Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Asphalt
2.1.2. Snowmelt Salt
2.1.3. Asphalt Sample Preparation
- Step 1:
- Step 2:
- Step 3:
2.2. Experimental Methods
2.2.1. High-Temperature Performance by Softening Point and Viscosity Tests
2.2.2. Low-Temperature Performance by Ductility and Equivalent Brittle Point Tests
2.2.3. Temperature Sensitivity by Penetration Index
2.2.4. Asphalt–Aggregate Adhesion Property Test
2.2.5. Fourier Transform Infrared Spectroscopy Test
3. Results and Discussion
3.1. High-Temperature Performance
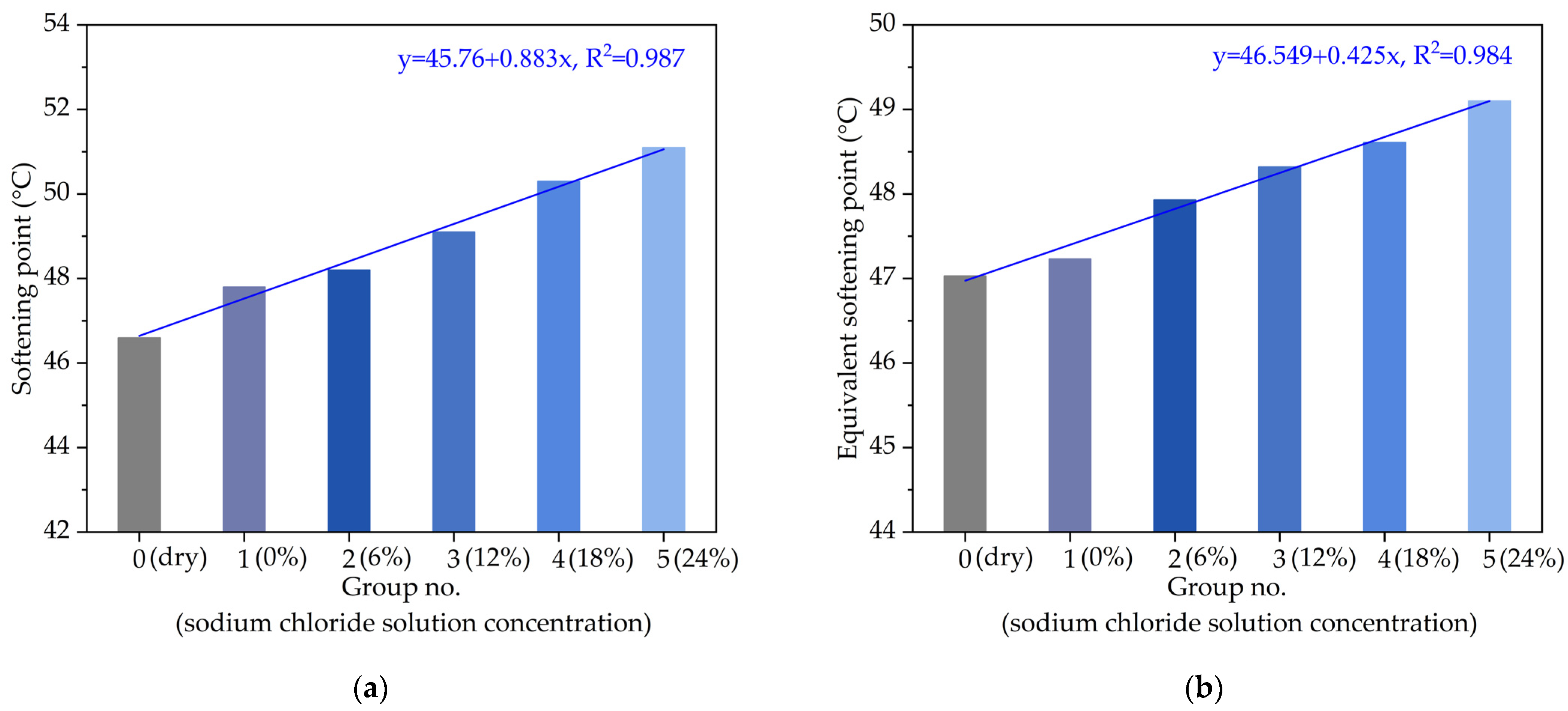
3.1.1. Softening Point
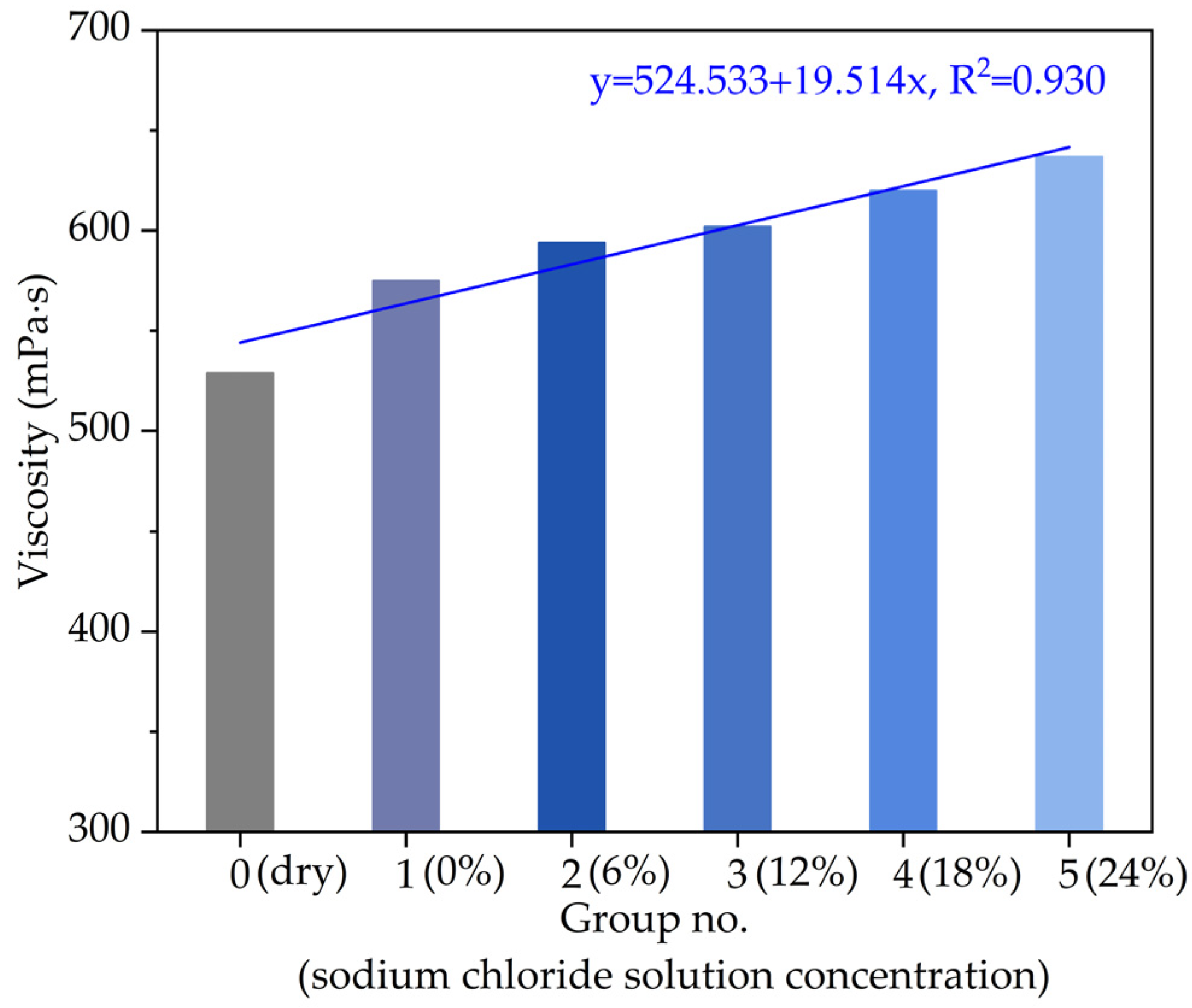
3.1.2. Viscosity
3.2. Low-Temperature Performance
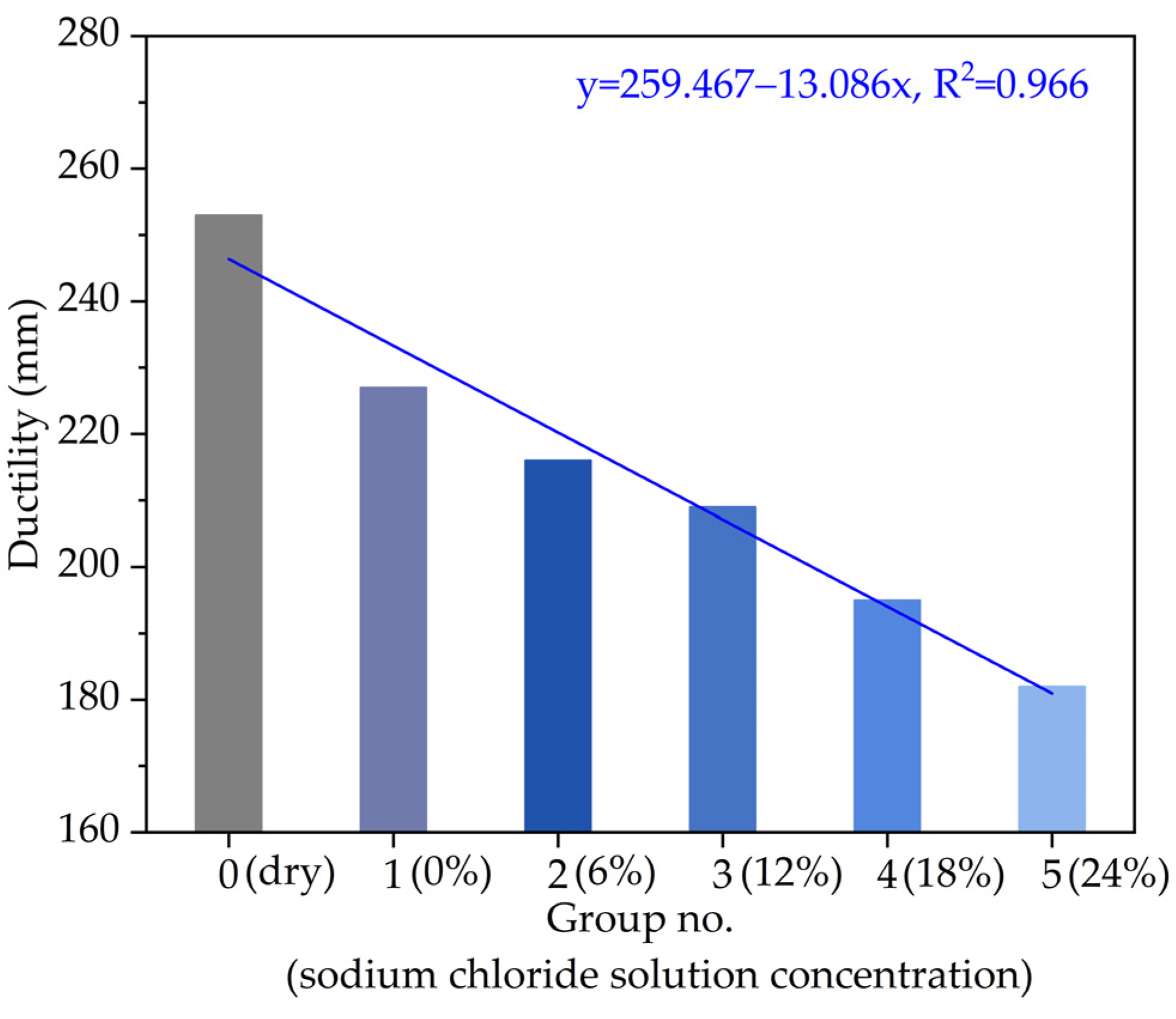
3.2.1. Ductility
3.2.2. Equivalent Brittle Point
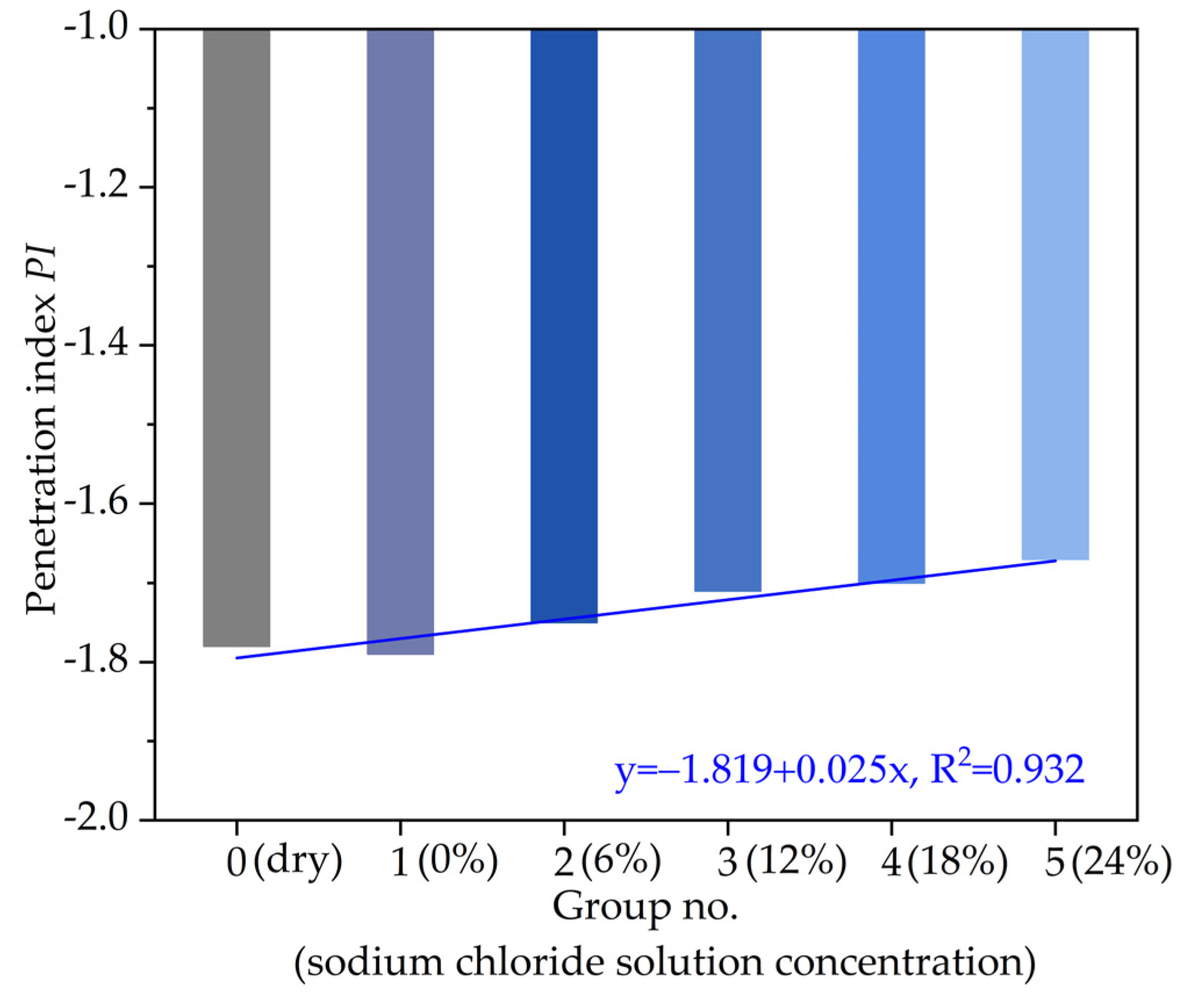
3.3. Temperature Sensitivity
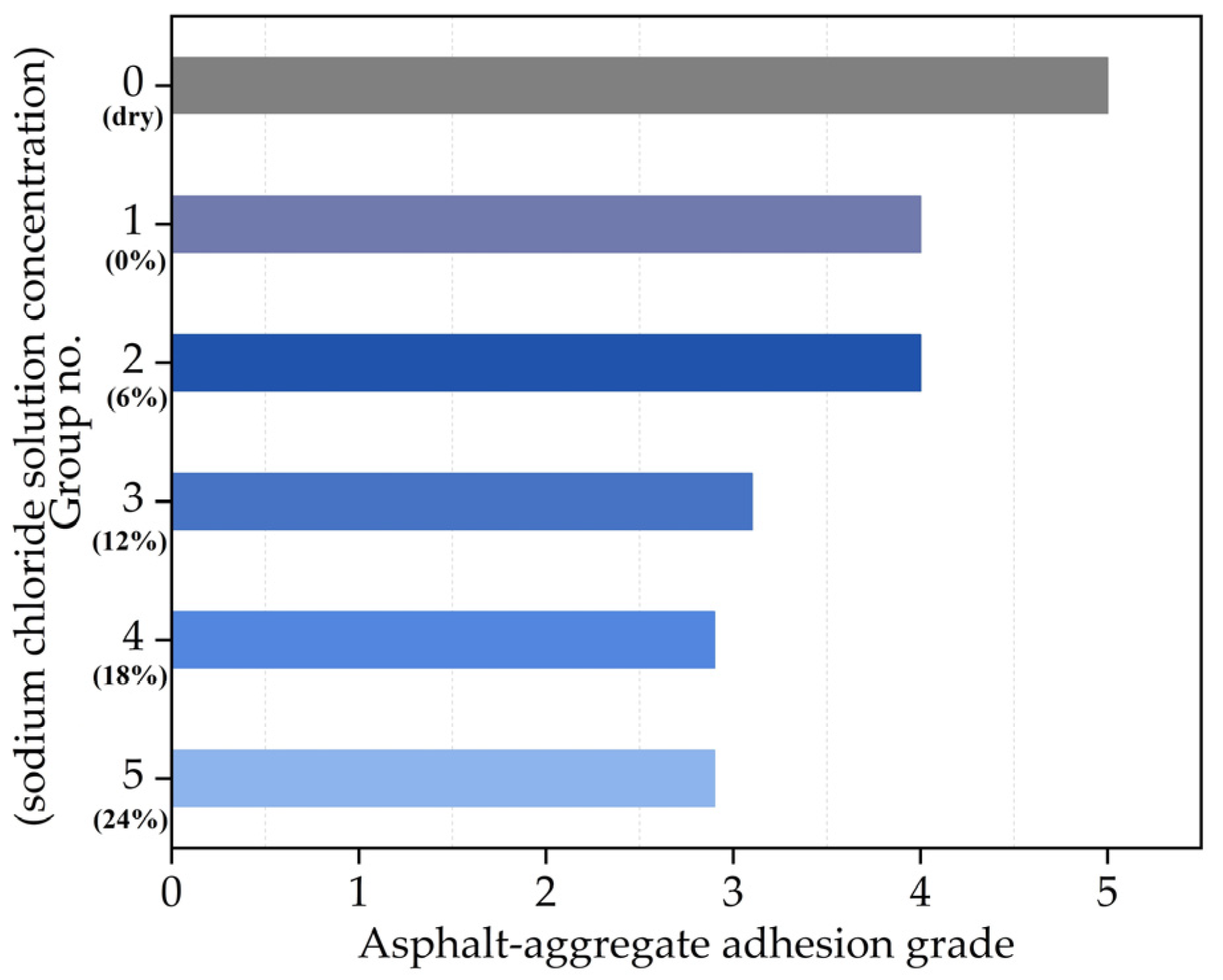
3.4. Asphalt–Aggregate Adhesion Property
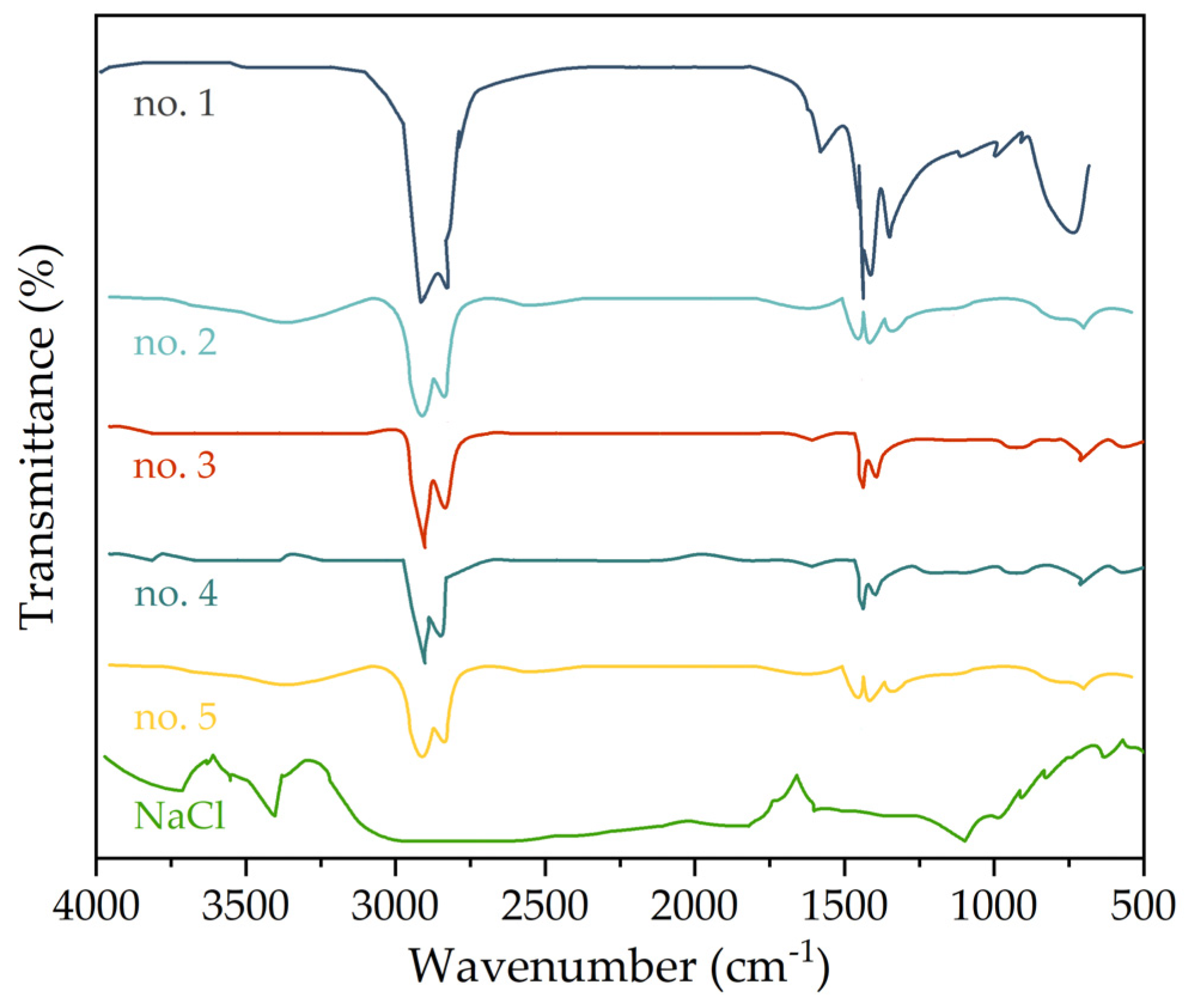
3.5. Fourier Transform Infrared Spectroscopy Test
4. Conclusions
- The linear variation relationships of the softening point, equivalent softening point and viscosity with chloride salt concentrations showed that the high-temperature performance of asphalt would be improved by chloride snowmelt salt.
- According to the ductility and equivalent brittle point, with the increase in the chloride salt solution concentration (i.e., the chloride ions increase), the low-temperature performance of asphalt becomes worse, and the chloride salt solution concentration should be recommended as less than 12~18%.
- The temperature sensitivity of asphalt presents an increasing linear relationship with the increase in the chloride salt solution concentration, and the temperature sensitivity increases by soaking in chloride salt solution concentration.
- After the effect of the chloride salt solution, the asphalt–aggregate adhesion property decreases with the increase in the chloride salt solution concentration. It is necessary to control the amount of chloride snowmelt salt in the actual snow removal projects.
- Based on Fourier transform infrared spectroscopy, the main reason for the performances of asphalt caused by chloride salt might be attributed to the change of asphalt components. With the increase in the chloride salt solution concentration, the proportion of light components (saturated fraction, aromatic fraction) in asphalt decreases, and the proportion of heavy components (resin and asphaltene) with good thermal stability increases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, T.; Geng, L.; Ding, X.H.; Zhang, D.Y.; Huang, X.M. Experimental study of deicing asphalt mixture with anti-icing additives. Constr. Build. Mater. 2016, 127, 653–662. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Ma, T.; Fan, J.W.; Fang, Z.Y.; Chen, T.; Zhou, Y. Experimental study of high modulus asphalt mixture containing reclaimed asphalt pavement. J. Clean. Prod. 2020, 263. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.P.; Cao, Y.B.; Hu, Q.S.; Guo, X.D. Design and evaluation of light-transmitting concrete (ltc) using waste tempered glass: A novel concrete for future photovoltaic road. Constr. Build. Mater. 2021, 280. [Google Scholar] [CrossRef]
- Radhakrishnan, V.; Sri, M.R.; Reddy, K.S. Evaluation of asphalt binder rutting parameters. Constr. Build. Mater. 2018, 173, 298–307. [Google Scholar] [CrossRef]
- Yalghouzaghaj, M.N.; Sarkar, A.; Hamedi, G.H.; Hayati, P. Application of the surface free energy method on the mechanism of low-temperature cracking of asphalt mixtures. Constr. Build. Mater. 2021, 268. [Google Scholar] [CrossRef]
- Wei, H.; Hu, B.; Wang, F.Y.; Zheng, J.L.; Jin, J.; Liu, C.C. Temporal-spatial evolution characteristics of acoustic emission in asphalt concrete cracking process under low temperature. Constr. Build. Mater. 2020, 248. [Google Scholar] [CrossRef]
- Saeed, F.; Rahman, M.; Chamberlain, D.; Collins, P. Asphalt surface damage due to combined action of water and dynamic loading. Constr. Build. Mater. 2019, 196, 530–538. [Google Scholar] [CrossRef]
- Wang, W.S.; Cheng, Y.C.; Ma, G.R.; Tan, G.J.; Sun, X.; Yang, S.T. Further investigation on damage model of eco-friendly basalt fiber modified asphalt mixture under freeze-thaw cycles. Appl. Sci. 2019, 9, 60. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Li, H.; Wang, W.S.; Li, L.D.; Wang, H.T. Laboratory evaluation on the performance degradation of styrene-butadiene-styrene-modified asphalt mixture reinforced with basalt fiber under freeze-thaw cycles. Polymers 2020, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Cheraghian, G.; Wistuba, M.P. Effect of fumed silica nanoparticles on ultraviolet aging resistance of bitumen. Nanomaterials 2021, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.Y.; Li, Z.W.; Tian, D.; Tan, Y.Q. Effect of anti-icing additives on the stability of emulsified asphalt binders. Constr. Build. Mater. 2021, 275. [Google Scholar] [CrossRef]
- Xiong, R.; Chu, C.; Qiao, N.; Wang, L.; Yang, F.; Sheng, Y.P.; Guan, B.W.; Niu, D.Y.; Geng, J.G.; Chen, H.X. Performance evaluation of asphalt mixture exposed to dynamic water and chlorine salt erosion. Constr. Build. Mater. 2019, 201, 121–126. [Google Scholar] [CrossRef]
- Guo, Q.L.; Li, G.Y.; Gao, Y.; Wang, K.Y.; Dong, Z.Z.; Liu, F.C.; Zhu, H. Experimental investigation on bonding property of asphalt-aggregate interface under the actions of salt immersion and freeze-thaw cycles. Constr. Build. Mater. 2019, 206, 590–599. [Google Scholar] [CrossRef]
- Zheng, M.L.; Zhou, J.L.; Wu, S.J.; Yuan, H.T.; Meng, J.D. Evaluation of long-term performance of anti-icing asphalt pavement. Constr. Build. Mater. 2015, 84, 277–283. [Google Scholar] [CrossRef]
- Xiong, R.; Jiang, W.Y.; Yang, F.; Li, K.H.; Guan, B.W.; Zhao, H. Investigation of voids characteristics in an asphalt mixture exposed to salt erosion based on ct images. Materials 2019, 12, 3774. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, T.; Shao, M.Y.; Ai, T.; Zhao, P. Investigation on snow-melting performance of asphalt mixtures incorporating with salt-storage aggregates. Constr. Build. Mater. 2017, 142, 187–198. [Google Scholar] [CrossRef]
- Wu, S.P.; Pan, P.; Chen, M.Y.; Zhang, Y. Analysis of characteristics of electrically conductive asphalt concrete prepared by multiplex conductive materials. J. Mater. Civil Eng. 2013, 25, 871–879. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wu, S.P.; Liu, Q.T.; Hu, J.F.; Yuan, Y.; Ye, Q.S. Snow and ice melting properties of self-healing asphalt mixtures with induction heating and microwave heating. Appl. Therm. Eng. 2018, 129, 871–883. [Google Scholar] [CrossRef]
- Wu, S.J.; Zheng, M.L.; Chen, W.; Bi, S.T.; Wang, C.T.; Li, Y.F. Salt-dissolved regularity of the self-ice-melting pavement under rainfall. Constr. Build. Mater. 2019, 204, 371–383. [Google Scholar] [CrossRef]
- Behbahani, H.; Hamedi, G.H.; Gilani, V.N.M. Predictive model of modified asphalt mixtures with nano hydrated lime to increase resistance to moisture and fatigue damages by the use of deicing agents. Constr. Build. Mater. 2020, 265. [Google Scholar] [CrossRef]
- Amini, B.; Tehrani, S.S. Simultaneous effects of salted water and water flow on asphalt concrete pavement deterioration under freeze-thaw cycles. Int. J. Pavement Eng. 2014, 15, 383–391. [Google Scholar] [CrossRef]
- Feng, D.C.; Yi, J.Y.; Wang, D.S.; Chen, L.L. Impact of salt and freeze-thaw cycles on performance of asphalt mixtures in coastal frozen region of china. Cold Reg. Sci. Technol. 2010, 62, 34–41. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.F.; Xie, W.; Wu, J.J. Evaluation of road performance and adhesive characteristic of asphalt binder in salt erosion environment. Mater. Today Commun. 2020, 25. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Huang, Z.Y. Investigation of the microcharacteristics of asphalt mastics under dry-wet and freeze-thaw cycles in a coastal salt environment. Materials 2019, 12, 2627. [Google Scholar] [CrossRef]
- Technical Specifications for Construction of Highway Asphalt Pavements (JTG F40-2004); Ministry of Transport of the People’s Republic of China: Beijing, China, 2004.
- Salt of Ice and Snow Melting for Road (GB/T 23851-2009); Ministry of Transport of the People’s Republic of China: Beijing, China, 2009.
- Fu, J. Effect of Coated Chlorine-Based Deicing Additive on Ice-Melting and Pavement Properties of Asphalt Concrete. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2015. [Google Scholar]
- Ma, C. Research on Influence of Chloride Deicing Salt’s Concentration on Performances of Asphalt and Asphalt Mixture. Master’s Thesis, Jilin University, Changchun, China, 2017. [Google Scholar]
- Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering (JTG E20-2011); Ministry of Transport of the People’s Republic of China: Beijing, China, 2011.
- Wang, W.S.; Cheng, Y.C.; Tan, G.J.; Liu, Z.Y.; Shi, C.L. Laboratory investigation on high- and low-temperature performances of asphalt mastics modified by waste oil shale ash. J. Mater. Cycles Waste 2018, 20, 1710–1723. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Wang, W.S.; Tan, G.J.; Shi, C.L. Assessing high- and low-temperature properties of asphalt pavements incorporating waste oil shale as an alternative material in jilin province, china. Sustainability 2018, 10, 2179. [Google Scholar] [CrossRef]
- Cala, A.; Caro, S.; Lleras, M.; Rojas-Agramonte, Y. Impact of the chemical composition of aggregates on the adhesion quality and durability of asphalt-aggregate systems. Constr. Build. Mater. 2019, 216, 661–672. [Google Scholar] [CrossRef]
- Guo, F.C.; Pei, J.Z.; Zhang, J.P.; Xue, B.; Sun, G.Q.; Li, R. Study on the adhesion property between asphalt binder and aggregate: A state-of-the-art review. Constr. Build. Mater. 2020, 256. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, Z.Y.; Tan, L.J.; Xu, Y.; Wang, C.H.; Liu, P.F.; Yu, H.Y.; Oeser, M. Laboratory evaluation of emulsified asphalt reinforced with glass fiber treated with different methods. J. Clean. Prod. 2020, 274. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.L.; You, Z.P. Fourier transform infrared spectroscopy characterization of aging-related properties of original and nano-modified asphalt binders. Constr. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- General Rules for Infrared Analysis (GB/T 6040-2002); General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2002.



| Test Items | Units | Index | Standards | Requirements |
|---|---|---|---|---|
| Penetration @ 25 °C | 0.1 mm | 76 | T0604 | 60~80 |
| Penetration index (PI) | - | −1.78 | T0604 | −1.8~+1.0 |
| Ductility @ 10 °C | cm | 25.3 | T0605 | ≥20 |
| Ductility @ 15 °C | cm | >100 | T0605 | ≥100 |
| Softening point (R and B) | °C | 46.6 | T0606 | ≥43 |
| Brookfield viscosity @ 135 °C | Pa∙s | 0.529 | T0625 | - |
| Brookfield viscosity @ 60 °C | Pa∙s | 0.794 | T0625 | - |
| Group No. | 0 (Dry) | 1 (0%) | 2 (6%) | 3 (12%) | 4 (18%) | 5 (24%) |
|---|---|---|---|---|---|---|
| K | 0.05302 | 0.05318 | 0.05268 | 0.05256 | 0.05238 | 0.05211 |
| AlgP | 0.41102 | 0.39052 | 0.37755 | 0.37223 | 0.36230 | 0.34891 |
| R2 | 0.997 | 0.999 | 0.999 | 0.998 | 0.999 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Wang, W.; Zhu, L.; Wang, H.; Ai, Y. Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion. Materials 2021, 14, 3089. https://doi.org/10.3390/ma14113089
Zhou P, Wang W, Zhu L, Wang H, Ai Y. Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion. Materials. 2021; 14(11):3089. https://doi.org/10.3390/ma14113089
Chicago/Turabian StyleZhou, Peilei, Wensheng Wang, Lili Zhu, Haoyun Wang, and Yongming Ai. 2021. "Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion" Materials 14, no. 11: 3089. https://doi.org/10.3390/ma14113089
APA StyleZhou, P., Wang, W., Zhu, L., Wang, H., & Ai, Y. (2021). Study on Performance Damage and Mechanism Analysis of Asphalt under Action of Chloride Salt Erosion. Materials, 14(11), 3089. https://doi.org/10.3390/ma14113089







