Application of Ionic Liquids for Batteries and Supercapacitors
Abstract
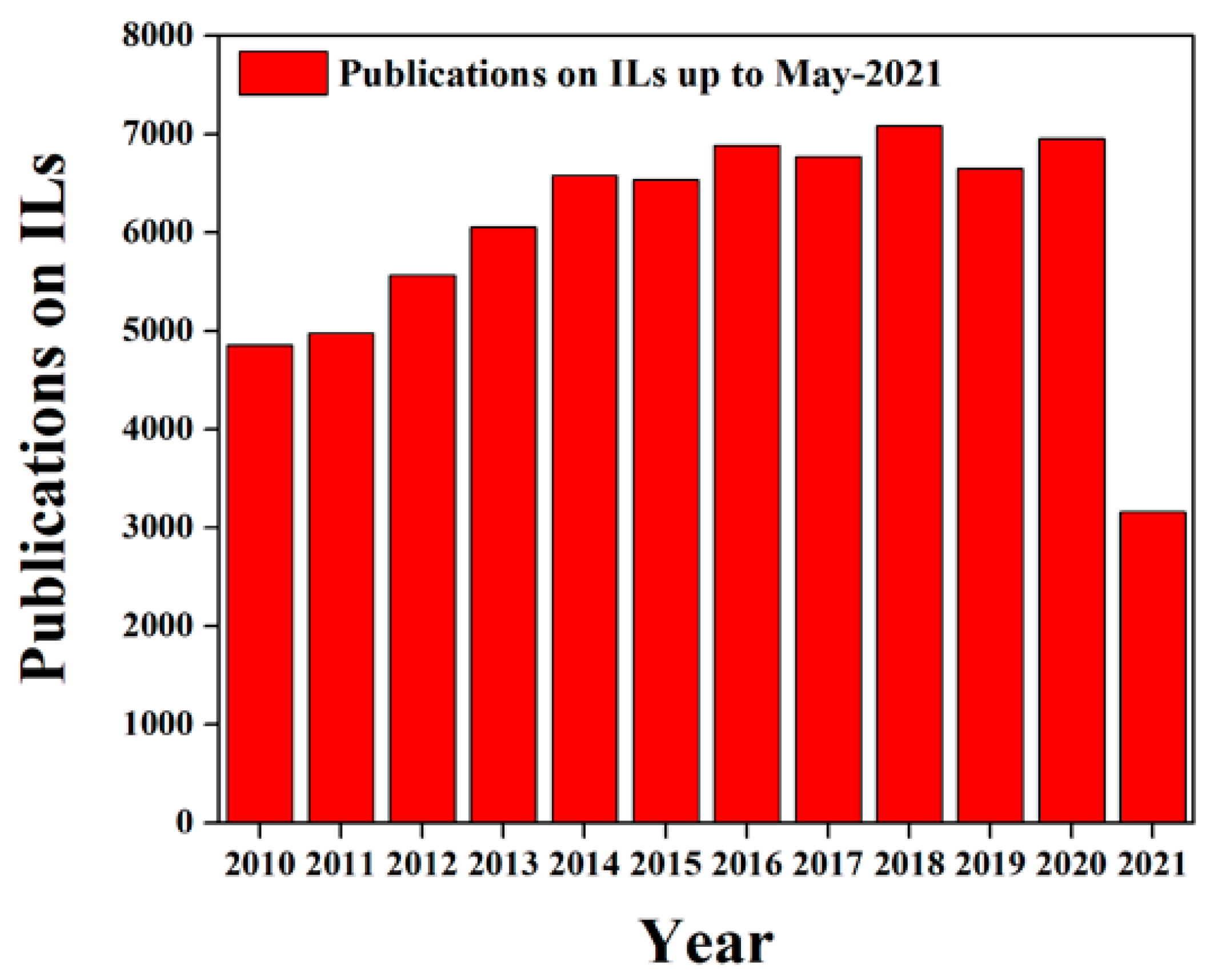
1. Introduction
2. Types of Ionic Liquids (ILs)
2.1. Imidazolium-Based ILs
2.2. Pyrrolidinum-Based ILs
2.3. Quaternary Ammonium-Based ILs
2.4. Pyridinium-Based ILs
2.5. Phosphonium-Based ILs
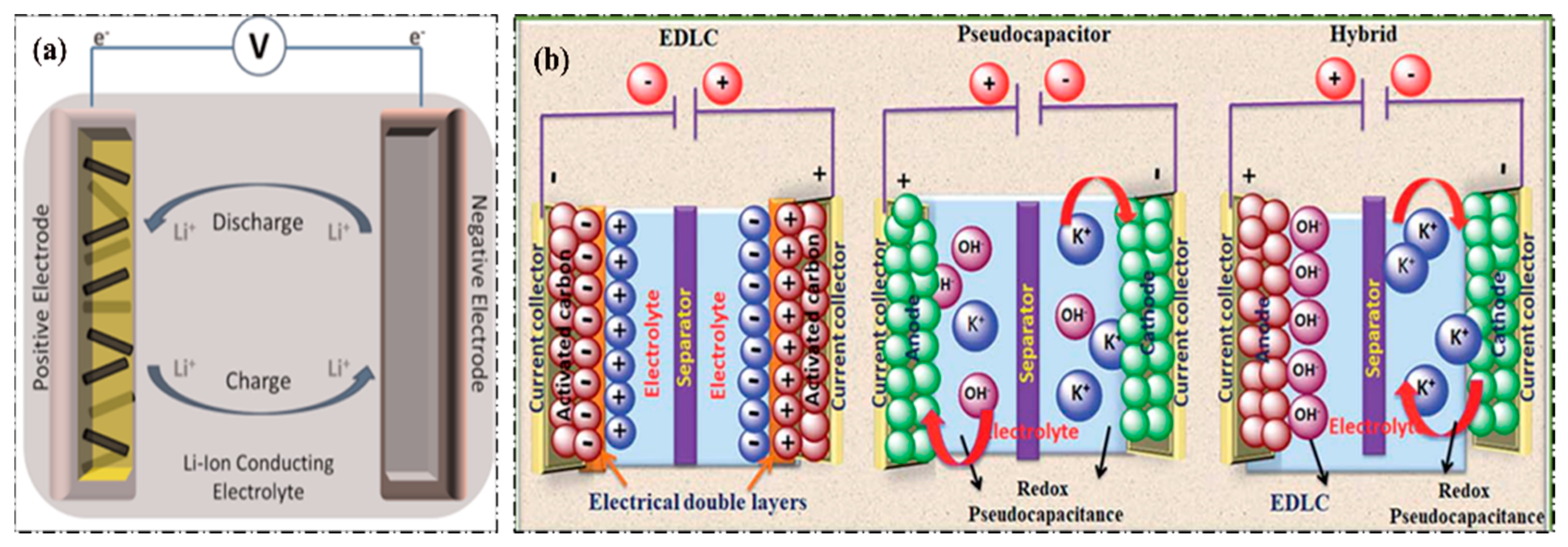
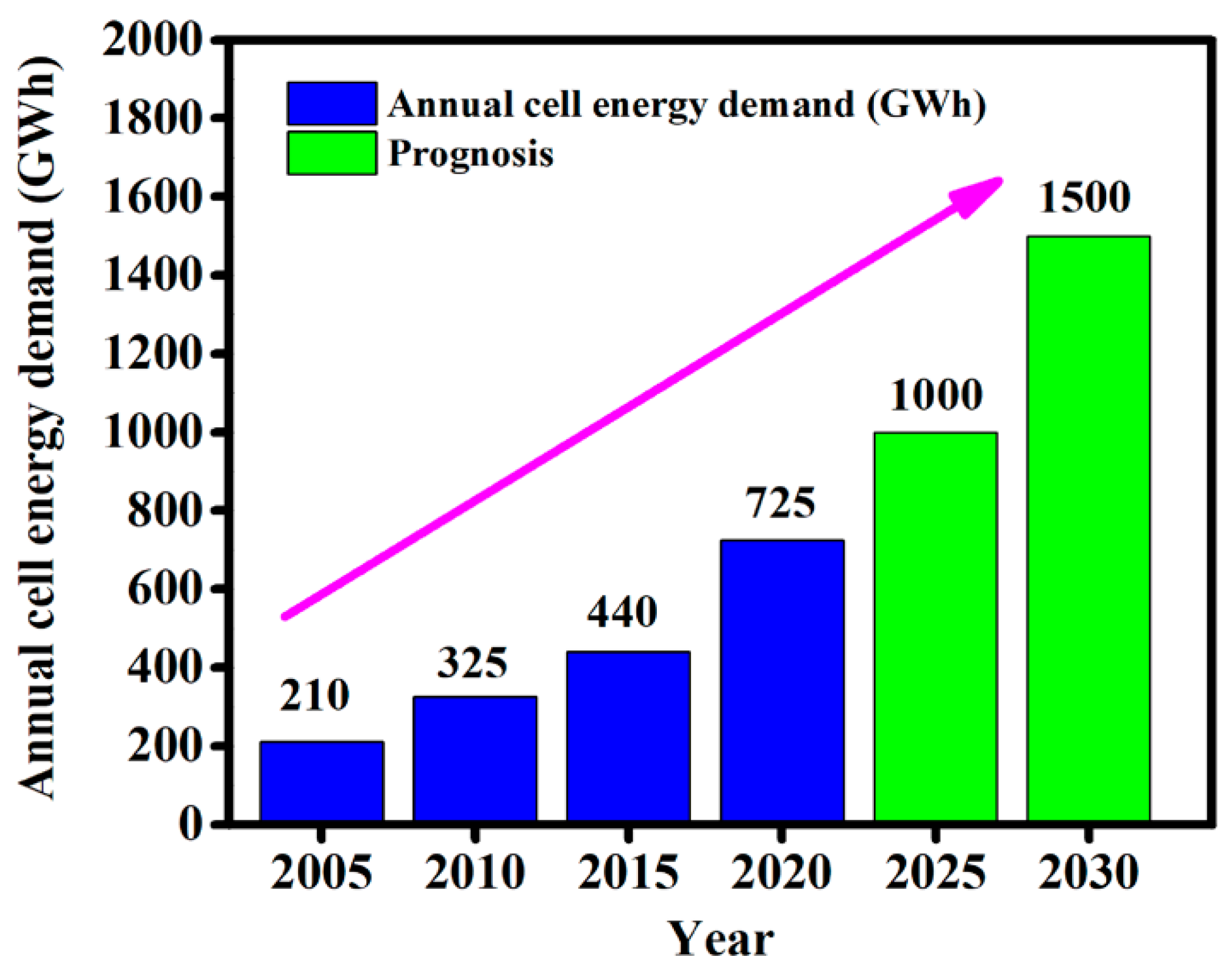
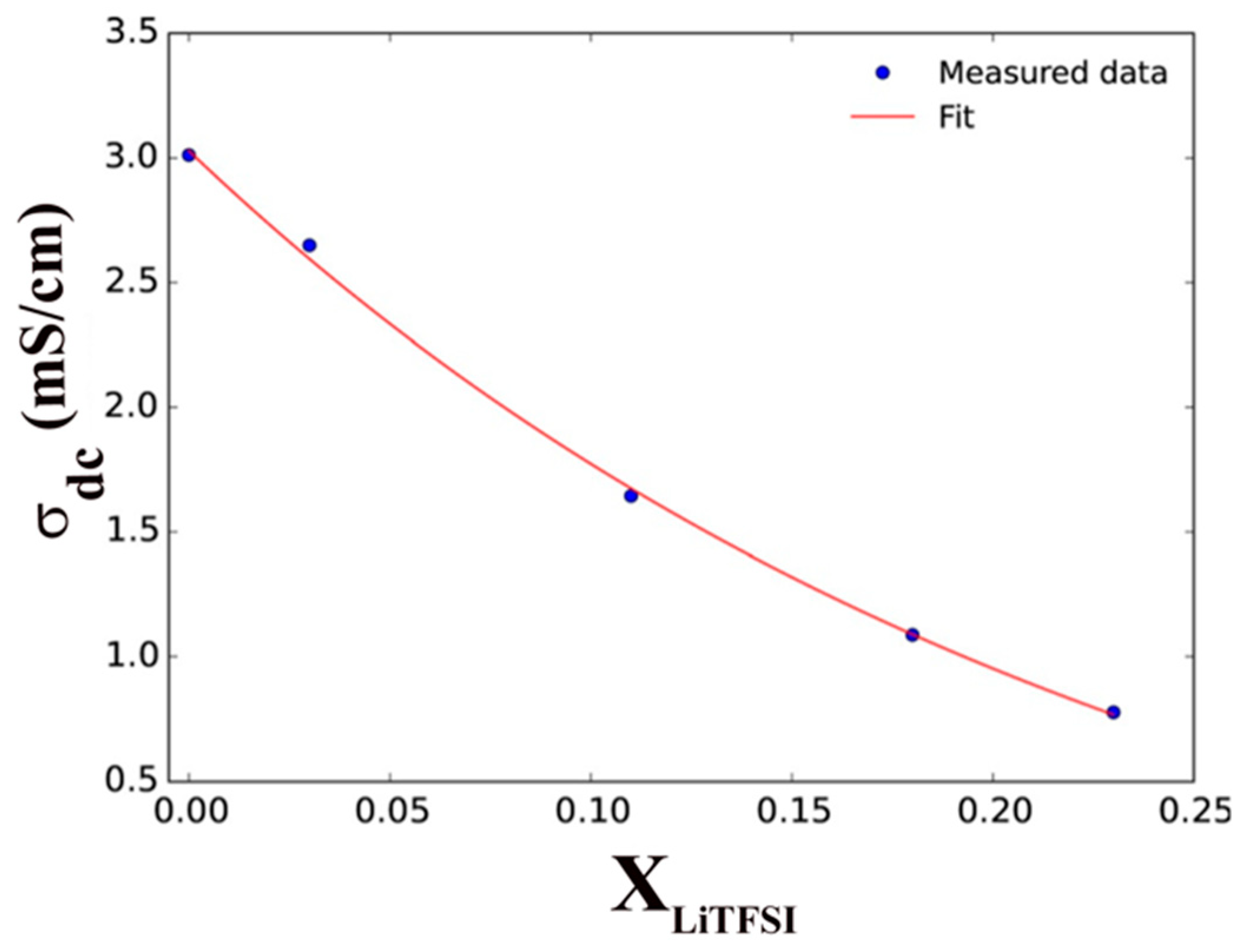
3. Ionic Liquids (ILs) for Li-ion Batteries
 Al), low cost, most abundant and most significantly high volumetric capacity (8040 mAh/g), and high gravimetric capacity (2980 mAh/g), just in the second position next to LIBs (3870 mAh/g) [99,112]. However, many studies are still ongoing, and state of the art of AIBs, carbon-based electrodes and ILs electrolytes combinations have proved to be one of the most suitable combinations due to their synergistic effect and many research groups also focus on AlCl3-type ILs electrolytes in AIBs [117,118]. Furthermore, inorganic material (e.g., V2O5) cathodes and ILs electrolytes such as AlCl3/(EMIm)Cl can be useful in AIBs. However, to address the corrosive effect of AlCl3-type ILs to Al foil, different combinations of ILs such as AlCl3/(BMIm)Cl, Al(TfO)3/(BMIm)TfO, etc., have been examined to develop the advantages of AIBs in real-life applications over LIBs. From a practical point of view, the progress of AIBs is still lagging due to challenges and difficulties in finding high-performance electrode materials and electrolytes [99,100,112,119].
Al), low cost, most abundant and most significantly high volumetric capacity (8040 mAh/g), and high gravimetric capacity (2980 mAh/g), just in the second position next to LIBs (3870 mAh/g) [99,112]. However, many studies are still ongoing, and state of the art of AIBs, carbon-based electrodes and ILs electrolytes combinations have proved to be one of the most suitable combinations due to their synergistic effect and many research groups also focus on AlCl3-type ILs electrolytes in AIBs [117,118]. Furthermore, inorganic material (e.g., V2O5) cathodes and ILs electrolytes such as AlCl3/(EMIm)Cl can be useful in AIBs. However, to address the corrosive effect of AlCl3-type ILs to Al foil, different combinations of ILs such as AlCl3/(BMIm)Cl, Al(TfO)3/(BMIm)TfO, etc., have been examined to develop the advantages of AIBs in real-life applications over LIBs. From a practical point of view, the progress of AIBs is still lagging due to challenges and difficulties in finding high-performance electrode materials and electrolytes [99,100,112,119].4. Ionic Liquids (ILs) for Supercapacitors (SCs)
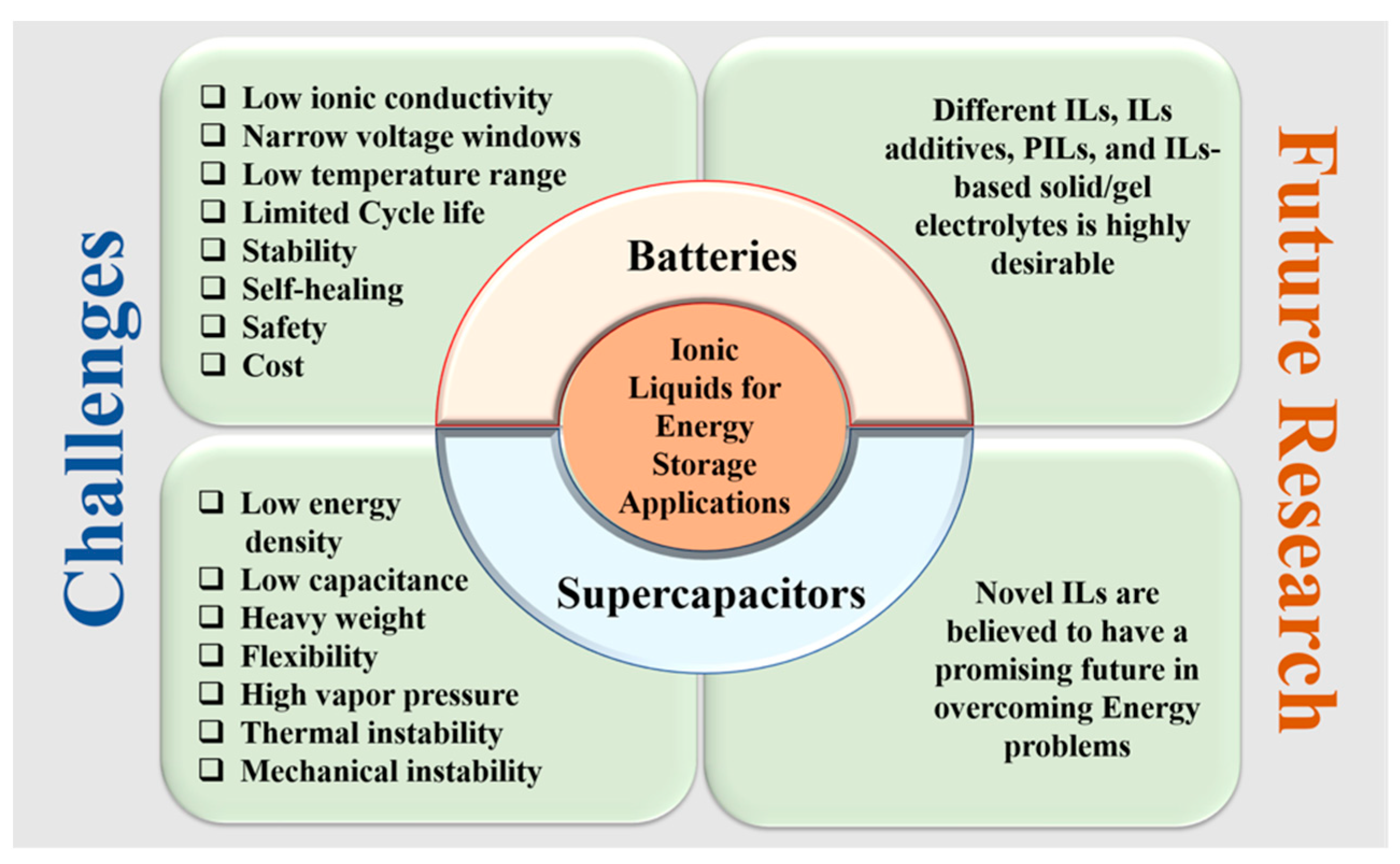
5. Challenges and Future Motivation for ILs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Seol, M.L.; Nam, I.; Sadatian, E.; Dutta, N.; Han, J.W.; Meyyappan, M. Printable gel polymer electrolytes for solid-state printed supercapacitors. Materials 2021, 14, 316. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Roy, A.; Ghosh, M.; Ramos-ramón, J.A.; Saha, S. Applied Surface Science Study on charge storage mechanism in working electrodes fabricated by sol- gel derived spinel NiMn2O4 nanoparticles for supercapacitor application. Appl. Surf. Sci. 2019, 463, 513–525. [Google Scholar] [CrossRef]
- Du, Y.; Mo, Y.; Chen, Y. Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors. Materials 2021, 14, 1908. [Google Scholar] [CrossRef]
- Yin, C.; Yang, C.; Jiang, M.; Deng, C.; Yang, L.; Li, J.; Qian, D. A Novel and Facile One-Pot Solvothermal Synthesis of PEDOT-PSS/Ni-Mn-Co-O Hybrid as an Advanced Supercapacitor Electrode Material. ACS Appl. Mater. Interfaces 2016, 8, 2741–2752. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, C.; Wang, J.; Cao, L.; Ma, Z.; Yuan, Q.; Lin, Z.; Zhang, P. Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors. Materials 2021, 14, 1714. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, H.M.R.; Pereira, R.F.P.; Lepleux, E.; Pacheco, L.; Valente, A.J.M.; Duarte, A.J.; de Zea Bermudez, V. Non-Newtonian Thermosensitive Nanofluid Based on Carbon Dots Functionalized with Ionic Liquids. Small 2020, 16. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, H.; Yang, Z.; Li, J. Synthesis and Electrochemical Properties of LiNi0.5Mn1.5O4 for Li-Ion Batteries by the Metal-Organic Framework Method. ACS Appl. Mater. Interfaces 2018, 10, 13624–13634. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, H.; Li, J. Influence of doped anions on PEDOT/Ni-Mn-Co-O for supercapacitor electrode material. Appl. Surf. Sci. 2019, 464, 220–228. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Feng, Z.; Li, X.; Yi, R.; Sun, W.; Zhao, C.; Yang, L. Nitrogen-doped hierarchical porous activated carbon derived from paddy for high-performance supercapacitors. Materials 2021, 14, 318. [Google Scholar] [CrossRef]
- Maji, P.; Ray, A.; Sadhukhan, P.; Roy, A.; Das, S. Fabrication of symmetric supercapacitor using cesium lead iodide (CsPbI3) microwire. Mater. Lett. 2018, 227, 268–271. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Ray, A.; Das, T.; Nandi, M.; Ghosh, B.; Das, S. Effect of particle morphology on the electrochemical performance of hydrothermally synthesized NiMn2O. Electrochim. Acta 2020, 353, 136515. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Das, S. Investigation on energy storage and conversion properties of multifunctional PANI-MWCNT composite. Int. J. Hydrog. Energy 2018, 43, 7128–7139. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Saha, S.; Das, S. Transition Metal Oxide-Based Nano-materials for Energy Storage Application. In Science, Technology and Advanced Application of Supercapacitors; IntechOpen: Rijeka, Croatia, 2018; pp. 89–106. [Google Scholar]
- Saha, S.; Sadhukhan, P.; Roy Chowdhury, S.; Das, S. Rotary-Jet spin assisted fabrication of MnO2 microfiber for supercapacitor electrode application. Mater. Lett. 2020, 277, 128342. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Sadhukhan, P.; Naskar, K.; Lal, G. Polyaniline-multiwalled carbon nanotube (PANI-MWCNT): Room temperature resistive carbon monoxide (CO) sensor. Synth. Met. 2018, 245, 182–189. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Sadhukhan, P.; Roy, S.; Maji, P.; Kumar, S.; Das, S. Applied Surface Science Electrochemical properties of TiO2-V2 O5 nanocomposites as a high performance supercapacitors electrode material. Appl. Surf. Sci. 2018, 443, 581–591. [Google Scholar] [CrossRef]
- Shen, F.; Sun, Z.; He, Q.; Sun, J.; Kaner, R.B.; Shao, Y. Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage. Mater. Horiz. 2021, 8, 1130–1152. [Google Scholar] [CrossRef]
- Banda, H.; Dou, J.H.; Chen, T.; Libretto, N.J.; Chaudhary, M.; Bernard, G.M.; Miller, J.T.; Michaelis, V.K.; Dincǎ, M. High-Capacitance Pseudocapacitors from Li+ Ion Intercalation in Nonporous, Electrically Conductive 2D Coordination Polymers. J. Am. Chem. Soc. 2021, 143, 2285–2292. [Google Scholar] [CrossRef]
- Sankar, K.V.; Lee, S.C.; Seo, Y.; Ray, C.; Liu, S.; Kundu, A.; Jun, S.C. Binder-free cobalt phosphate one-dimensional nanograsses as ultrahigh-performance cathode material for hybrid supercapacitor applications. J. Power Sources 2018, 373, 211–219. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Nandi, M.; Lal, G.; Das, S. Influence of electrochemical active surface area on the oxygen evolution reaction and energy storage performance of MnO2-multiwalled carbon nanotube composite. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Deb, D.; Bose, P.; Bhattacharya, S. Gel-polymer electrolytes plasticized with pyrrolidinium-based ionanofluid for lithium battery applications. Ionics 2021, 27, 123–136. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Kwak, H.; Lyoo, J.; Park, J.; Han, Y.; Asakura, R.; Remhof, A.; Battaglia, C.; Kim, H.; Hong, S.T.; Jung, Y.S. Na2ZrCl6 enabling highly stable 3 V all-solid-state Na-ion batteries. Energy Storage Mater. 2021, 37, 47–54. [Google Scholar] [CrossRef]
- Kwak, H.; Han, D.; Lyoo, J.; Park, J.; Jung, S.H.; Han, Y.; Kwon, G.; Kim, H.; Hong, S.T.; Nam, K.W.; et al. New Cost-Effective Halide Solid Electrolytes for All-Solid-State Batteries: Mechanochemically Prepared Fe3+-Substituted Li2ZrCl. Adv. Energy Mater. 2021, 11, 2003190. [Google Scholar] [CrossRef]
- Gupta, S.; Varshney, P.K. Effect of plasticizer concentration on structural and electrical properties of hydroxyethyl cellulose (HEC)-based polymer electrolyte. Ionics 2017, 23, 1613–1617. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Huang, S.S.; Lin, Y.H.; Lin, Y.C.; Shih, B.Y.; Sheu, H.S.; Liao, Y.F.; Wu, N.L. High-performance carbon-coated ZnMn2O4 nanocrystallite supercapacitors with tailored microstructures enabled by a novel solution combustion method. J. Power Sources 2018, 378, 90–97. [Google Scholar] [CrossRef]
- Jaumaux, P.; Wu, J.; Shanmukaraj, D.; Wang, Y.; Zhou, D.; Sun, B.; Kang, F.; Li, B.; Armand, M.; Wang, G. Non-Flammable Liquid and Quasi-Solid Electrolytes toward Highly-Safe Alkali Metal-Based Batteries. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Aziz, S.B.; Nofal, M.M.; Hamsan, M.H.; Brza, M.A.; Yusof, Y.M.; Abdilwahid, R.T.; Muzakir, S.K.; Kadir, M.F.Z. Glycerolized Li+ ion conducting chitosan-based polymer electrolyte for energy storage EDLC device applications with relatively high energy density. Polymers 2020, 12, 1433. [Google Scholar] [CrossRef]
- Oldiges, K.; Diddens, D.; Ebrahiminia, M.; Hooper, J.B.; Cekic-Laskovic, I.; Heuer, A.; Bedrov, D.; Winter, M.; Brunklaus, G. Understanding transport mechanisms in ionic liquid/carbonate solvent electrolyte blends. Phys. Chem. Chem. Phys. 2018, 20, 16579–16591. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Comparing triflate and hexafluorophosphate anions of ionic liquids in polymer electrolytes for supercapacitor applications. Materials 2014, 7, 4019–4033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, Y.; Montazami, R. Ionic liquid-doped gel polymer electrolyte for flexible lithium-ion polymer batteries. Materials 2015, 8, 2735–2748. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of ionic liquids in hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Flieger, J.; Grushka, E.B.; Czajkowska-Żelazko, A. Ionic Liquids as Solvents in Separation Processes. Austin J. Anal. Pharm. Chem. 2014, 1, 1009–1016. [Google Scholar]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.S. Ionic liquid-based electrolytes for energy storage devices: A brief review on their limits and applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef]
- Wishart, J.F. Energy applications of ionic liquids. Energy Environ. Sci. 2009, 2, 956–961. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Chiarotto, I.; Mattiello, L.; Pandolfi, F.; Rocco, D.; Feroci, M. NHC in imidazolium acetate ionic liquids: Actual or potential presence? Front. Chem. 2018, 6, 355. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Design of Stretchable and Self-Healing Gel Electrolytes via Fully Zwitterionic Polymer Networks in Solvate Ionic Liquids for Li-Based Batteries. Chem. Mater. 2019, 31, 2913–2922. [Google Scholar] [CrossRef]
- Weingarth, D.; Czekaj, I.; Fei, Z.; Foelske-Schmitz, A.; Dyson, P.J.; Wokaun, A.; Kötz, R. Electrochemical Stability of Imidazolium Based Ionic Liquids Containing Cyano Groups in the Anion: A Cyclic Voltammetry, XPS and DFT Study. J. Electrochem. Soc. 2012, 159, H611–H615. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, Z.; Wang, H.; Sun, X.; Lu, L.; Zhao, X.S. A high-energy-density supercapacitor with graphene-CMK-5 as the electrode and ionic liquid as the electrolyte. J. Mater. Chem. A 2013, 1, 2313–2321. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghamouss, F.; Ghosh, A.; Achak, O.; Chafik, T. Activated carbon with exceptionally high surface area and tailored nanoporosity obtained from natural anthracite and its use in supercapacitors. J. Power Sources 2019, 436, 226882. [Google Scholar] [CrossRef]
- Momodu, D.; Sylla, N.F.; Mutuma, B.; Bello, A.; Masikhwa, T.; Lindberg, S.; Matic, A.; Manyala, N. Stable ionic-liquid-based symmetric supercapacitors from Capsicum seed-porous carbons. J. Electroanal. Chem. 2019, 838, 119–128. [Google Scholar] [CrossRef]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated graphene-based carbons as supercapacitor electrodes with macro- and mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef]
- Pham, D.T.; Lee, T.H.; Luong, D.H.; Yao, F.; Ghosh, A.; Le, V.T.; Kim, T.H.; Li, B.; Chang, J.; Lee, Y.H. Carbon nanotube-bridged graphene 3D building blocks for ultrafast compact supercapacitors. ACS Nano 2015, 9, 2018–2027. [Google Scholar] [CrossRef]
- Que, M.; Tong, Y.; Wei, G.; Yuan, K.; Wei, J.; Jiang, Y.; Zhu, H.; Chen, Y. Safe and flexible ion gel based composite electrolyte for lithium batteries. J. Mater. Chem. A 2016, 4, 14132–14140. [Google Scholar] [CrossRef]
- Kerner, M.; Johansson, P. Pyrrolidinium FSI and TFSI-based polymerized ionic liquids as electrolytes for high-temperature lithium-ion batteries. Batteries 2018, 4, 10. [Google Scholar] [CrossRef]
- Vogl, T.; Menne, S.; Kühnel, R.S.; Balducci, A. The beneficial effect of protic ionic liquids on the lithium environment in electrolytes for battery applications. J. Mater. Chem. A 2014, 2, 8258–8265. [Google Scholar] [CrossRef]
- Kirchner, B.; Perlt, E. Ionic Liquids II, 1st ed.; Springer: Cham, Switzerland, 2018; p. 289. ISBN 9783319897936. [Google Scholar] [CrossRef]
- Asha, S.; Vijayalakshmi, K.P.; George, B.K. Pyrrolidinium-based ionic liquids as electrolytes for lithium batteries: A Computational Study. Int. J. Quantum Chem. 2019, 119, e26014. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Zhou, J.; Liu, J.; Zhang, Q. Pyrrolidinium-based ionic liquid electrolyte with organic additive and LiTFSI for high-safety lithium-ion batteries. Electrochim. Acta 2014, 148, 39–45. [Google Scholar] [CrossRef]
- Arbizzani, C.; Beninati, S.; Lazzari, M.; Soavi, F.; Mastragostino, M. Electrode materials for ionic liquid-based supercapacitors. J. Power Sources 2007, 174, 648–652. [Google Scholar] [CrossRef]
- Lazzari, M.; Mastragostino, M.; Soavi, F. Capacitance response of carbons in solvent-free ionic liquid electrolytes. Electrochem. Commun. 2007, 9, 1567–1572. [Google Scholar] [CrossRef]
- Xu, N.; Klein, J.M.; Huang, P.; Alwusaydi, H.A.; Mann, E.K.; Gurkan, B.E. Improved accessibility of porous carbon electrodes with surfactant ionic liquids for supercapacitors. J. Appl. Electrochem. 2019, 49, 151–162. [Google Scholar] [CrossRef]
- Quezada, D.; Honores, J.; Ruiz-León, D. Zinc Stannate as anode and Pyrrolidinium-Based Room Temperature Ionic Liquid as electrolyte for Lithium-ion Cells. Int. J. Electrochem. Sci. 2021, 16. [Google Scholar] [CrossRef]
- Kalinova, R.; Dimitrov, I.; Novakov, C.; Veleva, S. Modular Platform for Synthesis of Poly (Ionic Liquid) Electrolytes for Electrochemical Applications in Supercapacitors. Chem. Sel. 2021, 6, 3795–3801. [Google Scholar] [CrossRef]
- Seki, S.; Ohno, Y.; Miyashiro, H.; Kobayashi, Y.; Usami, A.; Mita, Y.; Terada, N.; Hayamizu, K.; Tsuzuki, S.; Watanabe, M. Quaternary Ammonium Room-Temperature Ionic Liquid/Lithium Salt Binary Electrolytes: Electrochemical Study. J. Electrochem. Soc. 2008, 155, A421. [Google Scholar] [CrossRef]
- Sato, T.; Masuda, G.; Takagi, K. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim. Acta 2004, 49, 3603–3611. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Gunawan, C.A.; Suryanto, B.H.R.; Zhao, C. Electrochemical Study of Copper in Room Temperature Protic Ionic Liquids Ethylammonium Nitrate and Triethylammonium Methylsulfonate. J. Electrochem. Soc. 2012, 159, D611–D615. [Google Scholar] [CrossRef]
- Cho, S.D.; Im, J.K.; Kim, H.K.; Kim, H.S.; Park, H.S. Functionalization of reduced graphene oxides by redox-active ionic liquids for energy storage. Chem. Commun. 2012, 48, 6381–6383. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; De Visscher, A.; Gates, I.D. Comparison of Electronic and Physicochemical Properties between Imidazolium-Based and Pyridinium-Based Ionic Liquids. J. Phys. Chem. B 2018, 122, 6771–6780. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Vikraman, D.; Hwang, I.T.; Kim, H.J.; Nichelson, A.; Bose, R.; Kim, H.S. Nonaqueous liquid electrolytes based on novel 1-ethyl-3-methylimidazolium bis (nonafluorobutane-1-sulfonyl imidate) ionic liquid for energy storage devices. J. Mater. Res. Technol. 2020, 9, 1251–1260. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Liu, G.; Li, J.; Tian, H.; Li, J.; Ma, Y. Scalable Self-Propagating High-Temperature Synthesis of Graphene for Supercapacitors with Superior Power Density and Cyclic Stability. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Chen, M.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 2020, 19, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Patra, J.; Hsieh, C.T.; Li, J.; Dong, Q.F.; Chang, J.K. Supercapacitive Properties of Micropore- and Mesopore-Rich Activated Carbon in Ionic-Liquid Electrolytes with Various Constituent Ions. ChemSusChem 2019, 12, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Young, K.H.; Wong, D.F.; Nei, J. Ionic liquid-based non-aqueous electrolytes for nickel/metal hydride batteries. Batteries 2017, 3, 4. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, J.H.; Lee, S.Y.; Shim, E.G.; Kim, D.W. Cycling performance and thermal stability of lithium polymer cells assembled with ionic liquid-containing gel polymer electrolytes. J. Power Sources 2011, 196, 6750–6755. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of Ionic Liquids as High-Voltage Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, X.; Xu, J.; Kamali, A.; Shi, Z. Temperature dependence on density, viscosity, and electrical conductivity of ionic liquid 1-ethyl-3-methylimidazolium fluoride. Appl. Sci. 2018, 8, 356. [Google Scholar] [CrossRef]
- Ababtain, K.; Babu, G.; Lin, X.; Rodrigues, M.T.F.; Gullapalli, H.; Ajayan, P.M.; Grinstaff, M.W.; Arava, L.M.R. Ionic Liquid-Organic Carbonate Electrolyte Blends to Stabilize Silicon Electrodes for Extending Lithium Ion Battery Operability to 100 °C. ACS Appl. Mater. Interfaces 2016, 8, 15242–15249. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, G. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Khalid, S.; Cao, C.; Naveed, M.; Younas, W. 3D hierarchical MnO2 microspheres: A prospective material for high performance supercapacitors and lithium-ion batteries. Sustain. Energy Fuels 2017, 1, 1795–1804. [Google Scholar] [CrossRef]
- Wei, J.H.; Yi, J.W.; Han, M.L.; Li, B.; Liu, S.; Wu, Y.P.; Ma, L.F.; Li, D.S. A Water-Stable Terbium(III)—Organic Framework as a Chemosensor for Inorganic Ions, Nitro-Containing Compounds and Antibiotics in Aqueous Solutions. Chem. Asian J. 2019, 14, 3694–3701. [Google Scholar] [CrossRef]
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.; Mouritz, A.P. Energy Storage Structural Composites with Integrated Lithium-Ion Batteries: A Review. Adv. Mater. Technol. 2021, 2001059. [Google Scholar] [CrossRef]
- Inoue, T.; Mukai, K. Are all-solid-state lithium-ion batteries really safe?—Verification by differential scanning calorimetry with an all-inclusive microcell. ACS Appl. Mater. Interfaces 2017, 9, 1507–1515. [Google Scholar] [CrossRef]
- Bartsch, T.; Strauss, F.; Hatsukade, T.; Schiele, A.; Kim, A.Y.; Hartmann, P.; Janek, J.; Brezesinski, T. Gas Evolution in All-Solid-State Battery Cells. ACS Energy Lett. 2018, 3, 2539–2543. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Pillot, C. Micro hybrid, HEV, P-HEV and EV market 2012–2025 impact on the battery business. In Proceedings of the World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Murphin Kumar, P.S.; Al-Muhtaseb, A.H.; Kumar, G.; Vinu, A.; Cha, W.; Villanueva Cab, J.; Pal, U.; Krishnan, S.K. Piper longum Extract-Mediated Green Synthesis of Porous Cu2O:Mo Microspheres and Their Superior Performance as Active Anode Material in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 14557–14567. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Zhou, C.; Guo, S.; Li, S.; Yang, Y.; Wu, J.; Yu, D.; Chen, H.; Zhao, M.; et al. Polypyrrole-derived nitrogen-doped carbon coated hierarchical MnO porous microspheres for highly reversible lithium storage. J. Electroanal. Chem. 2020, 856, 113733. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Wang, M.; Tang, Y. A Review on the Features and Progress of Dual-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703320. [Google Scholar] [CrossRef]
- Wang, W.Y.; Shang, S.L.; Wang, Y.; Han, F.; Darling, K.A.; Wu, Y.; Xie, X.; Senkov, O.N.; Li, J.; Hui, X.D.; et al. Atomic and electronic basis for the serrations of refractory high-entropy alloys. NPJ Comput. Mater. 2017, 3, 23. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- Rothermel, S.; Meister, P.; Schmuelling, G.; Fromm, O.; Meyer, H.W.; Nowak, S.; Winter, M.; Placke, T. Dual-graphite cells based on the reversible intercalation of bis(trifluoromethanesulfonyl)imide anions from an ionic liquid electrolyte. Energy Environ. Sci. 2014, 7, 3412–3423. [Google Scholar] [CrossRef]
- Balabajew, M.; Kranz, T.; Roling, B. Ion-Transport Processes in Dual-Ion Cells Utilizing a Pyr1,4TFSI/LiTFSI Mixture as the Electrolyte. ChemElectroChem 2015, 2, 1991–2000. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Carrete, J.; Bouzón-Capelo, S.; Pérez-Rodríguez, M.; Cabeza, Ó.; Gallego, L.J.; Varela, L.M. MD simulations of the formation of stable clusters in mixtures of alkaline salts and imidazolium-based ionic liquids. J. Phys. Chem. B 2013, 117, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, W. Recent Advances in linear and nonlinear Raman spectroscopy I. J. Raman Spectrosc. 2007, 38, 1538–1553. [Google Scholar] [CrossRef]
- Marczewski, M.J.; Stanje, B.; Hanzu, I.; Wilkening, M.; Johansson, P. Ionic Liquids-in-Salt—A Promising Electrolyte Concept for High-Temperature Lithium Batteries? Phys. Chem. Chem. Phys. 2014, 16, 12341–12349. [Google Scholar] [CrossRef]
- Balo, L.; Gupta, H.; Singh, V.K.; Singh, R.K. Flexible gel polymer electrolyte based on ionic liquid EMIMTFSI for rechargeable battery application. Electrochim. Acta 2017, 230, 123–131. [Google Scholar] [CrossRef]
- Chatterjee, K.; Pathak, A.D.; Lakma, A.; Sharma, C.S.; Sahu, K.K.; Singh, A.K. Synthesis, characterization and application of a non-flammable dicationic ionic liquid in lithium-ion battery as electrolyte additive. Sci. Rep. 2020, 10, 9606. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhang, X.; Tang, Y. A Review of Emerging Dual-Ion Batteries: Fundamentals and Recent Advances. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Lei, X.; Zheng, Y.; Zhang, F.; Wang, Y.; Tang, Y. Highly stable magnesium-ion-based dual-ion batteries based on insoluble small-molecule organic anode material. Energy Storage Mater. 2020, 30, 34–41. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, K.; Wu, F.; Bai, Y.; Wu, C. Ionic Liquid-Based Electrolytes for Aluminum/Magnesium/Sodium-Ion Batteries. Energy Mater. Adv. 2021, 2021. [Google Scholar] [CrossRef]
- Giffin, G.A. Ionic liquid-based electrolytes for “beyond lithium” battery technologies. J. Mater. Chem. A 2016, 4, 13378–13389. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.; Thi, L.; Linh, M.; Thi, H.; Tuyen, K.; Van Hoang, N.; Thanh, V.D. Safe sodium-ion battery using hybrid electrolytes of organic solvent/pyrrolidinium ionic liquid. Vietnam J. Chem. 2021, 59, 17–26. [Google Scholar] [CrossRef]
- Xu, C.; Yang, G.; Wu, D.; Yao, M.; Xing, C.; Zhang, J.; Zhang, H.; Li, F.; Feng, Y.; Qi, S.; et al. Roadmap on Ionic Liquid Electrolytes for Energy Storage Devices. Chem. Asian J. 2021, 16, 549–562. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Lu, S.; Xiang, Y. Carbon Anode Materials: A Detailed Comparison between Na-ion and K-ion Batteries. Adv. Energy Mater. 2021, 11. [Google Scholar] [CrossRef]
- Lourenço, T.C.; Dias, L.G.; da Silva, J.L.F. Theoretical Investigation of the Na+ Transport Mechanism and Electrolyte Performance in Ionic Liquids Sodium-ion Electrolytes. ACS Appl. Energy Mater. 2021. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Ding, F.; Meng, Q.; Yu, P.; Wang, H.; Niu, Y.; Li, Y.; Yang, Y.; Rong, X.; Liu, X.; Lu, Y.; et al. Additive-Free Self-Presodiation Strategy for High-Performance Na-Ion Batteries. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsubara, R.; Nohira, T. Highly conductive ionic liquid electrolytes for potassium-ion batteries. J. Chem. Eng. Data 2021, 66, 1081–1088. [Google Scholar] [CrossRef]
- Yamamoto, T.; Matsumoto, K.; Hagiwara, R.; Nohira, T. Physicochemical and Electrochemical Properties of K[N(SO2F)2]-[N-Methyl-N-propylpyrrolidinium][N(SO2F)2] Ionic Liquids for Potassium-Ion Batteries. J. Phys. Chem. C 2017, 121, 18450–18458. [Google Scholar] [CrossRef]
- Fiore, M.; Wheeler, S.; Hurlbutt, K.; Capone, I.; Fawdon, J.; Ruffo, R.; Pasta, M. Paving the Way toward Highly Efficient, High-Energy Potassium-Ion Batteries with Ionic Liquid Electrolytes. Chem. Mater. 2020, 32, 7653–7661. [Google Scholar] [CrossRef]
- Liu, X.; Elia, G.A.; Gao, X.; Qin, B.; Zhang, H.; Passerini, S. Highly Concentrated KTFSI: Glyme Electrolytes for K/Bilayered-V2 O5 Batteries. Batter. Supercaps 2020, 3, 261–267. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, M.; Kong, X.; Huang, W.; Zhang, Q. Recent Advance in Ionic-Liquid-Based Electrolytes for Rechargeable Metal-Ion Batteries. Adv. Sci. 2021. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Yang, W.; Du, X.; Zhao, J.; Chen, Z.; Li, J.; Xie, J.; Zhang, Y.; Cui, Z.; Kong, Q.; Zhao, Z.; et al. Hydrated Eutectic Electrolytes with Ligand-Oriented Solvation Shells for Long-Cycling Zinc-Organic Batteries. Joule 2020, 4, 1557–1574. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.L.; Lei, H.; Yu, Z.; Chen, L.L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, S.; Bai, Y.; Chen, S.; Wu, F.; Wu, C. High-Voltage and Noncorrosive Ionic Liquid Electrolyte Used in Rechargeable Aluminum Battery. ACS Appl. Mater. Interfaces 2016, 8, 27444–27448. [Google Scholar] [CrossRef]
- Ray, A.; Korkut, D.; Saruhan, B. Efficient flexible all-solid supercapacitors with direct sputter-grown needle-like Mn/MnOx@ Graphite-foil electrodes and ppc-embedded ionic electrolytes. Nanomaterials 2020, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Ivol, F.; Porcher, M.; Ghosh, A.; Jacquemin, J. Phenylacetonitrile and Ionic Liquid Blends as Alternative Electrolytes for Safe and High-Performance Supercapacitors. Molecules 2020, 25, 2697. [Google Scholar] [CrossRef]
- Yadav, H.M.; Park, J.D.; Kang, H.C.; Kim, J.; Lee, J.J. Cellulose nanofiber composite with bimetallic zeolite imidazole framework for electrochemical supercapacitors. Nanomaterials 2021, 11, 395. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Ma, Y.; Song, H.; Pi, C.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. In-situ synthesis of heterostructured carbon-coated Co/MnO nanowire arrays for high-performance anodes in asymmetric supercapacitors. Molecules 2020, 25, 3218. [Google Scholar] [CrossRef]
- Roy, A.; Cancino-Gordillo, F.E.; Saha, S.; Pal, U.; Das, S. Performance of asymmetric supercapacitor fabricated with perovskite-type Sr2+-incorporated LaMnO3 (La0.7 Sr0.3 MnO3) nanostructures in neutral 1M Na2SO4 aqueous electrolyte. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Saha, S.; Ghosh, M.; Chowdhury, S.R.; Maiyalagan, T.; Bhattacharya, S.K.; Das, S. Electrochemical Energy Storage Properties of Ni-Mn-Oxide Electrodes for Advance Asymmetric Supercapacitor Application. Langmuir 2019, 35, 8257–8267. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. NiO-CNT composite for high performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Ray, A.; Roy, A.; Das, S. Water Splitting by Using Electrochemical Properties of Material. In Water Remediation. Energy, Environment, and Sustainability; Bhattacharya, S., Gupta, A., Gupta, A., Pandey, A., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Lin, R.; Taberna, P.-L.; Fantini, S.; Presser, V.; Pérez, C.R.; Malbosc, F.; Rupesinghe, N.L.; Teo, K.B.K.; Gogotsi, Y.; Simon, P. Capacitive Energy Storage from −50 to 100 °C Using an Ionic Liquid Electrolyte. J. Phys. Chem. Lett. 2011, 2, 2396–2401. [Google Scholar] [CrossRef]
- Mao, X.; Brown, P.; Červinka, C.; Hazell, G.; Li, H.; Ren, Y.; Chen, D.; Atkin, R.; Eastoe, J.; Grillo, I.; et al. Self-assembled nanostructures in ionic liquids facilitate charge storage at electrified interfaces. Nat. Mater. 2019, 18, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.F.L.; Pereira, R.G.; Salanne, M.; Siqueira, L.J.A. Molecular Dynamics Simulations of Ether-Modified Phosphonium Ionic Liquid Confined in between Planar and Porous Graphene Electrode Models. J. Phys. Chem. C 2019, 123, 10816–10825. [Google Scholar] [CrossRef]
- Burt, R.; Birkett, G.; Salanne, M.; Zhao, X.S. Molecular Dynamics Simulations of the Influence of Drop Size and Surface Potential on the Contact Angle of Ionic-Liquid Droplets. J. Phys. Chem. C 2016, 120, 15244–15250. [Google Scholar] [CrossRef]
- Navathe, G.J.; Patil, D.S.; Jadhav, P.R.; Awale, D.V.; Teli, A.M.; Bhise, S.C.; Kolekar, S.S.; Karanjkar, M.M.; Kim, J.H.; Patil, P.S. Rapid synthesis of nanostructured copper oxide for electrochemical supercapacitor based on novel [HPMIM][Cl] ionic liquid. J. Electroanal. Chem. 2015, 738, 170–175. [Google Scholar] [CrossRef]
- Newell, R.; Faure-Vincent, J.; Iliev, B.; Schubert, T.; Aradilla, D. A new high performance ionic liquid mixture electrolyte for large temperature range supercapacitor applications (−70 °C to 80 °C) operating at 3.5 V cell voltage. Electrochim. Acta 2018, 267, 15–19. [Google Scholar] [CrossRef]
Short Biography of Authors
 | Dr. Apurba Ray received his Ph.D. degree (2019) from the Department of Instrumentation Science at Jadavpur University, Kolkata in India. Currently, he is working as a DLR/DAAD Post-doctoral researcher at the Institute of Materials Research, German Aerospace Center (DLR), Cologne, Germany. His major research interest is focused on the fabrication of advanced, cost-effective, eco-friendly, high-performance electrode and electrolyte materials for electrochemical energy storage devices (Supercapacitors and Batteries) to be applied in large-scale aerospace, industrial, domestic, electronic and mobile applications. To date, he has published 34 research papers and 3 book chapters in different reputed International Journals. |
| Dr. Bilge Saruhan is a material scientist with a PhD degree from the University of Limerick in Ireland and Lecturing Qualification (Habilitation in German) from the University of Freiberg in Germany on high-performance functional ceramics and on nanomaterials and technologies. Prior to her PhD, she worked in the Turkish Automotive Industry as a quality-control engineer. She was awarded as Senior Scientist in 2019. Currently, she is the deputy head of the department High-Temperature and Functional Coatings and leads the Functional Coatings Group activities at the Institute of Materials Research of the German Aerospace Center (DLR) in Cologne. Her research fields cover thin film gas sensor materials and devices, energy storage systems and the development of catalysts and functional nanostructured coatings for energy applications. To date, she has published 126 papers, edited one book and holds nine patents. |


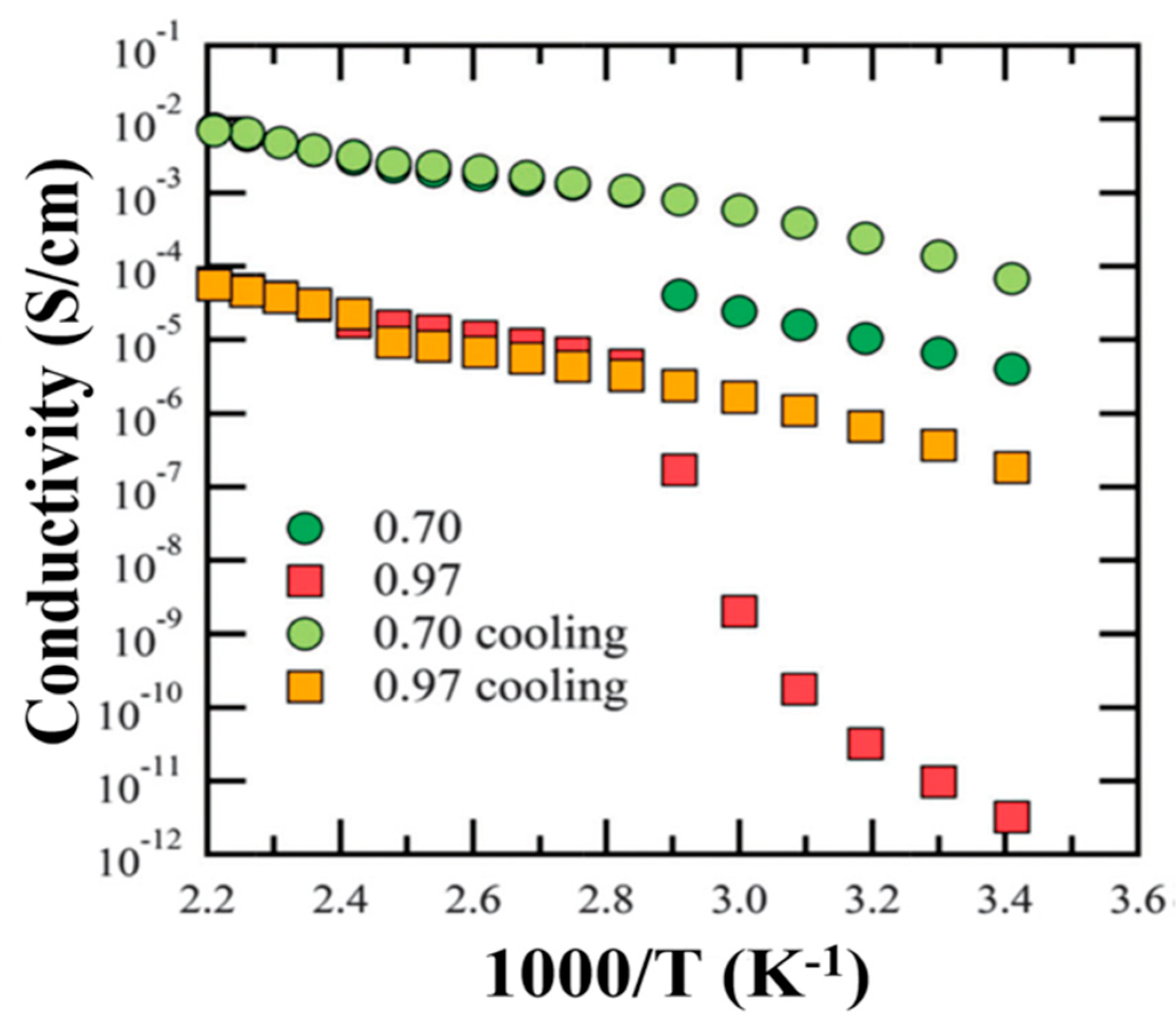


| Ionic Liquid (IL) | Abbreviation | Viscosity (mPa s) | Conductivity (σ) (mS/cm) | Electrochemical Potential Window (V) | Ref. |
|---|---|---|---|---|---|
| Ethyl-3-methylimidazolium bis-trifluoromethylsulfonylimide | (EMIM)(TFSI) | 34 | 10.8 | 4.0 | [68] |
| 1-ethyl-3-methyl imidazolium tetrafluoroborate | (EMIM)(BF4) | 32 | 16.01 | 4.0 | [69] |
| 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)imide | (EMIM)(FSI) | 19 | 17.74 | 3.5 | [70] |
| 1-ethyl-3-methylimidazolium trifluoromethanesulfonate | (EMIM)(TfO) | 45.7 | 8.5 | 4.1 | [71] |
| 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | PYR14TFSI | 85 | 2.2 | >4.8 | [72] |
| N-propyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide | PYR13TFSI | 58.7 | 4.92 | 5.0 | [73] |
| 1-ethyl-3-methylimidazolium chloride | (EMIM)(Cl) | - | 1.85 | 2.8 | [74] |
| N-methyl-N-propylpiperidinium bis(trifluoromethylsulfonyl)imide | Pip13TFSI | - | 10 | 4.35 | [75] |
| N,N-diethyl-N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate | DEME-BF4 | - | 4.8 | 6.0 | [76] |
| 1-ethyl-3-methylimidazolium hexafluorophosphate | (EMIM)(PF6) | - | 1.93 | 3.2 | [77] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | (BMIM)(PF6) | - | 2.1 | 3.0 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, A.; Saruhan, B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials 2021, 14, 2942. https://doi.org/10.3390/ma14112942
Ray A, Saruhan B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials. 2021; 14(11):2942. https://doi.org/10.3390/ma14112942
Chicago/Turabian StyleRay, Apurba, and Bilge Saruhan. 2021. "Application of Ionic Liquids for Batteries and Supercapacitors" Materials 14, no. 11: 2942. https://doi.org/10.3390/ma14112942
APA StyleRay, A., & Saruhan, B. (2021). Application of Ionic Liquids for Batteries and Supercapacitors. Materials, 14(11), 2942. https://doi.org/10.3390/ma14112942







