Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Fe3O4 and Fe3O4/POA Core–Shell Particles
2.2. Characterization
3. Results and Discussion
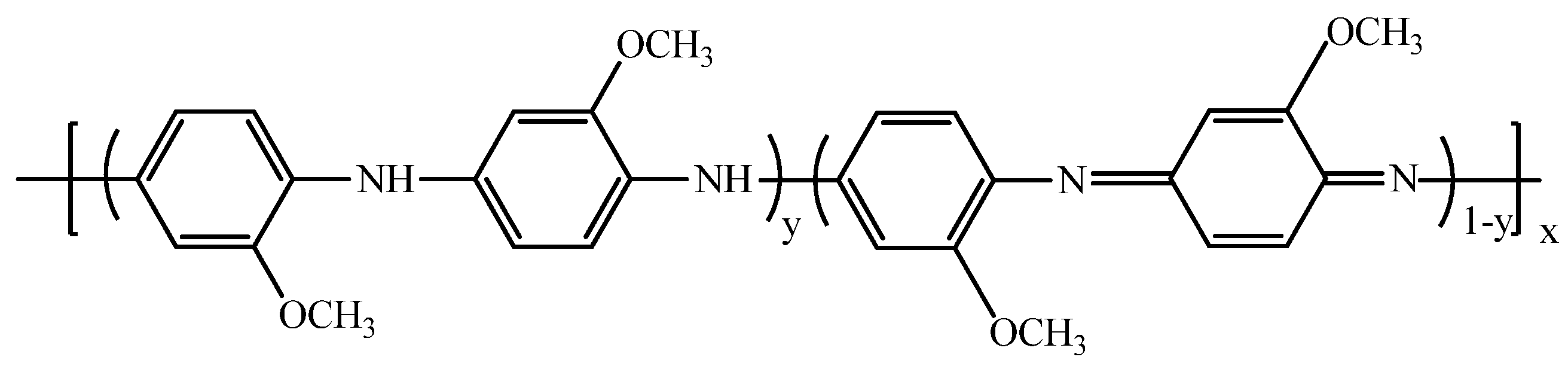
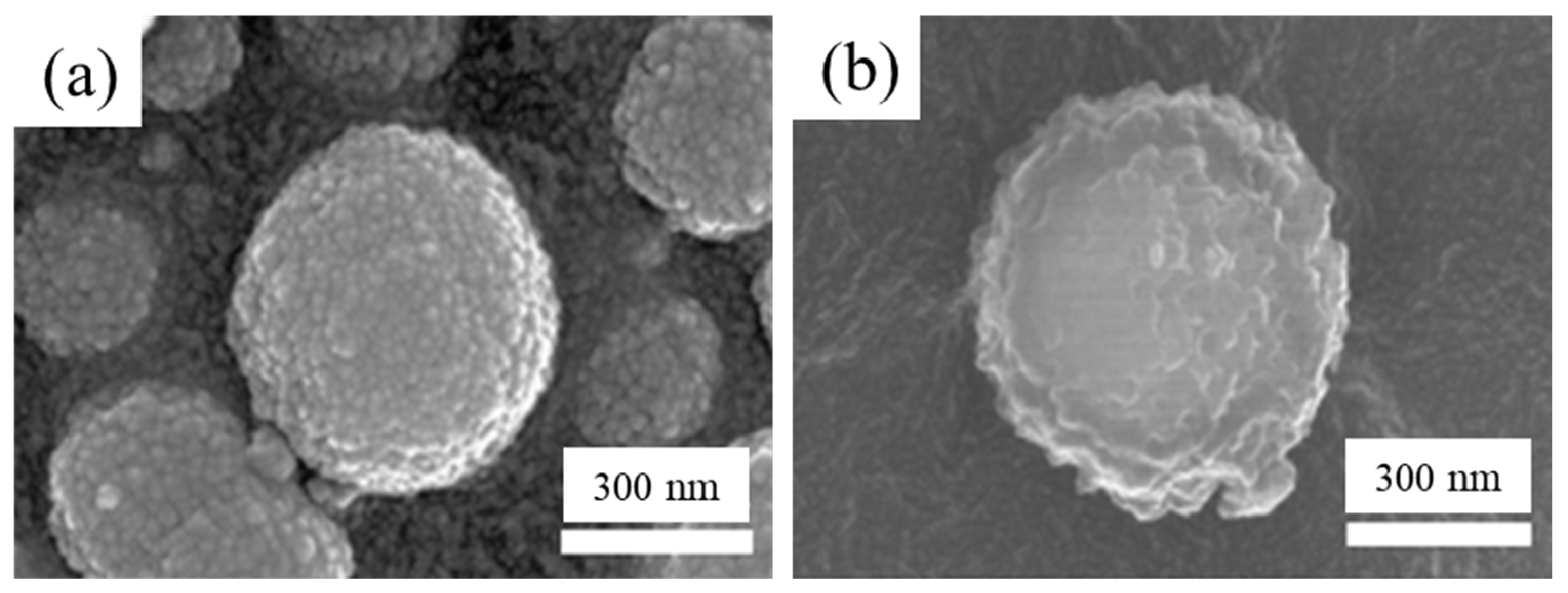
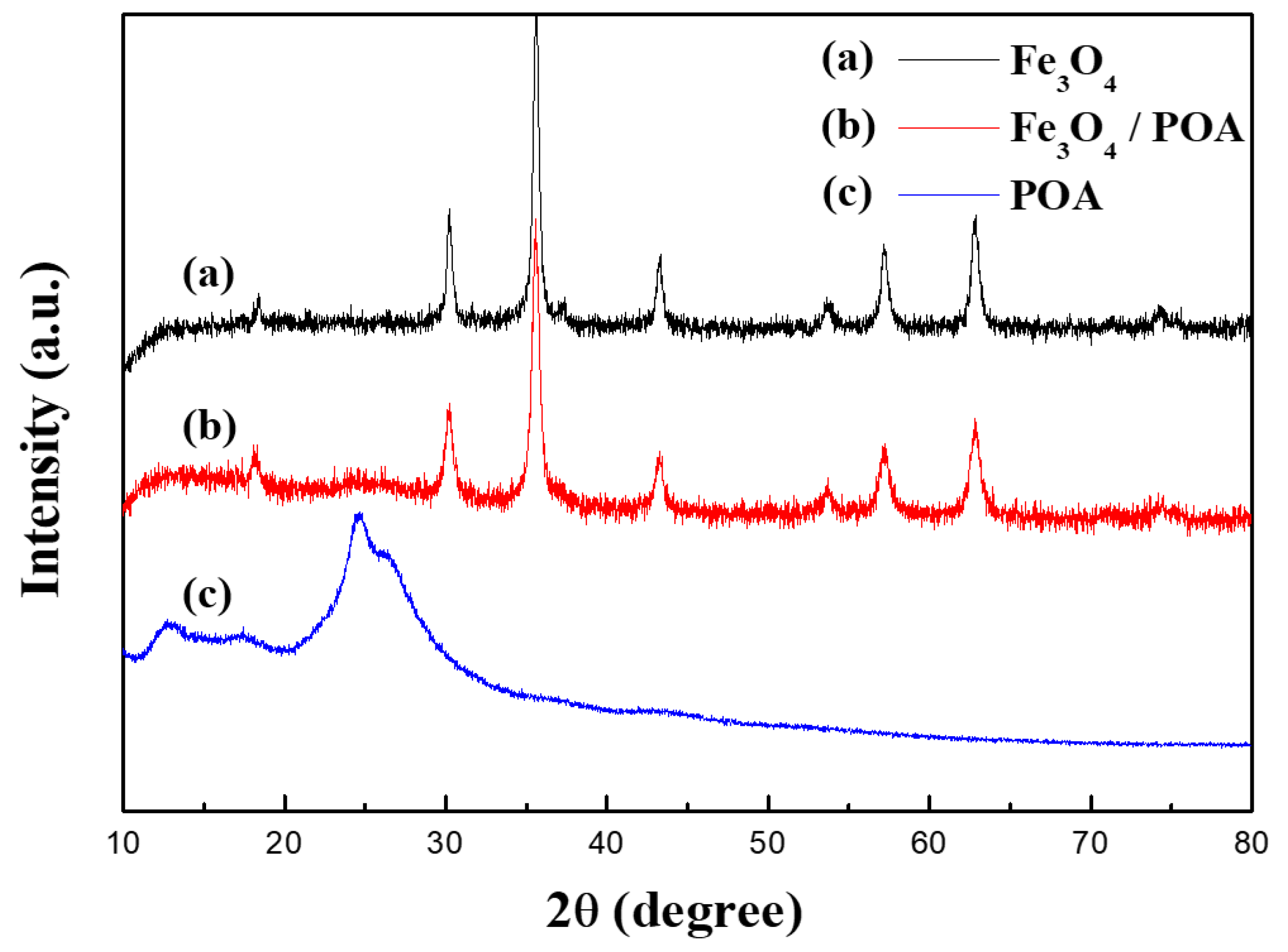
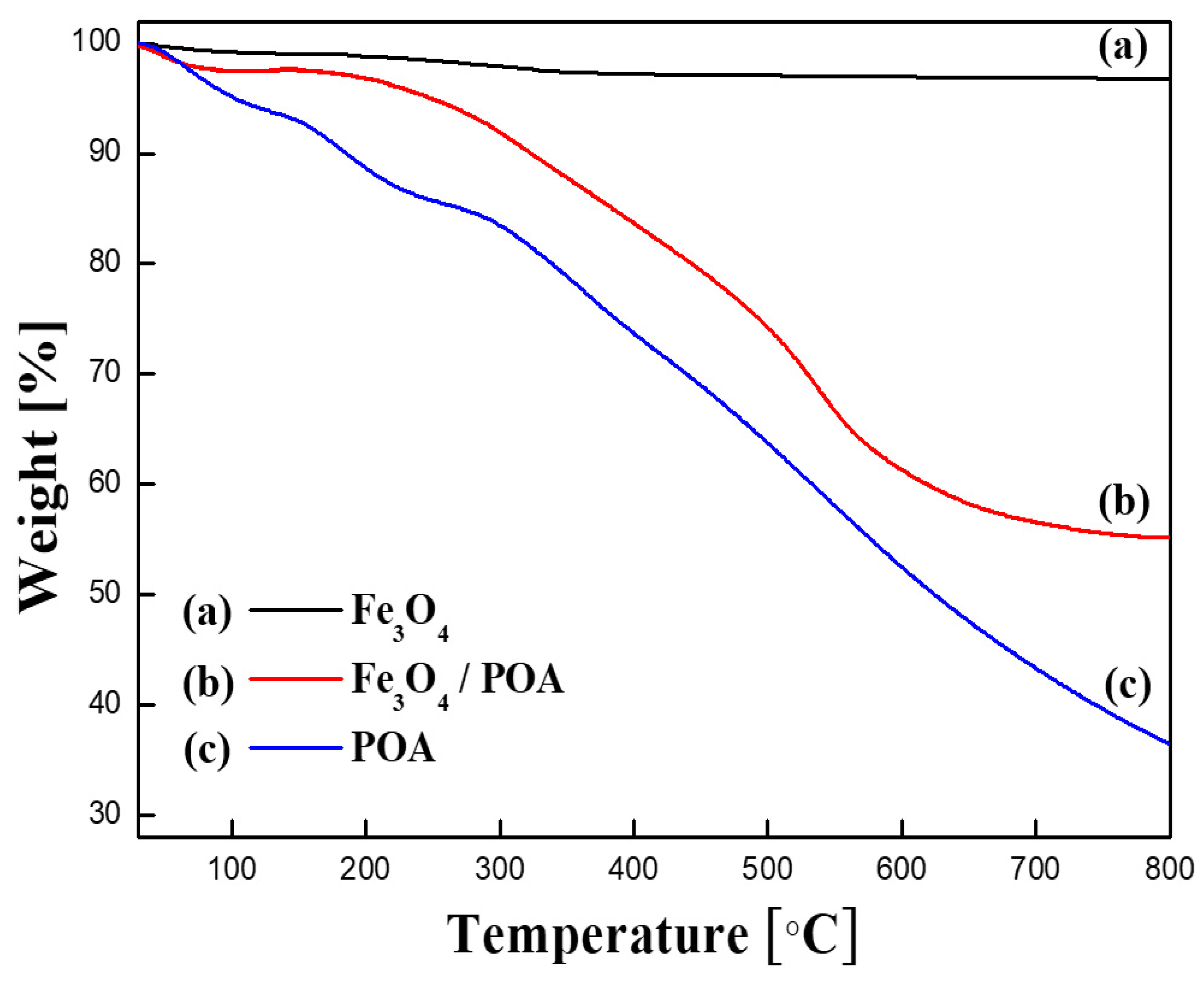
3.1. Material Properties
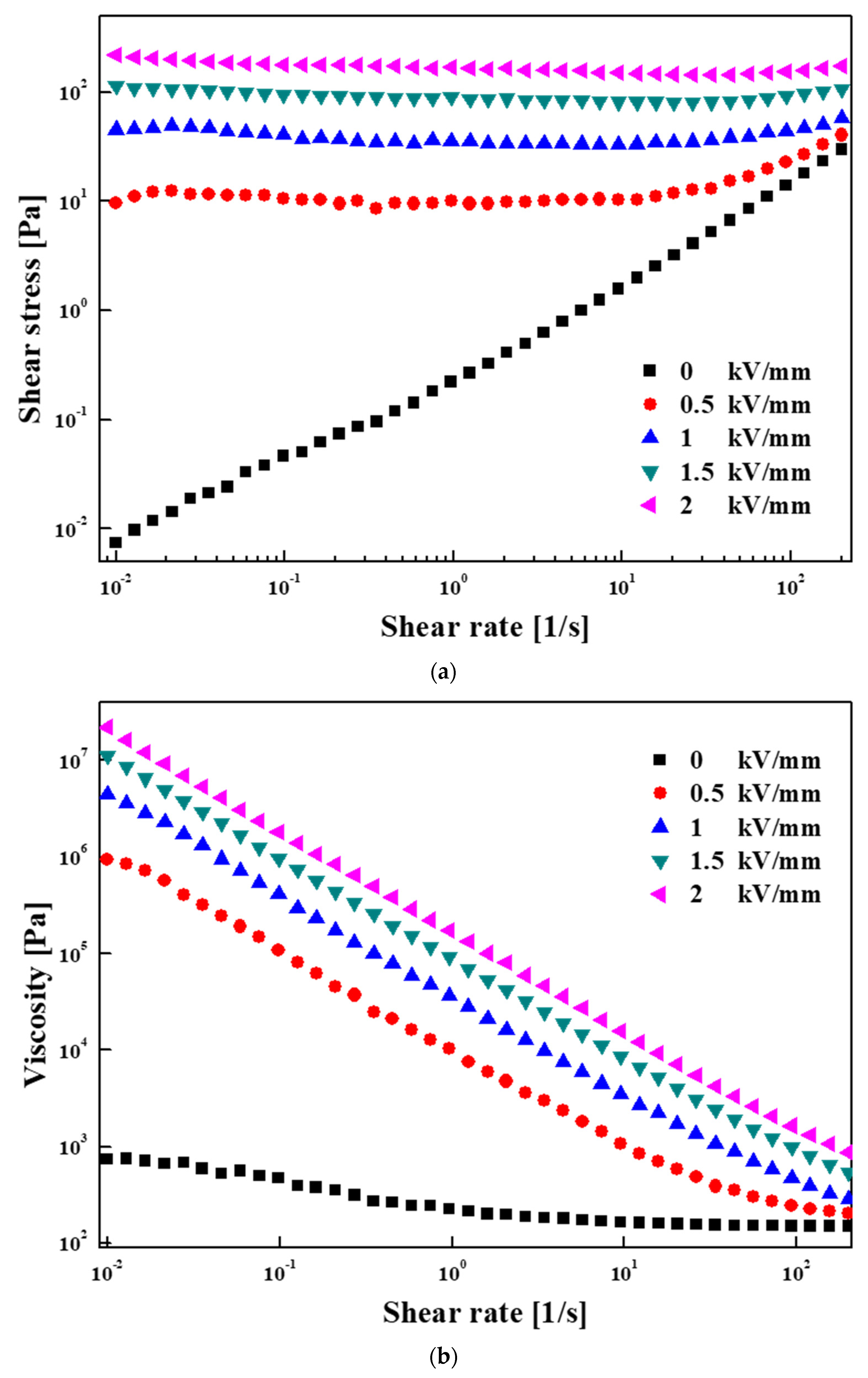
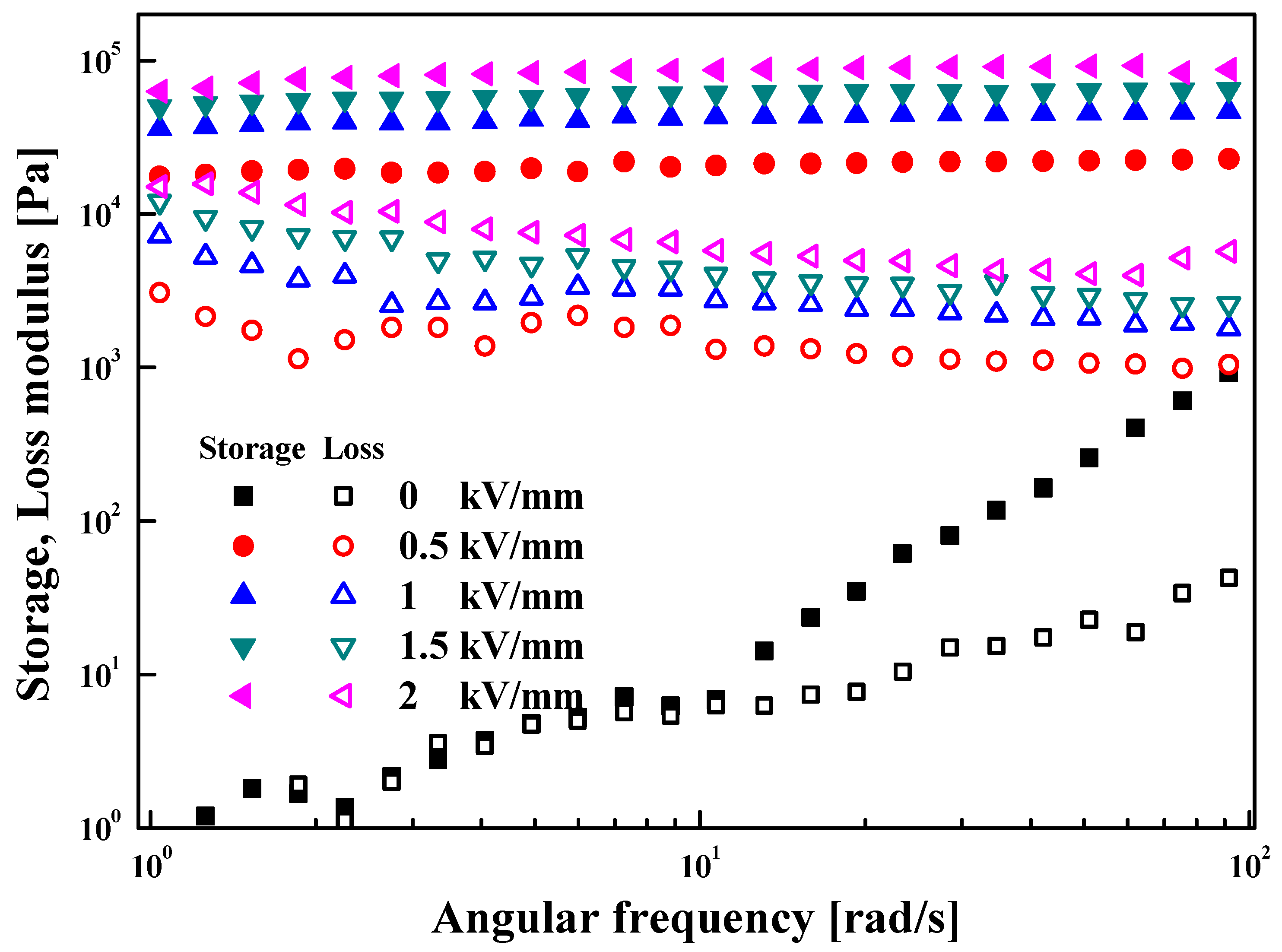
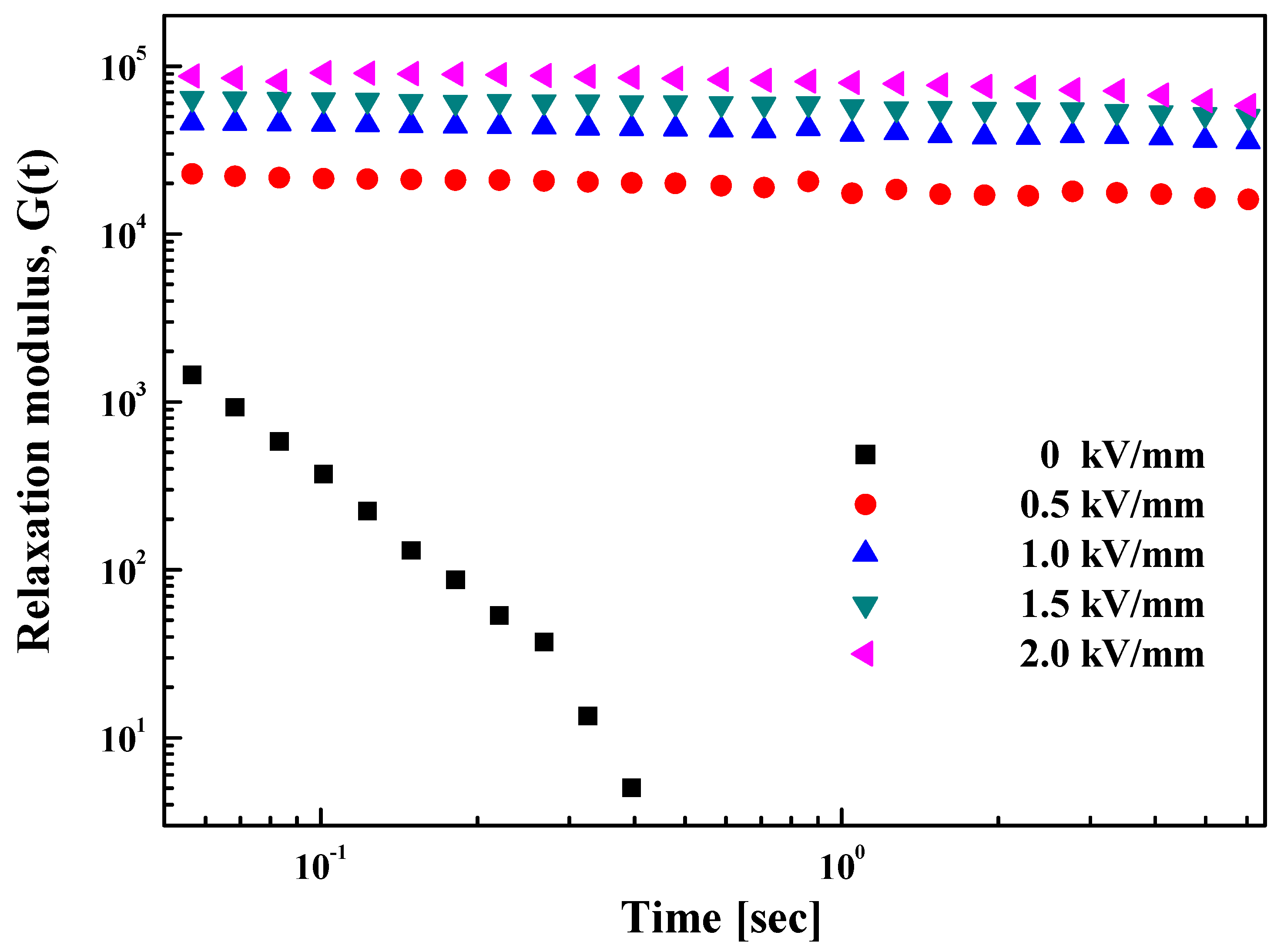
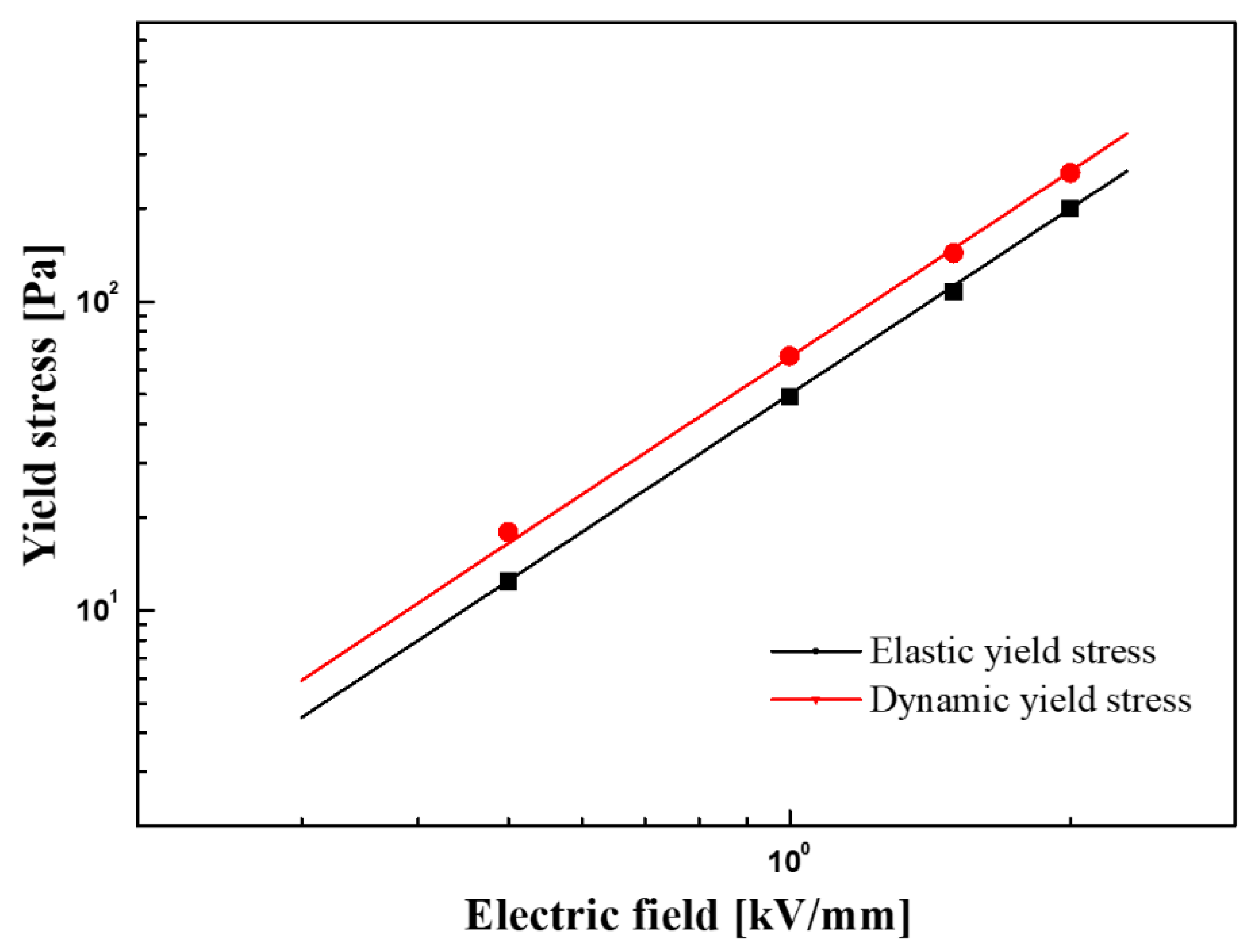
3.2. Electrorheological (ER) Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuznetsov, N.M.; Zagoskin, Y.D.; Vdovichenko, A.Y.; Bakirov, A.V.; Kamyshinsky, R.A.; Istomina, A.P.; Grigoriev, T.E.; Chvalun, S.N. Enhanced electrorheological activity of porous chitosan particles. Carbohydr. Polym. 2021, 256, 117530. [Google Scholar] [CrossRef] [PubMed]
- Bica, I.; Anitas, E.M.; Averis, L.M.E. Tensions and deformations in composites based on polyurethane elastomer and magnetorheological suspension: Effects of the magnetic field. J. Ind. Eng. Chem. 2015, 28, 86–90. [Google Scholar] [CrossRef]
- Shen, M.; Huang, Q. Acoustic velocity and attenuation coefficient of magnetorheological fluids under electromagnetic fields. Appl. Acoust. 2016, 107, 27–33. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Plachy, T.; Sedlacik, M.; Mosnacek, J.; Pavlinek, V. A facile controllable coating of carbonyl iron particles with poly(glycidyl methacrylate): A tool for adjusting MR response and stability properties. J. Mater. Chem. C 2015, 3, 4646–4656. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, X. Electrorheology of nanofiber suspensions. Nanoscale Res. Lett. 2011, 6, 1–17. [Google Scholar] [CrossRef]
- Plachy, T.; Sedlacik, M.; Pavlinek, V.; Stejskal, J. The observation of a conductivity threshold on the electrorheological effect of p-phenylenediamine oxidized with p-benzoquinone. J. Mater. Chem. C 2015, 3, 9973–9980. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Dong, Y.; He, F.; Zhao, X.; Yin, J. Low-Temperature Interfacial Polymerization and Enhanced Electro-Responsive Characteristic of Poly(ionic liquid)s@polyaniline Core-shell Microspheres. Macromol. Rapid Commun. 2019, 40, 1800351. [Google Scholar] [CrossRef] [PubMed]
- Kutalkova, E.; Plachy, T.; Sedlacik, M. On the enhanced sedimentation stability and electrorheological performance of intelligent fluids based on sepiolite particles. J. Mol. Liq. 2020, 309, 113120. [Google Scholar] [CrossRef]
- Cho, Y.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Electrorheological characterization of polyaniline-coated poly(methyl methacrylate) suspensions. Colloid Polym. Sci. 2002, 280, 1062–1066. [Google Scholar]
- Chen, P.; Cheng, Q.; Wang, L.-M.; Liu, Y.D.; Choi, H.J. Fabrication of dual-coated graphene oxide nanosheets by polypyrrole and poly(ionic liquid) and their enhanced electrorheological responses. J. Ind. Eng. Chem. 2019, 69, 106–115. [Google Scholar] [CrossRef]
- Do, T.; Lee, H.; Ko, Y.G.; Chun, Y.; Choi, U.S.; Kim, C.H. Influence of amine- and sulfonate-functional groups on electrorheological behavior of polyacrylonitrile dispersed suspension. Colloids Surf. A 2017, 514, 56–62. [Google Scholar] [CrossRef]
- Kuznetsov, N.M.; Belousov, S.I.; Kamyshinsky, R.A.; Vasiliev, A.L.; Chvalun, S.N.; Yudina, E.B.; Vul, A.Y. Detonation nanodiamonds dispersed in polydimethylsiloxane as a novel electrorheological fluid: Effect of nanodiamonds surface. Carbon 2021, 174, 138–147. [Google Scholar] [CrossRef]
- Plachy, T.; Masar, M.; Mrlik, M.; Machovsky, M.; Machovska, Z.; Kutalkova, E.; Kuritka, I. Switching between negative and positive electrorheological effect of g-C3N4 by copper ions doping. Adv. Powder Technol. 2019, 30, 714–723. [Google Scholar] [CrossRef]
- Xi, Z.; Ma, J.; Sun, W.; Wang, B.; Hao, C. Synthesis and electrorheological properties of hierarchical and core-shell MoS2@ TiO2 nanocomposite. J. Solid State Chem. 2020, 290, 121601. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Q.; He, F.; Zheng, C.; Zhao, X.; Yi, J. Influence of geometry of mobile countercations on conductivity, polarization and electrorheological effect of polymeric anionic liquids at ice point temperature. Polymer 2020, 205, 122826. [Google Scholar] [CrossRef]
- Gercek, B.; Yavuz, M.; Yilmaz, H.; Sari, B.; Unal, H.I. Comparison of electrorheological properties of some polyaniline derivatives. Colloids Surf. A 2007, 299, 124–132. [Google Scholar] [CrossRef]
- Gao, C.Y.; Kim, M.H.; Jin, H.-J.; Choi, H.J. Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers 2020, 12, 1984. [Google Scholar] [CrossRef]
- Ma, N.; Yao, Y.; Wang, Q.; Niu, C.; Dong, X. Properties and mechanical model of a stiffness tunable viscoelastic damper based on electrorheological elastomers. Smart Mater. Struct. 2020, 29, 045041. [Google Scholar] [CrossRef]
- Chiolerio, A.; Quadrelli, M.B. Smart fluid systems: The advent of autonomous liquid robotics. Adv. Sci. 2017, 4, 1700036. [Google Scholar] [CrossRef]
- Stejskal, J.; Mrlík, M.; Plachý, T.; Trchová, M.; Kovářová, J.; Li, Y. Molybdenum and tungsten disulfides surface-modified with a conducting polymer, polyaniline, for application in electrorheology. React. Funct. Polym. 2017, 120, 30–37. [Google Scholar] [CrossRef]
- Tian, X.; He, K.; Wang, C.; Wen, Q.; Wang, B.; Yu, S.; Hao, C.; Chen, K.; Lei, Q. Preparation and electrorheological behavior of anisotropic titanium oxide/polyaniline core/shell nanocomposite. Compos. Sci. Technol. 2016, 137, 118–129. [Google Scholar] [CrossRef]
- Kim, M.H.; Bae, D.H.; Choi, H.J.; Seo, Y. Synthesis of semiconducting poly(diphenylamine) particles and analysis of their electrorheological properties. Polymer 2017, 119, 40–49. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yoon, D.J. Electrorheological fluids of polypyrrole-tin oxide nanocomposite particles. Korea-Aust. Rheol. J. 2016, 28, 275–279. [Google Scholar] [CrossRef]
- Cabuk, S.; Unal, H.I. Enhanced electrokinetic, dielectric and electrorheological properties of covalently bonded particles-TiO2/polypyrrole nanocomposite. React. Funct. Polym. 2015, 95, 1–11. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Q.; Zhang, T.; Pang, X. Electrorheological Response of Novel polyaniline-Fe2O3 Nanocomposite Particles. Polym. Plast. Tech. Mater. 2019, 58, 573–577. [Google Scholar] [CrossRef]
- Erol, O.; Unal, H.I. Core/shell-structured, covalently bonded TiO2/poly(3,4-ethylenedioxythiophene) dispersions and their electrorheological response: The effect of anisotropy. RSC Adv. 2015, 5, 103159–103171. [Google Scholar] [CrossRef]
- Tian, X.; He, K.; Wang, B.; Yu, S.; Hao, C.; Chen, K.; Lei, Q. Flower-like Fe2O3/polyaniline core/shell nanocomposite and its electroheological properties. Colloids Surf. A 2016, 498, 185–193. [Google Scholar] [CrossRef]
- Piao, S.H.; Gao, C.Y.; Choi, H.J. Sulfonated polystyrene particles coated with conducting polyaniline and their electro-responsive suspension characteristics under electric fields. Polymer 2017, 127, 174–181. [Google Scholar] [CrossRef]
- Wang, X.; Qian, X.; Jiang, X.; Lu, Z.; Hou, L. Tunable electrorheological characteristics and mechanism of a series of graphene-like molybdenum disulfide coated core–shell structured polystyrene microspheres. RSC Adv. 2016, 6, 26096–26103. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Choi, H.J. Synthesis of organic–inorganic poly(diphenylamine)/magnetite composite particles and their magnetorheological response. IEEE Trans. Magn. 2018, 54, 1–4. [Google Scholar] [CrossRef]
- Mrlík, M.; Ilčíková, M.; Pavlínek, V.; Mosnáček, J.; Peer, P.; Filip, P. Improved thermooxidation and sedimentation stability of covalently-coated carbonyl iron particles with cholesteryl groups and their influence on magnetorheology. J. Colloid Interface Sci. 2013, 396, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Machovsky, M.; Mrlik, M.; Kuritka, I.; Pavlinek, V.; Babayan, V. Novel synthesis of core–shell urchin-like ZnO coated carbonyl iron microparticles and their magnetorheological activity. RSC Adv. 2014, 4, 996–1003. [Google Scholar] [CrossRef]
- Plachy, T.; Kutalkova, E.; Sedlacik, M.; Vesel, A.; Masar, M.; Kuritka, I. Impact of corrosion process of carbonyl iron particles on magnetorheological behavior of their suspensions. J. Ind. Eng. Chem. 2018, 66, 362–369. [Google Scholar] [CrossRef]
- Hong, C.H.; Kim, M.W.; Zhang, W.L.; Moon, I.J.; Choi, H.J. Fabrication of smart magnetite/reduced graphene oxide composite particles and their magnetic stimuli-response. J. Colloid Interface Sci. 2016, 481, 194–200. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Cui, G.; Li, N.; Dong, X. Two-dimensional Fe3O4/MoS2 nanocomposites for a magnetorheological fluid with enhanced sedimentation stability. Soft Matter 2018, 14, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Plachy, T.; Cvek, M.; Kozakova, Z.; Sedlacik, M.; Moucka, R. The enhanced MR performance of dimorphic MR suspensions containing either magnetic rods or their non-magnetic analogs. Smart Mater. Struct. 2017, 26, 025026. [Google Scholar] [CrossRef]
- Ruan, X.; Pei, L.; Xuan, S.; Yan, Q.; Gong, X. The rheological responds of the superparamagnetic fluid based on Fe3O4 hollow particles. J. Magn. Magn. Mater. 2017, 429, 1–10. [Google Scholar] [CrossRef]
- Chae, H.S.; Kim, S.D.; Piao, S.H.; Choi, H.J. Core-shell structured Fe3O4@ SiO2 particles fabricated by sol–gel method and their magnetorheology. Colloid Polym. Sci. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Guo, Z.; Henry, L.L.; Palshin, V.; Podlaha, E.J. Synthesis of poly(methyl methacrylate) stabilized colloidal zero-valence metallic particles. J. Mater. Chem. 2006, 16, 1772–1777. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Guo, C.; Li, J.; Xue, Q.; Feng, J.; Gong, X. Poly(methyl methacrylate)-coated carbonyl iron particles and their magnetorheological characteristics. Polym. Int. 2010, 59, 879–883. [Google Scholar] [CrossRef]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual external field-responsive polyaniline-coated magnetite/silica particles for smart fluid applications. Chem. Commun. 2017, 53, 6645–6648. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Choi, H.J. Synthesis of Smart Poly(diphenylamine)/Magnetic Particle Composites and Their Electric/Magnetic Stimuli-Response. Macromol. Res. 2018, 26, 667–670. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Ho, K.-S.; Bi, X.; Han, Y.-K.; Chen, Z.-L.; Hsu, C.-H.; Chang, Y.-C. Synthesis and electromagnetic properties of polyaniline-coated silica/maghemite particles. Eur. Polym. J. 2009, 45, 613–620. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Esmaeilnezhad, E.; Choi, H.J. Core–Shell Structured Magnetite-Poly(diphenylamine) Microspheres and Their Tunable Dual Response under Magnetic and Electric Fields. Langmuir 2021, 37, 2298–2311. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, M.S.; Choi, H.J.; Jhon, M.S. Effect of polymerization temperature on polyaniline based electrorheological suspensions. Colloid Polym. Sci. 1999, 277, 73–76. [Google Scholar] [CrossRef]
- Abaci, S.; Nessark, B. Characterization and corrosion protection properties of composite material (PANI+TiO2) coatings on A304 stainless steel. J. Coat. Technol. Res. 2015, 12, 107–120. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Andriianova, A.N.; Biglova, Y.N.; Mustafin, A.G. Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv. 2020, 10, 7468–7491. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, C.; Wang, B.; Yu, S.; Chen, K. The synthesis and properties of bifunctional and intelligent Fe3O4@titanium oxide core/shell nanoparticles. Dalton Trans. 2013, 42, 7233–7240. [Google Scholar] [CrossRef]
- Lee, J.H.; Lu, Q.; Lee, J.Y.; Choi, H.J. Polymer-Magnetic Composite Particles of Fe3O4/Poly(o-anisidine) and Their Suspension Characteristics under Applied Magnetic Fields. Polymers 2019, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Murali, M.; Deshpande, P.P.; Galphade, V.S.; More, M.A. Conducting poly(o-anisidine)-coated steel electrodes for supercapacitors. Chem. Pap. 2013, 67, 1066–1071. [Google Scholar] [CrossRef]
- Khan, A.A.; Shaheen, S.; Habiba, U. Synthesis and characterization of poly-o-anisidine Sn(IV) tungstate: A new and novel ‘organic–inorganic’ nano-composite material and its electro-analytical applications as Hg(II) ion-selective membrane electrode. J. Adv. Res. 2012, 3, 269–278. [Google Scholar] [CrossRef]
- Fang, F.F.; Choi, H.J.; Jhon, M.S. Magnetorheology of soft magnetic carbonyl iron suspension with single-walled carbon nanotube additive and its yield stress scaling function. Colloids Surf. A 2009, 351, 46–51. [Google Scholar] [CrossRef]
- Cabuk, M. Electrorheological response of mesoporous expanded perlite particles. Microporous Mesoporous Mater. 2017, 247, 60–65. [Google Scholar] [CrossRef]
- Schwarzl, F. Numerical calculation of stress relaxation modulus from dynamic data for linear viscoelastic materials. Rheol. Acta 1975, 14, 581–590. [Google Scholar] [CrossRef]
- Stenicka, M.; Pavlinek, V.; Saha, P.; Blinova, N.V.; Stejskal, J.; Quadrat, O. The electrorheological efficiency of polyaniline par ticles with various conductivities suspended in silicone oil. Colloid Polym. Sci. 2009, 287, 403–412. [Google Scholar] [CrossRef]
- Jang, W.H.; Kim, J.W.; Choi, H.J.; Jhon, M.S. Synthesis and electrorheology of camphorsulfonic acid doped polyaniline suspensions. Colloid Polym. Sci. 2001, 279, 823–827. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Lee, J.-H.; Lee, J.H.; Choi, H.J. Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response. Materials 2021, 14, 2900. https://doi.org/10.3390/ma14112900
Lu Q, Lee J-H, Lee JH, Choi HJ. Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response. Materials. 2021; 14(11):2900. https://doi.org/10.3390/ma14112900
Chicago/Turabian StyleLu, Qi, Jin-Hee Lee, Jin Hyun Lee, and Hyoung Jin Choi. 2021. "Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response" Materials 14, no. 11: 2900. https://doi.org/10.3390/ma14112900
APA StyleLu, Q., Lee, J.-H., Lee, J. H., & Choi, H. J. (2021). Magnetite/Poly(ortho-anisidine) Composite Particles and Their Electrorheological Response. Materials, 14(11), 2900. https://doi.org/10.3390/ma14112900





