Photoautotrophs–Bacteria Co-Cultures: Advances, Challenges and Applications
Abstract
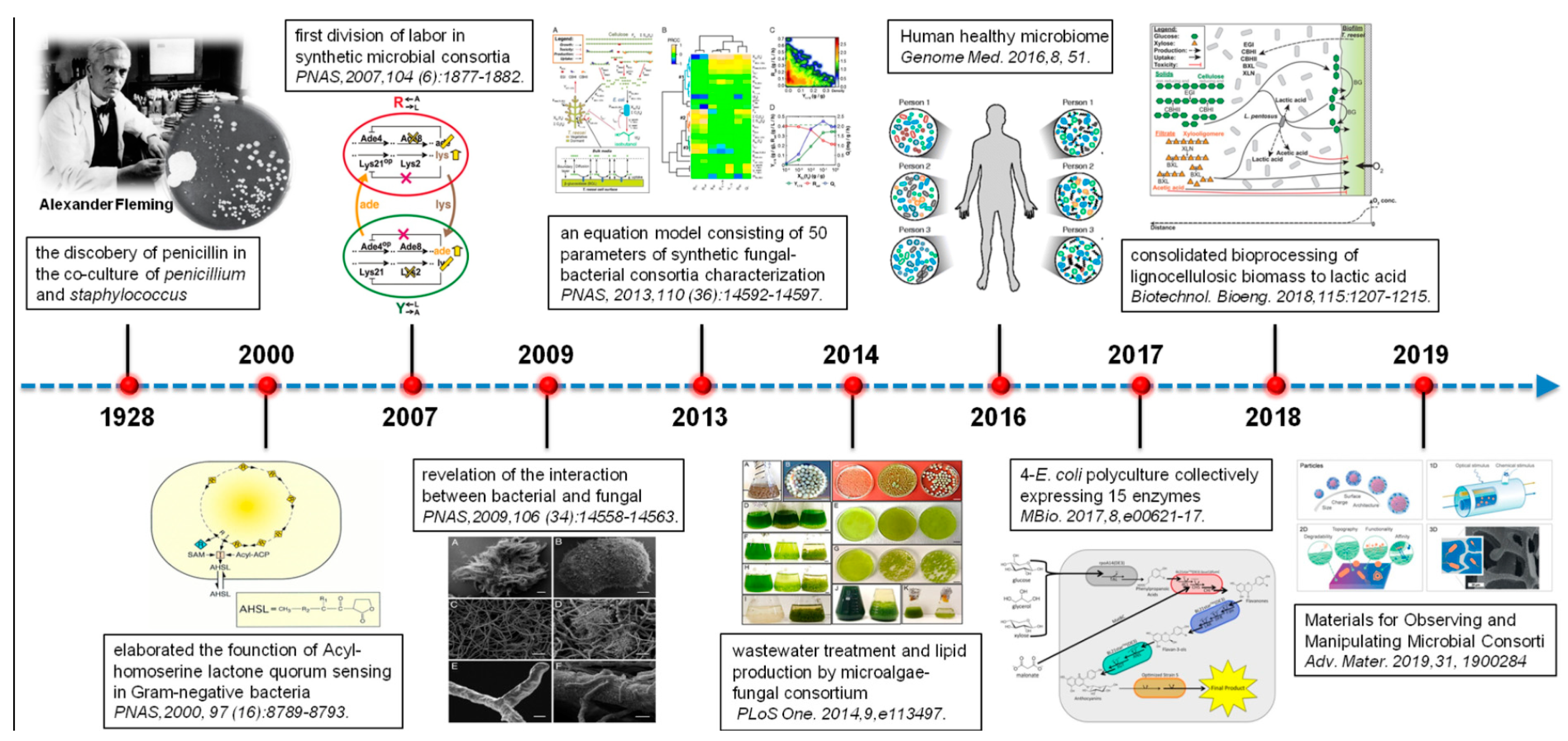
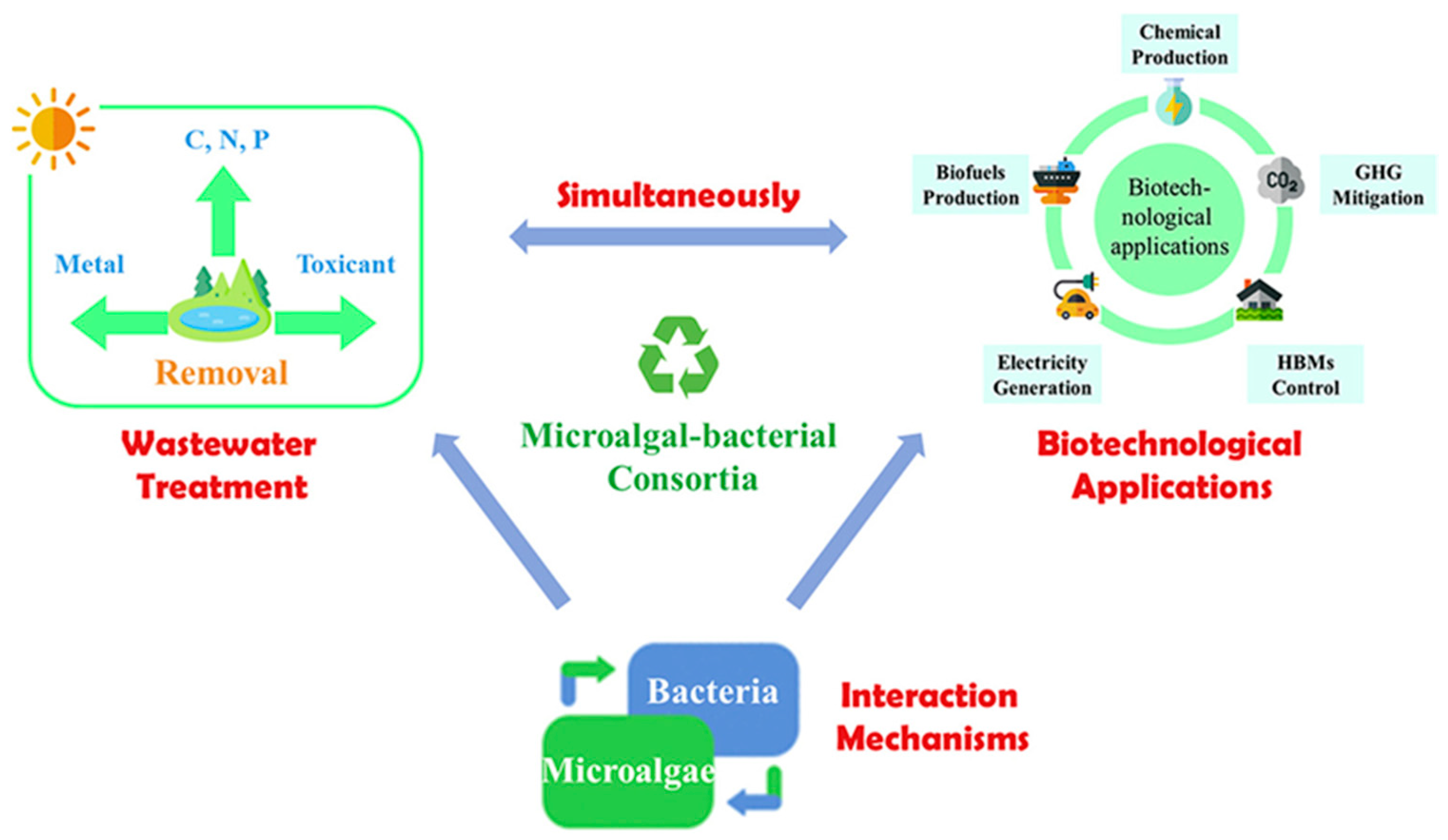
:1. Introduction
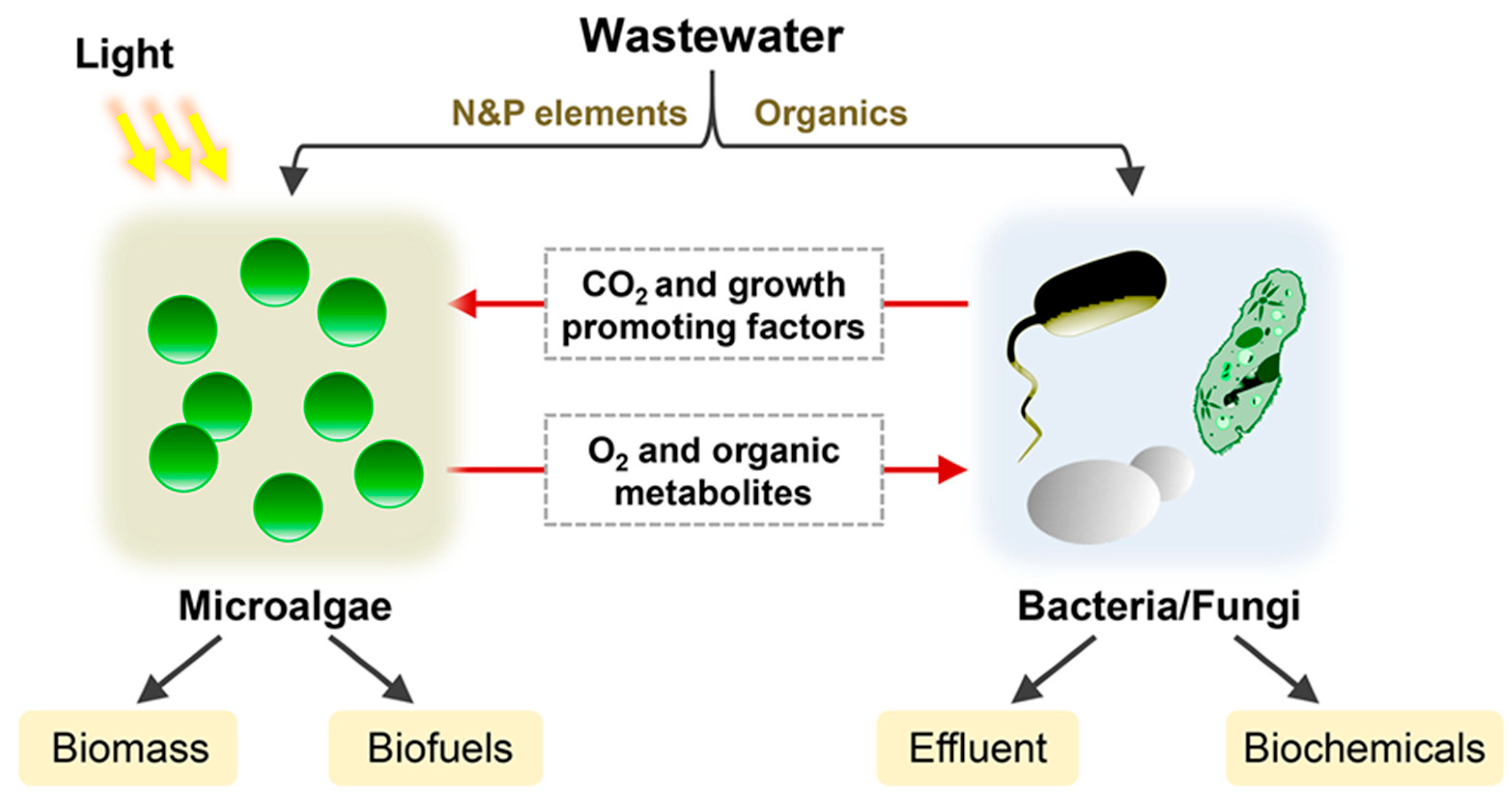
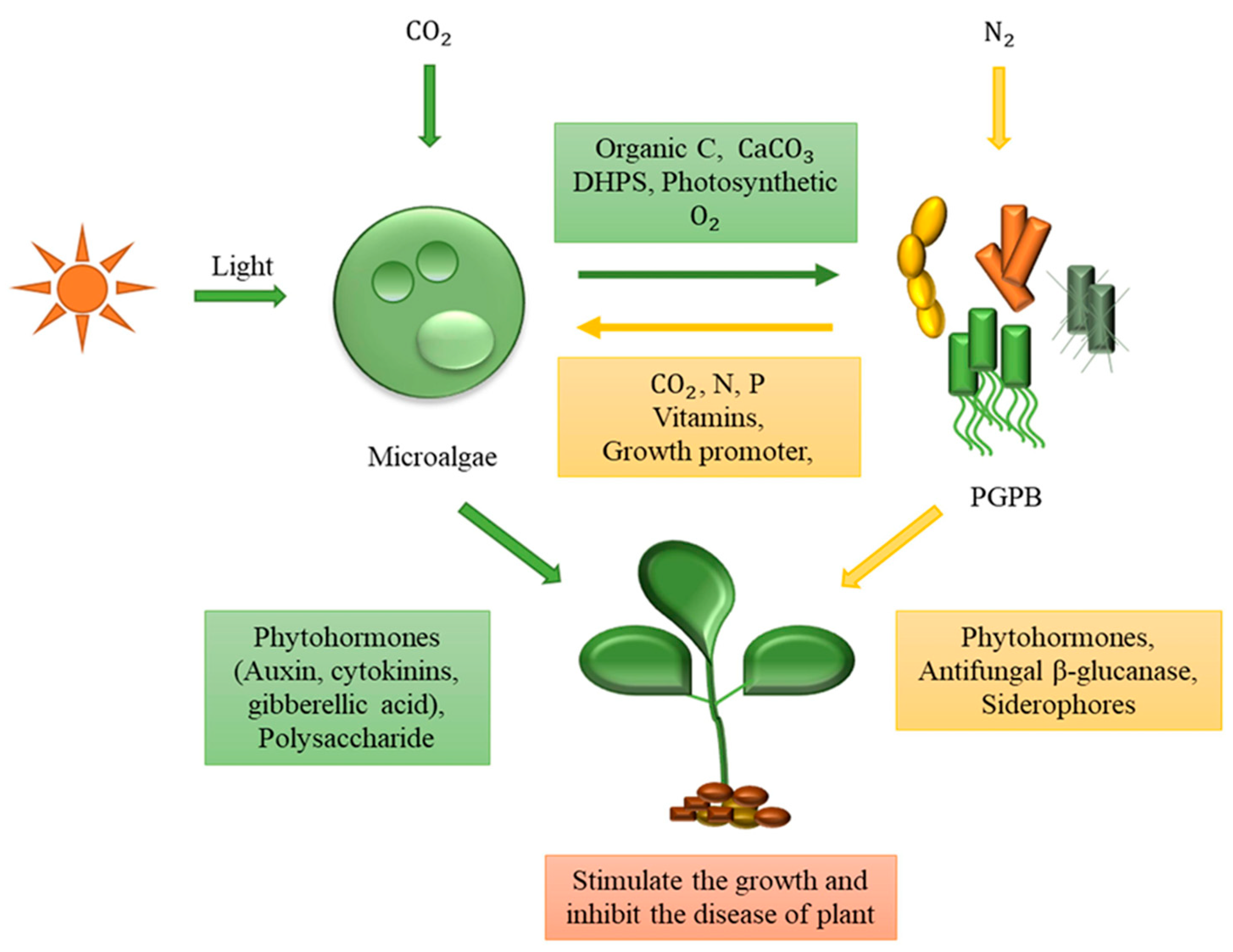
2. Microbial Consortium for Photosynthetic Biomanufacturing Materials
2.1. Manufacturing of Biomolecules
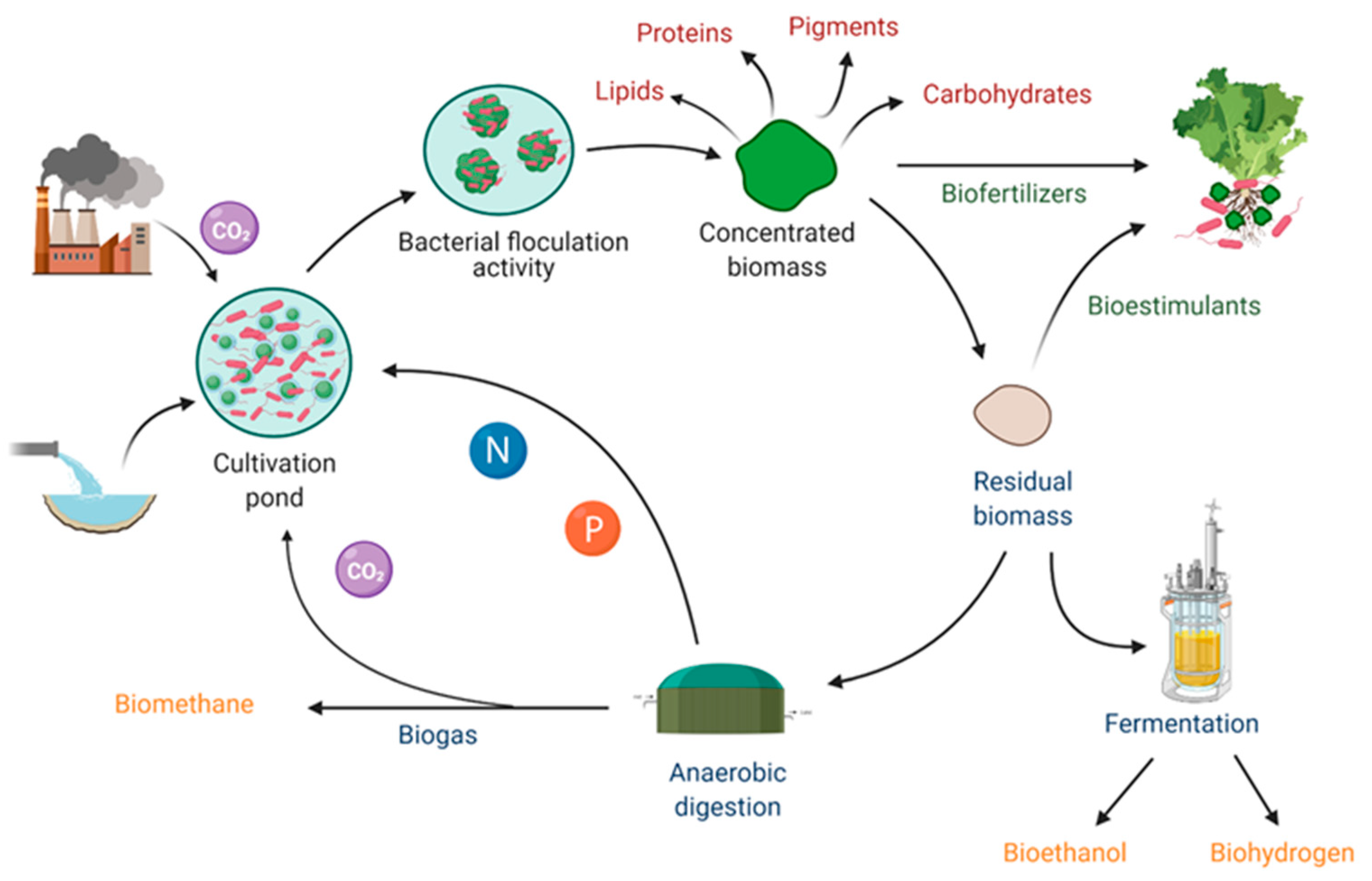
2.2. Spent Biomass: An Efficient and Economically Viable Biorefinery Feedstock
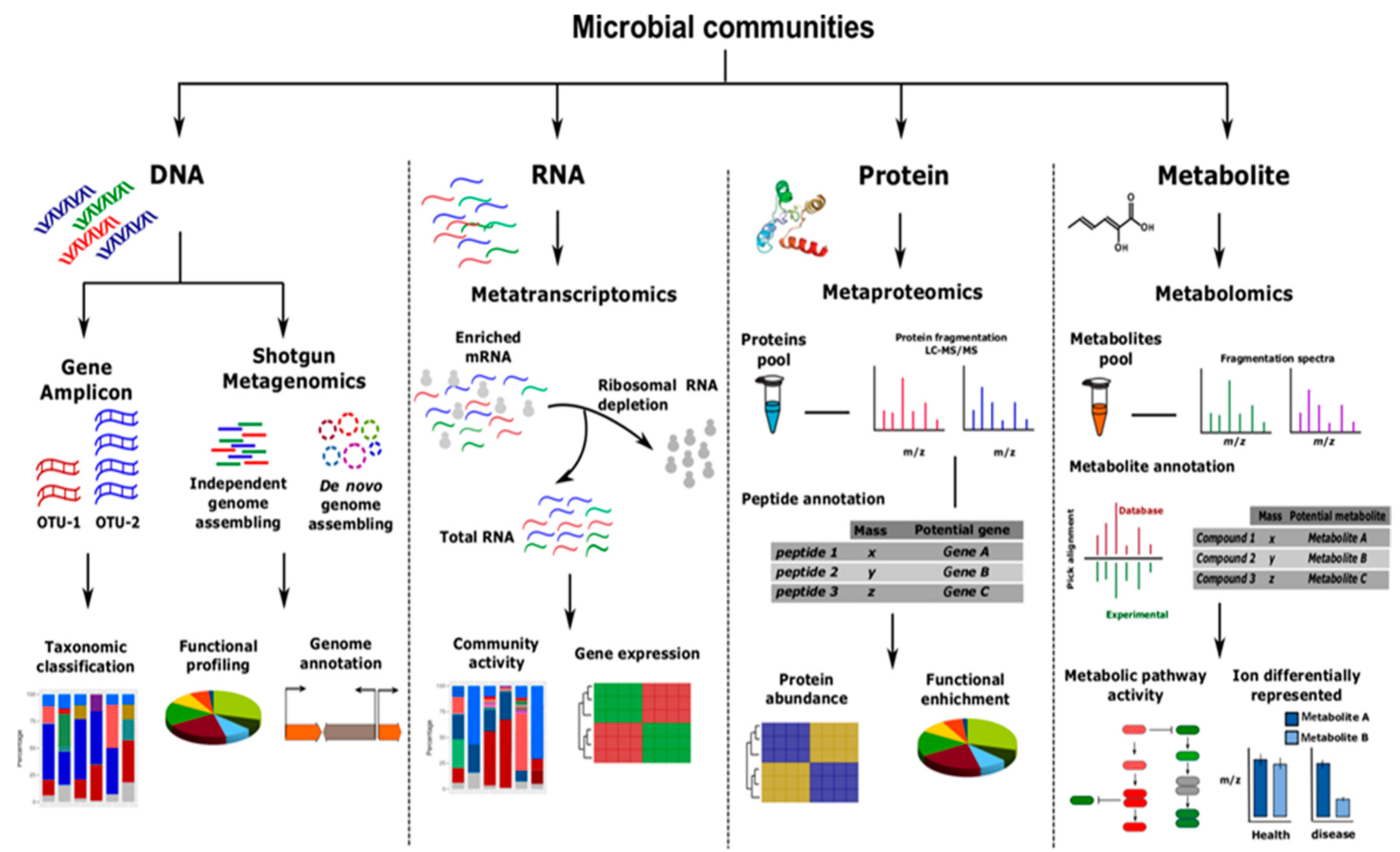
3. Improve the Consortia by Omics Approaches
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, B.D.; Mota, A.; Teixeira, J.A.; Vicente, A.A. Continuous cultivation of photosynthetic microorganisms: Approaches, applications and future trends. Biotechnol. Adv. 2015, 33, 1228–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlozzi, P.; Touloupakis, E. Bioplastic production by feeding the marine Rhodovulum sulfidophilum DSM-1374 with four different carbon sources under batch, fed-batch and semi-continuous growth regimes. New Biotechnol. 2021, 62, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rea, G.; Antonacci, A.; Lambreva, M.D.; Pastorelli, S.; Tibuzzi, A.; Ferrari, S.; Fischer, D.; Johanningmeier, U.; Oleszek, W.; Doroszewska, T.; et al. Integrated plant biotechnologies applied to safer and healthier food production: The Nutra-Snack manufacturing chain. Trends Food Sci. Technol. 2011, 22, 353–366. [Google Scholar] [CrossRef]
- Touloupakis, E.; Faraloni, C.; Benavides, A.M.S.; Masojídek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutrient starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Touloupakis, E.; Benavides, A.M.S.; Cicchi, B.; Torzillo, G. Growth and hydrogen production of outdoor cultures of Synechocystis PCC. Algal Res. 2016, 18, 78–85. [Google Scholar] [CrossRef]
- Kagermann, H.; Lukas, W.; Wahlster, W. Industrie 4.0–Mitdem Internet Er Dinge Auf Dem Wegzur 4. Industriellen Revolution [Industry 4.0: With the Internet of Things towards 4th Industrial Revolution]. VDI Nachrichten. 2011. Available online: http://www.wolfgang-wahlster.de/wordpress/wp-content/uploads/Industrie_4_0_Mit_dem_Internet_der_Dinge_auf_dem_Weg_zur_vierten_industriellen_Revolution_2.pdf (accessed on 20 April 2021).
- Ejsmont, K.; Gladysz, B.; Kluczek, A. Impact of Industry 4.0 on Sustainability—Bibliometric Literature Review. Sustainability 2020, 12, 5650. [Google Scholar] [CrossRef]
- Gangl, D.; Zedler, J.A.Z.; Rajakumar, P.D.; Martinez, E.M.R.; Riseley, A.; Wlodarczyk, A.; Purton, S.; Sakuragi, Y.; Howe, C.J.; Jensen, P.E.; et al. Biotechnological exploitation of microalgae. J. Exp. Bot. 2015, 66, 6975–6990. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef] [Green Version]
- Antonacci, A.; Scognamiglio, V. Biotechnological Advances in the Design of Algae-Based Biosensors. Trends Biotechnol. 2020, 38, 334–347. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Apel, A.C.; Weuster-Botz, D. Engineering solutions for open microalgae mass cultivation and realistic indoor simulation of outdoor environments. Bioprocess Biosyst. Eng. 2015, 38, 995–1008. [Google Scholar] [CrossRef]
- Shahab, R.L.; Brethauer, S.; Luterbacher, J.S.; Studer, M.H. Engineering of ecological niches to create stable artificial consortia for complex biotransformations. Curr. Opin. Biotechnol. 2020, 62, 129–136. [Google Scholar] [CrossRef]
- Magdouli, S.; Brar, S.; Blais, J. Co-culture for lipid production: Advances and challenges. Biomass Bioenergy 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Tsoi, R.; Dai, Z.; You, L. Emerging strategies for engineering microbial communities. Biotechnol. Adv. 2019, 37, 107372. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, N.S.; Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noack, S.; Baumgart, M. Communities of Niche-Optimized Strains: Small-Genome Organism Consortia in Bioproduction. Trends Biotechnol. 2019, 37, 126–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspe-cies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Diender, M.; Olm, I.P.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotechnol. 2021, 67, 72–79. [Google Scholar] [CrossRef]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.-H.; Oh, H.-M.; Kim, H.-S. Enhancing microalgal biomass productivity by engineering a microalgal–bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Zhu, S.; Qin, L.; Feng, P.; Shang, C.; Wang, Z.; Yuan, Z. Treatment of low C/N ratio wastewater and biomass production using co-culture of Chlorella vulgaris and activated sludge in a batch photobioreactor. Bioresour. Technol. 2019, 274, 313–320. [Google Scholar] [CrossRef]
- Decelle, J.; Colin, S.; Foster, R.A. Photosymbiosis in Marine Planktonic Protists. In Marine Protists: Diversity and Dynamics; Ohtsuka, S., Suzaki, T., Horiguchi, T., Suzuki, N., Not, F., Eds.; Springer: Tokyo, Japan, 2015; pp. 465–500. ISBN 978-4-431-55130-0. [Google Scholar]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial consortia: A critical look at microalgae co-cultures for enhanced biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae-bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhang, T.-Y.; Zhan, X.-M.; Hu, H.-Y. Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res. 2018, 33, 345–351. [Google Scholar] [CrossRef]
- Makut, B.B.; Goswami, G.; Das, D. Evaluation of bio-crude oil through hydrothermal liquefaction of microalgae-bacteria consortium grown in open pond using wastewater. Biomass Convers. Biorefinery 2020, 1–15. [Google Scholar] [CrossRef]
- Praveen, P.; Loh, K.-C. Photosynthetic aeration in biological wastewater treatment using immobilized microalgae-bacteria symbiosis. Appl. Microbiol. Biotechnol. 2015, 99, 10345–10354. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nat. Cell Biol. 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tong, Y.W. The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. Environ. Boil. Fishes 2014, 26, 1483–1492. [Google Scholar] [CrossRef]
- Mayfield, S.; Golden, S.S. Photosynthetic bio-manufacturing: Food, fuel, and medicine for the 21st century. Photosynth. Res. 2015, 123, 225–226. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 2020, 40, 107500. [Google Scholar] [CrossRef]
- Tinzl-Malang, S.K.; Rast, P.; Grattepanche, F.; Sych, J.; Lacroix, C. Exopolysaccharides from co-cultures of Weissella confusa 11GU-1 and Propionibacterium freudenreichii JS15 act synergistically on wheat dough and bread texture. Int. J. Food Microbiol. 2015, 214, 91–101. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Cheok, W.L.; de Toledo, R.A.; Shim, H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 2014, 173, 132–139. [Google Scholar] [CrossRef]
- Kitcha, S.; Cheirsilp, B. Enhanced Lipid Production by Co-cultivation and Co-encapsulation of Oleaginous Yeast Trichosporonoides spathulata with Microalgae in Alginate Gel Beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Berthold, D.E.; Shetty, K.G.; Jayachandran, K.; Laughinghouse, H.D.; Gantar, M. Enhancing algal biomass and lipid production through bacterial co-culture. Biomass Bioenergy 2019, 122, 280–289. [Google Scholar] [CrossRef]
- Xue, L.; Shang, H.; Ma, P.; Wang, X.; He, X.; Niu, J.; Wu, J. Analysis of growth and lipid production characteristics of Chlorella vulgaris in artificially constructed consortia with symbiotic bacteria. J. Basic Microbiol. 2018, 58, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, H.; Li, X.; Zhao, Q.; Yin, Y.; Xi, L.; Ge, B.; Qin, S. Enhanced biomass and lipid production by co-cultivation of Chlorella vulgaris with Mesorhizobium sangaii under nitrogen limitation. Environ. Boil. Fishes 2020, 32, 233–242. [Google Scholar] [CrossRef]
- Higgins, B.T.; VanderGheynst, J.S. Effects of Escherichia coli on Mixotrophic Growth of Chlorella minutissima and Production of Biofuel Precursors. PLoS ONE 2014, 9, e96807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-Bacteria Consortia as a Strategy to Enhance H2 Production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Ge, B.; He, J.; Zhang, Q.; Wei, Y.; Xi, L.; Khan, N.U.; Huang, F. Evaluation of various sulfides for enhanced photobiological H2 production by a dual-species co-culture system of Chlamydomonas reinhardtii and Thiomonas intermedia. Process. Biochem. 2019, 82, 110–116. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O. Improving hydrogen production using co-cultivation of bacteria with Chlamydomonas reinhardtii microalga. Mater. Sci. Energy Technol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Lakatos, G.; Balogh, D.; Farkas, A.; Ördög, V.; Nagy, P.T.; Bíró, T.; Maróti, G. Factors influencing algal photobiohydrogen production in algal-bacterial co-cultures. Algal Res. 2017, 28, 161–171. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef]
- He, J.; Xi, L.; Sun, X.; Ge, B.; Liu, D.; Han, Z.; Pu, X.; Huang, F. Enhanced hydrogen production through co-cultivation of Chlamydomonas reinhardtii CC-503 and a facultative autotrophic sulfide-oxidizing bacterium under sulfurated conditions. Int. J. Hydrogen Energy 2018, 43, 15005–15013. [Google Scholar] [CrossRef]
- Fakhimi, N.; Dubini, A.; Tavakoli, O.; González-Ballester, D. Acetic acid is key for synergetic hydrogen production in Chlamydomonas-bacteria co-cultures. Bioresour. Technol. 2019, 289, 121648. [Google Scholar] [CrossRef]
- Fakhimi, N.; Tavakoli, O.; Marashi, S.-A.; Moghimi, H.; Mehrnia, M.R.; Dubini, A.; González-Ballester, D. Acetic acid uptake rate controls H2 production in Chlamydomonas-bacteria co-cultures. Algal Res. 2019, 42, 101605. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Wang, Q.; Wu, S. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. Hydrogen Energy 2016, 41, 9276–9283. [Google Scholar] [CrossRef] [Green Version]
- Kao, P.-M.; Hsu, B.-M.; Chang, T.-Y.; Chiu, Y.-C.; Tsai, S.-H.; Huang, Y.-L.; Chang, C.-M. Biohydrogen production by Clostridium butyricum and Rhodopseudomonas palustris in Co-cultures. Int. J. Green Energy 2015, 13, 715–719. [Google Scholar] [CrossRef]
- Veeramalini, J.; Selvakumari, I.A.E.; Park, S.; Jayamuthunagai, J.; Bharathiraja, B. Continuous production of biohydrogen from brewery effluent using co-culture of mutated Rhodobacter M 19 and Enterobacter aerogenes. Bioresour. Technol. 2019, 286, 121402. [Google Scholar] [CrossRef] [PubMed]
- Laurinavichene, T.; Laurinavichius, K.; Shastik, E.; Tsygankov, A. Long-term H2 photoproduction from starch by co-culture of Clostridium butyricum and Rhodobacter sphaeroides in a repeated batch process. Biotechnol. Lett. 2017, 40, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Hitit, Z.Y.; Lazaro, C.Z.; Hallenbeck, P.C. Hydrogen production by co-cultures of Clostridium butyricum and Rhodospeudomonas palustris: Optimization of yield using response surface methodology. Int. J. Hydrogen Energy 2017, 42, 6578–6589. [Google Scholar] [CrossRef]
- Dong, Q.-L.; Zhao, X.-M. In situ carbon dioxide fixation in the process of natural astaxanthin production by a mixed culture of Haematococcus pluvialis and Phaffia rhodozyma. Catal. Today 2004, 98, 537–544. [Google Scholar] [CrossRef]
- Buzzini, P. Batch and fed-batch carotenoid production by Rhodotorula glutinis-Debaryomyces castellii co-cultures in corn syrup. J. Appl. Microbiol. 2001, 90, 843–847. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.E.; Bashan, Y. Increased Growth of the Microalga Chlorella vulgariswhen Coimmobilized and Cocultured in Alginate Beads with the Plant-Growth-Promoting Bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Bashan, L.E.; Bashan, Y.; Moreno, M.; Lebsky, V.K.; Bustillos, J.J. Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can. J. Microbiol. 2002, 48, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Angelis, S.; Novak, A.C.; Sydney, E.; Soccol, V.T.; Carvalho, J.C.; Pandey, A.; Noseda, M.D.; Tholozan, J.L.; Lorquin, J.; Soccol, C.R. Co-Culture of Microalgae, Cyanobacteria, and Macromycetes for Exopolysaccharides Production: Process Preliminary Optimization and Partial Characterization. Appl. Biochem. Biotechnol. 2012, 167, 1092–1106. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Advances in the technologies for studying consortia of bacteria and cyanobacteria/microalgae in wastewaters. Crit. Rev. Biotechnol. 2019, 39, 709–731. [Google Scholar] [CrossRef]
- Lin, Y.; de Kreuk, M.; van Loosdrecht, M.; Adin, A. Characterization of alginate-like exopolysaccharides isolated from aerobic granular sludge in pilot-plant. Water Res. 2010, 44, 3355–3364. [Google Scholar] [CrossRef]
- Snell, K.D.; Peoples, O.P. PHA bioplastic: A value-added coproduct for biomass biorefineries. Biofuels Bioprod. Biorefining 2009, 3, 456–467. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Monroy, I.; Buitrón, G. Production of polyhydroxybutyrate by pure and mixed cultures of purple non-sulfur bacteria: A review. J. Biotechnol. 2020, 317, 39–47. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria. Bioresour. Technol. 2020, 323, 124610. [Google Scholar] [CrossRef]
- Löwe, H.; Hobmeier, K.; Moos, M.; Kremling, A.; Pflüger-Grau, K. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.; Reis, M.; Oehmen, A. Beyond feast and famine: Selecting a PHA accumulating photosynthetic mixed culture in a permanent feast regime. Water Res. 2016, 105, 421–428. [Google Scholar] [CrossRef]
- Arumugam, A.; Sandhya, M.; Ponnusami, V. Biohydrogen and polyhydroxyalkanoate co-production by Enterobacter aerogenes and Rhodobacter sphaeroides from Calophyllum inophyllum oil cake. Bioresour. Technol. 2014, 164, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Policastro, G.; Luongo, V.; Fabbricino, M. Biohydrogen and poly-β-hydroxybutyrate production by winery wastewater photofermentation: Effect of substrate concentration and nitrogen source. J. Environ. Manag. 2020, 271, 111006. [Google Scholar] [CrossRef]
- Dinesh, G.H.; Nguyen, D.D.; Ravindran, B.; Chang, S.W.; Vo, D.-V.N.; Bach, Q.-V.; Tran, H.N.; Basu, M.J.; Mohanrasu, K.; Murugan, R.S.; et al. Simultaneous biohydrogen (H2) and bioplastic (poly-β-hydroxybutyrate-PHB) productions under dark, photo, and subsequent dark and photo fermentation utilizing various wastes. Int. J. Hydrogen Energy 2020, 45, 5840–5853. [Google Scholar] [CrossRef]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour. Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-J.; Han, T.H.; Yoo, G.; Cho, M.H.; Hwang, S.-J. Co-culture Consortium of Scenedesmus dimorphus and Nitrifiers Enhances the Removal of Nitrogen and Phosphorus from Artificial Wastewater. KSCE J. Civ. Eng. 2018, 22, 3215–3221. [Google Scholar] [CrossRef]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in algal-prokaryotic wastewater treatment: A review of nitrogen transformations, reactor configurations and molecular tools. J. Environ. Manag. 2018, 217, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Qi, Y.; Zhao, L.; Chen, G. Interactions Between Microalgae and Microorganisms for Wastewater Remediation and Biofuel Production. Waste Biomass Valorization 2019, 10, 3907–3919. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Bruni, L.; Andreottola, G. Bacteria and photosynthetic cells in a photobioreactor treating real municipal wastewater: Analysis and quantification using flow cytometry. Algal Res. 2020, 50, 101969. [Google Scholar] [CrossRef]
- Fito, J.; Alemu, K. Microalgae–bacteria consortium treatment technology for municipal wastewater management. Nanotechnol. Environ. Eng. 2018, 4, 4. [Google Scholar] [CrossRef]
- da Silva Rodrigues, D.A.; da Cunha, C.C.R.F.; Freitas, M.G.; de Barros, A.L.C.; Castro, P.B.N.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cássia Franco Afonso, R.J. Biodegradation of sulfamethoxazole by microalgae-bacteria consortium in wastewater treatment plant effluents. Sci. Total. Environ. 2020, 749, 141441. [Google Scholar] [CrossRef] [PubMed]
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239. [Google Scholar] [CrossRef]
- García, D.; Alcántara, C.; Blanco, S.; Pérez, R.; Bolado, S.; Muñoz, R. Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem. Eng. J. 2017, 313, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Mhedhbi, E.; Khelifi, N.; Foladori, P.; Smaali, I. Real-Time Behavior of a Microalgae–Bacteria Consortium Treating Wastewater in a Sequencing Batch Reactor in Response to Feeding Time and Agitation Mode. Water 2020, 12, 1893. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total. Environ. 2020, 710, 135583. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; González-Martínez, A.; Rodelas, B.; González-López, J. Full-scale photobioreactor for biotreatment of olive washing water: Structure and diversity of the microalgae-bacteria consortium. Bioresour. Technol. 2017, 238, 389–398. [Google Scholar] [CrossRef]
- López-Serna, R.; García, D.; Bolado, S.; Jiménez, J.J.; Lai, F.Y.; Golovko, O.; Gago-Ferrero, P.; Ahrens, L.; Wiberg, K.; Muñoz, R. Photobioreactors based on microalgae-bacteria and purple phototrophic bacteria consortia: A promising technology to reduce the load of veterinary drugs from piggery wastewater. Sci. Total. Environ. 2019, 692, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; Marín, D.; Blanco, S.; Lebrero, R.; Muñoz, R. Simultaneous biogas upgrading and centrate treatment in an outdoors pilot scale high rate algal pond. Bioresour. Technol. 2017, 232, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; Garcia-Encina, P.A.; Muñoz, R. Influence of Biogas Flow Rate on Biomass Composition During the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Posadas, E.; Grajeda, C.; Blanco, S.; Martínez-Páramo, S.; Acién, G.; Garcia-Encina, P.A.; Bolado, S.; Muñoz, R. Comparative evaluation of piggery wastewater treatment in algal-bacterial photobioreactors under indoor and outdoor conditions. Bioresour. Technol. 2017, 245, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Sun, L.; Sun, Z.; Li, D. Screening of a Chlorella-bacteria consortium and research on piggery wastewater purification. Algal Res. 2020, 47, 101840. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. An overview of microdiesel—A sustainable future source of renewable energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [Green Version]
- Stiles, W.A.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [Green Version]
- González-González, L.; De-Bashan, L. Toward the Enhancement of Microalgal Metabolite Production through Microalgae–Bacteria Consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour. Technol. 2013, 135, 598–603. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Alcántara, C.; Domínguez, J.M.; García, D.; Blanco, S.; Pérez, R.; Garcia-Encina, P.A.; Muñoz, R. Evaluation of wastewater treatment in a novel anoxic–aerobic algal–bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour. Technol. 2015, 191, 173–186. [Google Scholar] [CrossRef]
- Wicker, R.; Bhatnagar, A. Application of Nordic microalgal-bacterial consortia for nutrient removal from wastewater. Chem. Eng. J. 2020, 398, 125567. [Google Scholar] [CrossRef]
- Goswami, G.; Makut, B.B.; Das, D. Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2016, 24, 3315–3335. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, J.; Arora, N.K. Plant Growth-Promoting Rhizobacteria: Diversity and Applications. In Environmental Biotechnology: For Sustainable Future; Sobti, R.C., Arora, N.K., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 129–173. ISBN 978-981-10-7284-0. [Google Scholar]
- Gou, J.-Y.; Suo, S.-Z.; Shao, K.-Z.; Zhao, Q.; Yao, D.; Li, H.-P.; Zhang, J.-L.; Rensing, C. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J. Microbiol. Biotechnol. 2020, 36, 86. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, M.; Shim, C.; Bae, S.; Jang, S. Potential of Algae–Bacteria Synergistic Effects on Vegetable Production. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Prasanna, R.; Joshi, M.; Rana, A.; Shivay, Y.S.; Nain, L. Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C–N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 2011, 28, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Kopta, T.; Pavlíková, M.; Sękara, A.; Pokluda, R.; Maršálek, B. Effect of Bacterial-algal Biostimulant on the Yield and Internal Quality of Lettuce (Lactuca sativa L.) Produced for Spring and Summer Crop. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Horácio, E.H.; Zucareli, C.; Gavilanes, F.Z.; Yunes, J.S.; Sanzovo, A.W.D.S.; Andrade, D.S. Co-inoculation of rhizobia, azospirilla and cyanobacteria for increasing common bean production. Semina: Ciências Agrárias 2020, 41, 2015–2028. [Google Scholar] [CrossRef]
- Gavilanes, F.Z.; Andrade, D.S.; Zucareli, C.; Horácio, E.H.; Yunes, J.S.; Barbosa, A.P.; Alves, L.A.R.; Cruzatty, L.G.; Maddela, N.R.; Guimarães, M.D.F. Co-inoculation of Anabaena cylindrica with Azospirillum brasilense increases grain yield of maize hybrids. Rhizosphere 2020, 15, 100224. [Google Scholar] [CrossRef]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef]
- Mishra, A.; Medhi, K.; Malaviya, P.; Thakur, I.S. Omics approaches for microalgal applications: Prospects and challenges. Bioresour. Technol. 2019, 291, 121890. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, Y.; Richlen, M.L.; Anderson, D.M.; Cai, Z. Quorum Sensing Is a Language of Chemical Signals and Plays an Ecological Role in Algal-Bacterial Interactions. Crit. Rev. Plant Sci. 2016, 35, 81–105. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, Y.M.; Park, J.M. Enhanced microalgal biomass and lipid production from a consortium of indigenous microalgae and bacteria present in municipal wastewater under gradually mixotrophic culture conditions. Bioresour. Technol. 2017, 228, 290–297. [Google Scholar] [CrossRef]
- González-Ballester, D.; Casero, D.; Cokus, S.; Pellegrini, M.; Merchant, S.S.; Grossman, A.R. RNA-Seq Analysis of Sulfur-Deprived Chlamydomonas Cells Reveals Aspects of Acclimation Critical for Cell Survival. Plant Cell 2010, 22, 2058–2084. [Google Scholar] [CrossRef] [Green Version]
- VerBerkmoes, N.C.; Denef, V.J.; Hettich, R.L.; Banfield, J.F. Functional analysis of natural microbial consortia using community proteomics. Nat. Rev. Genet. 2009, 7, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Ravindran, A.; Cheow, E.S.H.; Sze, S.K. Quantitative proteomic analysis of secretome of microbial consortium during saw dust utilization. J. Proteom. 2012, 75, 5590–5603. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.J.; Maruthamuthu, M.; Van Elsas, J.D. Metasecretome analysis of a lignocellulolytic microbial consortium grown on wheat straw, xylan and xylose. Biotechnol. Biofuels 2015, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tan, N.G.J.; Li, S.F.Y. NMR-based metabolomics and LC-MS/MS quantification reveal metal-specific tolerance and redox homeostasis in Chlorella vulgaris. Mol. BioSyst. 2014, 10, 149–160. [Google Scholar] [CrossRef]
- Ortiz-Marquez, J.C.F.; Nascimento, M.D.; Dublan, M.D.L.A.; Curatti, L. Association with an Ammonium-Excreting Bacterium Allows Diazotrophic Culture of Oil-Rich Eukaryotic Microalgae. Appl. Environ. Microbiol. 2012, 78, 2345–2352. [Google Scholar] [CrossRef] [Green Version]
- Weiss, T.L.; Young, E.J.; Ducat, D.C. A synthetic, light-driven consortium of cyanobacteria and heterotrophic bacteria enables stable polyhydroxybutyrate production. Metab. Eng. 2017, 44, 236–245. [Google Scholar] [CrossRef]
- Zuñiga, C.; Zaramela, L.; Zengler, K. Elucidation of complexity and prediction of interactions in microbial communities. Microb. Biotechnol. 2017, 10, 1500–1522. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Zou, X.; Xue, Y.; Qu, Y.; Li, Y. The impact of seasonal variations about temperature and photoperiod on the treatment of municipal wastewater by algae-bacteria system in lab-scale. Algal Res. 2021, 54, 102175. [Google Scholar] [CrossRef]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: Enhancement of nutrients removal and influence of algal biomass generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, W.; Wang, Q.; Guo, S.; Tu, R.; Han, S.-F.; Chen, C.; Xie, G.; Qu, F.; Wang, Q. Enhancement of productivity of Chlorella pyrenoidosa lipids for biodiesel using co-culture with ammonia-oxidizing bacteria in municipal wastewater. Renew. Energy 2020, 151, 598–603. [Google Scholar] [CrossRef]
- Casagli, F.; Zuccaro, G.; Bernard, O.; Steyer, J.-P.; Ficara, E. ALBA: A comprehensive growth model to optimize algae-bacteria wastewater treatment in raceway ponds. Water Res. 2021, 190, 116734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scognamiglio, V.; Giardi, M.T.; Zappi, D.; Touloupakis, E.; Antonacci, A. Photoautotrophs–Bacteria Co-Cultures: Advances, Challenges and Applications. Materials 2021, 14, 3027. https://doi.org/10.3390/ma14113027
Scognamiglio V, Giardi MT, Zappi D, Touloupakis E, Antonacci A. Photoautotrophs–Bacteria Co-Cultures: Advances, Challenges and Applications. Materials. 2021; 14(11):3027. https://doi.org/10.3390/ma14113027
Chicago/Turabian StyleScognamiglio, Viviana, Maria Teresa Giardi, Daniele Zappi, Eleftherios Touloupakis, and Amina Antonacci. 2021. "Photoautotrophs–Bacteria Co-Cultures: Advances, Challenges and Applications" Materials 14, no. 11: 3027. https://doi.org/10.3390/ma14113027
APA StyleScognamiglio, V., Giardi, M. T., Zappi, D., Touloupakis, E., & Antonacci, A. (2021). Photoautotrophs–Bacteria Co-Cultures: Advances, Challenges and Applications. Materials, 14(11), 3027. https://doi.org/10.3390/ma14113027









