Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GONs and o-SWNTs
2.3. Preparation of GONs- and o-SWNT-TFC Membranes
2.4. Characterization of GONs and o-SWNTs
2.5. Characterization of GONs- and o-SWNT-TFC Membranes
2.6. Evaluation on the Performance of GONs- and o-SWNT-TFC Membranes
3. Results
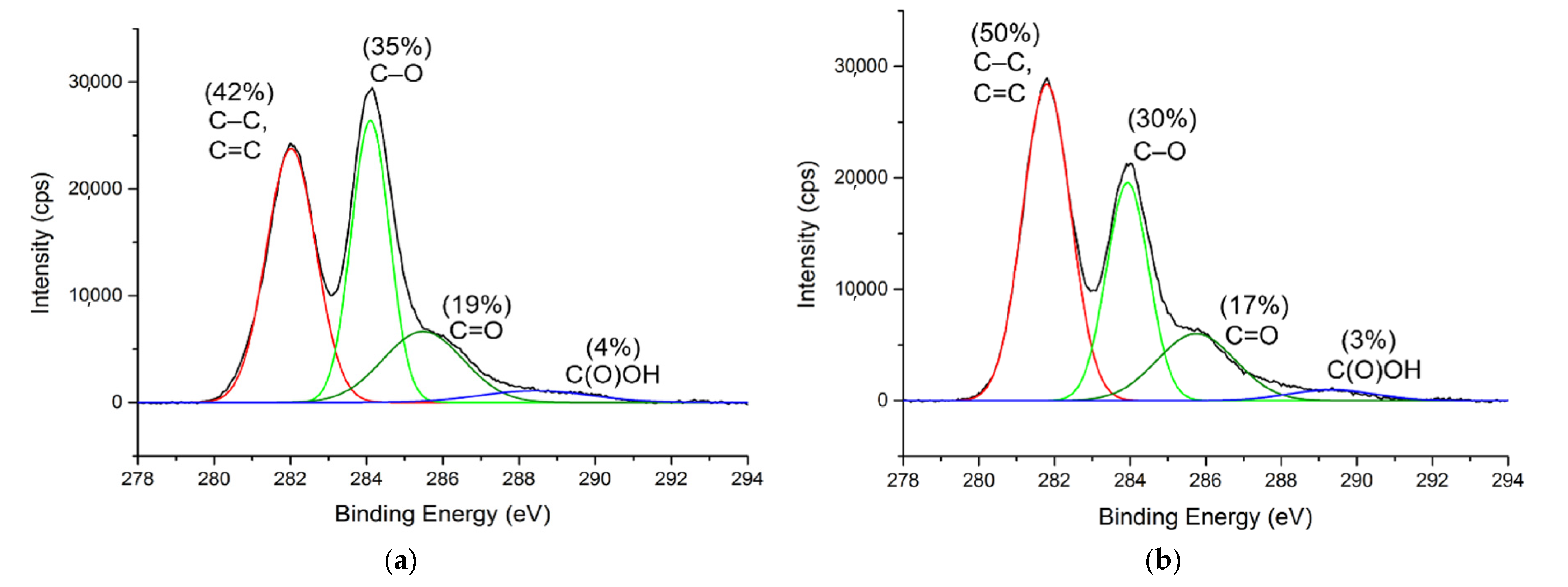
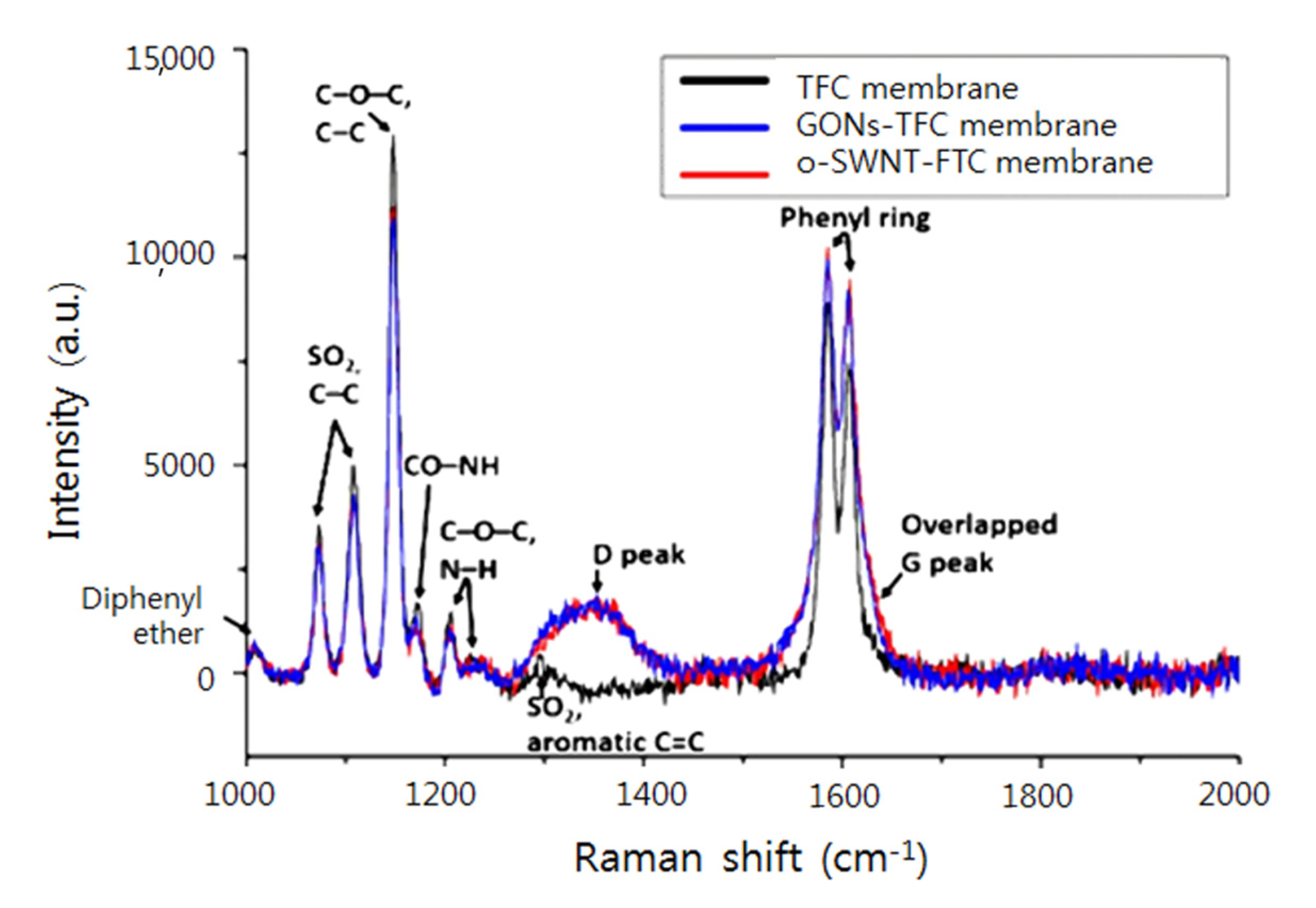
3.1. Characterization of GONs and o-SWNTs
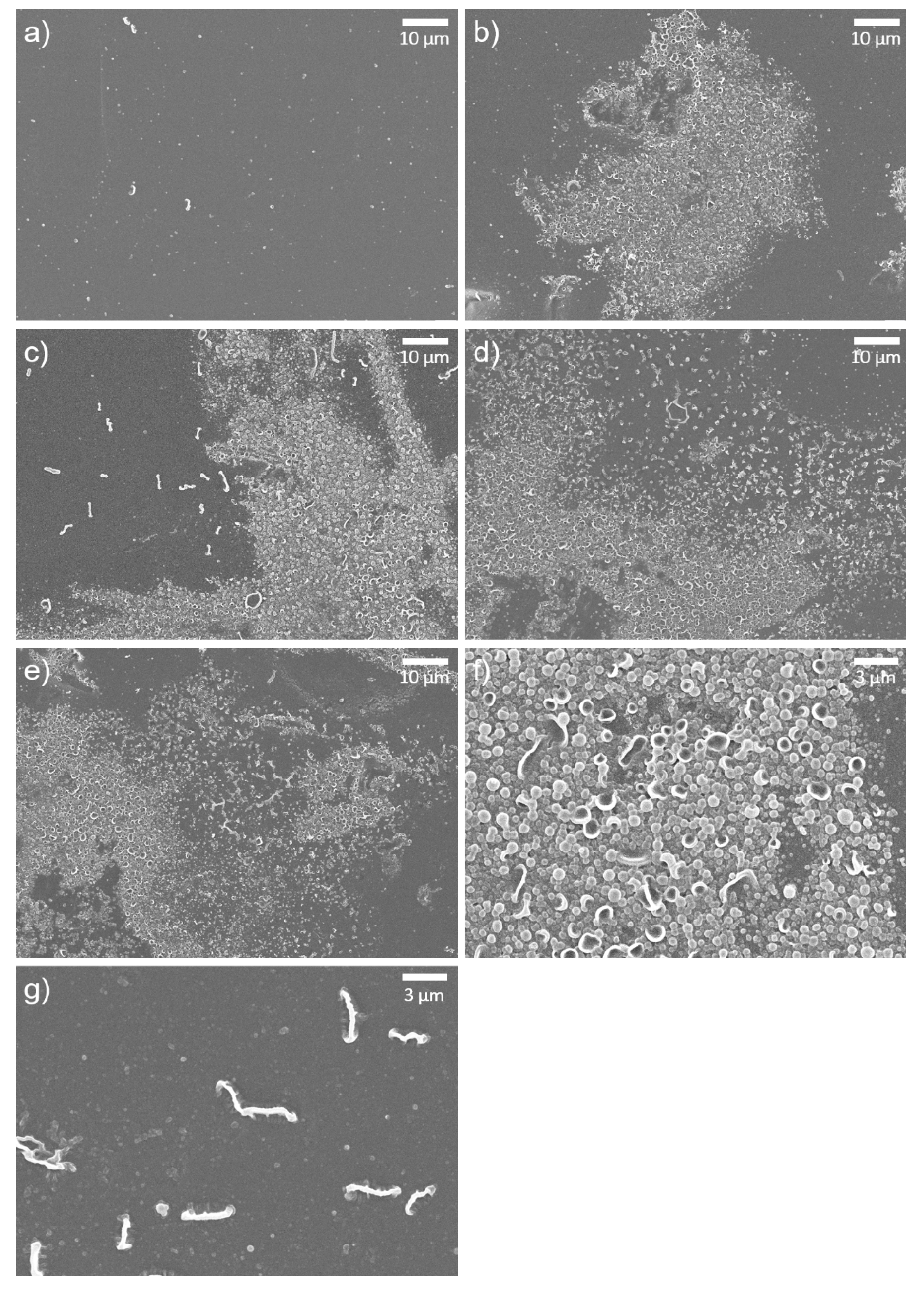
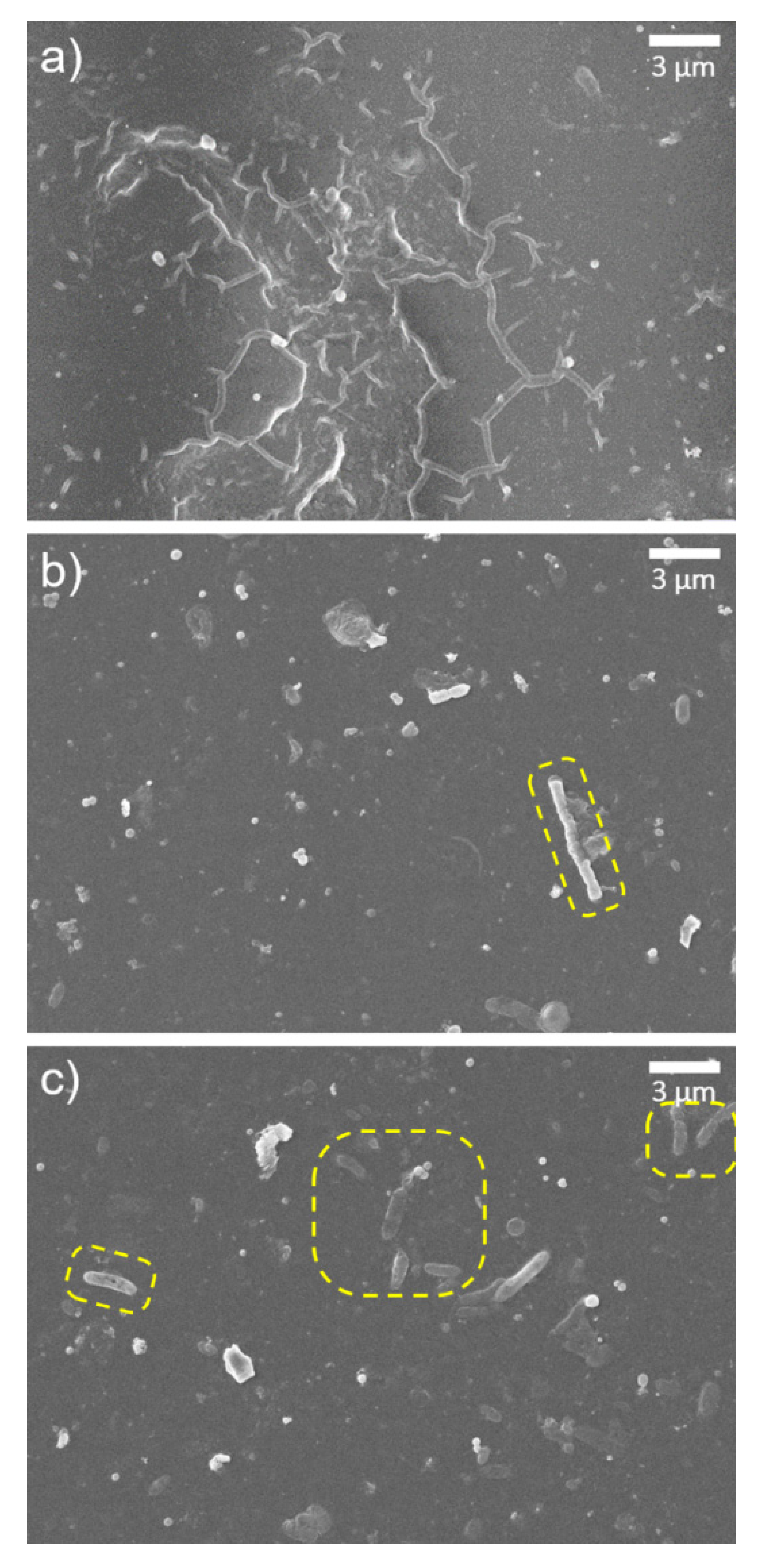
3.2. Characterization of GONs- and o-SWNTs-TFC Membranes
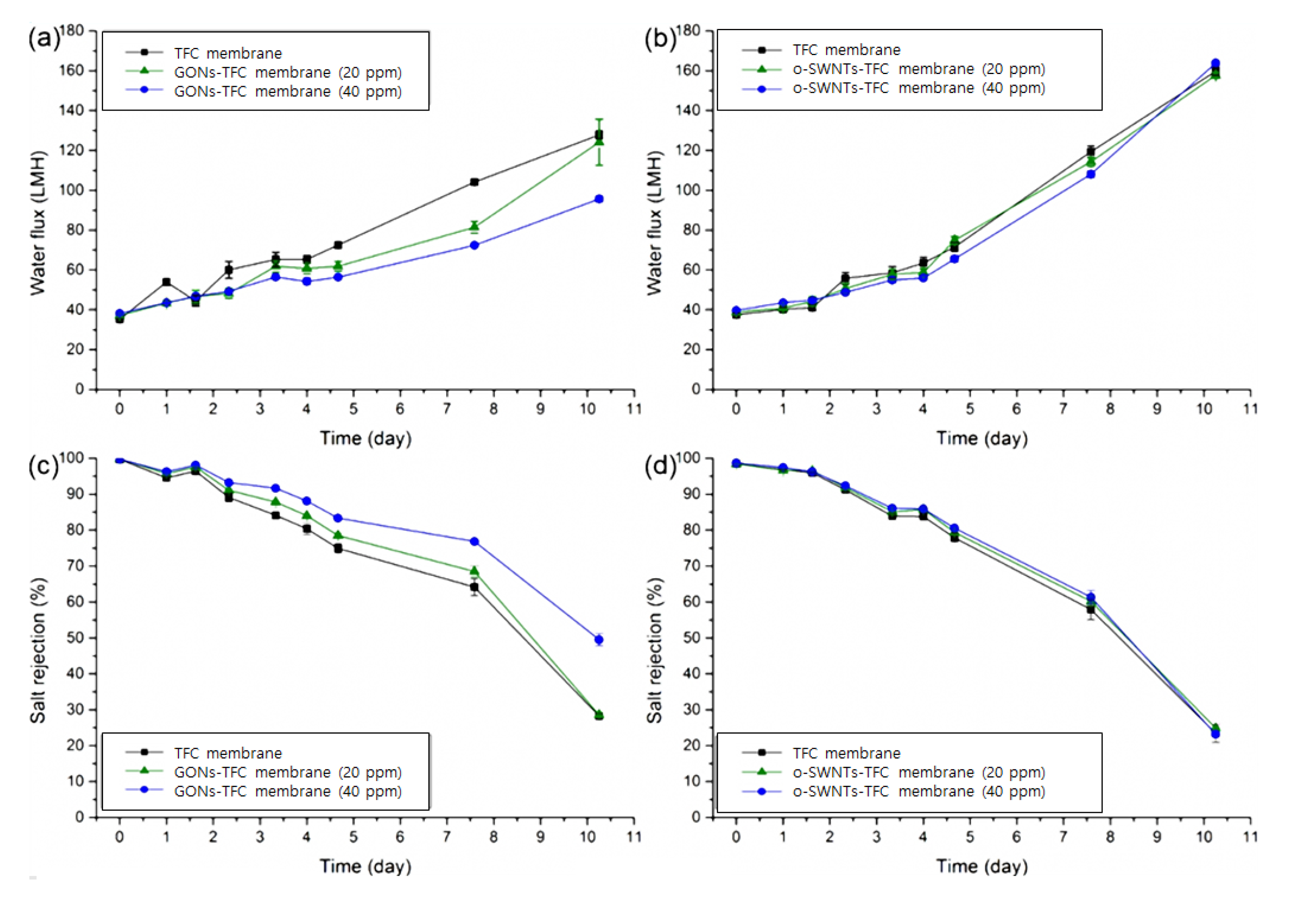
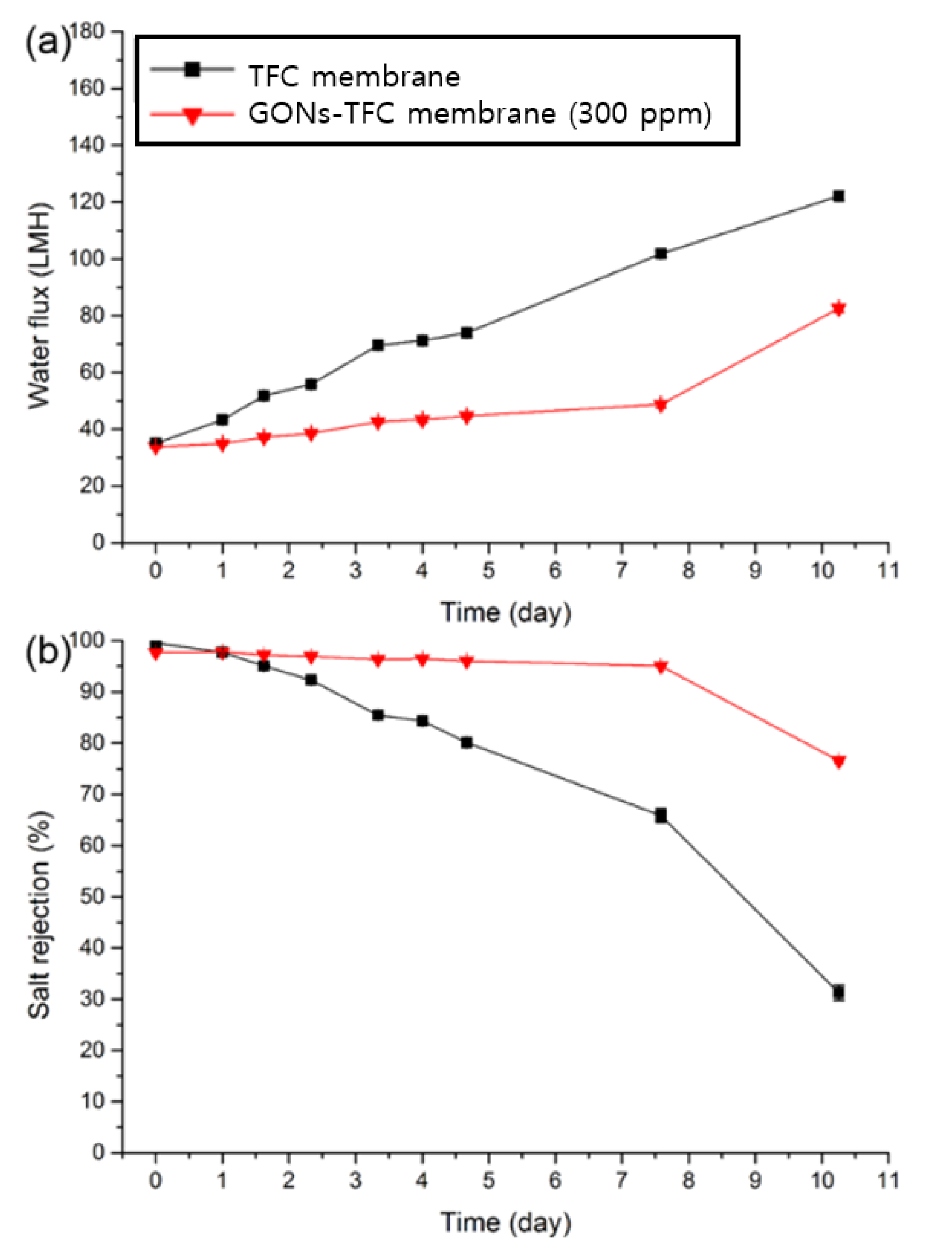
3.3. Evaluation of the Performance of GONs- and o-SWNTs-TFC Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh, S.J.; Moon, S.-H.; Davis, T. Effects of metal ions on diffusion dialysis of inorganic acids. J. Membr. Sci. 2000, 169, 95–105. [Google Scholar] [CrossRef]
- Mänttäri, M.; Nuortila-Jokinen, J.; Nyström, M. Evaluation of nanofiltration membranes for filtration of paper mill total effluent. Filtr. Sep. 1997, 34, 275–280. [Google Scholar] [CrossRef]
- Räsänen, E.; Nyström, M.; Sahlstein, J.; Tossavainen, O. Purification and regeneration of diluted caustic and acidic washing solutions by membrane filtration. Desalination 2002, 149, 185–190. [Google Scholar] [CrossRef]
- Musale, D.A.; Kumar, A. Solvent and pH resistance of surface crosslinked chitosan/poly(acrylonitrile) composite nanofiltration membranes. J. Appl. Polym. Sci. 2000, 77, 1782–1793. [Google Scholar] [CrossRef]
- Tanninen, J.; Nyström, M. Separation of ions in acidic conditions using NF. Desalination 2002, 147, 295–299. [Google Scholar] [CrossRef]
- Yoon, S.-H. Membrane Bioreactor Processes: Principles and Applications; CRC Press: London, UK, 2015; p. 378. [Google Scholar]
- Jun, B.-M.; Kim, S.H.; Kwak, S.K.; Kwon, Y.-N. Effect of acidic aqueous solution on chemical and physical properties of polyamide NF membranes. Appl. Surf. Sci. 2018, 444, 387–398. [Google Scholar] [CrossRef]
- Jun, B.-M.; Lee, H.K.; Kwon, Y.-N. Acid-catalyzed hydrolysis of semi-aromatic polyamide NF membrane and its application to water softening and antibiotics enrichment. Chem. Eng. J. 2018, 332, 419–430. [Google Scholar] [CrossRef]
- Tanninen, J.; Platt, S.; Weis, A.; Nyström, M. Long-term acid resistance and selectivity of NF membranes in very acidic conditions. J. Membr. Sci. 2004, 240, 11–18. [Google Scholar] [CrossRef]
- Platt, S.; Nyström, M.; Bottino, A.; Capannelli, G. Stability of NF membranes under extreme acidic conditions. J. Membr. Sci. 2004, 239, 91–103. [Google Scholar] [CrossRef]
- Freger, V.; Bottino, A.; Capannelli, G.; Perry, M.; Gitis, V.; Belfer, S. Characterization of novel acid-stable NF membranes before and after exposure to acid using ATR-FTIR, TEM and AFM. J. Membr. Sci. 2005, 256, 134–142. [Google Scholar] [CrossRef]
- Lee, K.P.; Zheng, J.; Bargeman, G.; Kemperman, A.J.B.; Benes, N.E. pH stable thin film composite polyamine nanofiltration membranes by interfacial polymerisation. J. Membr. Sci. 2015, 478, 75–84. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Z.; Yu, S.; Wu, D.; Gao, C. Thin-film composite polyamide reverse osmosis membranes with improved acid stability and chlorine resistance by coating N-isopropylacrylamide-co-acrylamide copolymers. Desalination 2011, 270, 248–257. [Google Scholar] [CrossRef]
- Jin, L.M.; Wang, Z.Y.; Zheng, S.X.; Mi, B.X. Polyamide-crosslinked graphene oxide membrane for forward osmosis. J. Membr. Sci. 2018, 545, 11–18. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin, K.; Iskandar, F.; Wenten, I.G. Functionalized carbon nanotube (CNT) membrane: Progress and challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film Composite Polyamide Membranes Functionalized with Biocidal Graphene Oxide Nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, S.; Hwang, J. Graphene-Based Two-Dimensional Mesoporous Materials: Synthesis and Electrochemical Energy Storage Applications. Materials 2021, 14, 2597. [Google Scholar] [CrossRef] [PubMed]
- Kamedulski, P.; Lukaszewicz, J.P.; Witczak, L.; Szroeder, P.; Ziolkowski, P. The Importance of Structural Factors for the Electrochemical Performance of Graphene/Carbon Nanotube/Melamine Powders towards the Catalytic Activity of Oxygen Reduction Reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.R.; Lee, J.; Lee, C.H.; Kim, I.C.; Park, P.K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, T.; Gurzadyan, G.G. The Origin of Fluorescence from Graphene Oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef]
- Bader, M.; Maenz, T.; Schmiederer, D.; Kuehnert, I.; Leuteritz, A.; Heinrich, G. Correlation between morphological properties and thermal conductivity of injection-molded polyamide 46. Polym. Eng. Sci. 2015, 55, 2231–2236. [Google Scholar] [CrossRef]
- Deimede, V.; Voyiatzis, G.A.; Kallitsis, J.K.; Qingfeng, L.; Bjerrum, N.J. Miscibility Behavior of Polybenzimidazole/Sulfonated Polysulfone Blends for Use in Fuel Cell Applications. Macromolecules 2000, 33, 7609–7617. [Google Scholar] [CrossRef]
- Ferreiro, V.; Depecker, C.; Laureyns, J.; Coulon, G. Structures and morphologies of cast and plastically strained polyamide 6 films as evidenced by confocal Raman microspectroscopy and atomic force microscopy. Polymer 2004, 45, 6013–6026. [Google Scholar] [CrossRef]
- Voicu, S.I.; Pandele, M.A.; Vasije, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Kim, H.J.; Fouda, A.E.; Jonasson, K. In situ study on kinetic behavior during asymmetric membrane formation via phase inversion process using Raman spectroscopy. J. Appl. Polym. Sci. 1999, 75, 135–141. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araujo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef]
- Song, Y.; Sun, P.; Henry, L.L.; Sun, B. Mechanisms of structure and performance controlled thin film composite membrane formation via interfacial polymerization process. J. Membr. Sci. 2005, 251, 67–79. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A Novel Approach Toward Fabrication of High Performance Thin Film Composite Polyamide Membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13rd ed.; Merck Research Laboratories: Whitehouse Station, NJ, USA, 2001; p. 1337. [Google Scholar]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Xie, J.; Spallas, J.P. Different Contrast Mechanisms in SEM Imaging of Graphene. 5991-0782EN, 2012. Available online: http://www.toyo.co.jp/files/user/img/product/microscopy/pdf/5991-0782EN.pdf (accessed on 8 June 2021).
- Physical and Chemical Properties of Carbon Nanotubes; NTT Basic Research Laboratories: Kanagawa, Japan, 2013.
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Hirose, M.; Ito, H.; Kamiyama, Y. Effect of skin layer surface structures on the flux behaviour of RO membranes. J. Membr. Sci. 1996, 121, 209–215. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Park, N.; Kwon, B.; Kim, I.S.; Cho, J. Biofouling potential of various NF membranes with respect to bacteria and their soluble microbial products (SMP): Characterizations, flux decline, and transport parameters. J. Membr. Sci. 2005, 258, 43–54. [Google Scholar] [CrossRef]
- Xue, S.-M.; Xu, Z.-L.; Tang, Y.-J.; Ji, C.-H. Polypiperazine-amide Nanofiltration Membrane Modified by Different Functionalized Multiwalled Carbon Nanotubes (MWCNTs). ACS Appl. Mater. Interfaces 2016, 8, 19135–19144. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.-H. Layer-by-Layer Assembly of Graphene Oxide Nanosheets on Polyamide Membranes for Durable Reverse-Osmosis Applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef]
- Kim, S.G.; Hyeon, D.H.; Chun, J.H.; Chun, B.-H.; Kim, S.H. Novel thin nanocomposite RO membranes for chlorine resistance. Desalination Water Treat. 2013, 51, 6338–6345. [Google Scholar] [CrossRef]
- Petty, M.C. Langmuir-Blodgett Films; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir−Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.H. Organic Chemistry, 2nd ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Huang, H.; Qu, X.; Ji, X.; Gao, X.; Zhang, L.; Chen, H.; Hou, L. Acid and multivalent ion resistance of thin film nanocomposite RO membranes loaded with silicalite-1 nanozeolites. J. Mater. Chem. A 2013, 1, 11343–11349. [Google Scholar] [CrossRef]
- Silva-Tapia, A.B.; García-Carmona, X.; Radovic, L.R. Similarities and differences in O2 chemisorption on graphene nanoribbon vs. carbon nanotube. Carbon 2012, 50, 1152–1162. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, H.-R.; Kim, I.-C.; Kwon, Y.-N. Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets. Materials 2021, 14, 3151. https://doi.org/10.3390/ma14123151
Chae H-R, Kim I-C, Kwon Y-N. Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets. Materials. 2021; 14(12):3151. https://doi.org/10.3390/ma14123151
Chicago/Turabian StyleChae, Hee-Ro, In-Chul Kim, and Young-Nam Kwon. 2021. "Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets" Materials 14, no. 12: 3151. https://doi.org/10.3390/ma14123151
APA StyleChae, H.-R., Kim, I.-C., & Kwon, Y.-N. (2021). Acid-Resistance Enhancement of Thin-Film Composite Membrane Using Barrier Effect of Graphene Oxide Nanosheets. Materials, 14(12), 3151. https://doi.org/10.3390/ma14123151




