Degradation Mechanisms of Metal-Supported Solid Oxide Cells and Countermeasures: A Review
Abstract
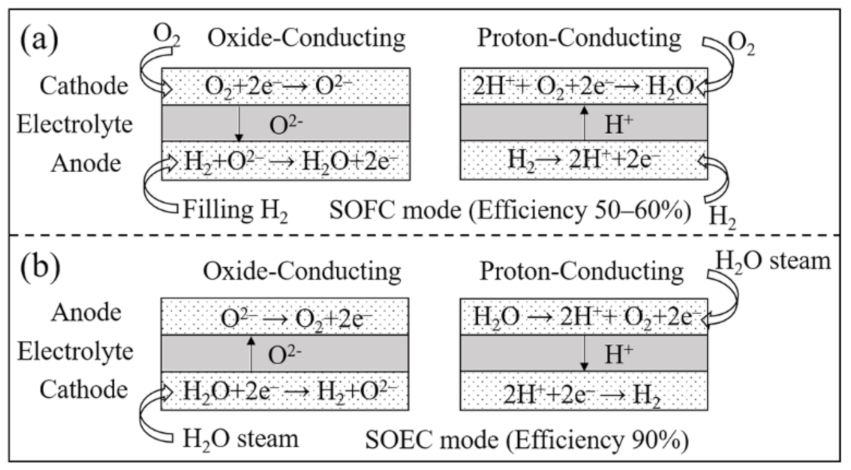
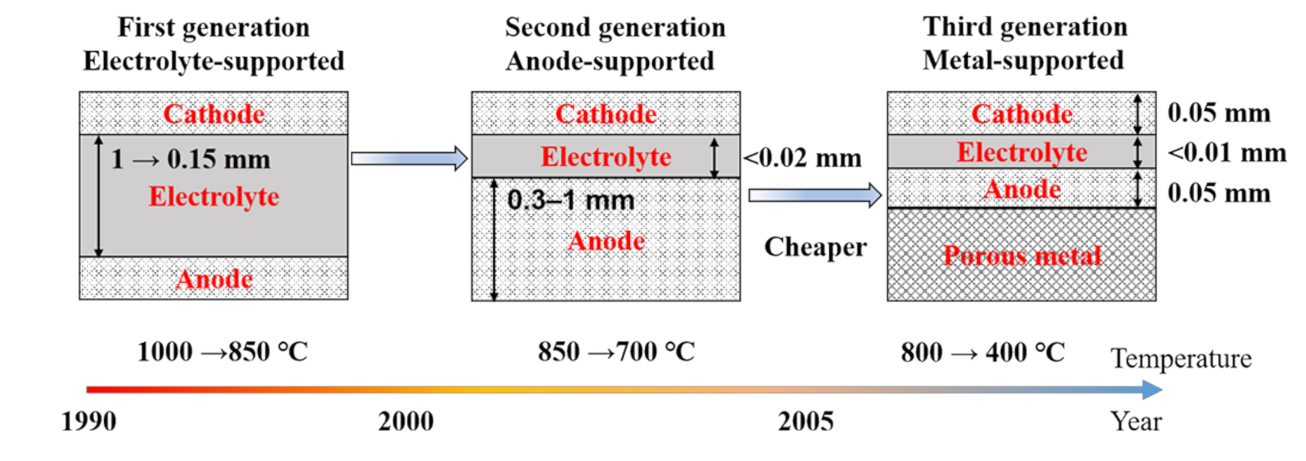
1. Introduction
2. Degradation Mechanism and Countermeasures
2.1. The Degradation of the Anode
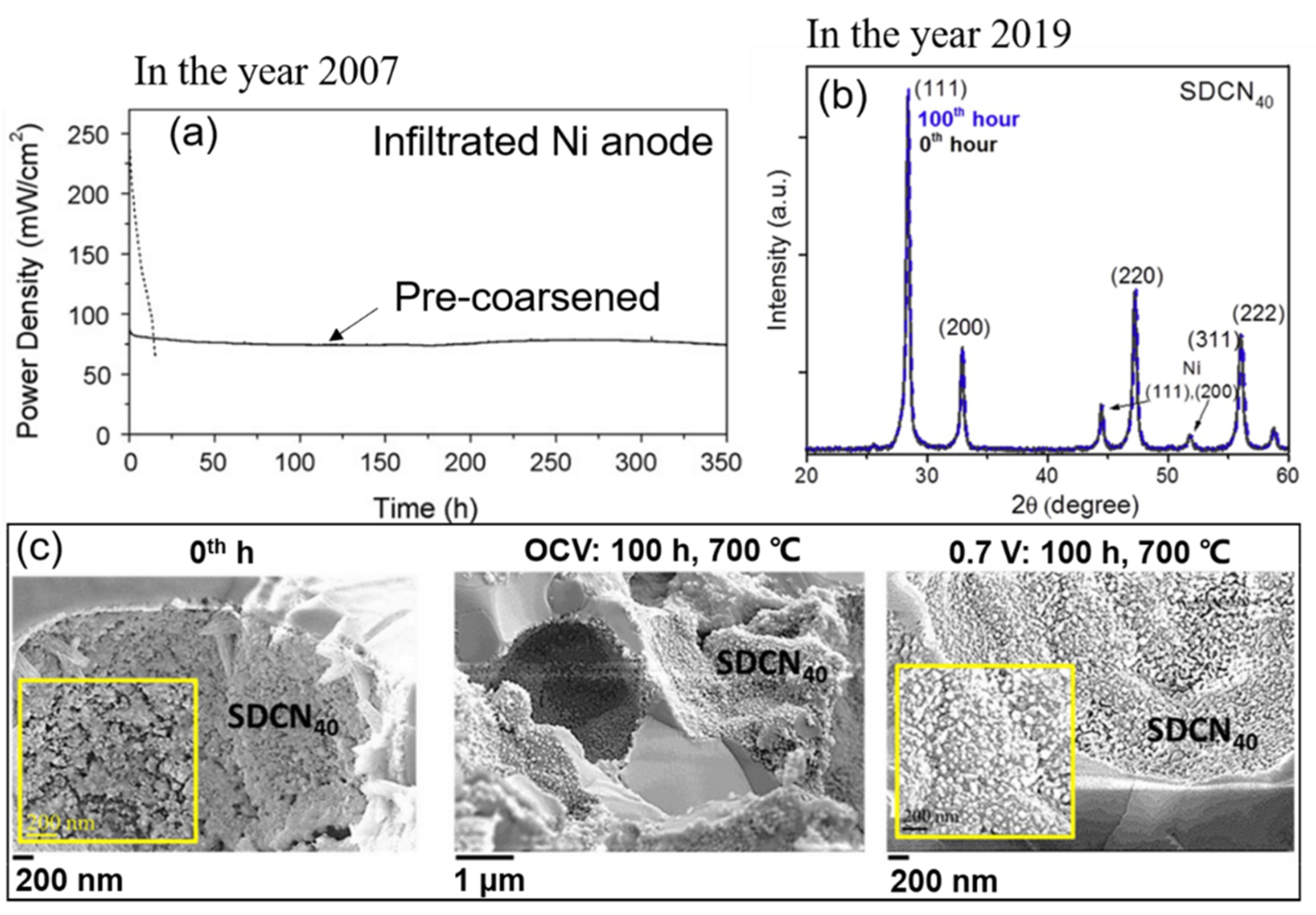
2.1.1. Coarsening and Migration of Nickel
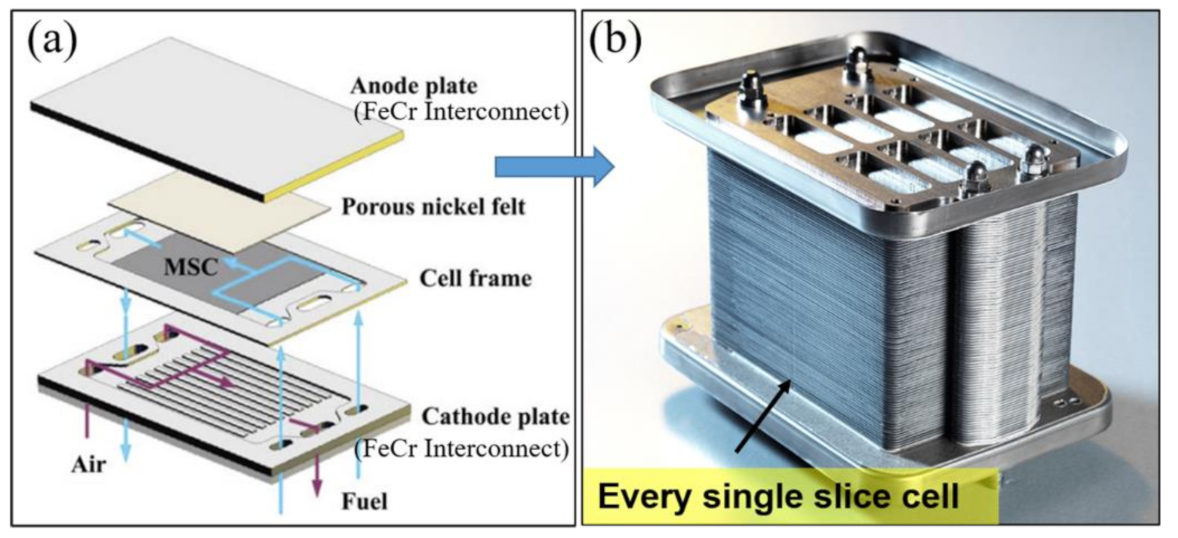
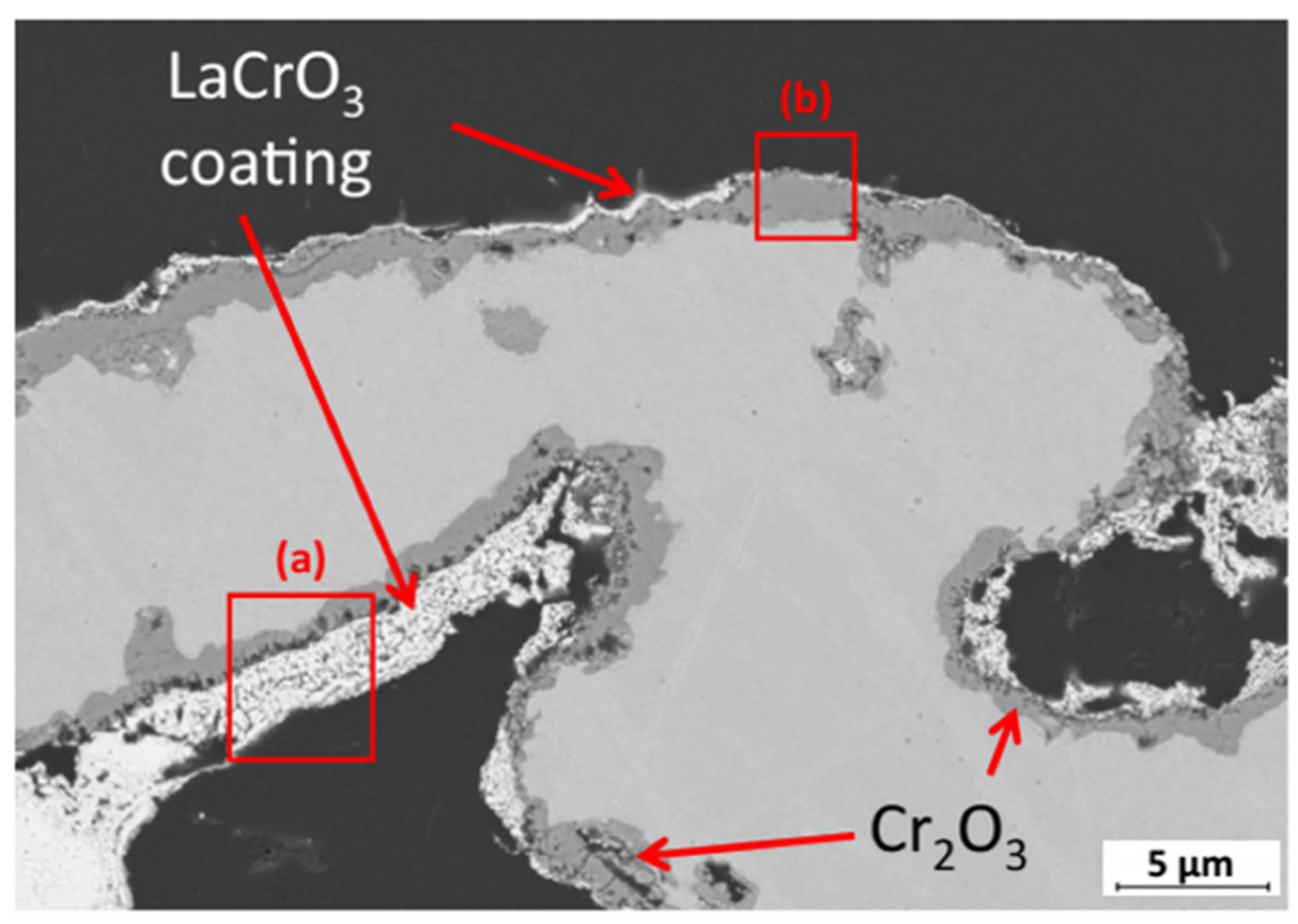
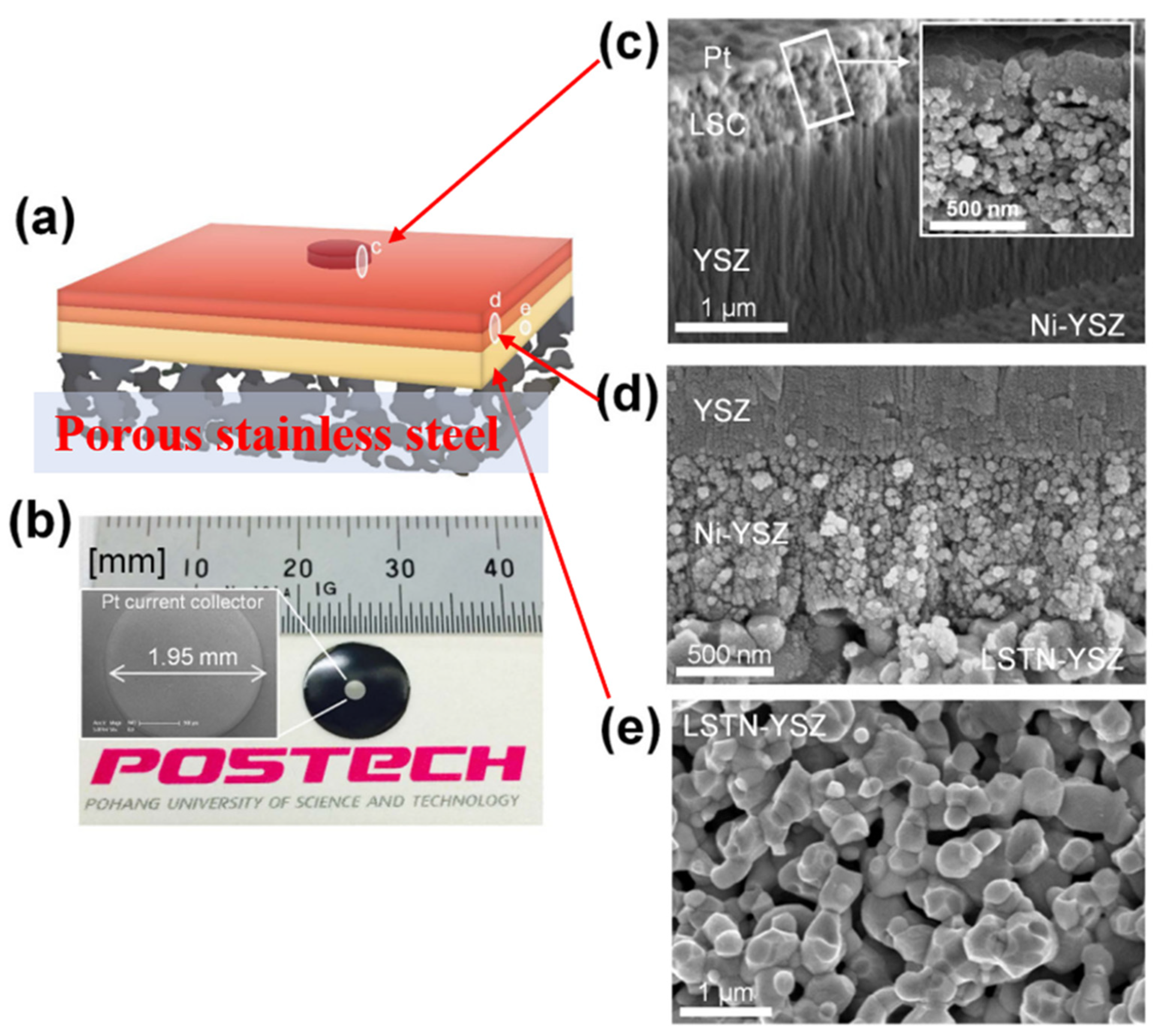
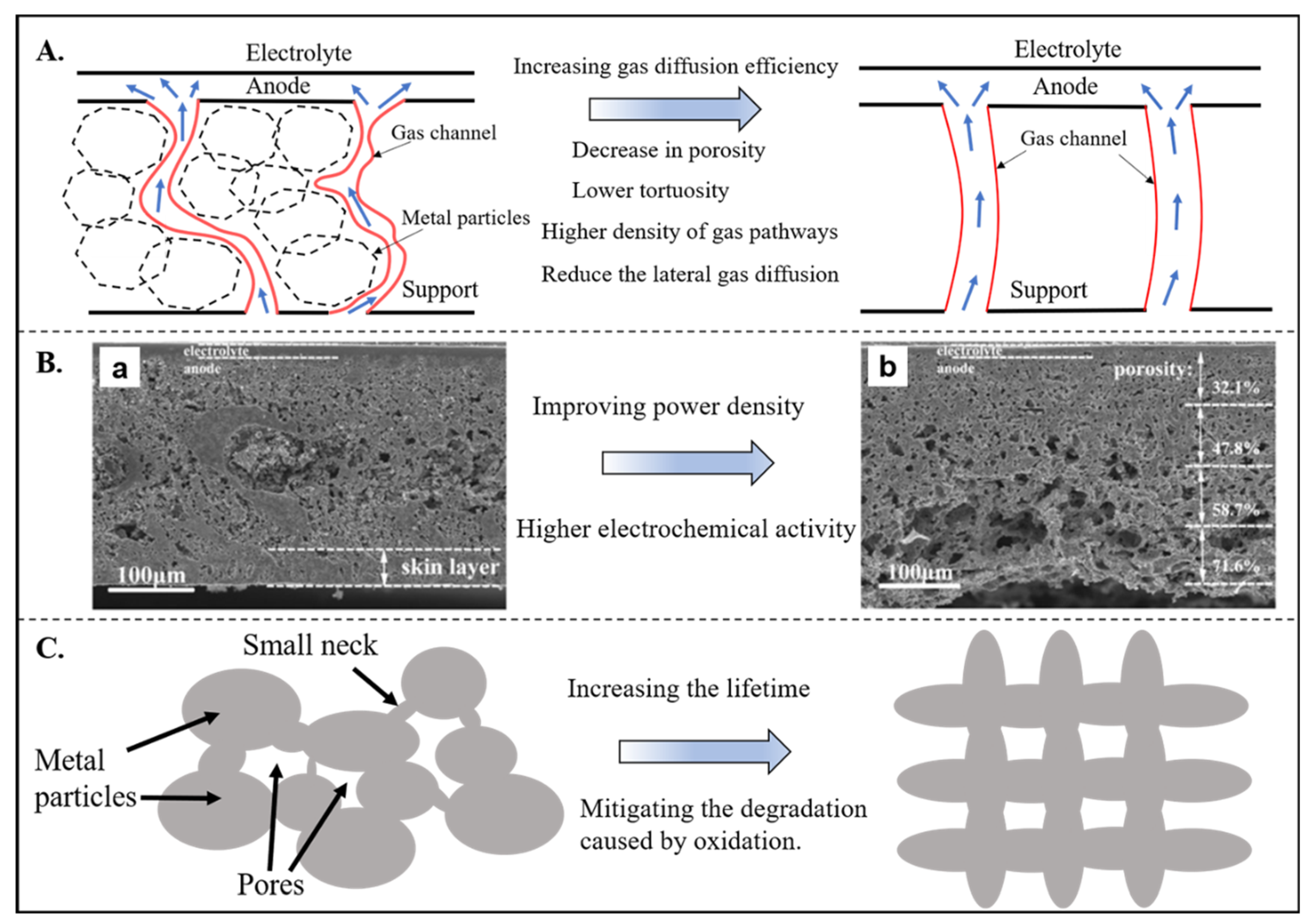
2.1.2. Metal Support Issues
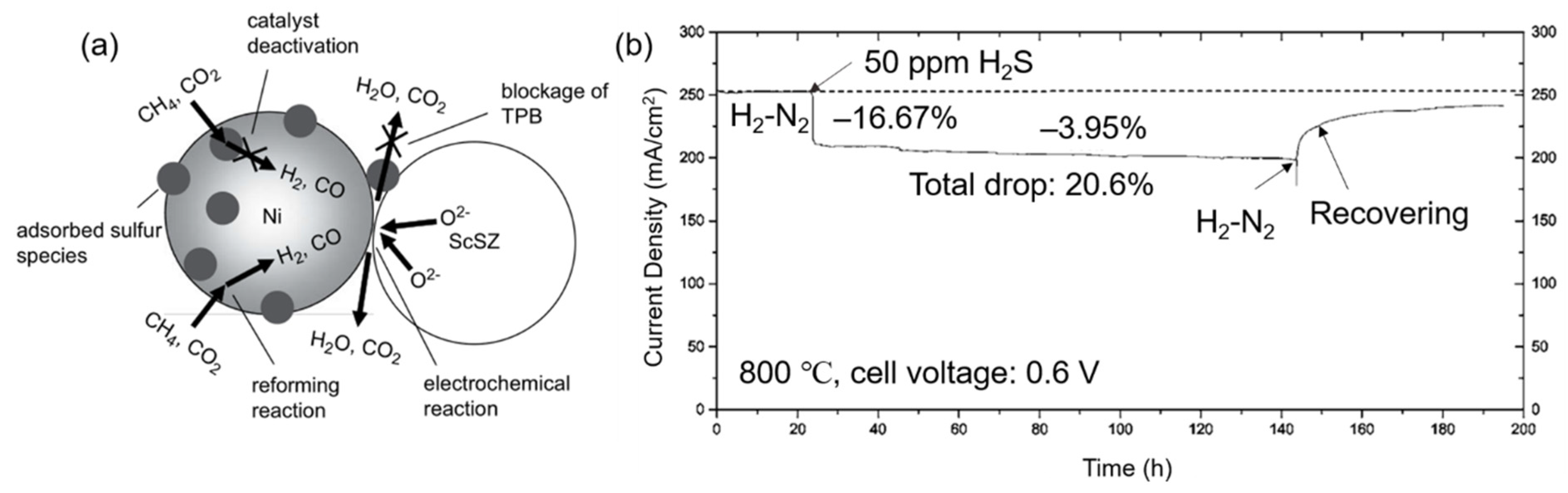
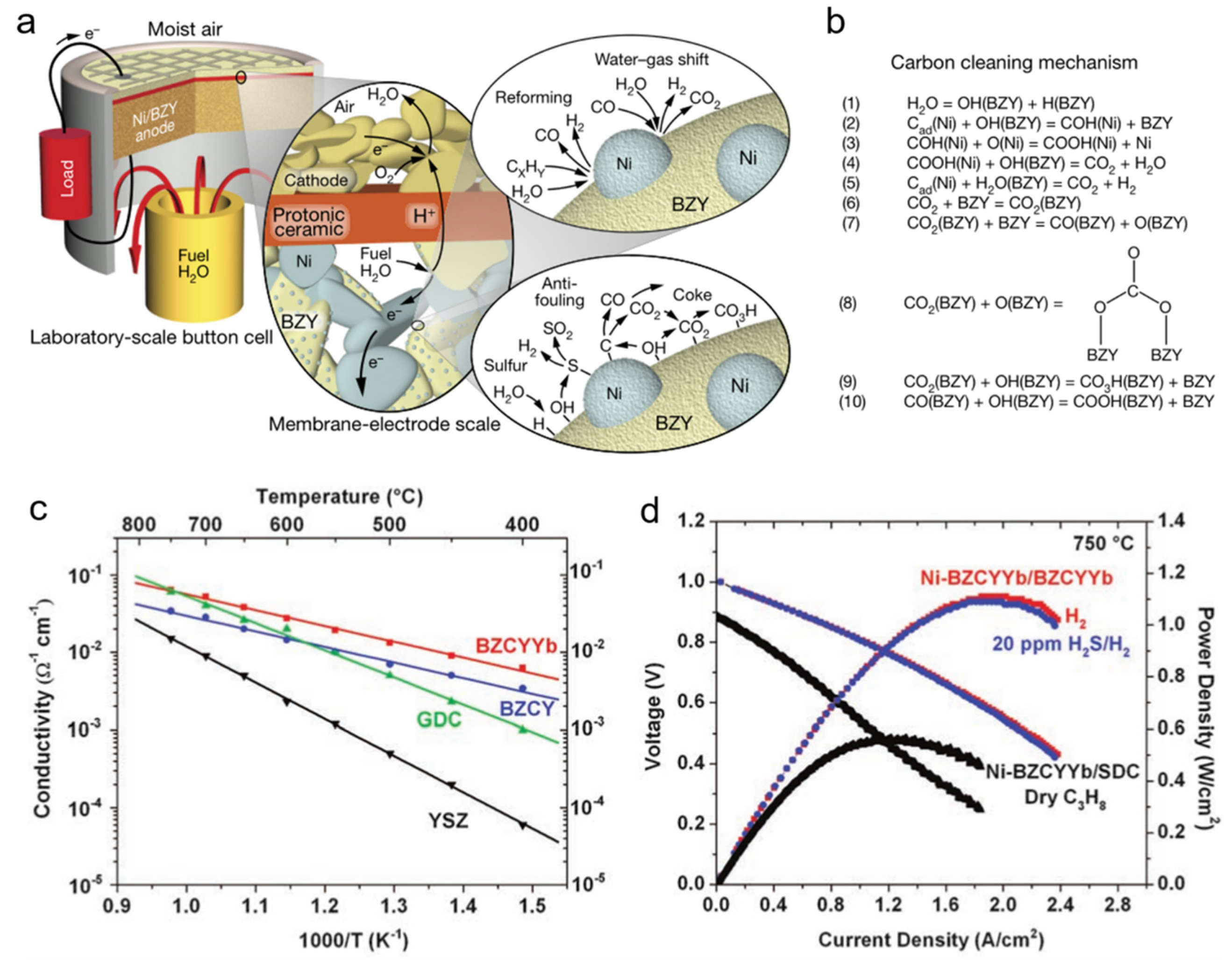
2.1.3. Carbon Deposition and Sulfur Poisoning
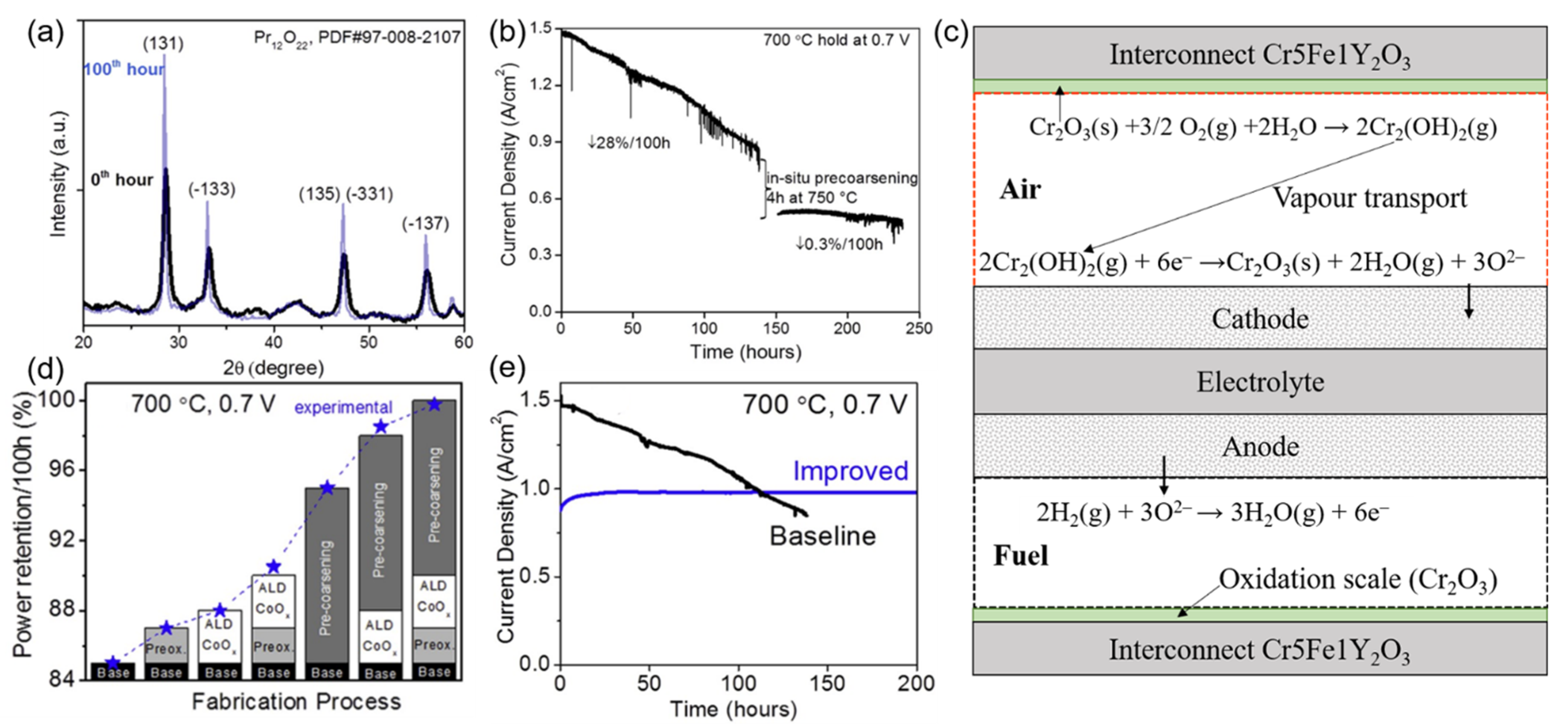
2.2. The Degradation of the Cathode
- (1)
- (2)
- (3)
- (4)
- (5)
3. The Degradation of Cell Stacks
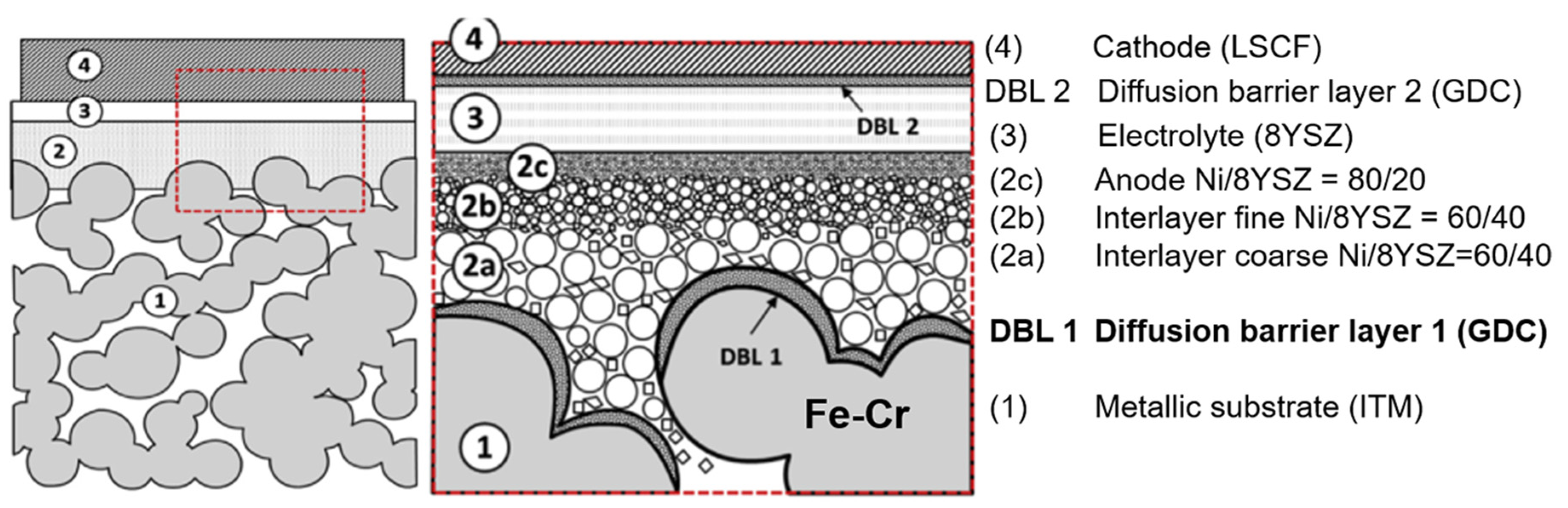
4. Thin-Film Electrolyte Metal-Supported SOCs and Issues
5. R&D Opportunities and Recommendations
6. Conclusions
- (a)
- The use of infiltrated anode completely avoids the coarsening during the co-sintering.
- (b)
- The application of DBL removes the limitation of the use of ferritic stainless-steel supports, and replacing nickel supports with ferritic stainless steels results in improved oxidation resistance and improved compatibility with ceramics.
- (c)
- The use of thin-film electrolytes reduces the operating temperature to below 600 °C.
- (d)
- The cermet anodes such as nano Ni/CGO and Ni/SDC are designed to suppress the nano-nickel coarsening, which improves the stability of infiltrated anodes during the operation.
- (e)
- Proton conductors such as BZY, BZCY, and BZCYYb are highly resistant to chromium and sulfur poisoning, which are promising to be used in MSCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Tanveer, W.H.; Sayed, E.T.; Assad, M.E.H.; Allagui, A.; Cha, S.W. On the technical challenges affecting the performance of direct internal reforming biogas solid oxide fuel cells. Renew. Sustain. Energy Rev. 2019, 101, 361–375. [Google Scholar] [CrossRef]
- Gardner, F.J.; Day, M.J.; Brandon, N.P.; Pashley, M.N.; Cassidy, M. SOFC technology development at Rolls-Royce. J. Power Sources 2000, 86, 122–129. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- De Angelis, S.; Jørgensen, P.S.; Esposito, V.; Tsai, E.H.R.; Holler, M.; Kreka, K.; Abdellahi, E.; Bowen, J.R. Ex-situ tracking solid oxide cell electrode microstructural evolution in a redox cycle by high resolution ptychographic nanotomography. J. Power Sources 2017, 360, 520–527. [Google Scholar] [CrossRef]
- Martinez-Frias, J.; Pham, A.-Q.; Aceves, S.M. A natural gas-assisted steam electrolyzer for high-efficiency production of hydrogen. Int. J. Hydrog. Energy 2003, 28, 483–490. [Google Scholar] [CrossRef]
- Hauch, A.; Ebbesen, S.D.; Jensen, S.H.; Mogensen, M. Highly efficient high temperature electrolysis. J. Mater. Chem. 2008, 18. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Connor, P. Solid Oxide Fuels Cells: Facts and Figures; Springer: London, UK, 2013. [Google Scholar]
- Minh, N.Q.; Takahashi, T. Science and Technology of Ceramic Fuel Cells; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Will, J.; Mitterdorfer, A.; Kleinlogel, C.; Perednis, D.; Gauckler, L.J. Fabrication of thin electrolytes for second-generation solid oxide fuel cells. Solid State Ion. 2000, 131, 79–96. [Google Scholar] [CrossRef]
- Chelmehsara, M.E.; Mahmoudimehr, J. Techno-economic comparison of anode-supported, cathode-supported, and electrolyte-supported SOFCs. Int. J. Hydrog. Energy 2018, 43, 15521–15530. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Foger, K. Solid oxide electrolyte fuel cell review. Ceram. Int. 1996, 22, 257–265. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Inagaki, T.; Ida, S.; Ishihara, T. Sm(Sr)CoO3 cone cathode on LaGaO3 thin film electrolyte for IT-SOFC with high power density. J. Electrochem. Soc. 2011, 158, B825–B830. [Google Scholar] [CrossRef]
- Kesler, O.; Cuglietta, M.; Harris, J.; Kuhn, J.; Marr, M.; Metcalfe, C. Progress in metal-supported SOFCs using hydrogen and methane fuels. ECS Trans. 2013, 57, 491–501. [Google Scholar] [CrossRef]
- Tucker, M.C. Development of high power density metal-supported solid oxide fuel cells. Energy Technol. 2017, 5, 2175–2181. [Google Scholar] [CrossRef]
- Reolon, R.P.; Sanna, S.; Xu, Y.; Lee, I.; Bergmann, C.P.; Pryds, N.; Esposito, V. Effects of accelerated degradation on metal supported thin film-based solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 7887–7896. [Google Scholar] [CrossRef]
- Gadea, C.; Hanniet, Q.; Lesch, A.; Marani, D.; Jensen, S.H.; Esposito, V. Aqueous metal–organic solutions for YSZ thin film inkjet deposition. J. Mater. Chem. C 2017, 5, 6021–6029. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, B.H.; Kim, S.J.; Lee, Y.; Bae, H.; Choi, G.M. Micro solid oxide fuel cell fabricated on porous stainless steel: A new strategy for enhanced thermal cycling ability. Sci. Rep. 2016, 6, 22443. [Google Scholar] [CrossRef] [PubMed]
- Roehrens, D.; Packbier, U.; Fang, Q.; Blum, L.; Sebold, D.; Bram, M.; Menzler, N. Operation of thin-film electrolyte metal-supported solid oxide fuel cells in lightweight and stationary stacks: Material and microstructural aspects. Materials 2016, 9, 762. [Google Scholar] [CrossRef]
- Xu, M.; Yu, J.; Song, Y.; Ran, R.; Wang, W.; Shao, Z. Advances in ceramic thin films fabricated by pulsed laser deposition for intermediate-temperature solid oxide fuel cells. Energy Fuels 2020, 34, 10568–10582. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, I.; Cho, G.Y.; Park, J.; Yu, W.; Tanveer, W.H.; Cha, S.W. Thin film solid oxide fuel cells operating below 600 °C: A review. Int. J. Precis. Eng. Manuf. Green Technol. 2018, 5, 441–453. [Google Scholar] [CrossRef]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Stability and robustness of metal-supported SOFCs. J. Power Sources 2008, 175, 447–451. [Google Scholar] [CrossRef]
- Tucker, M.C. Durability of symmetric-structured metal-supported solid oxide fuel cells. J. Power Sources 2017, 369, 6–12. [Google Scholar] [CrossRef]
- Tucker, M.C. Dynamic-temperature operation of metal-supported solid oxide fuel cells. J. Power Sources 2018, 395, 314–317. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Shi, Y.; Li, X. Numerical model of direct internal reforming SOFC: A comparison between anode-support and metal-support. ECS Trans. 2019, 91, 2013–2022. [Google Scholar] [CrossRef]
- Franco, T.; Brandner, M.; Rüttinger, M.; Kunschert, G.; Venskutonis, A.; Sigl, L. Recent development aspects of metal supported thin-film SOFC. ECS Trans. 2019, 25, 681–688. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Tucker, M.C. High performance metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 410, 91–98. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Lee, S.H.; Shin, H.Y.; Bae, J. Development and evaluation of 3-layer metal supported solid oxide fuel cell short stack. ECS Trans. 2017, 78, 2045–2050. [Google Scholar] [CrossRef]
- Sudireddy, B.R.; Nielsen, J.; Persson, Å.H.; Thydén, K.; Brodersen, K.; Ramousse, S.; Neagu, D.; Stefan, E.; Irvine, J.T.S.; Geisler, H.; et al. Development of robust metal-supported SOFCs and stack components in EU METSAPP Consortium. Fuel Cells 2017, 17, 508–516. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrog. Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Tucker, M.C.; Carreon, B.; Charyasatit, J.; Langston, K.; Taylor, C.; Manjarrez, J.; Burton, N.; LaBarbera, M.; Jacobson, C.P. R&D and commercialization of metal-supported SOFC personal power products at point source power. ECS Trans. 2013, 57, 503–509. [Google Scholar]
- Tucker, M.C.; Taylor, C.; LaBarbera, M.; Jacobson, C.P. Operation of metal-supported SOFC with charcoal fuel. ECS Trans. 2013, 57, 2929–2937. [Google Scholar] [CrossRef]
- Brandon, N.P.; Blake, A.; Corcoran, D.; Cumming, D.; Duckett, A.; El-Koury, K.; Haigh, D.; Kidd, C.; Leah, R.; Lewis, G.; et al. Development of metal supported solid oxide fuel cells for operation at 500–600 °C. J. Fuel Cell Sci. Technol. 2004, 1, 61–65. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hwang, C.S.; Chang, C.L.; Wu, S.H.; Lin, H.H.; Shiu, W.H.; Lin, J.K.; Yang, S.F.; Fu, C.Y.; Yang, C.S. Performances of plasma sprayed metal-supported solid oxide fuel cell and stack. Fuel Cells 2018, 18, 800–808. [Google Scholar] [CrossRef]
- Ceres Power. Available online: https://www.ceres.tech/ (accessed on 10 May 2021).
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Performance of metal-supported SOFCs with infiltrated electrodes. J. Power Sources 2007, 171, 477–482. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Yu, H.-C.; Scott Cronin, J.; Wilson, J.R.; Barnett, S.A.; Thornton, K. Simulation of coarsening in three-phase solid oxide fuel cell anodes. J. Power Sources 2011, 196, 1333–1337. [Google Scholar] [CrossRef]
- Khan, M.S.; Lee, S.-B.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J. Fundamental mechanisms involved in the degradation of nickel–yttria stabilized zirconia (Ni–YSZ) anode during solid oxide fuel cells operation: A review. Ceram. Int. 2016, 42, 35–48. [Google Scholar] [CrossRef]
- De Angelis, S.; Jørgensen, P.S.; Tsai, E.H.R.; Holler, M.; Kreka, K.; Bowen, J.R. Three dimensional characterization of nickel coarsening in solid oxide cells via ex-situ ptychographic nano-tomography. J. Power Sources 2018, 383, 72–79. [Google Scholar] [CrossRef]
- Sumi, H.; Lee, Y.-H.; Muroyama, H.; Matsui, T.; Kamijo, M.; Mimuro, S.; Yamanaka, M.; Nakajima, Y.; Eguchi, K. Effect of carbon deposition by carbon monoxide disproportionation on electrochemical characteristics at low temperature operation for solid oxide fuel cells. J. Power Sources 2011, 196, 4451–4457. [Google Scholar] [CrossRef]
- Ferlauto, A.S.; de Florio, D.Z.; Fonseca, F.C.; Esposito, V.; Muccillo, R.; Traversa, E.; Ladeira, L.O. Chemical vapor deposition of multi-walled carbon nanotubes from nickel/yttria-stabilized zirconia catalysts. Appl. Phys. A 2006, 84, 271–276. [Google Scholar] [CrossRef]
- Chun, C.M.; Ramanarayanan, T.A. Mechanism and control of carbon deposition on high temperature alloys. J. Electrochem. Soc. 2007, 154, C465–C471. [Google Scholar] [CrossRef][Green Version]
- Chun, C.M.; Mumford, J.D.; Ramanarayanan, T.A. Carbon-induced corrosion of nickel anode. J. Electrochem. Soc. 2000, 147, 3680–3686. [Google Scholar] [CrossRef]
- Chun, C.M.; Mumford, J.D.; Ramanarayanan, T.A. Mechanisms of metal dusting corrosion of iron. J. Electrochem. Soc. 2002, 149, B348–B355. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Jia, L.; Chi, B.; Pu, J.; Li, J. High performance and carbon-deposition resistance metal-supported solid oxide fuel cell with a nickel–manganese spinel modified anode. Mater. Today Energy 2020, 17, 100473. [Google Scholar] [CrossRef]
- Nielsen, J.; Sudireddy, B.R.; Hagen, A.; Persson, Å.H. Performance factors and sulfur tolerance of metal supported solid oxide fuel cells with nanostructured Ni:GDC infiltrated anodes. J. Electrochem. Soc. 2016, 163, F574–F580. [Google Scholar] [CrossRef]
- Skafte, T.L.; Blennow, P.; Hjelm, J.; Graves, C. Carbon deposition and sulfur poisoning during CO2 electrolysis in nickel-based solid oxide cell electrodes. J. Power Sources 2018, 373, 54–60. [Google Scholar] [CrossRef]
- Trembly, J.P.; Marquez, A.I.; Ohrn, T.R.; Bayless, D.J. Effects of coal syngas and H2S on the performance of solid oxide fuel cells: Single-cell tests. J. Power Sources 2006, 158, 263–273. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.Z.; Blinn, K.; Liu, M.F.; Liu, Z.; Cheng, Z.; Liu, M.L. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2−xYbxO3−δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.J.; Zhu, H.; Karakaya, C.; Chen, Y.; Ricote, S.; Jarry, A.; Crumlin, E.J.; Hook, D.; Braun, R.; et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018, 557, 217–222. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Karimaghaloo, A.; Lee, M.H.; Tucker, M.C. Progress in durability of metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2019, 437, 226935. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X. Chromium deposition and poisoning of cathodes of solid oxide fuel cells—A review. Int. J. Hydrog. Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Reisert, M.; Berova, V.; Aphale, A.; Singh, P.; Tucker, M.C. Oxidation of porous stainless steel supports for metal-supported solid oxide fuel cells. Int. J. Hydrog. Energy 2020, 45, 30882–30897. [Google Scholar] [CrossRef]
- Xu, N.; Chen, M.; Han, M. Oxidation behavior of a Ni-Fe support in SOFC anode atmosphere. J. Alloy. Compd. 2018, 765, 757–763. [Google Scholar] [CrossRef]
- Krishnan, V.V. Recent developments in metal-supported solid oxide fuel cells. Wiley Interdiscip. Rev. Energy Environ. 2017, 6. [Google Scholar] [CrossRef]
- Rojek-Wöckner, V.A.; Opitz, A.K.; Brandner, M.; Mathé, J.; Bram, M. A novel Ni/ceria-based anode for metal-supported solid oxide fuel cells. J. Power Sources 2016, 328, 65–74. [Google Scholar] [CrossRef]
- Villarreal, I.; Jacobson, C.; Leming, A.; Matus, Y.; Visco, S.; De Jonghe, L. Metal-supported solid oxide fuel cells. Electrochem. Solid State Lett. 2003, 6, A178–A179. [Google Scholar] [CrossRef]
- Bao, W.; Chang, Q.; Meng, G. Effect of NiO/YSZ compositions on the co-sintering process of anode-supported fuel cell. J. Membr. Sci. 2005, 259, 103–109. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, T.; Li, J.; Yuan, C.; Xin, X.; Chen, G.; Miao, G.; Zhan, W.; Zhan, Z.; Wang, S. Long-term stability of metal-supported solid oxide fuel cells employing infiltrated electrodes. J. Power Sources 2015, 295, 67–73. [Google Scholar] [CrossRef]
- Ovtar, S.; Tong, X.; Bentzen, J.J.; Thyden, K.T.S.; Simonsen, S.B.; Chen, M. Boosting the performance and durability of Ni/YSZ cathode for hydrogen production at high current densities via decoration with nano-sized electrocatalysts. Nanoscale 2019, 11, 4394–4406. [Google Scholar] [CrossRef]
- Ni, D.W.; Andersen, K.B.; Esposito, V. Sintering and grain growth kinetics in La0.85Sr0.15MnO3–Ce0.9Gd0.1O1.95 (LSM–CGO) porous composite. J. Eur. Ceram. Soc. 2014, 34, 3769–3778. [Google Scholar] [CrossRef]
- Esposito, V.; Castelli, I.E. Metal oxides interfaces: Metastability at defective metal oxide interfaces and nanoconfined structures. Adv. Mater. Interfaces 2020, 7, 2070071. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemenso, T.; Persson, A.; Brodersen, K.; Srivastava, A.; Frandsen, H.; Lundberg, M.; Ramousse, S.; Mogensen, M. Development of planar metal supported SOFC with novel cermet anode. ECS Trans. 2009, 25, 701–710. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Ramousse, S.; Kromp, A.; Leonide, A.; Weber, A. Manufacturing and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2011, 196, 7117–7125. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.; Behnamian, Y.; Luo, J. A-site deficient perovskite: The parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes. J. Mater. Chem. A 2015, 3, 11048–11056. [Google Scholar] [CrossRef]
- Tan, J.; Lee, D.; Ahn, J.; Kim, B.; Kim, J.; Moon, J. Thermally drivenin situexsolution of Ni nanoparticles from (Ni, Gd)CeO2 for high-performance solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 18133–18142. [Google Scholar] [CrossRef]
- Ni, C.; Zeng, Q.; He, D.; Peng, L.; Xie, D.; Irvine, J.T.S.; Duan, S.; Ni, J. A B-site doped perovskite ferrate as an efficient anode of a solid oxide fuel cell with in situ metal exsolution. J. Mater. Chem. A 2019, 7, 26944–26953. [Google Scholar] [CrossRef]
- Kim, K.J.; Rath, M.K.; Kwak, H.H.; Kim, H.J.; Han, J.W.; Hong, S.-T.; Lee, K.T. A highly active and redox-stable SrGdNi0.2Mn0.8O4±δ anode with in situ exsolution of nanocatalysts. ACS Catal. 2019, 9, 1172–1182. [Google Scholar] [CrossRef]
- Bahout, M.; Managutti, P.B.; Dorcet, V.; Le Gal La Salle, A.; Paofai, S.; Hansen, T.C. In situ exsolution of Ni particles on the PrBaMn2O5 SOFC electrode material monitored by high temperature neutron powder diffraction under hydrogen. J. Mater. Chem. A 2020, 8, 3590–3597. [Google Scholar] [CrossRef]
- Kong, Y.; Hua, B.; Pu, J.; Chi, B.; Jian, L. A cost-effective process for fabrication of metal-supported solid oxide fuel cells. Int. J. Hydrog. Energy 2010, 35, 4592–4596. [Google Scholar] [CrossRef]
- Hwang, C.; Tsai, C.-H.; Lo, C.-H.; Sun, C.-H. Plasma sprayed metal supported YSZ/Ni–LSGM–LSCF ITSOFC with nanostructured anode. J. Power Sources 2008, 180, 132–142. [Google Scholar] [CrossRef]
- Cho, H.J.; Choi, G.M. Fabrication and characterization of Ni-supported solid oxide fuel cell. Solid State Ion. 2009, 180, 792–795. [Google Scholar] [CrossRef]
- Mineshige, A.; Fukushima, K.; Okada, S.; Kikuchi, T.; Kobune, M.; Yazawa, T.; Kikuchi, K.; Inaba, M.; Ogumi, Z. Porous metal tubular support for solid oxide fuel cell design. Electrochem. Solid State Lett. 2006, 9, A427–A429. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, J.; Shinagawa, M.; Matsumoto, H. Ni–Fe bimetallic anode as an active anode for intermediate temperature SOFC using LaGaO3 based electrolyte film. Electrochim. Acta 2006, 52, 1645–1650. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, Y.M.; Kim, H. Fabrication and evaluation of the thin NiFe supported solid oxide fuel cell by co-firing method. Energy 2010, 35, 5385–5390. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Eto, H.; Inagaki, T.; Ishihara, T. High power SOFC using LSGM film on NiFe porous Bi-metal substrate. ECS Trans. 2019, 25, 719–726. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Eto, H.; Inagaki, T.; Ida, S.; Ishihara, T. Preparation of Ni–Fe bimetallic porous anode support for solid oxide fuel cells using LaGaO3 based electrolyte film with high power density. J. Power Sources 2010, 195, 6294–6300. [Google Scholar] [CrossRef]
- Ju, Y.-W.; Ida, S.; Inagaki, T.; Ishihara, T. Improved reoxidation tolerance of Ni–Fe metal support for LaGaO3 thin film electrolyte cell. Solid State Ion. 2012, 216, 58–63. [Google Scholar] [CrossRef]
- Klemensø, T.; Nielsen, J.; Blennow, P.; Persson, Å.H.; Stegk, T.; Christensen, B.H.; Sønderby, S. High performance metal-supported solid oxide fuel cells with Gd-doped ceria barrier layers. J. Power Sources 2011, 196, 9459–9466. [Google Scholar] [CrossRef]
- Nielsen, J.; Persson, Å.H.; Muhl, T.T.; Brodersen, K. Towards high power density metal supported solid oxide fuel cell for mobile applications. J. Electrochem. Soc. 2018, 165, F90–F96. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, L.M.; Otaegi, L.; Alvarez, M.; Rivas, M.; Gomez, N.; Zabala, A.; Arizmendiarrieta, N.; Antepara, I.; Urriolabeitia, A.; Olave, M.; et al. Degradation studies on tubular metal supported SOFC. ECS Trans. 2019, 25, 745–752. [Google Scholar] [CrossRef]
- Haydn, M.; Bischof, C.; Udomsilp, D.; Opitz, A.K.; Bimashofer, G.; Schafbauer, W.; Brandner, M.; Bram, M. Metal supported SOFCs: Electrochemical performance under various testing conditions. ECS Trans. 2017, 78, 1993–2003. [Google Scholar] [CrossRef]
- Udomsilp, D.; Rechberger, J.; Neubauer, R.; Bischof, C.; Thaler, F.; Schafbauer, W.; Menzler, N.H.; de Haart, L.G.J.; Nenning, A.; Opitz, A.K.; et al. Metal-supported solid oxide fuel cells with exceptionally high power density for range extender systems. Cell Rep. Phys. Sci. 2020, 1, 100072. [Google Scholar] [CrossRef]
- Esposito, V.; Ni, D.W.; Marani, D.; Teocoli, F.; Sune Thydén, K.T.; De Florio, D.Z.; Fonseca, F.C. Accelerated ceria–zirconia solubilization by cationic diffusion inversion at low oxygen activity. J. Mater. Chem. A 2016, 4, 16871–16878. [Google Scholar] [CrossRef]
- Esposito, V.; Luong, B.H.; Di Bartolomeo, E.; Wachsman, E.D.; Traversa, E. Applicability of Bi2Ru2O7 pyrochlore electrodes for ESB and BIMEVOX electrolytes. J. Electrochem. Soc. 2006, 153, A2232–A2238. [Google Scholar] [CrossRef]
- Esposito, V.; Traversa, E.; Wachsman, E.D. Pb2Ru2O6.5 as a low-temperature cathode for bismuth oxide electrolytes. J. Electrochem. Soc. 2005, 152, A2300–A2305. [Google Scholar] [CrossRef]
- Schiller, G.; Ansar, A.; Lang, M.; Patz, O. High temperature water electrolysis using metal supported solid oxide electrolyser cells (SOEC). J. Appl. Electrochem. 2008, 39, 293–301. [Google Scholar] [CrossRef]
- Franco, T.; Haydn, M.; Mücke, R.; Weber, A.; Rüttinger, M.; Büchler, O.; Uhlenbruck, S.; Menzler, N.H.; Venskutonis, A.; Sigl, L.S. Development of metal-supported solid oxide fuel cells. ECS Trans. 2011, 35, 343–349. [Google Scholar] [CrossRef]
- Sarasketa-Zabala, E.; Otaegi, L.; Rodriguez-Martinez, L.M.; Alvarez, M.A.; Burgos, N.; Castro, F.; Villarreal, I. High temperature stability of porous metal substrates under highly humidified hydrogen conditions for metal supported solid oxide fuel cells. Solid State Ion. 2012, 222, 16–22. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Molin, S.; Zhang, Y.; Chen, M.; Han, M. High temperature oxidation behavior of SUS430 SOFC interconnects with Mn-Co spinel coating in air. J. Alloy. Compd. 2019, 787, 1327–1335. [Google Scholar] [CrossRef]
- Molin, S.; Kusz, B.; Gazda, M.; Jasinski, P. Evaluation of porous 430L stainless steel for SOFC operation at intermediate temperatures. J. Power Sources 2008, 181, 31–37. [Google Scholar] [CrossRef]
- Antepara, I.; Villarreal, I.; Rodríguez-Martínez, L.M.; Lecanda, N.; Castro, U.; Laresgoiti, A. Evaluation of ferritic steels for use as interconnects and porous metal supports in IT-SOFCs. J. Power Sources 2005, 151, 103–107. [Google Scholar] [CrossRef]
- Deng, X.; Wei, P.; Bateni, M.R.; Petric, A. Cobalt plating of high temperature stainless steel interconnects. J. Power Sources 2006, 160, 1225–1229. [Google Scholar] [CrossRef]
- Jeong, H.; Roehrens, D.; Bram, M. Facile route for reactive coating of LaCrO3 on high-chromium steels as protective layer for solid oxide fuel cell applications. Mater. Lett. 2020, 258. [Google Scholar] [CrossRef]
- Tao, Y.; Ebbesen, S.D.; Mogensen, M.B. Carbon deposition in solid oxide cells during co-electrolysis of H2O and CO2. J. Electrochem. Soc. 2014, 161, F337–F343. [Google Scholar] [CrossRef]
- Costa-Nunes, O.; Gorte, R.J.; Vohs, J.M. Comparison of the performance of Cu–CeO2–YSZ and Ni–YSZ composite SOFC anodes with H2, CO, and syngas. J. Power Sources 2005, 141, 241–249. [Google Scholar] [CrossRef]
- Kim, H.; Vohs, J.M.; Gorte, R.J. Direct oxidation of sulfur-containing fuels in a solid oxide fuel cell. Chem. Commun. 2001, 22, 2334–2335. [Google Scholar] [CrossRef]
- An, S.; Lu, C.; Worrell, W.L.; Gorte, R.J.; Vohs, J.M. Characterization of Cu–CeO2 direct hydrocarbon anodes in a solid oxide fuel cell with lanthanum gallate electrolyte. Solid State Ion. 2004, 175, 135–138. [Google Scholar] [CrossRef]
- Gorte, R.J.; Park, S.; Vohs, J.M.; Wang, C. Anodes for direct oxidation of dry hydrocarbons in a solid-oxide fuel cell. Adv. Mater. 2000, 12, 1465–1469. [Google Scholar] [CrossRef]
- Zha, S.; Cheng, Z.; Liu, M. Sulfur poisoning and regeneration of Ni-based anodes in solid oxide fuel cells. J. Electrochem. Soc. 2006, 154, B201–B206. [Google Scholar] [CrossRef]
- Shiratori, Y.; Oshima, T.; Sasaki, K. Feasibility of direct-biogas SOFC. Int. J. Hydrog. Energy 2008, 33, 6316–6321. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, M. Characterization of sulfur poisoning of Ni–YSZ anodes for solid oxide fuel cells using in situ Raman microspectroscopy. Solid State Ion. 2007, 178, 925–935. [Google Scholar] [CrossRef]
- Sasaki, K.; Susuki, K.; Iyoshi, A.; Uchimura, M.; Imamura, N.; Kusaba, H.; Teraoka, Y.; Fuchino, H.; Tsujimoto, K.; Uchida, Y.; et al. H2S poisoning of solid oxide fuel cells. J. Electrochem. Soc. 2006, 153, A2023–A2029. [Google Scholar] [CrossRef]
- Cheng, Z.; Zha, S.; Liu, M. Influence of cell voltage and current on sulfur poisoning behavior of solid oxide fuel cells. J. Power Sources 2007, 172, 688–693. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Hansen, J.B.; Helveg, S.; Christiansen, N.; Jannasch, A.K. Sites for catalysis and electrochemistry in solid oxide fuel cell (SOFC) anode. Appl. Phys. A 2006, 85, 427–430. [Google Scholar] [CrossRef]
- Wang, R.; Lau, G.Y.; Ding, D.; Zhu, T.; Tucker, M.C. Approaches for co-sintering metal-supported proton-conducting solid oxide cells with Ba(Zr, Ce, Y, Yb)O3−δ electrolyte. Int. J. Hydrog. Energy 2019, 44, 13768–13776. [Google Scholar] [CrossRef]
- Wang, R.; Byrne, C.; Tucker, M.C. Assessment of co-sintering as a fabrication approach for metal-supported proton-conducting solid oxide cells. Solid State Ion. 2019, 332, 25–33. [Google Scholar] [CrossRef]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental mechanisms limiting solid oxide fuel cell durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Brugnoni, C.; Ducati, U.; Scagliotti, M. SOFC cathode/electrolyte interface. Part I: Reactivity between La0.85Sr0.15MnO3 and ZrO2-Y2O3. Solid State Ion. 1995, 76, 177–182. [Google Scholar] [CrossRef]
- Mitterdorfer, A.; Gauckler, L.J. La2Zr2O7 formation and oxygen reduction kinetics of the La0.85Sr0.15MnyO3, O2(g)|YSZ system. Solid State Ion. 1998, 111, 185–218. [Google Scholar] [CrossRef]
- de Florio, D.Z.; Muccillo, R.; Esposito, V.; Di Bartolomeo, E.; Traversa, E. Preparation and electrochemical characterization of perovskite/YSZ ceramic films. J. Electrochem. Soc. 2005, 152, A88–A92. [Google Scholar] [CrossRef]
- Esposito, V.; Søgaard, M.; Hendriksen, P.V. Chemical stability of La0.6Sr0.4CoO3−δ in oxygen permeation applications under exposure to N2 and CO2. Solid State Ion. 2012, 227, 46–56. [Google Scholar] [CrossRef]
- Wang, Z.; Berghaus, J.O.; Yick, S.; Decès-Petit, C.; Qu, W.; Hui, R.; Maric, R.; Ghosh, D. Dynamic evaluation of low-temperature metal-supported solid oxide fuel cell oriented to auxiliary power units. J. Power Sources 2008, 176, 90–95. [Google Scholar] [CrossRef]
- Ni, D.-W.; Esposito, V.; Schmidt, C.G.; Molla, T.T.; Andersen, K.B.; Kaiser, A.; Ramousse, S.; Pryds, N. Camber evolution and stress development of porous ceramic bilayers during co-firing. J. Am. Ceram. Soc. 2013, 96, 972–978. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.H.; Weiß, R. Chromium vapor species over solid oxide fuel cell interconnect materials and their potential for degradation processes. J. Electrochem. Soc. 1996, 143, 3642–3647. [Google Scholar] [CrossRef]
- Shen, F.; Wang, R.; Tucker, M.C. Long term durability test and post mortem for metal-supported solid oxide electrolysis cells. J. Power Sources 2020, 474, 228618. [Google Scholar] [CrossRef]
- Wang, R.; Dogdibegovic, E.; Lau, G.Y.; Tucker, M.C. Metal-supported solid oxide electrolysis cell with significantly enhanced catalysis. Energy Technol. 2019, 7, 1801154. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Gao, W.; Xue, S.; Zhang, M. Enhanced reliability of Ni-Fe alloys supported SOFCs with ex-Situ sintered La0.8Sr0.2MnO3−δ Coated Ba0.5Sr0.5Co0.8Fe0.2O3–δ Cathode. J. Electrochem. Soc. 2021, 168, 034516. [Google Scholar] [CrossRef]
- Udomsilp, D.; Thaler, F.; Menzler, N.H.; Bischof, C.; de Haart, L.G.J.; Opitz, A.K.; Guillon, O.; Bram, M. Dual-phase cathodes for metal-supported solid oxide fuel cells: Processing, performance, durability. J. Electrochem. Soc. 2019, 166, F506–F510. [Google Scholar] [CrossRef]
- Udomsilp, D.; Roehrens, D.; Menzler, N.H.; Bischof, C.; de Haart, L.G.J.; Opitz, A.K.; Guillon, O.; Bram, M. High-performance metal-supported solid oxide fuel cells by advanced cathode processing. J. Electrochem. Soc. 2017, 164, F1375–F1384. [Google Scholar] [CrossRef]
- Bram, M.; Udomsilp, D.; Roehrens, D.; Menzler, N.H.; Haart, L.G.J.d.; Opitz, A.K.; Guillon, O. High performance (La,Sr)(Co,Fe)O3 cathodes with improved adherence for metal-supported fuel cells. ECS Trans. 2017, 78, 709–715. [Google Scholar] [CrossRef]
- Udomsilp, D.; Roehrens, D.; Menzler, N.H.; Opitz, A.K.; Guillon, O.; Bram, M. Novel processing of La0.58Sr0.4Co0.2Fe0.8O3−δ cathodes for metal-supported fuel cells. Mater. Lett. 2017, 192, 173–176. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Deller, R.; Foger, K.; Ramprakash, Y.; Zhang, J.P. Interaction between chromia forming alloy interconnects and air electrode of solid oxide fuel cells. Solid State Ion. 1997, 99, 297–310. [Google Scholar] [CrossRef]
- Konysheva, E.; Penkalla, H.; Wessel, E.; Mertens, J.; Seeling, U.; Singheiser, L.; Hilpert, K. Chromium poisoning of perovskite cathodes by the ODS alloy Cr5Fe1Y2O3 and the high chromium ferritic steel Crofer22APU. J. Electrochem. Soc. 2006, 153, A765–A773. [Google Scholar] [CrossRef]
- Opila, E.J.; Jacobson, N.S.; Myers, D.L.; Copland, E.H. Predicting oxide stability in high-temperature water vapor. JOM 2006, 58, 22–28. [Google Scholar] [CrossRef]
- Gindorf, C.; Singheiser, L.; Hilpert, K. Vaporisation of chromia in humid air. J. Phys. Chem. Solids 2005, 66, 384–387. [Google Scholar] [CrossRef]
- Gindorf, C.; Singheiser, L.; Hilpert, K. Chromium vaporisation from Fe,Cr base alloys used as interconnect in fuel cells. Steel Res. 2001, 72, 528–533. [Google Scholar] [CrossRef]
- Chen, X.; Zhen, Y.; Li, J.; Jiang, S.P. Chromium deposition and poisoning in dry and humidified air at (La0.8Sr0.2)0.9MnO3+δ cathodes of solid oxide fuel cells. Int. J. Hydrog. Energy 2010, 35, 2477–2485. [Google Scholar] [CrossRef]
- Komatsu, T.; Chiba, R.; Arai, H.; Sato, K. Chemical compatibility and electrochemical property of intermediate-temperature SOFC cathodes under Cr poisoning condition. J. Power Sources 2008, 176, 132–137. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Chen, X.; Jiang, S.P.; Bae, J. Effect of strontium content on chromium deposition and poisoning in Ba1−xSrxCo0.8Fe0.2O3−δ(0.3 ≤x ≤0.7) cathodes of solid oxide fuel cells. J. Electrochem. Soc. 2011, 159, B185–B194. [Google Scholar] [CrossRef]
- Zhen, Y.D.; Tok, A.I.Y.; Jiang, S.P.; Boey, F.Y.C. La(Ni,Fe)O3 as a cathode material with high tolerance to chromium poisoning for solid oxide fuel cells. J. Power Sources 2007, 170, 61–66. [Google Scholar] [CrossRef]
- Xiong, C.; Li, W.; Ding, D.; Pu, J.; Chi, B.; Li, J. Chromium poisoning effect on strontium-doped samarium manganite for solid oxide fuel cell. Int. J. Hydrog. Energy 2016, 41, 20660–20669. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Gong, Y.; Palacio, D.; Song, X.; Patel, R.L.; Liang, X.; Zhao, X.; Goodenough, J.B.; Huang, K. Stabilizing nanostructured solid oxide fuel cell cathode with atomic layer deposition. Nano Lett. 2013, 13, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Puranen, J.; Pihlatie, M.; Lagerbom, J.; Bolelli, G.; Laakso, J.; Hyvärinen, L.; Kylmälahti, M.; Himanen, O.; Kiviaho, J.; Lusvarghi, L.; et al. Post-mortem evaluation of oxidized atmospheric plasma sprayed Mn–Co–Fe oxide spinel coatings on SOFC interconnectors. Int. J. Hydrog. Energy 2014, 39, 17284–17294. [Google Scholar] [CrossRef]
- Malzbender, J.; Batfalsky, P.; Vaßen, R.; Shemet, V.; Tietz, F. Component interactions after long-term operation of an SOFC stack with LSM cathode. J. Power Sources 2012, 201, 196–203. [Google Scholar] [CrossRef]
- Ding, K.; Zhu, M.; Han, Z.; Kochetov, V.; Lu, L.; Chen, D. Momentum-species-heat-electrochemistry distribution characteristics within solid oxide fuel cell stack with complex inter-digital fuel channels. Ionics 2020, 26, 4567–4578. [Google Scholar] [CrossRef]
- Promsen, M.; Komatsu, Y.; Sciazko, A.; Kaneko, S.; Shikazono, N. Feasibility study on saturated water cooled solid oxide fuel cell stack. Appl. Energy 2020, 279. [Google Scholar] [CrossRef]
- Yahya, A.; Rabhi, R.; Dhahri, H.; Slimi, K. Numerical simulation of temperature distribution in a planar solid oxide fuel cell using lattice Boltzmann method. Powder Technol. 2018, 338, 402–415. [Google Scholar] [CrossRef]
- Kalib, N.S.; Muchtar, A.; Somalu, M.R.; Ariffin, A.K.; Ihsan, M.; Aman, N.A.M.N.; Mah, J.C.W. Influence of heat transfer on thermal stress development in solid oxide fuel Ccells: A review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 54, 175–184. [Google Scholar]
- Zeng, Z.; Qian, Y.; Zhang, Y.; Hao, C.; Dan, D.; Zhuge, W. A review of heat transfer and thermal management methods for temperature gradient reduction in solid oxide fuel cell (SOFC) stacks. Appl. Energy 2020, 280. [Google Scholar] [CrossRef]
- Leah, R.T.; Bone, P.A.; Selcuk, A.; Rahman, M.; Clare, A.; Lankin, M.; Felix, F.; Mukerjee, S.; Selby, M.A. Latest results and commercialization of the ceres power steelCell® technology platform. ECS Trans. 2019, 91, 51–61. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Talic, B.; Falk-Windisch, H.; Venkatachalam, V.; Hendriksen, P.V.; Wiik, K.; Lein, H.L. Effect of coating density on oxidation resistance and Cr vaporization from solid oxide fuel cell interconnects. J. Power Sources 2017, 354, 57–67. [Google Scholar] [CrossRef]
- Alvarez, E.; Meier, A.; Weil, K.S.; Yang, Z. Oxidation kinetics of manganese cobaltite spinel protection layers on Sanergy HT for solid oxide fuel cell interconnect applications. Int. J. Appl. Ceram. Technol. 2011, 8, 33–41. [Google Scholar] [CrossRef]
- Cheng, F.; Sun, J. Fabrication of a double-layered Co-Mn-O spinel coating on stainless steel via the double glow plasma alloying process and preoxidation treatment as SOFC interconnect. Int. J. Hydrog. Energy 2019, 44, 18415–18424. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Bianco, M.; Linder, M.; Larring, Y.; Greco, F.; Van herle, J. Lifetime Issues for Solid Oxide Fuel Cell Interconnects. In Solid Oxide Fuel Cell Lifetime and Reliability; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–144. [Google Scholar] [CrossRef]
- Petric, A.; Ling, H. Electrical conductivity and thermal expansion of spinels at elevated temperatures. J. Am. Ceram. Soc. 2007, 90, 1515–1520. [Google Scholar] [CrossRef]
- Haydn, M.; Ortner, K.; Franco, T.; Uhlenbruck, S.; Menzler, N.H.; Stöver, D.; Bräuer, G.; Venskutonis, A.; Sigl, L.S.; Buchkremer, H.-P.; et al. Multi-layer thin-film electrolytes for metal supported solid oxide fuel cells. J. Power Sources 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Chen, L.; Yao, M.; Xia, C. Anode substrate with continuous porosity gradient for tubular solid oxide fuel cells. Electrochem. Commun. 2014, 38, 114–116. [Google Scholar] [CrossRef]
- Epstein, N. On tortuosity and the tortuosity factor in flow and diffusion through porous media. Chem. Eng. Sci. 1989, 44, 777–779. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. A strategy for achieving high performance with SOFC ceramic anodes. Electrochem. Solid State Lett. 2007, 10, B65–B69. [Google Scholar] [CrossRef]
- Holtappels, P.; Sorof, C.; Verbraeken, M.C.; Rambert, S.; Vogt, U. Preparation of porosity-graded SOFC anode substrates. Fuel Cells 2006, 6, 113–116. [Google Scholar] [CrossRef]
- Leah, R.; Bone, A.; Lankin, M.; Selcuk, A.; Rahman, M.; Clare, A.; Rees, L.; Phillip, S.; Mukerjee, S.; Selby, M. Ceres power steel cell technology: Rapid progress towards a truly commercially viable SOFC. ECS Trans. 2015, 68, 95–107. [Google Scholar] [CrossRef]
- Leah, R.; Bone, A.; Selcuk, A.; Corcoran, D.; Lankin, M.; Dehaney-Steven, Z.; Selby, M.; Whalen, P. Development of highly robust, volume-manufacturable metal-supported SOFCs for operation below 600 °C. ECS Trans. 2011, 35, 351–367. [Google Scholar] [CrossRef]
- Mercadelli, E.; Gondolini, A.; Pinasco, P.; Sanson, A. Stainless steel porous substrates produced by tape casting. Met. Mater. Int. 2017, 23, 184–192. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Sumi, H.; Takahashi, H.; Mori, R.; Shimizu, H. Development of metal-supported planar SOFCs fabricated by all wet process on metallurgical porous substrates. ECS Trans. 2019, 91, 909–915. [Google Scholar] [CrossRef]
- Yang, L.; Yan, C.; Cao, W.; Liu, Z.; Song, B.; Wen, S.; Zhang, C.; Shi, Y.; Yang, S. Compression–compression fatigue behaviour of gyroid-type triply periodic minimal surface porous structures fabricated by selective laser melting. Acta Mater. 2019, 181, 49–66. [Google Scholar] [CrossRef]
- Yang, L.; Mertens, R.; Ferrucci, M.; Yan, C.; Shi, Y.; Yang, S. Continuous graded Gyroid cellular structures fabricated by selective laser melting: Design, manufacturing and mechanical properties. Mater. Des. 2019, 162, 394–404. [Google Scholar] [CrossRef]
- Downing, D.; Jones, A.; Brandt, M.; Leary, M. Increased efficiency gyroid structures by tailored material distribution. Mater. Des. 2021, 197. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Shishkovsky, I.; Bertrand, P.; Smurov, I. Manufacturing of fine-structured 3D porous filter elements by selective laser melting. Appl. Surf. Sci. 2009, 255, 5523–5527. [Google Scholar] [CrossRef]



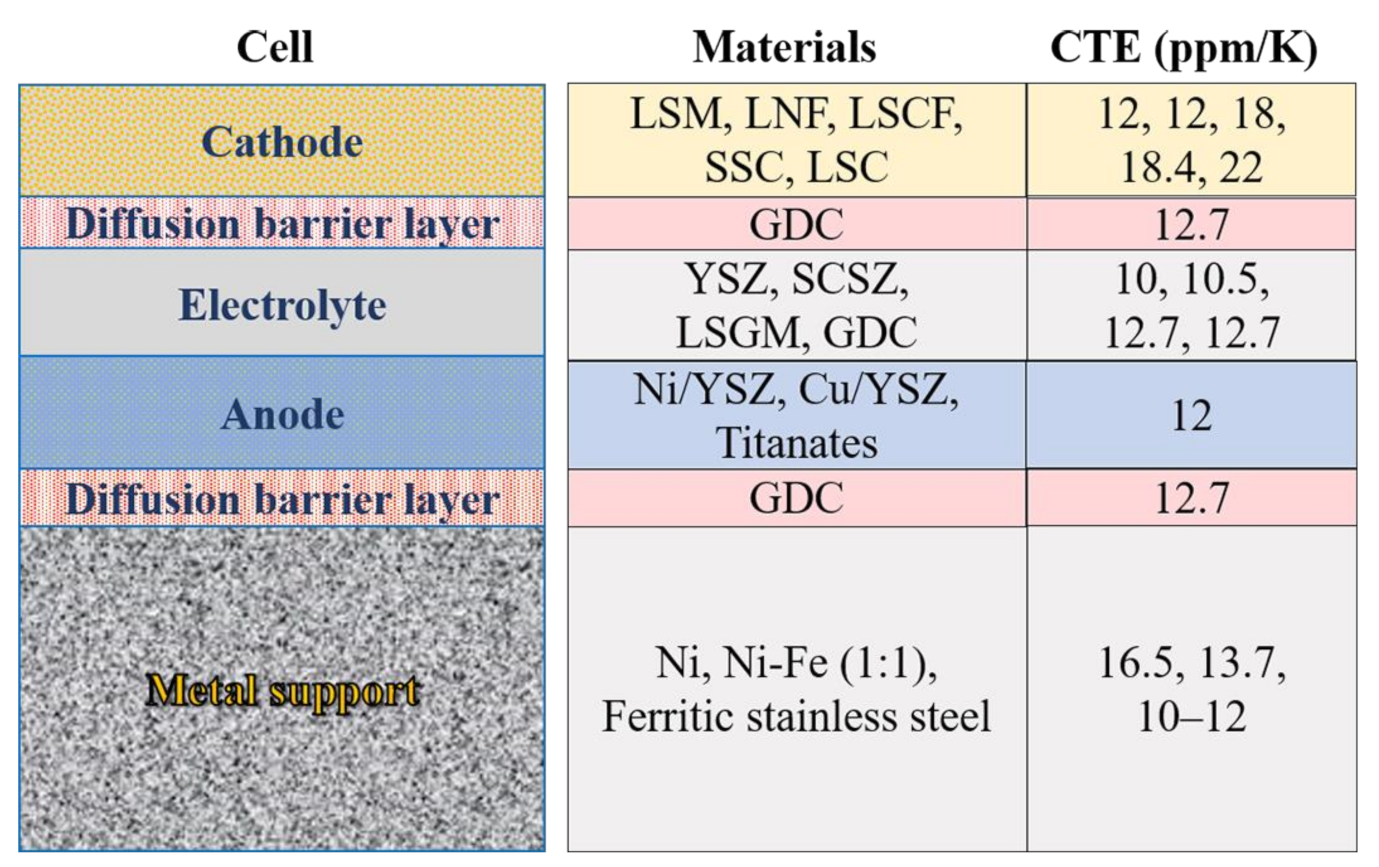

| Metal | CTE (ppm/K) | Relative Oxidation Resistance |
|---|---|---|
| Ni | 16.5 | Poor |
| Ni-Fe (1:1) | 13.7 | Poor |
| 400-series stainless steel | 10–12 | Good |
| Family | Candidate | Representative Composition | Survives Sintering in Reducing Atmosphere? | Survives Re-Oxidation? | React with Metal? | Densifies at 1450 °C or Lower? | Evaporation during Sintering? |
|---|---|---|---|---|---|---|---|
| Pyrochlore | LCZ | La1.95Ca0.05Zr2O7 | No | No | - | - | - |
| LCO | La2Ce2O7 | No | Yes | Yes-Cr, Si | - | - | |
| Perovskite | BCN | Ba3Ca1.18Nb1.82O9 | Yes | Yes | Yes-Cr, Si | Falls apart | Yes |
| BZCY | BaZr0.7Ce0.2Y0.1O3 | Yes | Yes | Yes-Cr, Si | Marginal | Yes | |
| SZCY | SrZr0.5Ce0.4Y0.1O3 | Yes | Yes | Yes-Si | Yes | Yes | |
| Acceptor doped rare-earth Orthoniobate | LCN | La0.99Ca0.01NbO4 | Yes | Yes | No | Yes | No |
| Interconnects | Coating | Testing Conditions | KP (g2 cm−4 s−1) | ASR (mΩ) | Year/Ref. |
|---|---|---|---|---|---|
| SUS430 | Mn-Co/PVD | 800 °C/air/1250 h | 1.22 × 10−14 | 28.6 | 2019/[91] |
| Crofer22APU | MnCo1.7Fe0.3O4/APS | 700 °C/air/1000 h | No data | 50 | 2014/[136] |
| Crofer22APU | MnCo1.7Fe0.3O4/EPD | 800 °C/air/5000 h | 0.34 × 10−14 | No data | 2017/[145] |
| Sanergy HT | (Mn,Co)3O4/Screen priting | 800 °C/air/1500 h | 2 × 10−14 | No data | 2011/[146] |
| AISI 430 | Mn-Co/DGPA | 800 °C/air | 0.25 × 10−14 (750 h) | 29 (408 h) | 2019/[147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Nadimpalli, V.K.; Pedersen, D.B.; Esposito, V. Degradation Mechanisms of Metal-Supported Solid Oxide Cells and Countermeasures: A Review. Materials 2021, 14, 3139. https://doi.org/10.3390/ma14113139
Zhou Z, Nadimpalli VK, Pedersen DB, Esposito V. Degradation Mechanisms of Metal-Supported Solid Oxide Cells and Countermeasures: A Review. Materials. 2021; 14(11):3139. https://doi.org/10.3390/ma14113139
Chicago/Turabian StyleZhou, Zhipeng, Venkata Karthik Nadimpalli, David Bue Pedersen, and Vincenzo Esposito. 2021. "Degradation Mechanisms of Metal-Supported Solid Oxide Cells and Countermeasures: A Review" Materials 14, no. 11: 3139. https://doi.org/10.3390/ma14113139
APA StyleZhou, Z., Nadimpalli, V. K., Pedersen, D. B., & Esposito, V. (2021). Degradation Mechanisms of Metal-Supported Solid Oxide Cells and Countermeasures: A Review. Materials, 14(11), 3139. https://doi.org/10.3390/ma14113139






