Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CaCO3 Polymorphs
2.2. In-Situ XRD Analysis during Setting and Hardening
2.3. Rheological Characterization
2.3.1. Sample Preparation
2.3.2. Measurements Protocols
Viscoelastic Measurements during Setting Reaction: Aging Experiment
Viscoelastic Properties and Yield Stress of Initial Pastes
3. Results and Discussion
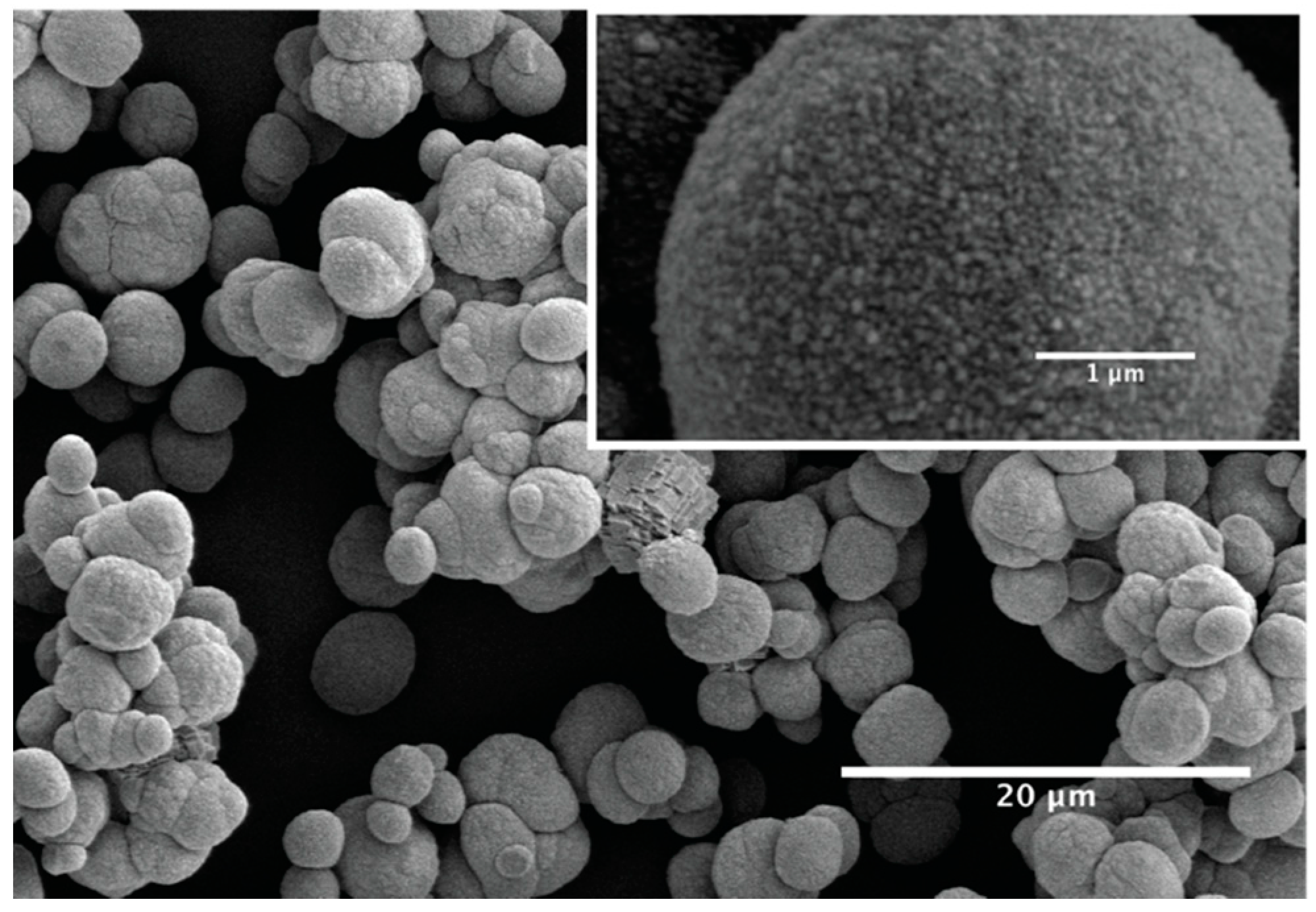
3.1. Characterization of CaCO3 Precipitates
3.2. Phase Transformation during Setting and Hardening
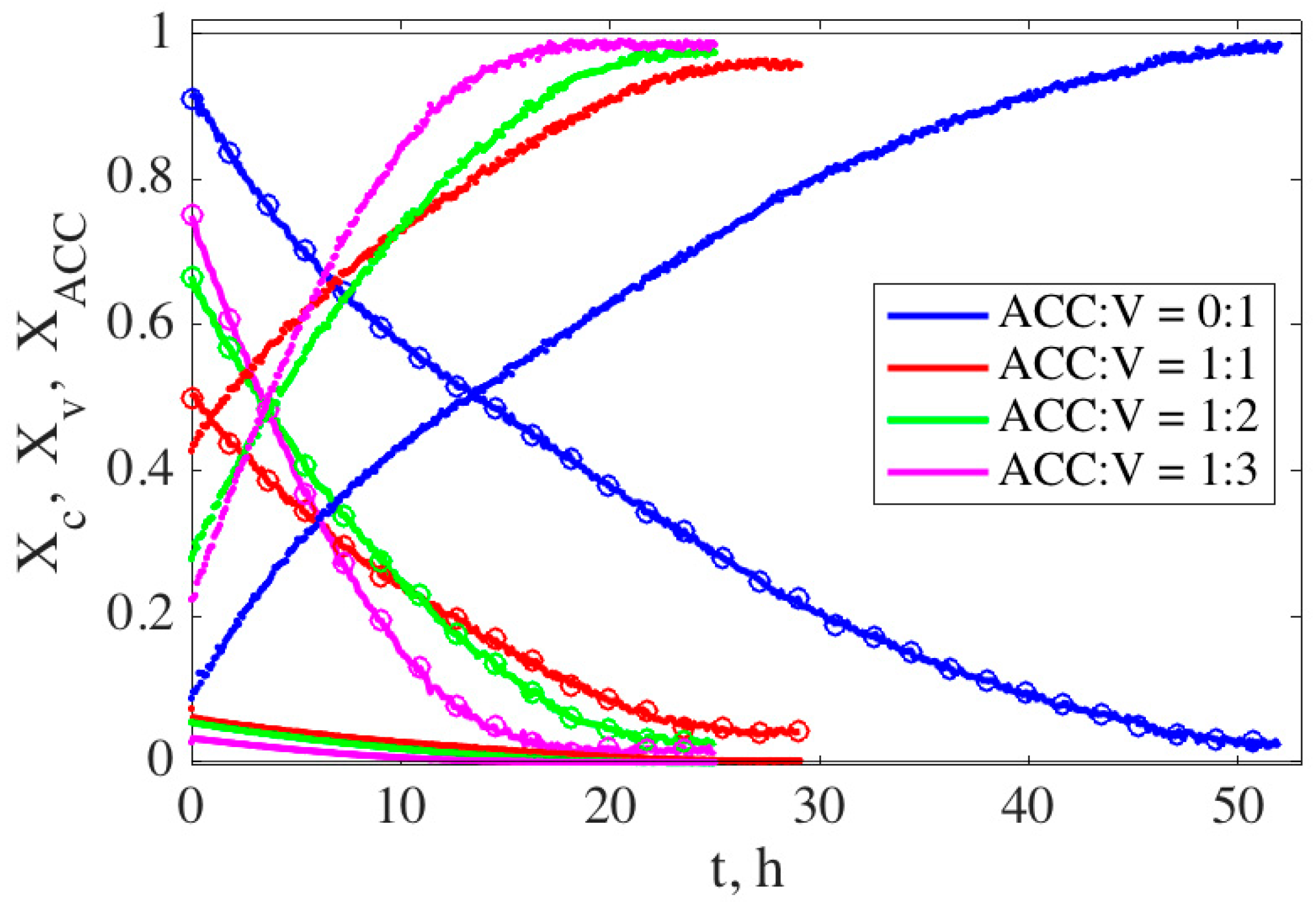
3.2.1. Measurement of Time Evolution of Different Phases
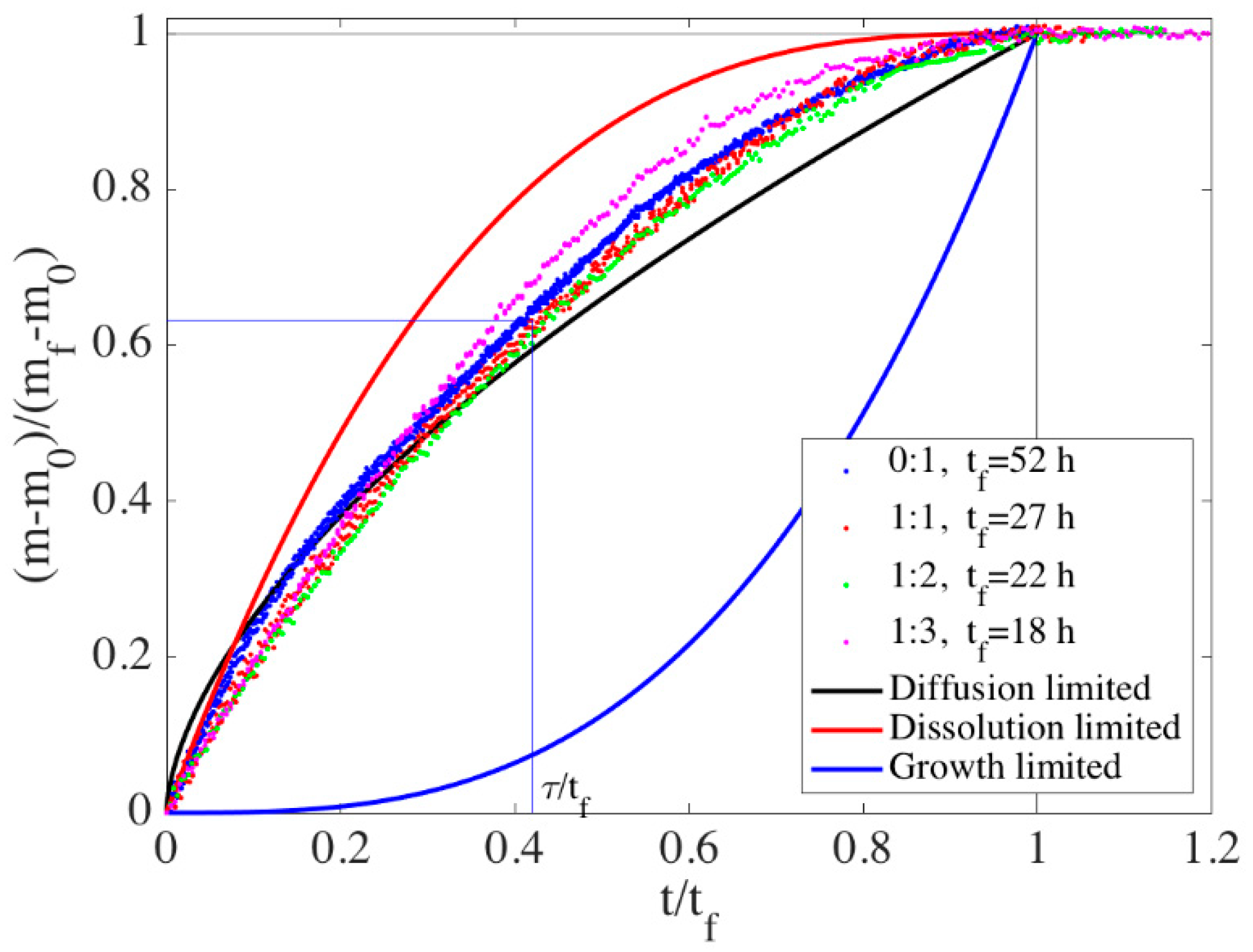
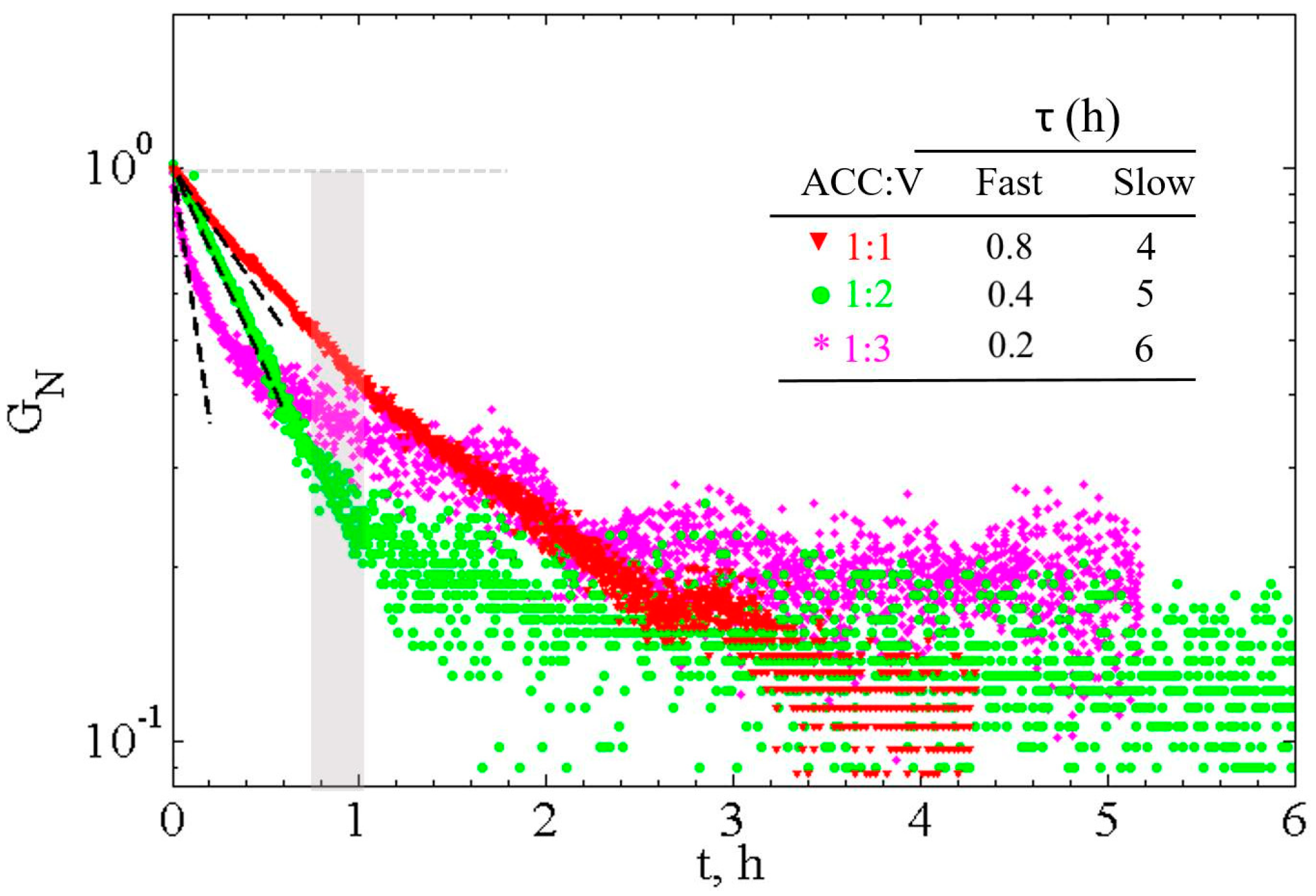
3.2.2. Analysis of Phase Transformation Kinetics: Rate-Limiting Step
3.3. Viscoelasticity of CaCO3 Pastes
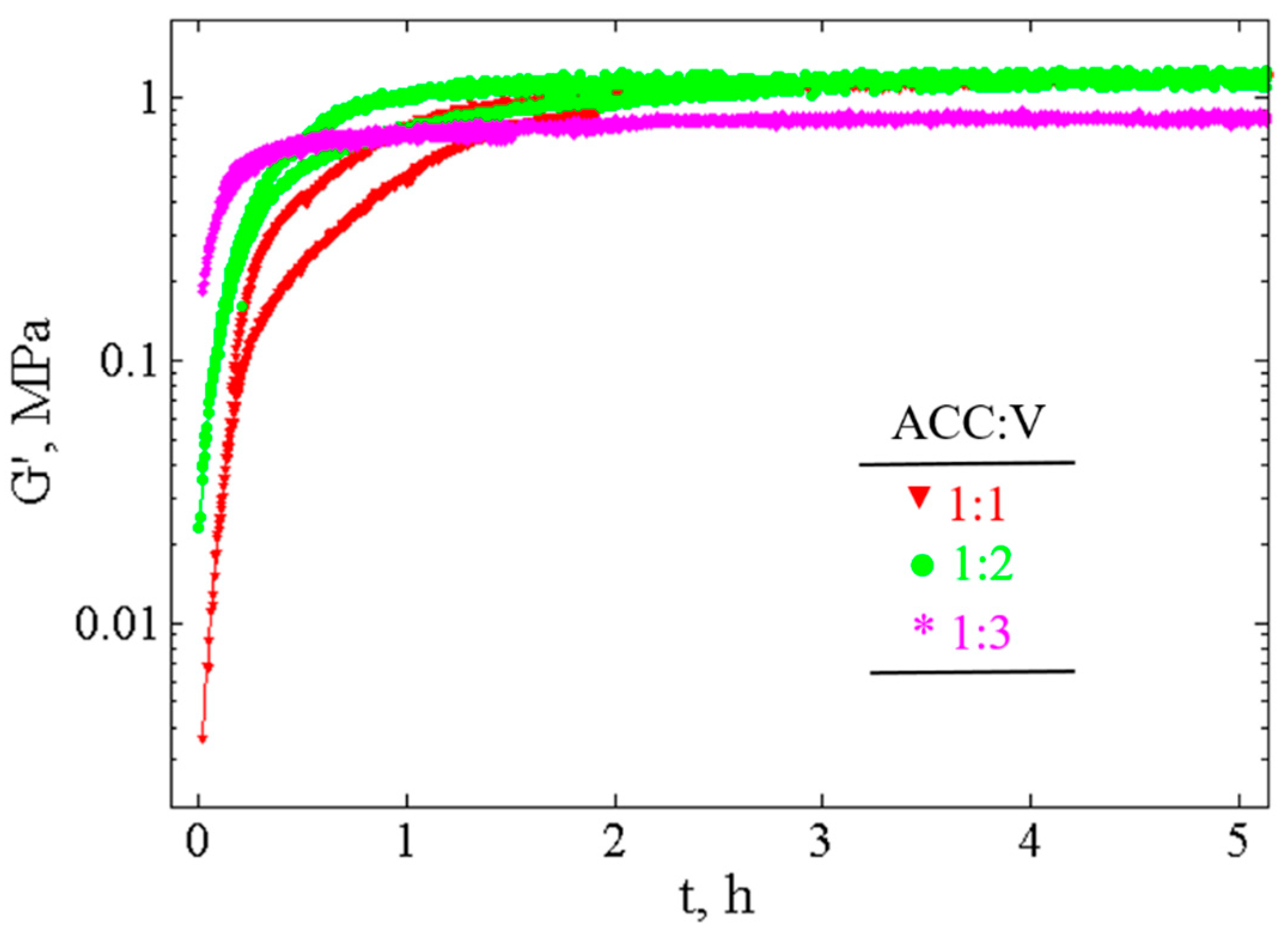
3.3.1. Measurement of Time Evolution of Viscoelastic Properties
3.3.2. Fast Increase of Elastic Modulus: Smoothing of Grains
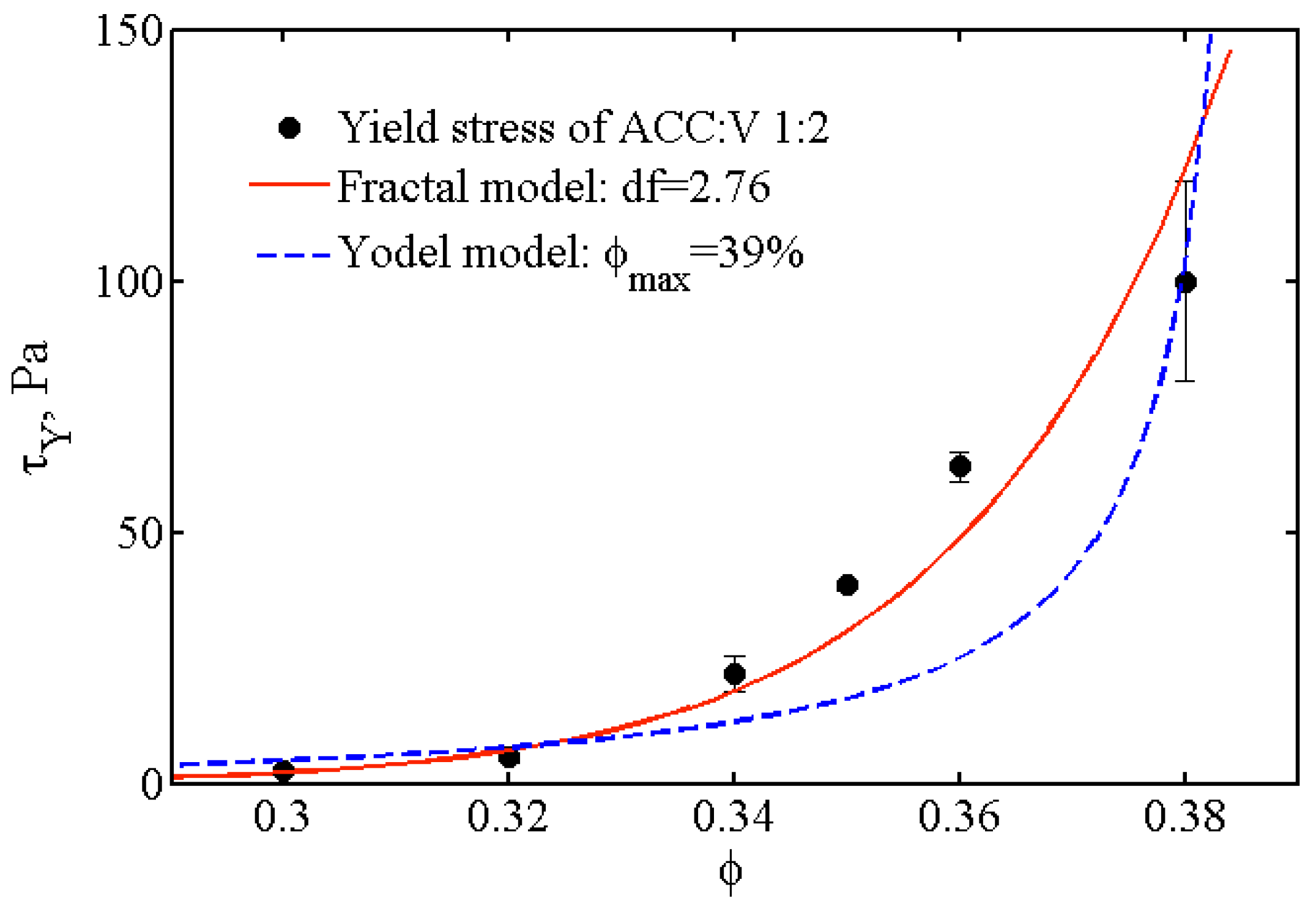
3.3.3. Structure of the Pastes
3.3.4. Discussion of Final Hardening of Cement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gartner, E.M.; Macphee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749. [Google Scholar] [CrossRef]
- Provis, J.L.; Deventer, J.S.J.V. Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7672-2. [Google Scholar]
- Zhang, T.; Cheeseman, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Justnes, H. Alternative Low-CO2 “Green” Clinkering Processes. Rev. Mineral. Geochem. 2012, 74, 83–99. [Google Scholar] [CrossRef]
- Fontaine, M.-L.; Combes, C.; Sillam, T.; Dechambre, G.; Rey, C. New Calcium Carbonate-Based Cements for Bone Reconstruction. Key Eng. Mater. 2005, 284–286, 105–108. [Google Scholar] [CrossRef]
- Combes, C.; Miao, B.; Bareille, R.; Rey, C. Preparation, physical-chemical characterisation and cytocompatibility of calcium carbonate cements. Biomaterials 2006, 27, 1945–1954. [Google Scholar] [CrossRef]
- Zaelke, D.; Young, O.; Andersen, S.O. Scientific synthesis of Calera carbon sequestration and carbonaceous by-product applications. Consensus findings of the scientific synthesis team. Inst. Gov. Sustain. Dev. 2011. [Google Scholar]
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochim. Cosmochim. Acta 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Wang, J.; Becker, U. Energetics and kinetics of carbonate orientational ordering in vaterite calcium carbonate. Am. Mineral. 2012, 97, 1427–1436. [Google Scholar] [CrossRef]
- Andreassen, J.P. Formation mechanism and morphology in precipitation of vaterite—Nano-aggregation or crystal growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Konrad, F.; Gallien, F.; Gerard, D.E.; Dietzel, M. Transformation of Amorphous Calcium Carbonate in Air. Cryst. Growth Des. 2016, 16, 6310–6317. [Google Scholar] [CrossRef]
- Wolf, G.; Günther, C. Thermophysical investigations of the polymorphous phases of calcium carbonate. J. Therm. Anal. Calorim. 2001, 65, 687–698. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Aloni, S.; De Yoreo, J.J. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 2014, 345, 1158–1162. [Google Scholar] [CrossRef]
- Schroeder, B.B.; Harris, D.D.; Smith, S.T.; Lignell, D.O. Theoretical framework for multiple-polymorph particle precipitation in highly supersaturated systems. Cryst. Growth Des. 2014, 14, 1756–1770. [Google Scholar] [CrossRef]
- Carr, F.P.; Frederick, D.K. Calcium carbonate. In Kirk-Othmer Encyclopedia of Chemical Technology, Volumes 1–26, 5th ed.; Kirk Othmer, Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1967; Volume 4, pp. 551–556. [Google Scholar]
- Banner, J.L. Application of the trace element and isotope geochemistry of strontium to studies of carbonate diagenesis. Int. Assoc. Sedimentol. 1995, 42, 805–824. [Google Scholar] [CrossRef]
- Colfen, H. Precipitation of carbonates: Recent progress in controlled production of complex shapes. Curr. Opin. Colloid Interface Sci. 2003, 8, 23–31. [Google Scholar] [CrossRef]
- Roehl, P.O.; Choquette, P.W. Carbonate Petroleum Reservoirs; Springer: New York, NY, USA, 1985; ISBN 978-1-4612-9536-5. [Google Scholar]
- Larson, R.G. The Structure and Rheology of Complex Fluids; Oxford University Press: Oxford, UK; New York, NY, USA, 1999. [Google Scholar]
- Flatt, R.J.; Bowen, P. Yodel: A yield stress model for suspensions. J. Am. Ceram. Soc. 2006, 89, 1244–1256. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A. Scaling properties of mech properties of colloidal gel.pdf. Phys. Rev. E 1990, 42, 4772–4779. [Google Scholar] [CrossRef]
- Shih, W.Y.; Shih, W.-H.; Aksay, I.A. Elastic and Yield Behavior of Strongly Flocculated Colloids. J. Am. Ceram. Soc. 1999, 82, 616–624. [Google Scholar] [CrossRef]
- Kirby, G.H.; Lewis, J.A. Rheological Property Evolution in Concentrated Cement-Polyelectrolyte Suspensions. J. Am. Ceram. Soc. 2004, 85, 2989–2994. [Google Scholar] [CrossRef]
- Flatt, R.J.; Martys, N.S.; Bergström, L. The Rheology of Cementitious Materials. MRS Bull. 2004, 29, 314–318. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, S.; Hanehara, S. Controlling of the adsorption and dispersing force of polycarboxylate-type superplasticizer by sulfate ion concentration in aqueous phase. Cem. Concr. Res. 2001, 31, 375–383. [Google Scholar] [CrossRef]
- Liberto, T.; Le Merrer, M.; Barentin, C.; Bellotto, M.; Colombani, J. Elasticity and yielding of a calcite paste: Scaling laws in a dense colloidal suspension. Soft Matter 2017, 13, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Weitz, D.; Pine, D. Diffusing Wave Spectroscopy. In Dynamic Light Scattering: The Method and Some Applications; Oxford University Press: Oxford, UK, 1993; pp. 652–720. [Google Scholar]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO-H2O solutions between 0 and 9OC, and an evaluation of the aqueous model for the system CaCO3-CO2-H20. Gcochlmica Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Cubillas, P.; Köhler, S.; Prieto, M.; Chaïrat, C.; Oelkers, E.H. Experimental determination of the dissolution rates of calcite, aragonite, and bivalves. Chem. Geol. 2005, 216, 59–77. [Google Scholar] [CrossRef]
- Li, L.; Sanchez, J.R.; Kohler, F.; Røyne, A.; Dysthe, D.K. Microfluidic Control of Nucleation and Growth of CaCO3. Cryst. Growth Des. 2018, 18, 4528–4535. [Google Scholar] [CrossRef]
- Reddy, M.M.; Plummer, L.N.; Busenberg, E. Crystal growth of calcite from calcium bicarbonate solutions at constant PCO2and 25°C: A test of a calcite dissolution model. Geochim. Cosmochim. Acta 1981, 45, 1281–1289. [Google Scholar] [CrossRef]
- Hernández, L.; Gurruchaga, M.; Goñi, I. Influence of powder particle size distribution on complex viscosity and other properties of acrylic bone cement for vertebroplasty and kyphoplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 77, 98–103. [Google Scholar] [CrossRef]
- Larsen, B.E.; Bjørnstad, J.; Pettersen, E.O.; Tønnesen, H.H.; Melvik, J.E. Rheological characterization of an injectable alginate gel system. BMC Biotechnol. 2015, 15, 1–12. [Google Scholar] [CrossRef]
- Brekke-Svaland, G.; Bresme, F. Interactions between Hydrated Calcium Carbonate Surfaces at Nanoconfinement Conditions. J. Phys. Chem. C 2018, 122, 7321–7330. [Google Scholar] [CrossRef]
- Parsons, D.F.; Walsh, R.B.; Craig, V.S.J. Surface forces: Surface roughness in theory and experiment. J. Chem. Phys. 2014, 140, 164701. [Google Scholar] [CrossRef] [PubMed]
- Olarte-Plata, J.D.; Brekke-Svaland, G.; Bresme, F. Dependence of the phase behavior of suspensions of mineral nanoparticles on the particle surface roughness. arXiv 2019, arXiv:1905.07254. [Google Scholar]
- Dziadkowiec, J.; Javadi, S.; Bratvold, J.E.; Nilsen, O.; Røyne, A. Surface Forces Apparatus Measurements of Interactions between Rough and Reactive Calcite Surfaces. Langmuir 2018, 34, 7248–7263. [Google Scholar] [CrossRef]
- Roussel, N.; Lemaître, A.; Flatt, R.J.; Coussot, P. Cement and Concrete Research Steady state fl ow of cement suspensions: A micromechanical state of the art. Cem. Concr. Res. 2010, 40, 77–84. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Zhang, Q.; Dysthe, D.K. Microstructure and relative humidity effects on long-term indentation creep properties of calcium carbonate cement. Preprints 2018, 1–25. [Google Scholar] [CrossRef]
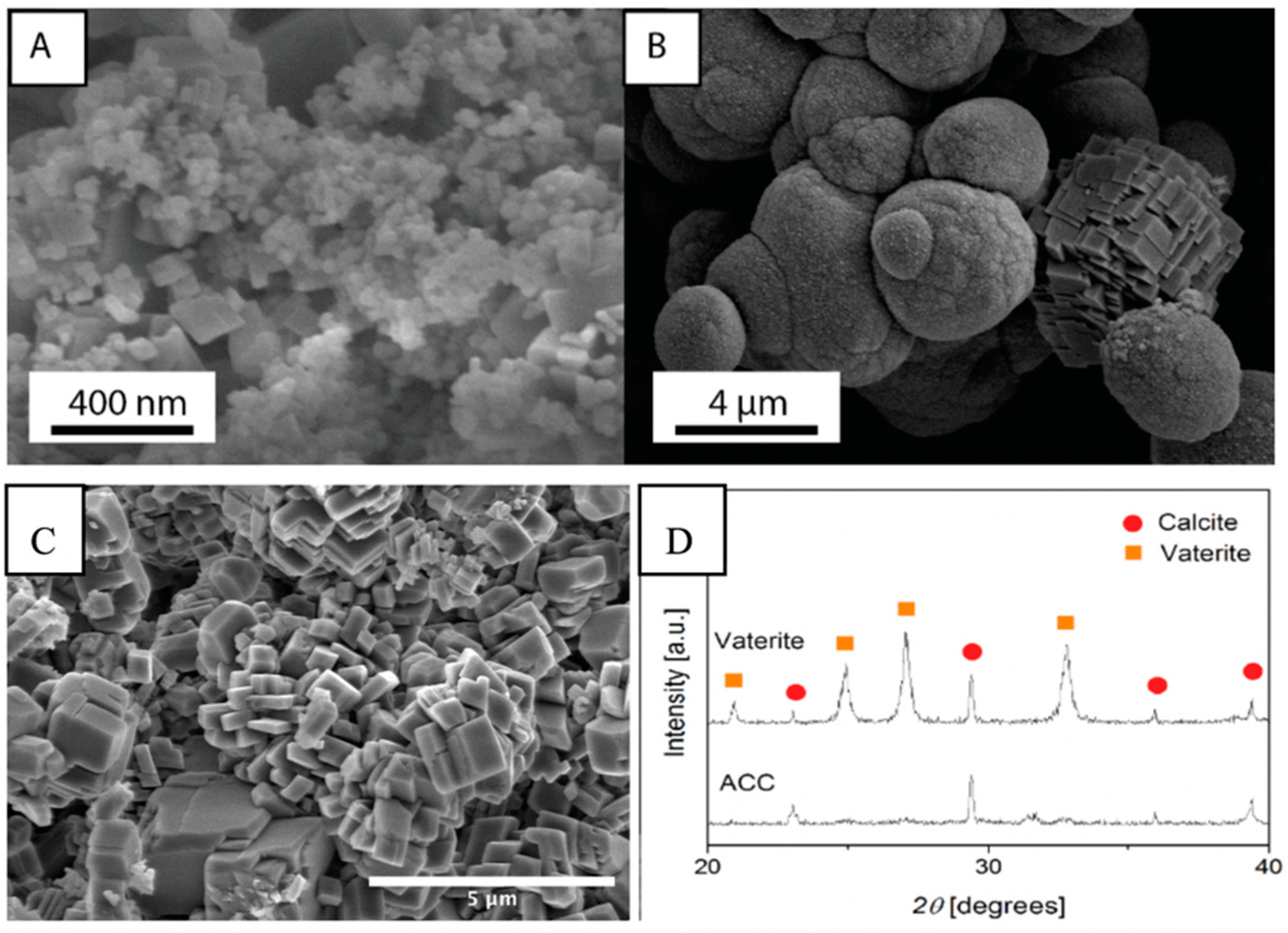

| Weight Ratio (%) | Water/Solid Ratio | |||
|---|---|---|---|---|
| Mixture Nomenclature | ACC 1 | V 2 | For XRD Tests | For Rheological Tests |
| ACC:V 1:0 | 100 | 0 | 0.43 | (not tested) |
| ACC:V 1:1 | 50 | 50 | 0.43 | 0.65 |
| ACC:V 1:2 | 33 | 66 | 0.43 | 0.65 |
| ACC:V 1:3 | 25 | 75 | 0.43 | 0.65 |
| ACC:V 0:1 | 0 | 100 | 0.43 | (not tested) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez, J.; Liberto, T.; Barentin, C.; Dysthe, D.K. Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement. Materials 2020, 13, 3582. https://doi.org/10.3390/ma13163582
Rodríguez-Sánchez J, Liberto T, Barentin C, Dysthe DK. Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement. Materials. 2020; 13(16):3582. https://doi.org/10.3390/ma13163582
Chicago/Turabian StyleRodríguez-Sánchez, Jesús, Teresa Liberto, Catherine Barentin, and Dag Kristian Dysthe. 2020. "Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement" Materials 13, no. 16: 3582. https://doi.org/10.3390/ma13163582
APA StyleRodríguez-Sánchez, J., Liberto, T., Barentin, C., & Dysthe, D. K. (2020). Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement. Materials, 13(16), 3582. https://doi.org/10.3390/ma13163582







