A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Setting Time Measurements
2.2.2. Rheology Measurements
- (i)
- Rotational measurements are used to determine yield stress. Shear stress was applied to the sample by presetting the shear rate in a logarithmic ramp. Logarithmic shear rate increasing and decreasing ramps were applied to the sample, from 10−4 s−1 to 300 s−1, with a constant measuring time per point of 5 s. Static yield stress (τS) is evaluated as the stress measured at the lowest shear rate in the increasing shear rate ramp, and dynamic yield stress (τD) is evaluated as the lowest stress measured during the decreasing shear rate ramp. The difference between the two types of yield stress is that τS reflects the connected structure at rest while τD measures interactions across the broken interparticle links [30,35]. To quantify the difference between τS and τD, a thixotropy index (T.I.) can be calculated as T.I. = (τS − τD)/τD to evaluate the degree of build-up/breakdown of the structure at different resting times. The thixotropy index (T.I.) is the difference between τS, which measures the cohesion of the system at rest, and τD, which measures the cohesion when the system’s structure is broken down by the applied stress. This microstructural change is, however, a complex phenomenon, whose extent depends on the elapsed time at rest. Therefore, T.I. indicates the degree of the structural build-up after a given aging time.
- (ii)
- Amplitude sweep oscillation measurements are used to determine viscoelastic properties. Constant frequency oscillation with a logarithmically increasing amplitude strain (γ) is applied to the sample. This measurement provides the determination of the extension of the linear viscoelastic region (LVE), where the stress/strain relationship is linear, and the measurement of the storage (G′) and loss (G′′) moduli within this region.The preshear, recovery, and measurement protocols are reported in detail in Table 3. The measurements were performed immediately after the recovery step (0 min) and after 30 thirty minutes of aging. One measurement was performed for each formulation only after verifying the reproducibility of the measurements with preliminary tests (the variation was less than 1%). Following the indication obtained from the setting time measurements, τS, τD, and G′ were measured at the sample ages of 0, 30, and 60 min, as reported in Table 3. The resting time of “60 min” refers to the second 30 min step of aging after shear at the first step of “30 min” resting time. During the resting times, the sample builds up the structure that is destroyed during the increasing shear ramps and the large amplitude oscillation of the oscillatory amplitude sweeps. Therefore, the recovery response is quantified by the increase in G′.
2.2.3. Plastic Shrinkage
3. Results and Discussions
3.1. Setting Time
3.2. Rheological Properties
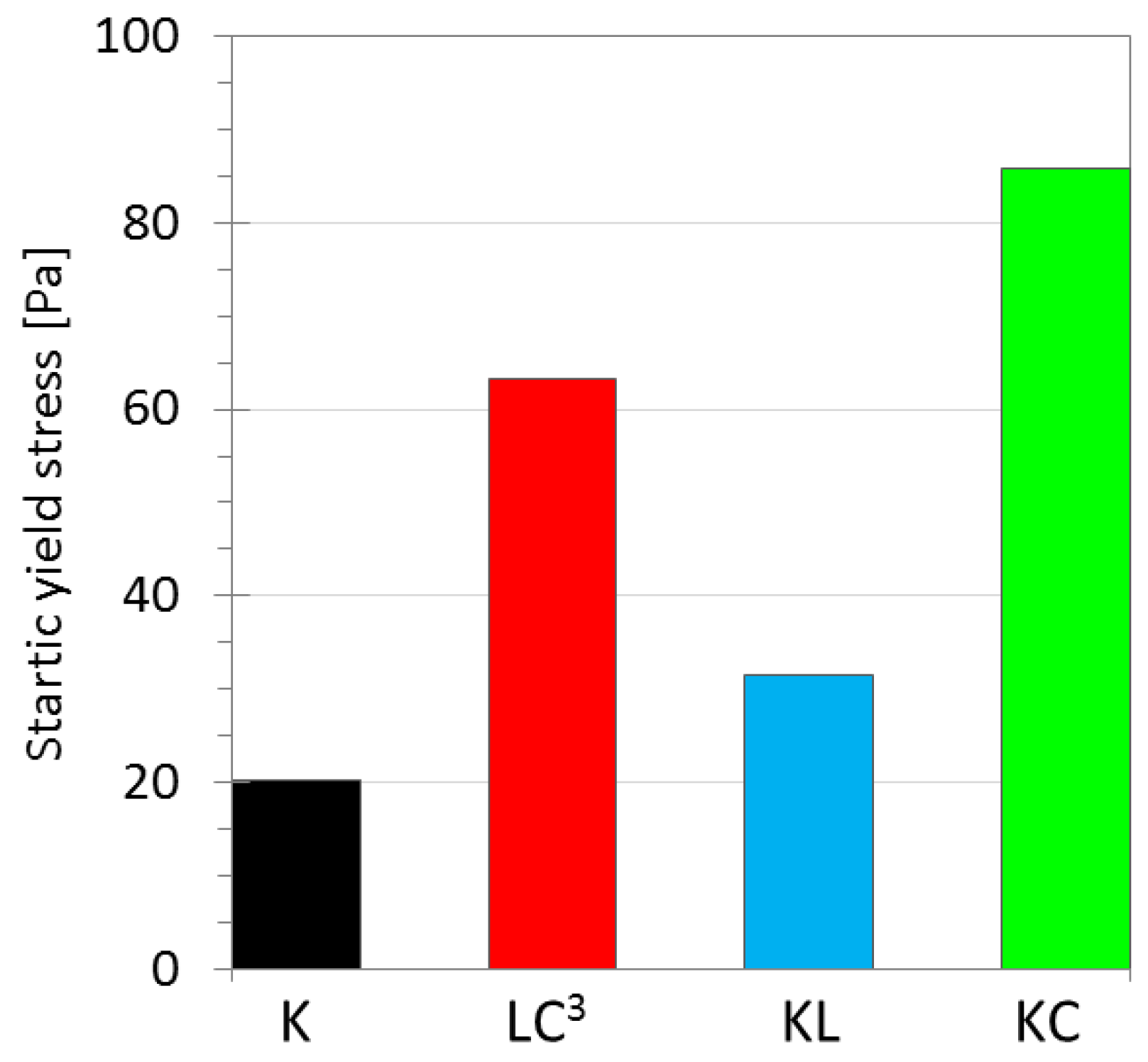
3.2.1. Yield Stress
3.2.2. Viscoelastic Properties
3.3. Plastic Shrinkage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liberto, T. Physico-Chemical Study of Calcite Colloidal Suspensions: From Macroscopic Rheology to Microscopic Interaction. Ph.D. Thesis, Université Claude Bernard Lyon 1, Villeurbanne, France, 19 September 2018. [Google Scholar]
- Bouregba, A.; Ez-zaki, H.; Diouri, A.; Sassi, O. Dicalcium silicate hydration behavior in the presence of Na2CO3 and water glass. Asian J. Civ. Eng. 2019, 20, 857–867. [Google Scholar] [CrossRef]
- Ashby, M.F. Materials and the Environment: Eco-Informed Material Choice, 1st ed.; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 2009; ISBN 978-1-85617-608-8. [Google Scholar]
- Yang, K.H.; Jung, Y.B.; Cho, M.S.; Tae, S.H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
- Miller, S.A. Supplementary cementitious materials to mitigate greenhouse gas emissions from concrete: Can there be too much of a good thing? J. Clean. Prod. 2018, 178, 587–598. [Google Scholar] [CrossRef]
- Bishnoi, S. Calcined Clays for Sustainable Concrete. In Proceedings of the 3rd International Conference on Calcined Clays for Sustainable Concrete, New Delhi, India, 15–17 October 2019; RILEM Bookseries, Ed.; Springer Nature: Singapore, 2020. ISBN 978-981-15-2806-4. [Google Scholar]
- Marangu, J.M. Physico-chemical properties of Kenyan made calcined Clay -Limestone cement (LC3). Case Stud. Constr. Mater. 2020, 12, e00333. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Cancio Díaz, Y.; Sánchez Berriel, S.; Heierli, U.; Favier, A.R.; Sánchez Machado, I.R.; Scrivener, K.L.; Martirena Hernández, J.F.; Habert, G. Limestone calcined clay cement as a low-carbon solution to meet expanding cement demand in emerging economies. Dev. Eng. 2017, 2, 82–91. [Google Scholar] [CrossRef]
- Scrivener, K.; Dekeukelaere, A.; Avet, F.; Grimmeissen, L. Financial Attractiveness of LC3; École Polytechnique Fédérale de Lausanne: Lausanne, Switzerland, 2019. [Google Scholar]
- Nickovic, S.; Vukovic, A.; Vujadinovic, M.; Djurdjevic, V.; Pejanovic, G. Technical Note: High-resolution mineralogical database of dust-productive soils for atmospheric dust modeling. Atmos. Chem. Phys. 2012, 12, 845–855. [Google Scholar] [CrossRef]
- Joseph, S.; Bishnoi, S.; Maity, S. An economic analysis of the production of limestone calcined clay cement in India. Indian Concr. J. 2016, 90, 22–27. [Google Scholar]
- Maraghechi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with various kaolinite contents with respect to chloride transport. Mater. Struct. Constr. 2018, 51, 1–17. [Google Scholar] [CrossRef]
- Beigh, M.A.B.; Nerella, V.N.; Schröfl, C.; Mechtcherine, V. Studying the Rheological Behavior of Limestone Calcined Clay Cement (LC3) Mixtures in the Context of Extrusion-Based 3D-Printing; Springer Nature: Singapore, 2020; pp. 229–236. [Google Scholar] [CrossRef]
- Nair, N.; Mohammed Haneefa, K.; Santhanam, M.; Gettu, R. A study on fresh properties of limestone calcined clay blended cementitious systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Figueiredo, S.C.; Çopuroğlu, O.; Veer, F.; Schlangen, E. Limestone and Calcined Clay-Based Sustainable Cementitious Materials for 3D Concrete Printing: A Fundamental Study of Extrudability and Early-Age Strength Development. Appl. Sci. 2019, 9, 1809. [Google Scholar] [CrossRef]
- Scrivener, K.; Favier, A. Calcined Clays for Sustainable Concrete. In Proceedings of the 1st International Conference on Calcined Clays for Sustainable Concrete, Lausanne, Switzerland, 23–25 June 2015; Springer: Dordrecht, The Netherlands, 2015. ISBN 9789401799386. [Google Scholar]
- Cassagnabère, F.; Diederich, P.; Mouret, M.; Escadeillas, G.; Lachemi, M. Impact of metakaolin characteristics on the rheological properties of mortar in the fresh state. Cem. Concr. Compos. 2013, 37, 95–107. [Google Scholar] [CrossRef]
- Madejová, J.; Barlog, M.; Jankovič, Ľ.; Slaný, M.; Pálková, H. Comparative study of alkylammonium- and alkylphosphonium-based analogues of organo-montmorillonites. Appl. Clay Sci. 2021, 200. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Mahaut, F.; Mokéddem, S.; Chateau, X.; Roussel, N.; Ovarlez, G. Effect of coarse particle volume fraction on the yield stress and thixotropy of cementitious materials. Cem. Concr. Res. 2008, 38, 1276–1285. [Google Scholar] [CrossRef]
- Koehler, E.P. Thixotropy of SCC and Its Effects on Formwork Pressure. Web Session: International Concrete Abstracts Portal. April 2013. Available online: https://www.concrete.org/publications/internationalconcreteabstractsportal/m/details/id/51685952 (accessed on 15 April 2021).
- Landrou, G.; Brumaud, C.; Winnefeld, F.; Flatt, R.J.; Habert, G. Lime as an anti-plasticizer for self-compacting clay concrete. Materials 2016, 9, 330. [Google Scholar] [CrossRef]
- Moller, P.; Fall, A.; Chikkadi, V.; Derks, D.; Bonn, D. An attempt to categorize yield stress fluid behaviour. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 5139–5155. [Google Scholar] [CrossRef]
- Coussot, P.; Ragouilliaux, A.; Ovarlez, G.; Herzhaft, B. Transition from a simple yield stress fluid to a thixotropic material. AIP Conf. Proc. 2008, 1027, 713–715. [Google Scholar] [CrossRef]
- Coussot, P.; Roussel, N.; Jarny, S.; Chanson, H. Continuous or catastrophic solid-liquid transition in jammed systems. Phys. Fluids 2005, 17. [Google Scholar] [CrossRef]
- Bekkour, K.; Leyama, M.; Benchabane, A.; Scrivener, O. Time-dependent rheological behavior of bentonite suspensions: An experimental study. J. Rheol. 2005, 49, 1329–1345. [Google Scholar] [CrossRef]
- Pignon, F.; Magnin, A.; Piau, J.-M. Thixotropic behavior of clay dispersions: Combinations of scattering and rheometric techniques. J. Rheol. 1998, 42, 1349–1373. [Google Scholar] [CrossRef]
- Hu, L.B.; Péron, H.; Hueckel, T.; Laloui, L. Mechanisms and critical properties in drying shrinkage of soils: Experimental and numerical parametric studies. Can. Geotech. J. 2013, 50, 536–549. [Google Scholar] [CrossRef]
- Muzenda, T.R.; Hou, P.; Kawashima, S.; Sui, T.; Cheng, X. The role of limestone and calcined clay on the rheological properties of LC3. Cem. Concr. Compos. 2020, 107, 103516. [Google Scholar] [CrossRef]
- Hou, P.; Muzenda, T.R.; Li, Q.; Chen, H.; Kawashima, S.; Sui, T.; Yong, H.; Xie, N.; Cheng, X. Mechanisms dominating thixotropy in limestone calcined clay cement (LC3). Cem. Concr. Res. 2021, 140, 106316. [Google Scholar] [CrossRef]
- Hill, R.J.; Howard, C.J. Quantitative phase analysis from neutron powder diffraction data using the Rietveld method. J. Appl. Crystallogr. 1987, 20, 467–474. [Google Scholar] [CrossRef]
- EAS 148-3. East African Standard: EAS 148-3 Determination of Setting Time and Soundness; East African Community: Arusha, Tanzania, 2000. [Google Scholar]
- Sleiman, H.; Perrot, A.; Amziane, S. A new look at the measurement of cementitious paste setting by Vicat test. Cem. Concr. Res. 2010, 40, 681–686. [Google Scholar] [CrossRef]
- Kolawole, J.T.; Combrinck, R.; Boshoff, W.P. Measuring the thixotropy of conventional concrete: The influence of viscosity modifying agent, superplasticiser and water. Constr. Build. Mater. 2019, 225, 853–867. [Google Scholar] [CrossRef]
- Ghourchian, S.; Wyrzykowski, M.; Lura, P. A poromechanics model for plastic shrinkage of fresh cementitious materials. Cem. Concr. Res. 2018, 109, 120–132. [Google Scholar] [CrossRef]
- Valentini, L.; Mascarin, L. Assessing the dimensional stability of alkali-activated calcined clays in the fresh state: A time-lapse X-ray imaging approach. Mater. Struct. 2021, 54. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211. [Google Scholar] [CrossRef]
- Courard, L.; Darimont, A.; Schouterden, M.; Ferauche, F.; Willem, X.; Degeimbre, R. Durability of mortars modified with metakaolin. Cem. Concr. Res. 2003, 33, 1473–1479. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.M.; Jones, A. Relative strength, pozzolanic activity and cement hydration in superplasticised metakaolin concrete. Cem. Concr. Res. 1996, 26, 1537–1544. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Haha, M.B.; Zajac, M.; Ludwig, H.M. Early hydration of SCM-blended Portland cements: A pore solution and isothermal calorimetry study. Cem. Concr. Res. 2017, 93, 71–82. [Google Scholar] [CrossRef]
- Kevin, D.; Kenneth, E. A review of limestone additions to Portland cement and concrete. Cem. Concr. Compos. 1991, 13, 165–170. [Google Scholar]
- Feldman, R.F.; Ramachandran, V.S.; Sereda, P.J. Influence of CaCO3 on the Hydration of 3CaO·Al2O3. J. Am. Ceram. Soc. 1965, 48, 25–30. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Hydration of C 3A-gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Skalny, J.; Tadros, M.E. Retardation of Tricalcium Aluminate Hydration by Sulfates. J. Am. Ceram. Soc. 1977, 60, 174–175. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Roussel, N.; Bessaies-Bey, H.; Kawashima, S.; Marchon, D.; Vasilic, K.; Wolfs, R. Recent advances on yield stress and elasticity of fresh cement-based materials. Cem. Concr. Res. 2019, 124. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, D.; Khayat, K.H.; Feys, D.; Shi, C. On the measurement of evolution of structural build-up of cement paste with time by static yield stress test vs. small amplitude oscillatory shear test. Cem. Concr. Res. 2017, 99, 183–189. [Google Scholar] [CrossRef]
- Ma, S.; Qian, Y.; Kawashima, S. Experimental and modeling study on the non-linear structural build-up of fresh cement pastes incorporating viscosity modifying admixtures. Cem. Concr. Res. 2018, 108, 1–9. [Google Scholar] [CrossRef]
- Bellotto, M. Cement paste prior to setting: A rheological approach. Cem. Concr. Res. 2013, 52, 161–168. [Google Scholar] [CrossRef]
- Bentz, D.P.; Ferraris, C.F.; Jones, S.Z.; Lootens, D.; Zunino, F. Limestone and silica powder replacements for cement: Early-age performance. Cem. Concr. Compos. 2017, 78, 43–56. [Google Scholar] [CrossRef]
- Vance, K.; Kumar, A.; Sant, G.; Neithalath, N. The rheological properties of ternary binders containing Portland cement, limestone, and metakaolin or fly ash. Cem. Concr. Res. 2013, 52, 196–207. [Google Scholar] [CrossRef]
- Sant, G.; Ferraris, C.F.; Weiss, J. Rheological properties of cement pastes: A discussion of structure formation and mechanical property development. Cem. Concr. Res. 2008, 38, 1286–1296. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Khayat, K.; Secrieru, E. Rheology and Processing of Construction Materials; Springer: Cham, Switzerland, 2020; Volume 23, ISBN 978-3-030-22565-0. [Google Scholar]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Academic Press: London, UK, 1990; ISBN 012683900X. [Google Scholar]
- Nachbaur, L.; Mutin, J.C.; Nonat, A.; Choplin, L. Dynamic mode rheology of cement and tricalcium silicate pastes from mixing to setting. Cem. Concr. Res. 2001, 31, 183–192. [Google Scholar] [CrossRef]
- Lootens, D.; Hébraud, P.; Lécolier, E.; Van Damme, H. Gelation, shear-thinning and shear-thickening in cement slurries. Oil Gas Sci. Technol. 2004, 59, 31–40. [Google Scholar] [CrossRef]
- Aikaterini, I. Precipitation, Gelation and Mechanical Properties of Calcium—Silicate-Hydrate Gels; ETH Zurich: Zürich, Switzerland, 2014. [Google Scholar]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Tregger, N.A.; Pakula, M.E.; Shah, S.P. Influence of clays on the rheology of cement pastes. Cem. Concr. Res. 2010, 40, 384–391. [Google Scholar] [CrossRef]
- Kawashima, S.; Kim, J.H.; Corr, D.J.; Shah, S.P. Study of the mechanisms underlying the fresh-state response of cementitious materials modified with nanoclays. Constr. Build. Mater. 2012, 36, 749–757. [Google Scholar] [CrossRef]
- Mehdipour, I.; Kumar, A.; Khayat, K.H. Rheology, hydration, and strength evolution of interground limestone cement containing PCE dispersant and high volume supplementary cementitious materials. Mater. Des. 2017, 127, 54–66. [Google Scholar] [CrossRef]
- Liberto, T.; Le Merrer, M.; Barentin, C.; Bellotto, M.; Colombani, J. Elasticity and yielding of a calcite paste: Scaling laws in a dense colloidal suspension. Soft Matter 2017, 13, 2014–2023. [Google Scholar] [CrossRef]
- Ettehadi, A.; Tezcan, M.; Altun, G. Rheological behavior of water-clay suspensions under large amplitude oscillatory shear. Rheol. Acta 2020. [Google Scholar] [CrossRef]
- Conte, T.; Chaouche, M. Rheological behavior of cement pastes under Large Amplitude Oscillatory Shear. Cem. Concr. Res. 2016, 89, 332–344. [Google Scholar] [CrossRef]
- Lura, P.; Pease, B.; Mazzotta, G.B.; Rajabipour, F.; Weiss, J. Influence of shrinkage-reducing admixtures on development of plastic shrinkage cracks. ACI Mater. J. 2007, 104, 187–194. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of pore structure evolution in the limestone calcined clay cementitious system and its implications for performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Hu, L.B.; Hueckel, T.; Peron, H.; Laloui, L. Desiccation shrinkage of unconstrained soil in the saturated phase. In Proceedings of the 1st European Conference on Unsaturated Soils, Durham, UK, 2–4 July 2008; pp. 653–659. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. A Review and Comparative Study of Existing Shrinkage Prediction Models for Portland and Non-Portland Cementitious Materials. Adv. Mater. Sci. Eng. 2016, 2016, 10–14. [Google Scholar] [CrossRef]
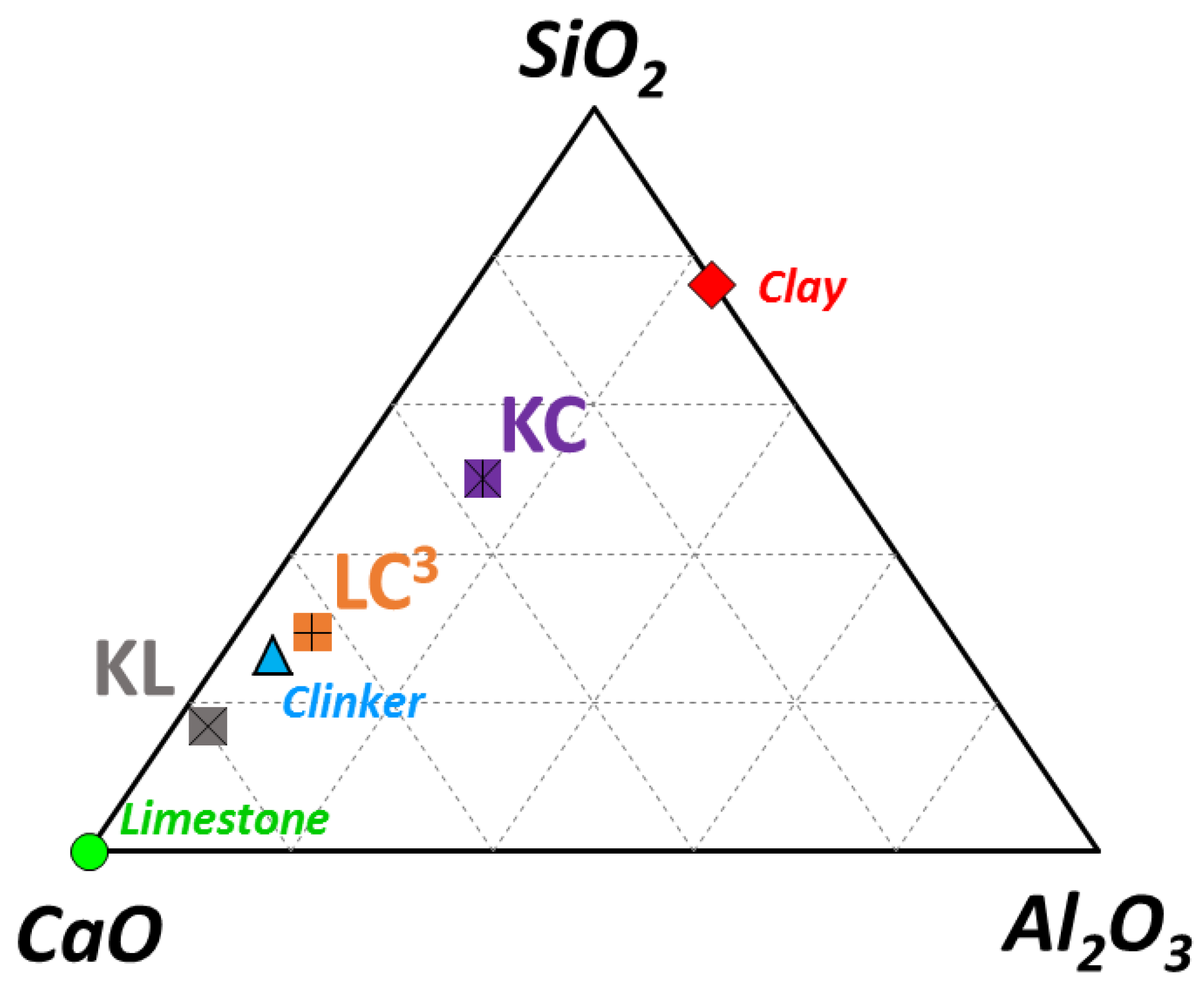






| Material | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay | 69.07 | 0.52 | 21.43 | 1.85 | 0.11 | 0.13 | 0.20 | 0.15 | 0.44 | 0.07 | 6.00 |
| Clinker | 23.67 | 0.35 | 4.69 | 4.06 | 0.10 | 2.07 | 61.43 | 0.69 | 1.53 | 0.00 | 1.41 |
| Limestone | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.60 | 55.90 | 0.00 | 0.01 | 0.00 | 43.4 |
| Binder | Clinker | Limestone | Calcined Clay | Gypsum |
|---|---|---|---|---|
| K | 95 | - | - | 5 |
| LC3 | 50 | 30 | 15 | 5 |
| KL | 50 | 45 | - | 5 |
| KC | 50 | - | 45 | 5 |
| Protocol Test | Rheological Properties | Action | Resting Time |
|---|---|---|---|
| Rotational regime | Static yield stress, Dynamic yield stress | Pre-shearing: Shear rate: 100 s−1, (30 s) Recovery: Strain: 0.0001%, Frequency: 1 Hz, (120 s) Shear rate (logarithmic ramps): Ramp up: 0.0001–300 s−1, (60 s) Ramp down: 300–0.0001 s−1, (60 s) | 0 min (after recovery), 30 min, 60 min |
| Oscillation regime | LVE region, Elastic modulus | Pre-shearing: Strain: 10%, Frequency: 1.5 Hz, (30 s) Recovery: Strain: 0.0001%, Frequency: 1 Hz, (120 s) Shear strain: Logarithmic ramp: 0.0001–100%, 1 Hz, (time set by device) | 0 min (after recovery), 30 min, 60 min |
| Binders | Initial Setting Time | Final Setting Time |
|---|---|---|
| K | 90 | 174 |
| LC3 | 114 | 234 |
| KL | 109 | 224 |
| KC | 130 | 280 |
| Resting Time | K | LC3 | KL | KC |
|---|---|---|---|---|
| 0 min | 0.97 | 0.49 | 1.02 | 0.27 |
| 30 min | 4.70 | 4.91 | 3.16 | 7.43 |
| 60 min | 3.06 | 6.47 | 3.04 | 11.10 |
| Binders | Rheological Properties | 0 min | Resting Time 30 min | 60 min |
|---|---|---|---|---|
| K | τcr (Pa) | 0.17 | 7.91 | 54.83 |
| γcr (%) | 3.10 × 10−3 | 3.11 × 10−3 | 5.63 × 10−3 | |
| G′ (Pa) | 5.27 × 103 | 2.52 × 105 | 9.50 × 105 | |
| γco (Pa) | 3.64 × 10−2 | 3.20 | 10.20 | |
| LC3 | τcr (Pa) | 0.12 | 11.83 | 32.61 |
| γcr (%) | 3.30 × 10−3 | 5.60 × 10−3 | 9.82 × 10−3 | |
| G′ (Pa) | 5.94 × 103 | 2.08 × 105 | 9.87 × 105 | |
| γco (Pa) | 2.14 × 10−2 | 5.78 | 10.50 | |
| KL | τcr (Pa) | 0.39 | 9.41 | 16.96 |
| γcr (%) | 5.60 × 10−3 | 5.71 × 10−3 | 9.98 × 10−3 | |
| G′ (Pa) | 6.89 × 103 | 1.63 × 105 | 5.62 × 105 | |
| γco (Pa) | 3.95 × 10−2 | 3.20 | 7.50 | |
| KC | τcr (Pa) | 0.24 | 35.74 | 42.15 |
| γcr (%) | 3.00 × 10−3 | 9.89 × 10−3 | 1.00 × 10−2 | |
| G′ (Pa) | 7.85 × 103 | 3.55 × 105 | 1.27 × 106 | |
| γco (%) | 2.21 × 10−2 | 10.30 | 18.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ez-zaki, H.; Marangu, J.M.; Bellotto, M.; Dalconi, M.C.; Artioli, G.; Valentini, L. A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes. Materials 2021, 14, 3037. https://doi.org/10.3390/ma14113037
Ez-zaki H, Marangu JM, Bellotto M, Dalconi MC, Artioli G, Valentini L. A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes. Materials. 2021; 14(11):3037. https://doi.org/10.3390/ma14113037
Chicago/Turabian StyleEz-zaki, Hassan, Joseph Mwiti Marangu, Maurizio Bellotto, Maria Chiara Dalconi, Gilberto Artioli, and Luca Valentini. 2021. "A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes" Materials 14, no. 11: 3037. https://doi.org/10.3390/ma14113037
APA StyleEz-zaki, H., Marangu, J. M., Bellotto, M., Dalconi, M. C., Artioli, G., & Valentini, L. (2021). A Fresh View on Limestone Calcined Clay Cement (LC3) Pastes. Materials, 14(11), 3037. https://doi.org/10.3390/ma14113037







