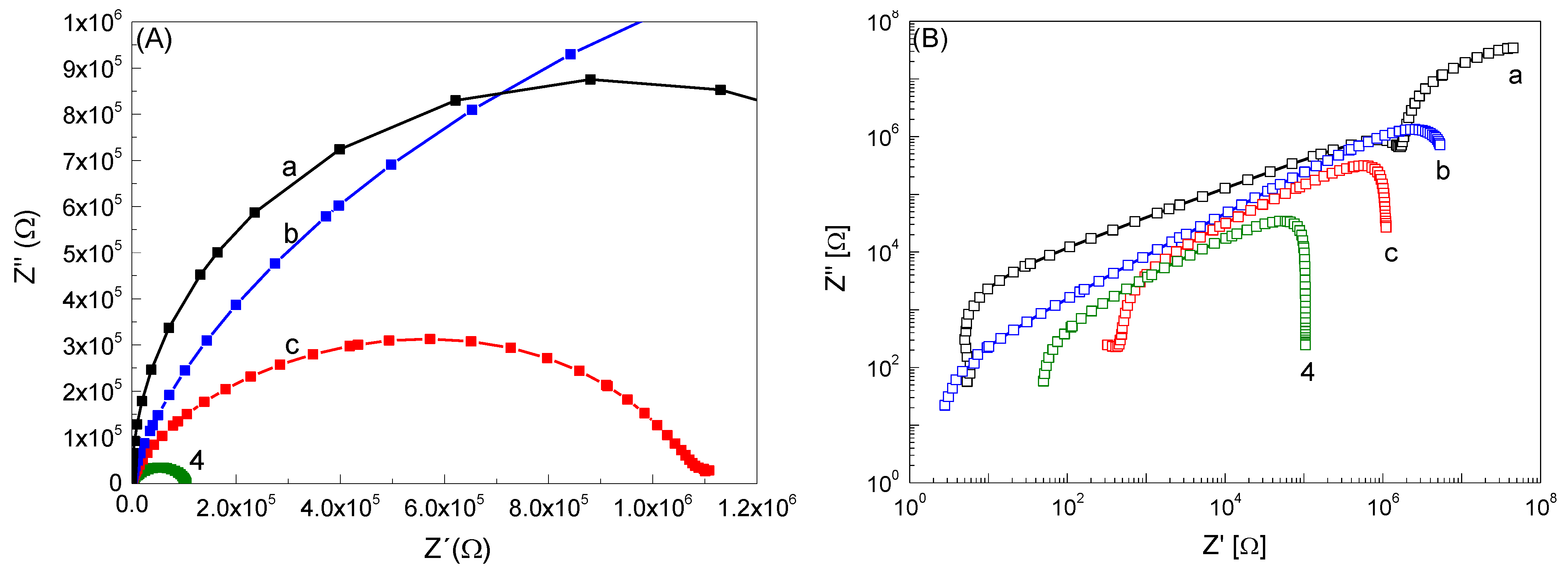
3.1. Characterisation of the Materials
3.2. Adsorption Experiments
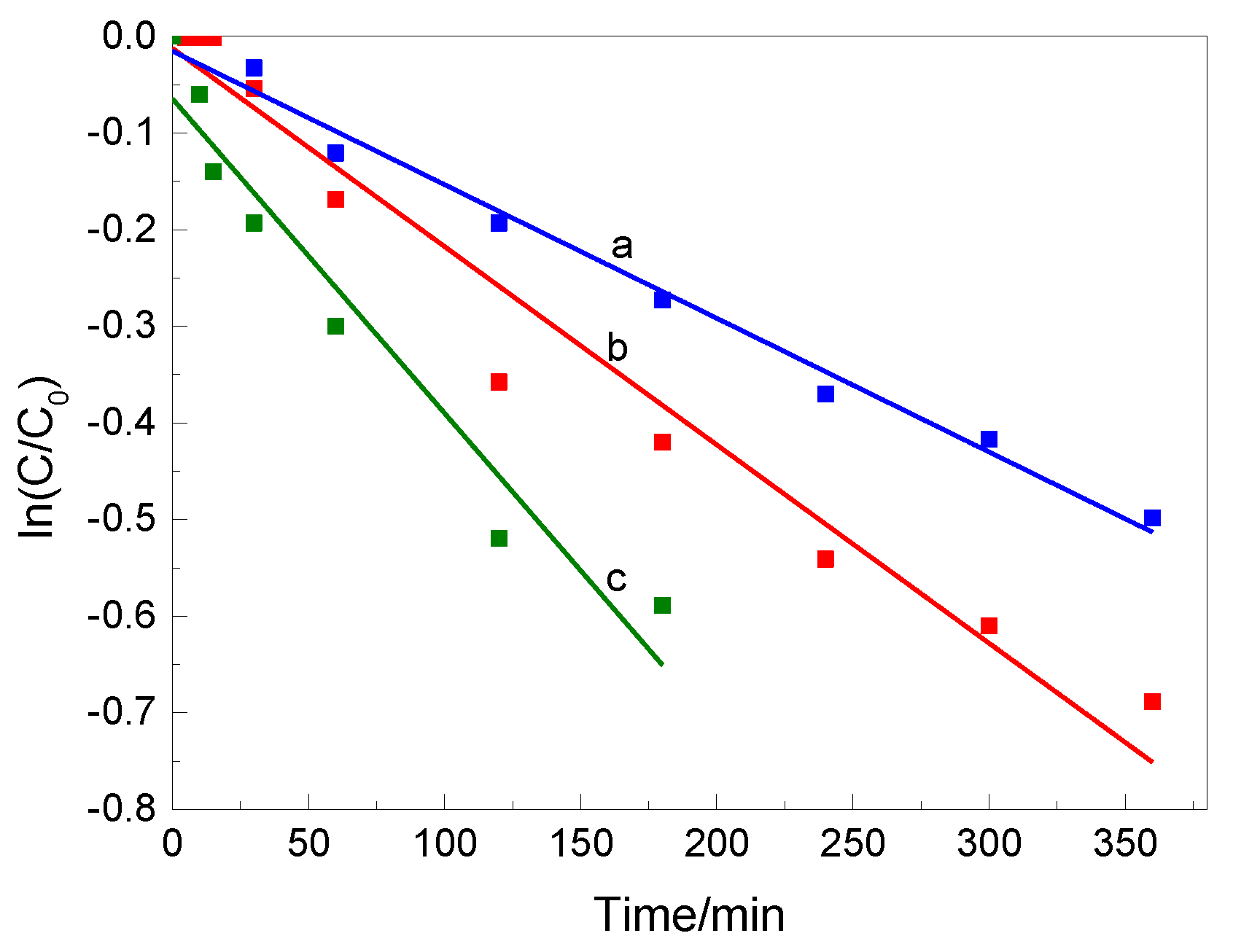
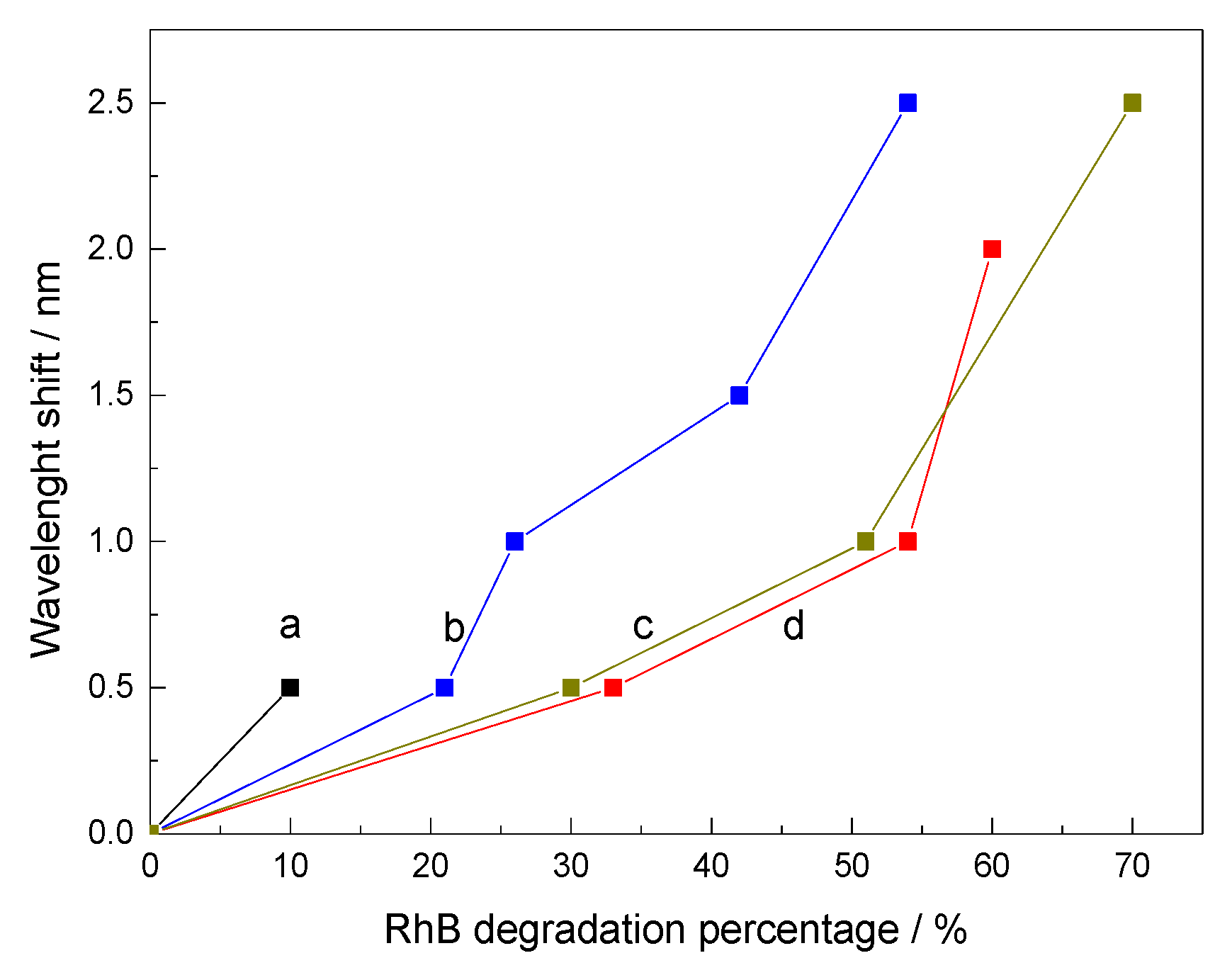
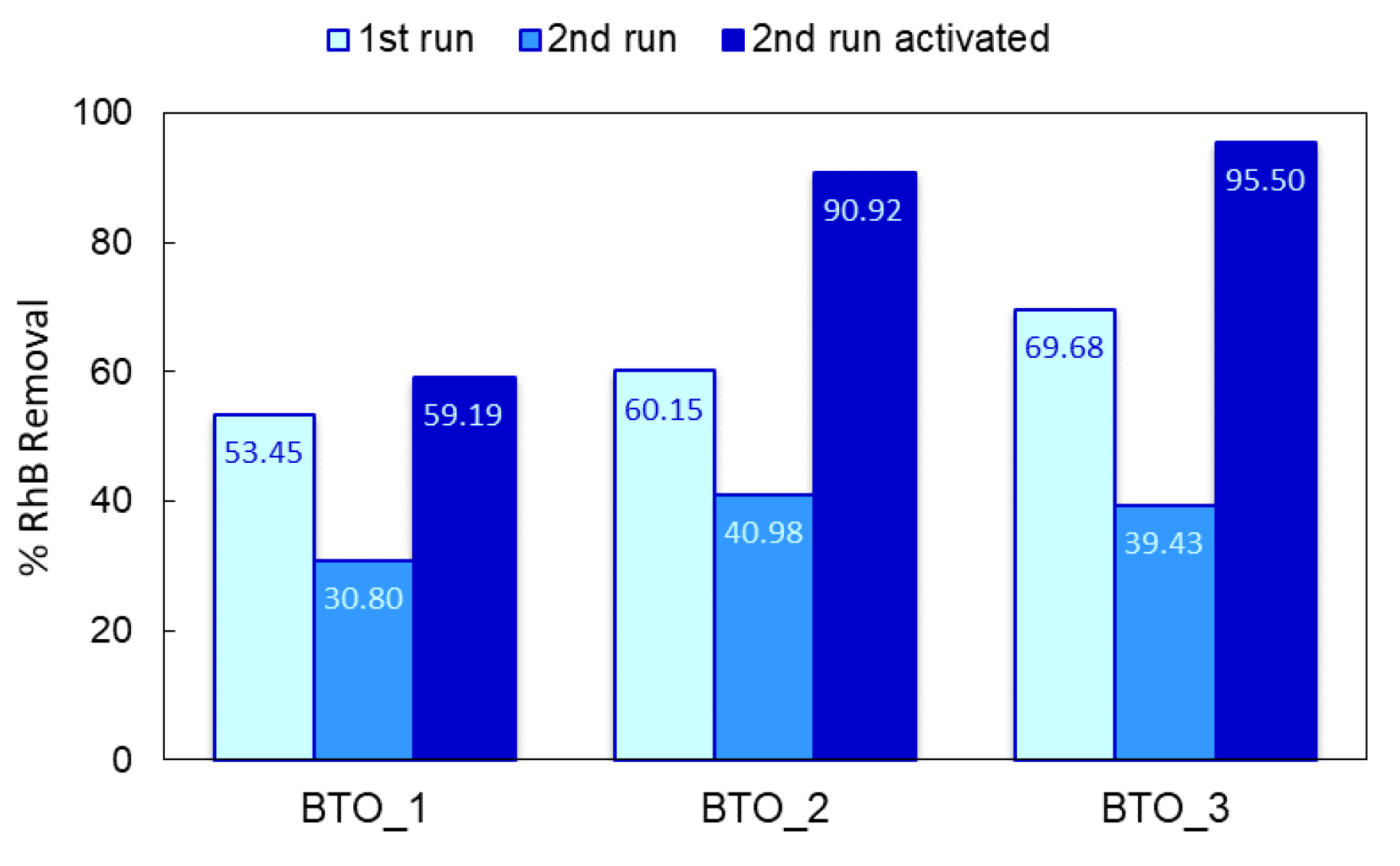
3.3. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.Q.; Cheng, H.-M. Synergistic Effects of B/N Doping on the Visible-Light Photocatalytic Activity of Mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 4516–4520. [Google Scholar] [CrossRef]
- Fan, H.; Li, H.; Liu, B.; Lu, Y.; Xie, T.; Wang, D. Photoinduced Charge Transfer Properties and Photocatalytic Activity in Bi2O3/BaTiO3 Composite Photocatalyst. ACS Appl. Mater. Interfaces 2012, 4, 4853–4857. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Han, S.; Cao, J.; Duan, L.; Zeng, T. Enhanced solar absorption and visible-light photocatalytic and photoelectrochemical properties of aluminium-reduced BaTiO3 nanoparticles. Chem. Commun. 2018, 54, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Sitko, D.; Bąk, W.; Garbarz-Glos, B.; Antonova, M.; Jankowska-Sumara, I. Effect of MnO2 doping on the dielectric properties of bariumtitanate ceramics. Ukr. J. Phys. Opt. 2012, 13, S34–S43. [Google Scholar] [CrossRef][Green Version]
- Cao, J.; Ji, Y.; Tian, C.; Yi, Z. Synthesis and enhancement of visible light activities of nitrogen-doped BaTiO3. J. Alloy. Compd. 2014, 615, 243–248. [Google Scholar] [CrossRef]
- Shen, H.; Ni, D.; Niu, P.; Zhou, Y.; Zhai, T.; Ma, Y. Enhancing photocatalytic H2 evolution from water on CuO-Co3O4/TiO2: The key roles of Co3O4 loading amounts. Int. J. Hydrog. Energy 2017, 42, 30559–30568. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Wang, M.; Kong, D.; Zhang, Y.; Chen, J.-F.; Dai, L. Uniform Two-Dimensional Co3O4 Porous Sheets: Facile Synthesis and Enhanced Photocatalytic Performance. Chem. Eng. Technol. 2016, 39, 891–898. [Google Scholar] [CrossRef]
- Li, H.; Han, J.; Guo, N.; Yu, H. The design of 3D artificial leaves with spatially separated active sites for H2 and O2 generation and their application to water splitting. Chem. Commun. 2016, 52, 4080–4083. [Google Scholar] [CrossRef]
- Dong, C.; Xing, M.; Zhang, J. Double-cocatalysts promote charge separation efficiency in CO2 photoreduction: Spatial location matters. Mater. Horiz. 2016, 3, 608–612. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Ravichandran, K.; Gracia, F.; Agarwal, S.; Gupta, V.K. Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photo catalysts for degradation of textile dyes. J. Photochem. Photobiol. B Biol. 2017, 173, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 Nanocomposite as a Direct Z-Scheme Photocatalyst for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 965–973. [Google Scholar] [CrossRef]
- Liu, C.; Tong, R.; Xu, Z.; Kuang, Q.; Xie, Z.; Zheng, L. Efficiently enhancing the photocatalytic activity of faceted TiO2 nanocrystals by selectively loading α-Fe2O3 and Pt co-catalysts. RSC Adv. 2016, 6, 29794–29801. [Google Scholar] [CrossRef]
- Guan, W.; Gao, X.; Ji, G.; Xing, Y.; Du, C.; Liu, Z. Fabrication of a magnetic nanocomposite photocatalysts Fe3O4@ZIF-67 for degradation of dyes in water under visible light irradiation. J. Solid State Chem. 2017, 255, 150–156. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C.S. Selective photocatalytic reduction of CO2 into CH4 over Pt-Cu2O TiO2 nanocrystals: The interaction between Pt and Cu2O cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Q.; Long, J.; Wang, X.; Gao, Z.; Gu, Q. Amorphous NiO as co-catalyst for enhanced visible-light-driven hydrogen generation over g-C3N4 photocatalyst. Appl. Catal. B Environ. 2018, 222, 35–43. [Google Scholar] [CrossRef]
- Wada, S.; Narahara, M.; Hoshina, T.; Kakemoto, H.; Tsurumi, T. Preparation of nm-sized BaO3 particles using a new 2-step thermal decomposition of barium titanyl oxalate. J. Mater. Sci. 2003, 38, 2655–2660. [Google Scholar] [CrossRef]
- Aal, A.A.; Hammad, T.R.; Zawrah, M.; Battisha, I.K.; Hammad, A.B.A. FTIR Study of Nanostructure Perovskite BaTiO3 Doped with Both Fe3+ and Ni2+ Ions Prepared by Sol-Gel Technique. Acta Phys. Pol. A 2014, 126, 1318–1321. [Google Scholar]
- Yang, R.; Wang, Z.; Dai, L.; Chen, L. Synthesis and characterization of single-crystalline nanorods of α-MnO2 and γ-MnOOH. Mater. Chem. Phys. 2005, 93, 149–153. [Google Scholar] [CrossRef]
- Mendelovici, E.; Villalba, R.; Sagarzazu, A.; Carias, O. The 1640 cm−1 infrared band, monitor for the gain and thermal stability of water produced in ground kaolinites. Clay Miner. 1995, 30, 307–313. [Google Scholar] [CrossRef]
- Pasierb, P.; Komornicki, S.; Rokita, M.; Rȩkas, M. Structural properties of Li2CO3–BaCO3 system derived from IR and Raman spectroscopy. J. Mol. Struct. 2001, 596, 151–156. [Google Scholar] [CrossRef]
- Bargar, J.R.; Kubicki, J.D.; Reitmeyer, R.; Davis, J.A. ATR-FTIR spectroscopic characterization of coexisting carbonate surface complexes on hematite. Geochim. Et Cosmochim. Acta 2005, 69, 1527–1542. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Xue, D.; Kang, H.; Liu, C. Wet routes of high purity BaTiO3 nanopowders. J. Alloys Compd. 2007, 440, 78–83. [Google Scholar] [CrossRef]
- Perry, C.H.; Khanna, B.N.; Rupprecht, G. Infrared Studies of Perovskite Titanates. Phys. Rev. 1964, 135, A408–A412. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Tizfahm, J.; Aghazadeh, M.; Maragheh, M.G.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Electrochemical preparationandevaluationofthesupercapacitive performance ofMnO2 nanoworms. Mater. Lett. 2016, 167, 153–156. [Google Scholar] [CrossRef]
- Alzahrani, S.A.; Al-Thabaiti, S.A.; Al-Arjan, W.S.; Malik, M.A.; Khan, Z. Preparation of ultra long α-MnO2 and Ag@MnO2 nanoparticles by seedless approach and their photocatalytic performance. J. Mol. Struct. 2017, 1137, 495–505. [Google Scholar] [CrossRef]
- Park, Y.; Na, Y.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption and UV/Visible photocatalytic performance of BiOI for methyl orange, Rhodamine B and methylene blue: Ag and Ti-loading effects. CrystEngComm 2014, 16, 3155–3167. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Dunn, S. Effect of Ferroelectricity on Solar-Light-Driven Photocatalytic Activity of BaTiO3—Influence on the Carrier Separation and Stern Layer Formation. Chem. Mater. 2013, 25, 4215–4223. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, H.; Shen, G.; Jing, P.; Pu, Y. Effect of Dual-Cocatalyst Surface Modification on Photodegradation Activity, Pathway, and Mechanisms with Highly Efficient Ag/BaTiO3/MnOx. Langmuir 2020, 36, 498–509. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; He, H.; Sun, C.; Gu, C.; Ju, Y. Visible Light-Driven Photocatalytic Degradation of Rhodamine B over NaBiO3: Pathways and Mechanism. J. Phys. Chem. A 2009, 113, 10024–10032. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric Relaxation in Solids; Chelsea Dielectric Press: London, UK, 1983. [Google Scholar]
- Macdonald, J.R. Impedance Spectroscopy; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Liu, X.; Lv, S.; Fan, B.; Xing, A.; Jia, B. Ferroelectric Polarization-Enhanced Photocatalysis in BaTiO3-TiO2 Core-Shell Heterostructures. Nanomaterials 2019, 9, 1116. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Wang, Y.; Tarakina, N.V.; Dunn, S. Enhanced Photocatalytic Activity of Heterostructured Ferroelectric BaTiO3/α-Fe2O3 and the Significance of Interface Morphology Control. ACS Appl. Mater. Interfaces 2017, 9, 24518–24526. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Li, C.; Yan, F.; Bai, H.; Shen, B.; Zeng, H.; Zhai, J. Piezophototronic effect in enhancing charge carrier separation and transfer in ZnO/BaTiO3 heterostructures for high-efficiency catalytic oxidation. Nano Energy 2019, 66, 104127. [Google Scholar] [CrossRef]
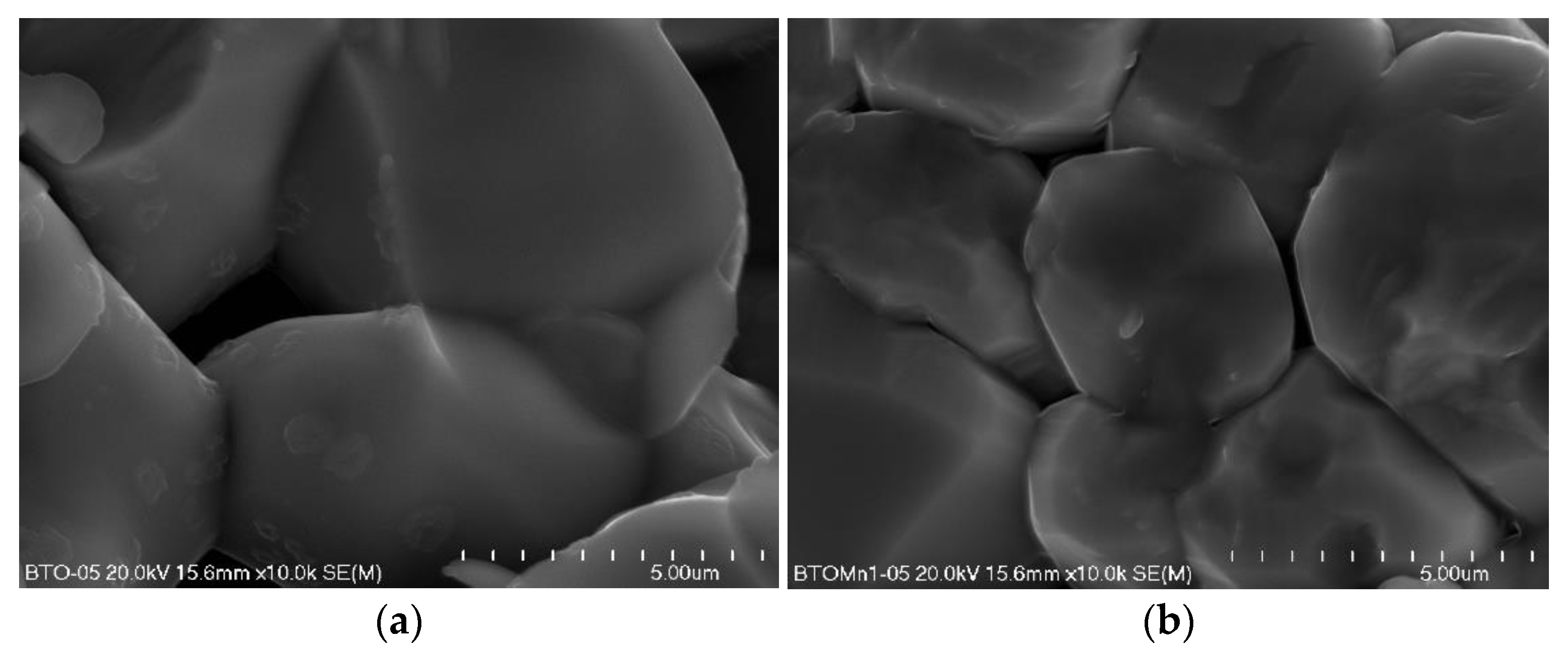
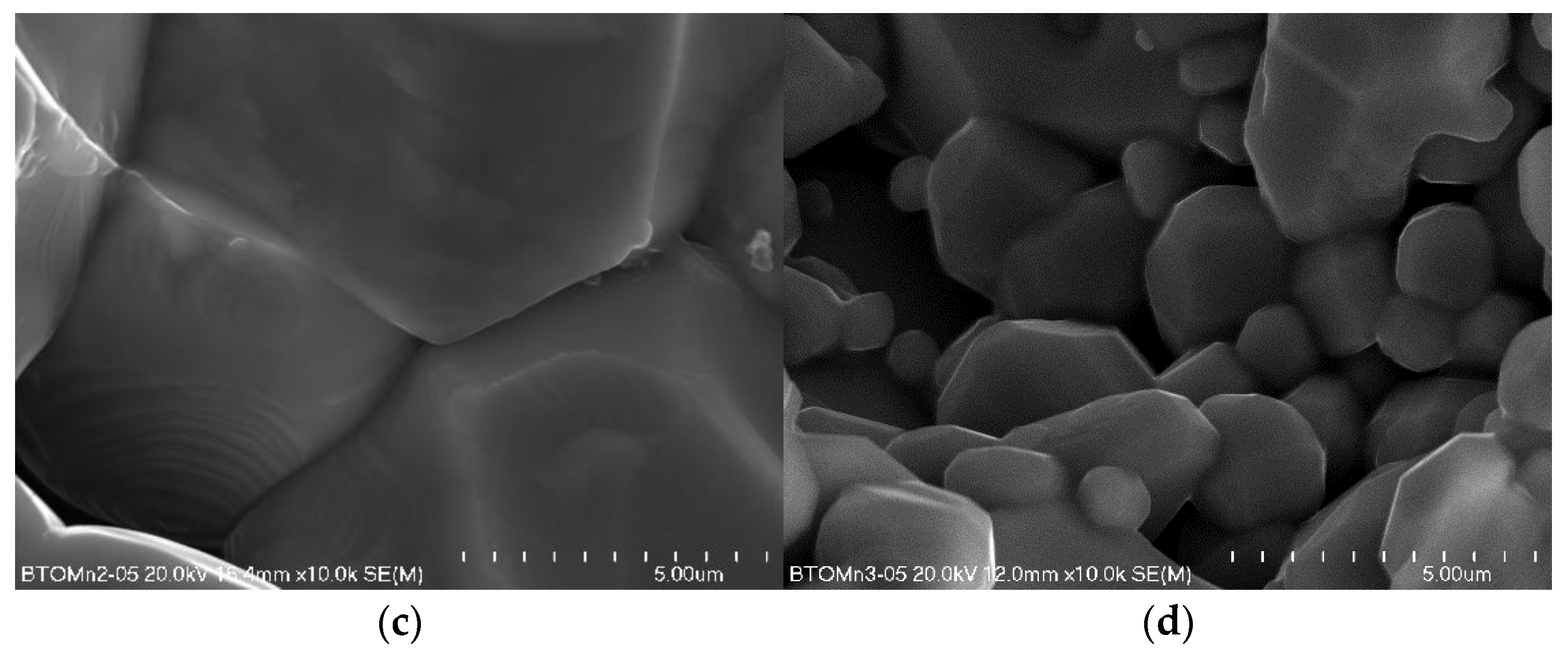

| Sample | MnO2 Content (%) | ||
|---|---|---|---|
| As Prepared | After Catalytic Test | ||
| BTO_1 | 0.94 1 | 0.98 2 | 0.96 1 |
| BTO_2 | 2.05 1 | 2.02 2 | 2.13 1 |
| BTO_3 | 2.77 1 | 2.82 2 | 2.98 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźniarska-Biernacka, I.; Garbarz-Glos, B.; Skiba, E.; Maniukiewicz, W.; Bąk, W.; Antonova, M.; Rebelo, S.L.H.; Freire, C. Evaluation of Rhodamine B Photocatalytic Degradation over BaTiO3-MnO2 Ceramic Materials. Materials 2021, 14, 3152. https://doi.org/10.3390/ma14123152
Kuźniarska-Biernacka I, Garbarz-Glos B, Skiba E, Maniukiewicz W, Bąk W, Antonova M, Rebelo SLH, Freire C. Evaluation of Rhodamine B Photocatalytic Degradation over BaTiO3-MnO2 Ceramic Materials. Materials. 2021; 14(12):3152. https://doi.org/10.3390/ma14123152
Chicago/Turabian StyleKuźniarska-Biernacka, Iwona, Barbara Garbarz-Glos, Elżbieta Skiba, Waldemar Maniukiewicz, Wojciech Bąk, Maija Antonova, Susana L. H. Rebelo, and Cristina Freire. 2021. "Evaluation of Rhodamine B Photocatalytic Degradation over BaTiO3-MnO2 Ceramic Materials" Materials 14, no. 12: 3152. https://doi.org/10.3390/ma14123152
APA StyleKuźniarska-Biernacka, I., Garbarz-Glos, B., Skiba, E., Maniukiewicz, W., Bąk, W., Antonova, M., Rebelo, S. L. H., & Freire, C. (2021). Evaluation of Rhodamine B Photocatalytic Degradation over BaTiO3-MnO2 Ceramic Materials. Materials, 14(12), 3152. https://doi.org/10.3390/ma14123152










