Enhancing Toughness and Reducing Volumetric Shrinkage for Bis-GMA/TEGDMA Resin Systems by Using Hyperbranched Thiol Oligomer HMDI-6SH
Abstract
1. Introduction
2. Materials and Methods
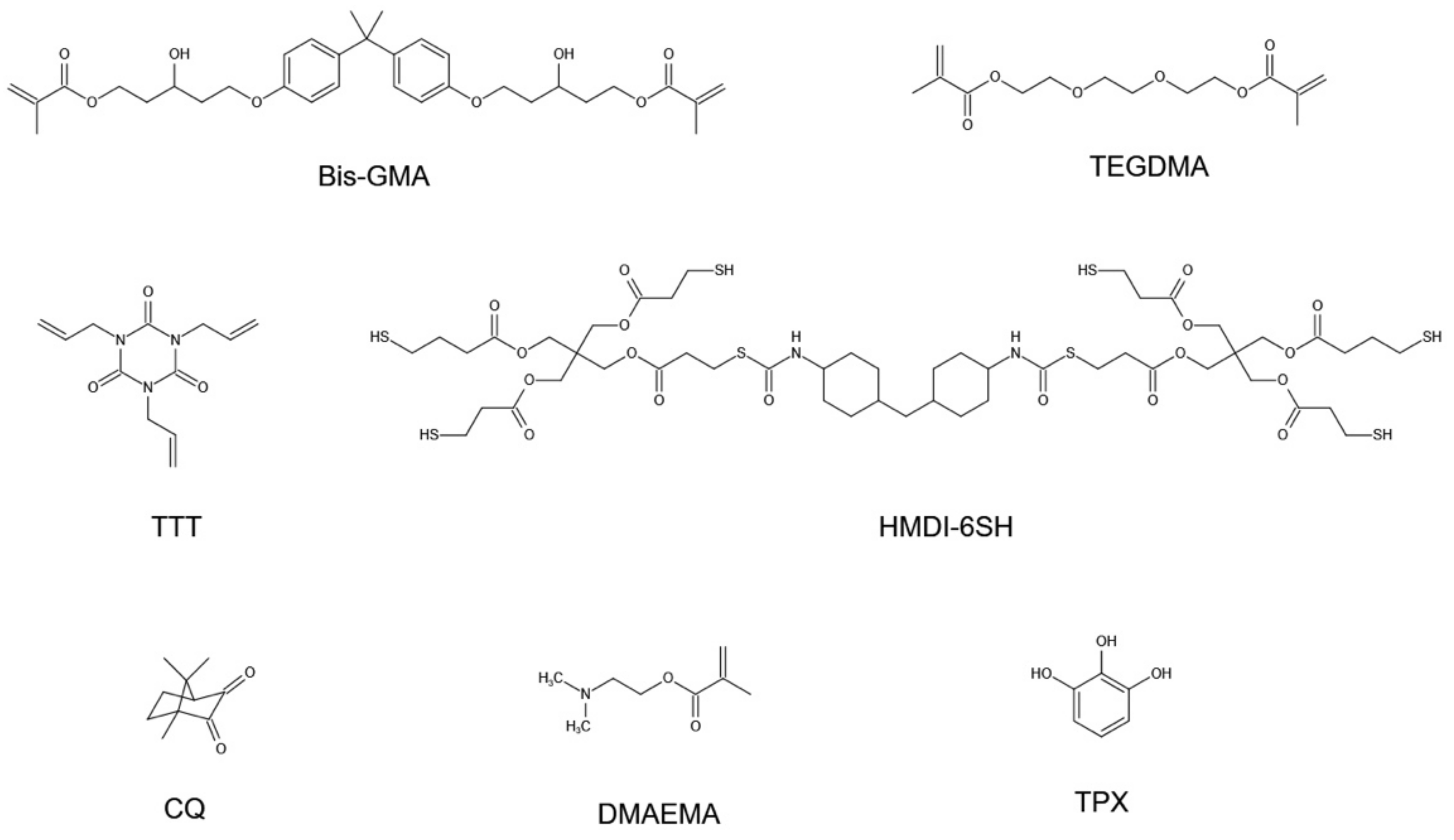
2.1. Materials
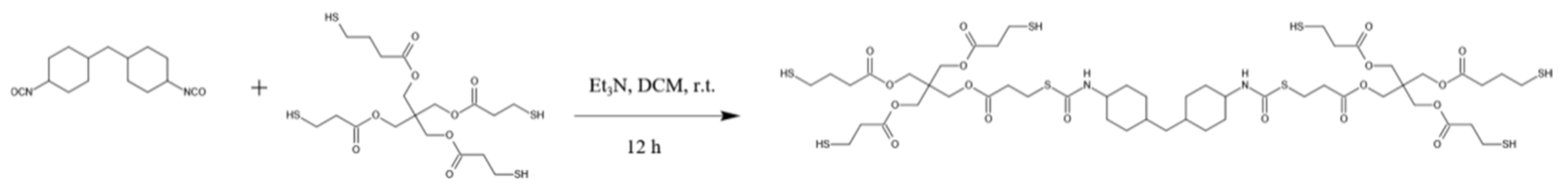
2.2. Synthesis of Hyperbranched Thiol Oligomer HMDI-6SH
2.3. Preparation of Resin Systems
2.4. Functional Groups Conversion Measurement
2.5. The Determination of Glass Transition Temperature (Tg)
2.6. Flexural Properties
2.7. Volumetric Shrinkage
2.8. Water Sorption and Water Solubility
2.9. Statistical Analysis
3. Result and Discussion
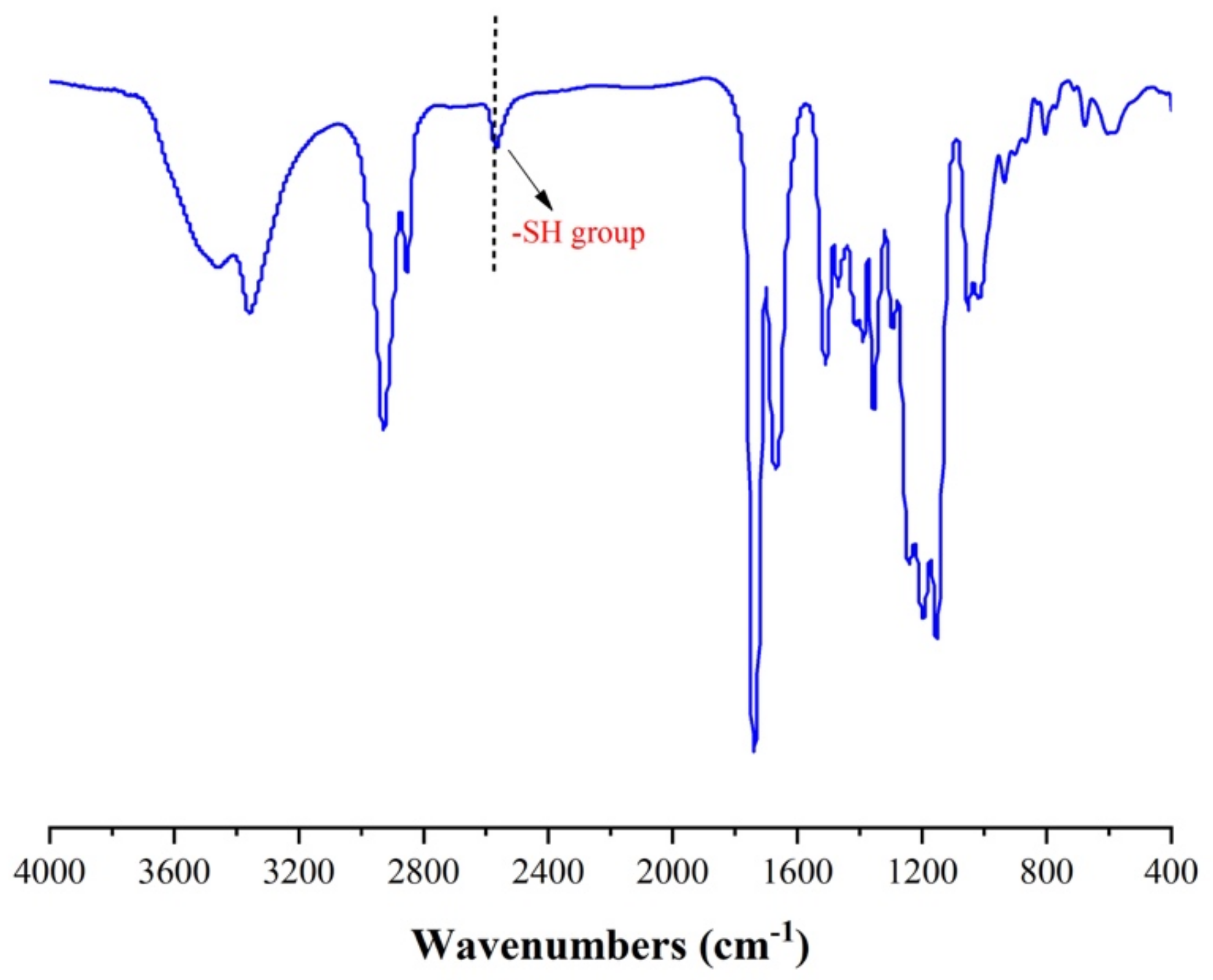
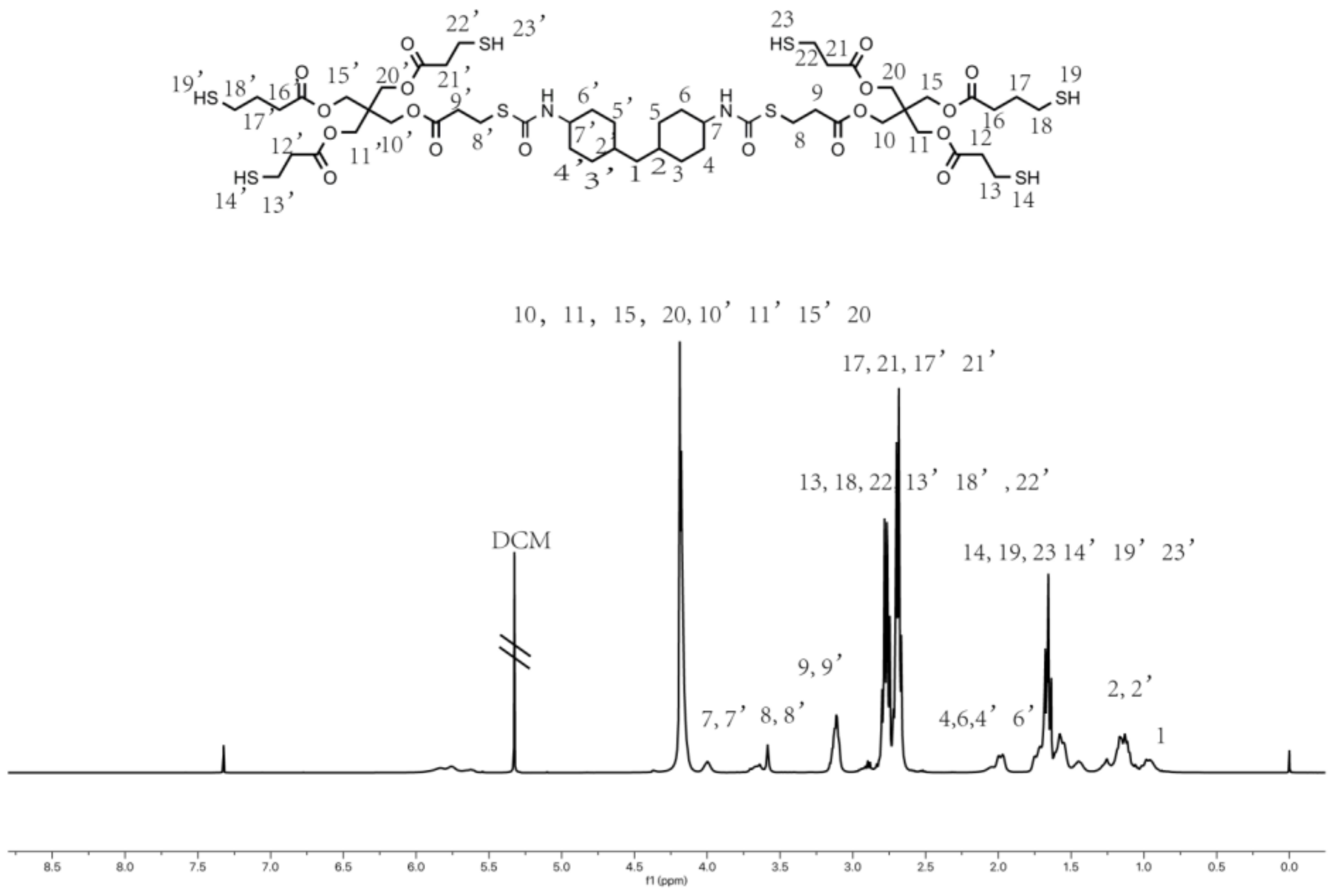
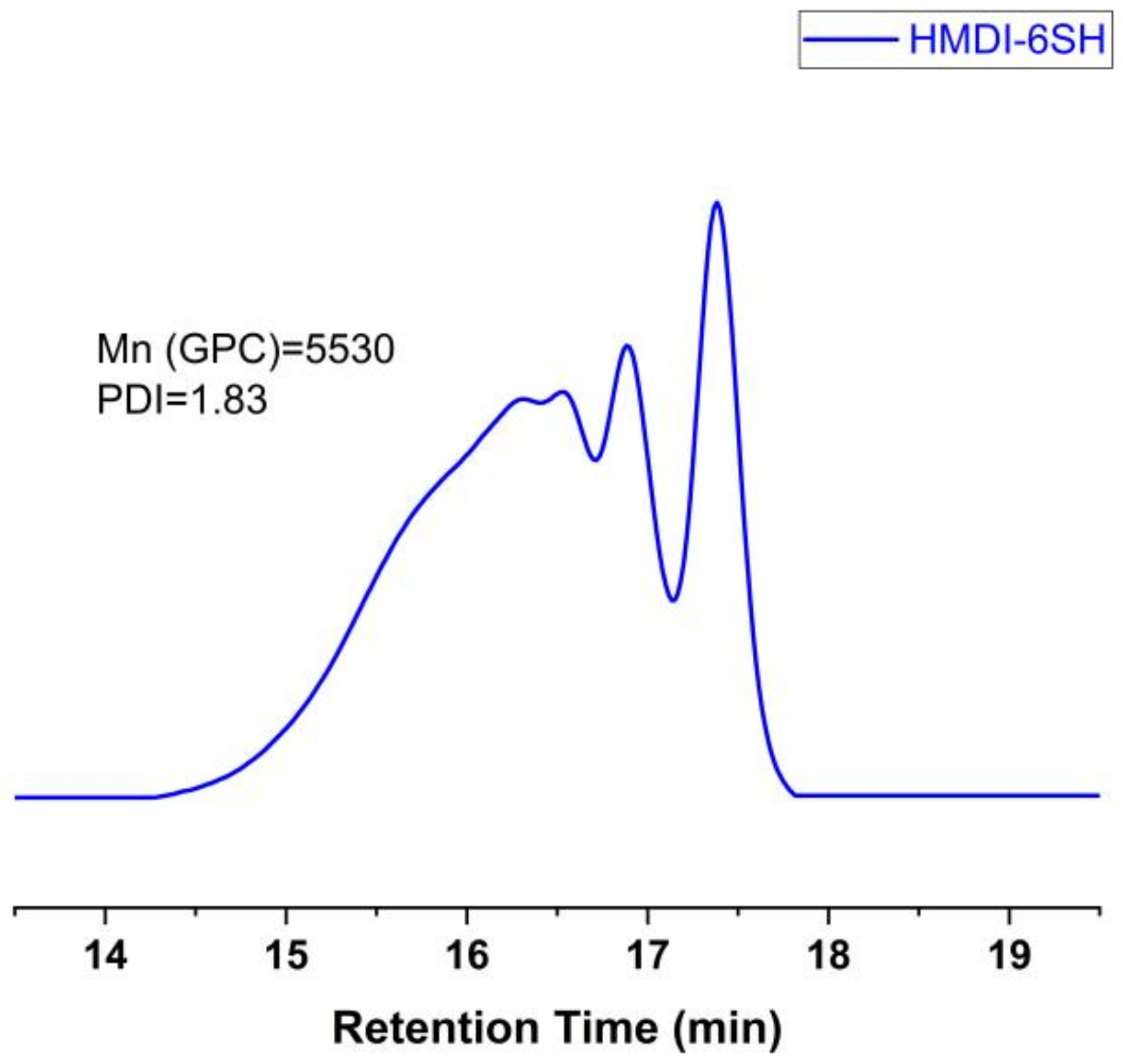
3.1. Hyperbranched Thiol Oligomer (HMDI-6SH) Synthesis
3.2. Degree of Conversion
3.3. Volumetric Shrinkage
3.4. Water Sorption and Water Solubility
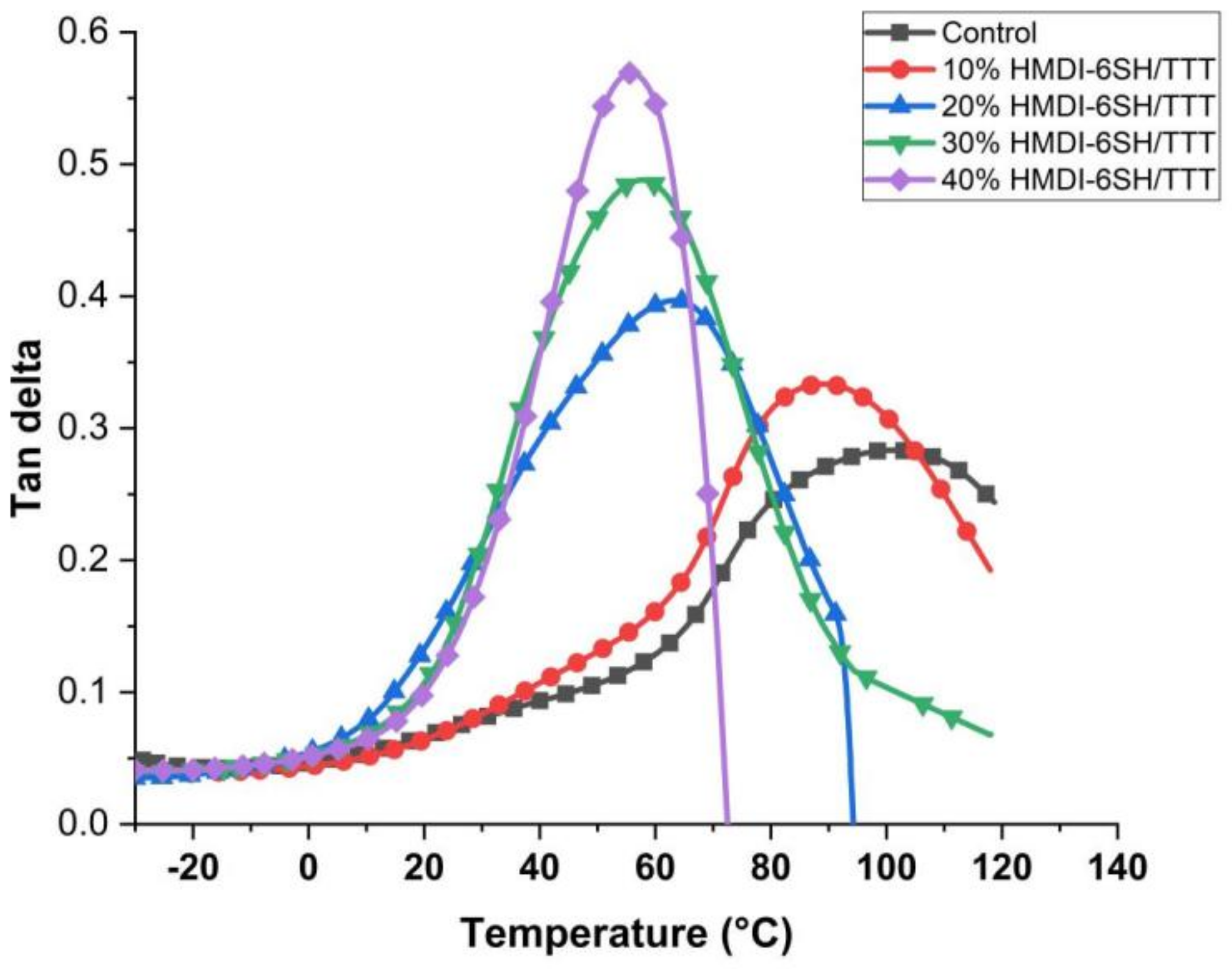
3.5. The Determination of Glass Transition Temperature (Tg)
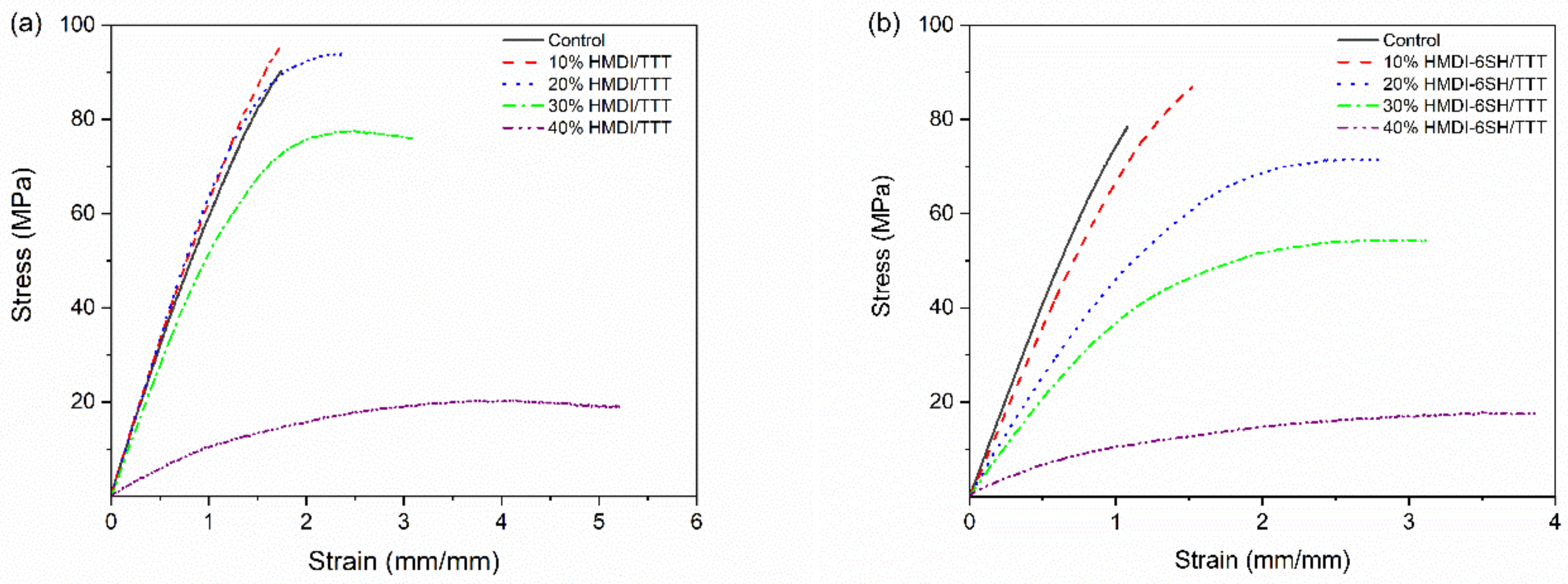
3.6. Flexural Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calheiros, F.C.; Sadek, F.T.; Braga, R.R.; Cardoso, P.E.C. Polymerization contraction stress of low-shrinkage composites and its correlation with microleakage in class V restorations. J. Dent. 2004, 32, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Versluis, A.; Tantbirojn, D.; Pintado, M.R.; DeLong, R.; Douglas, W.H. Residual shrinkage stress distributions in molars after composite restoration. Dent. Mater. 2004, 20, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.L.; Feilzer, A.J. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Gonçalves, F.; Azevedo, C.L.N.; Ferracane, J.L.; Braga, R.R. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent. Mater. 2011, 27, 520–526. [Google Scholar] [CrossRef]
- Labella, R.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent. Mater. 1999, 15, 128–137. [Google Scholar] [CrossRef]
- Klee, J.E.; Schneider, C.; Hölter, D.; Burgath, A.; Frey, H.; Mülhaupt, R. Hyperbranched polyesters and their application in dental composites: Monomers for low shrinking composites. Polym. Adv. Technol. 2001, 12, 346–354. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Cavalcante, L.M.; Silikas, N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J. Dent. Biomech. 2010, 2010, 131630. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Xiong, J.; Hu, X.; Chen, J. Shrinkage properties of a modified dental resin composites containing a novel spiro-orthocarbonate expanding monomer. Mater. Lett. 2011, 65, 3586–3589. [Google Scholar] [CrossRef]
- Moon, E.J.; Lee, J.Y.; Kim, C.K.; Cho, B.H. Dental restorative composites containing 2,2-bis-[4-(2-hydroxy-3-methacryloyloxy propoxy) phenyl] propane derivatives and spiro orthocarbonates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73B, 338–346. [Google Scholar] [CrossRef]
- Reed, B.B.; Stansbury, J.W.; Antonucci, J.M. Ring-Opening Dental Resin Systems Based on Cyclic Acetals. In Polymers of Biological and Biomedical Significance; American Chemical Society: Washington, DC, USA, 1993; Volume 540, pp. 184–190. [Google Scholar]
- Catel, Y.; Fassler, P.; Fischer, U.; Gorsche, C.; Liska, R.; Schorpf, S.; Tauscher, S.; Moszner, N. Synthesis and polymerization of vinylcyclopropanes bearing urethane groups for the development of low-shrinkage composites. Eur. Polym. J. 2018, 98, 439–447. [Google Scholar] [CrossRef]
- Childress, K.K.; Alim, M.D.; Hernandez, J.J.; Stansbury, J.W.; Bowman, C.N. Additive manufacture of lightly crosslinked semicrystalline thiol-enes for enhanced mechanical performance. Polym. Chem. 2020, 11, 39–46. [Google Scholar] [CrossRef]
- Podgórski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-free thiol–ene dental restoratives—Part A: Resin development. Dent. Mater. 2015, 31, 1255–1262. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Lu, H.; Stanbury, J.W.; Bowman, C.N. Thiol-ene oligomers as dental restorative materials. Dent. Mater. 2005, 21, 1137–1143. [Google Scholar] [CrossRef]
- Cramer, N.B.; Couch, C.L.; Schreck, K.M.; Boulden, J.E.; Wydra, R.; Stansbury, J.W.; Bowman, C.N. Properties of methacrylate-thiol-ene formulations as dental restorative materials. Dent. Mater. 2010, 26, 799–806. [Google Scholar] [CrossRef]
- Cramer, N.B.; Couch, C.L.; Schreck, K.M.; Carioscia, J.A.; Boulden, J.E.; Stansbury, J.W.; Bowman, C.N. Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials. Dent. Mater. 2010, 26, 21–28. [Google Scholar] [CrossRef]
- Podgorski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-free thiol-ene dental restoratives—Part B: Composite development. Dent. Mater. 2015, 31, 1263–1270. [Google Scholar] [CrossRef][Green Version]
- Fu, W.; Wang, L.; He, J.W. Evaluation of mechanical properties and shrinkage stress of thiol-ene-methacrylate dental composites with synthesized fluorinated allyl ether. J. Mech. Behav. Biomed. Mater. 2019, 95, 53–59. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Li, S.P.; Weng, Z.L.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Wan, Q.C.; Schricker, S.R.; Culbertson, B.M. Methacryloyl derivitized hyperbranched polyester. 2. Photo-polymerization and properties for dental resin systems. J. Macromol. Sci. A 2000, 37, 1317–1331. [Google Scholar] [CrossRef]
- Dewaele, M.; Leprince, J.G.; Fallais, I.; Devaux, J.; Leloup, G. Benefits and Limitations of Adding Hyperbranched Polymers to Dental Resins. J. Dent. Res. 2012, 91, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, F.; He, J. Preparation of low shrinkage methacrylate-based resin system without Bisphenol A structure by using a synthesized dendritic macromer (G-IEMA). J. Mech. Behav. Biomed. Mater. 2014, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, H.A.; Manso, A.P.; Leung, V.; do Valle, A.L.; Ko, F.; Carvalho, R.M. Flexural properties of experimental nanofiber reinforced composite are affected by resin composition and nanofiber/resin ratio. Dent. Mater. 2015, 31, 1132–1141. [Google Scholar] [CrossRef]
- Lu, H.; Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent. Mater. 2005, 21, 1129–1136. [Google Scholar] [CrossRef]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef]
- Senyurt, A.F.; Wei, H.; Hoyle, C.E.; Piland, S.G.; Gould, T.E. Ternary Thiol–Ene/Acrylate Photopolymers: Effect of Acrylate Structure on Mechanical Properties. Macromolecules 2007, 40, 4901–4909. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Hoyle, C.E. The effect of thiol and ene structures on thiol–ene networks: Photopolymerization, physical, mechanical and optical properties. Polymer 2009, 50, 2237–2245. [Google Scholar] [CrossRef]
- Beigi, S.; Yeganeh, H.; Atai, M. Evaluation of fracture toughness and mechanical properties of ternary thiol–ene–methacrylate systems as resin matrix for dental restorative composites. Dent. Mater. 2013, 29, 777–787. [Google Scholar] [CrossRef]
- Lee, T.Y.; Carioscia, J.; Smith, Z.; Bowman, C.N. Thiol-allyl ether-methacrylate ternary systems. Evolution mechanism of polymerization-induced shrinkage stress and mechanical properties. Macromolecules 2007, 40, 1473–1479. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Evaluation and control of thiol–ene/thiol–epoxy hybrid networks. Polymer 2007, 48, 1526–1532. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Guo, R.; Zhou, H.; Li, Z.; Chen, G.; Zhou, Z.; Li, Q. Preparation of novel UV-cured methacrylate hybrid materials with high thermal stability via thiol–ene photopolymerization. J. Mater. Sci. 2019, 54, 5877–5897. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.d.O. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, H.; Shi, W. Photopolymerization behavior and properties of highly branched polyester acrylate containing thioether linkage used for UV curing coatings. Prog. Org. Coat. 2011, 71, 48–55. [Google Scholar] [CrossRef]
- Fonseca, A.S.Q.S.; Labruna Moreira, A.D.; de Albuquerque, P.P.A.C.; de Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F.J. Effect of monomer type on the CC degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
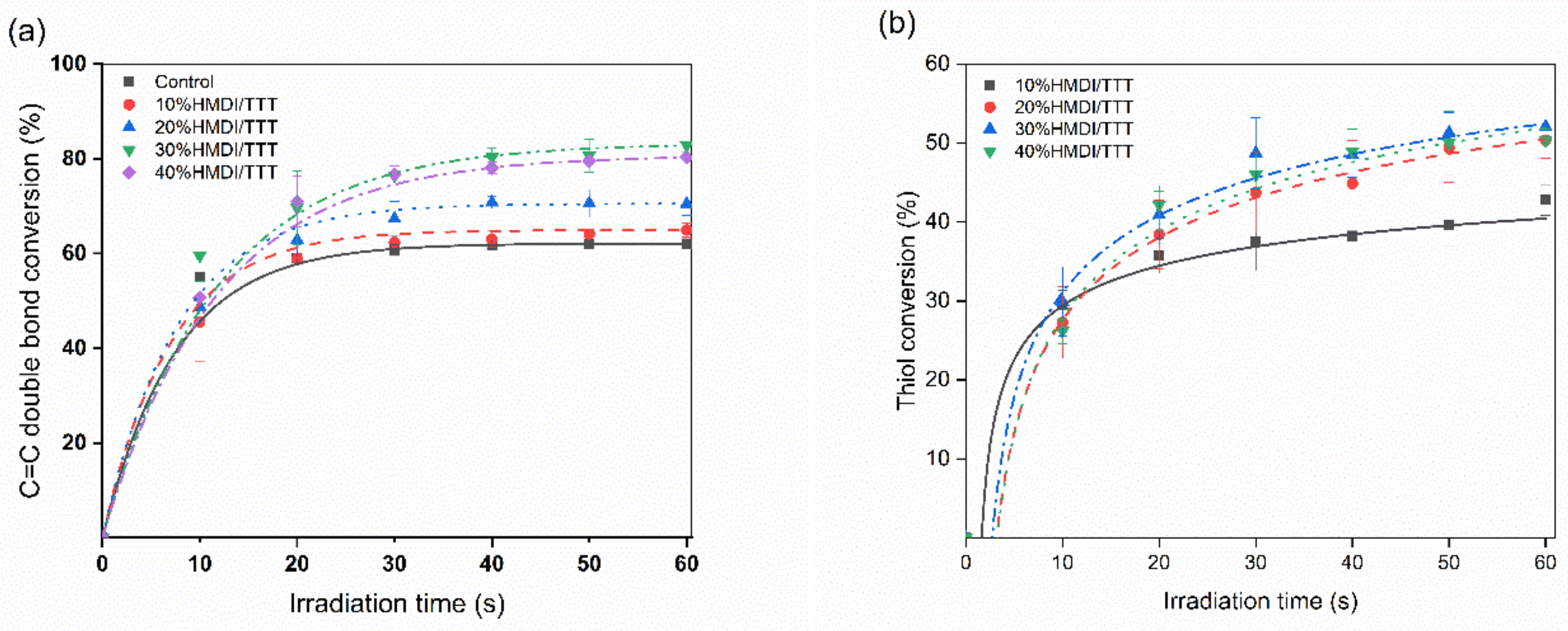
| Resins | C=C Double Bond Conversion at 60 s (%) | Thiol (-SH) Conversion at 60 s (%) |
|---|---|---|
| Control | 62.1 ± 1.1 a | - |
| 10%HMDI-6SH/TTT | 63.8 ± 1.8 a | 42.8 ± 2.0 a |
| 20%HMDI-6SH/TTT | 68.1 ± 2.0 b | 50.4 ± 2.3 b |
| 30%HMDI-6SH/TTT | 82.8 ± 1.5 c | 54.1 ± 1.4 b |
| 40%HMDI-6SH/TTT | 80.1 ± 1.9 c | 50.3 ± 1.4 b |
| Resins | Volumetric Shrinkage (%) |
|---|---|
| Control | 8.53 ± 0.22 a |
| 10%HMDI-6SH/TTT | 6.59 ± 0.25 b |
| 20%HMDI-6SH/TTT | 6.72 ± 0.23 b |
| 30%HMDI-6SH/TTT | 6.50 ± 0.26 b |
| 40%HMDI-6SH/TTT | 4.92 ± 0.13 c |
| Resins | Water Sorption (%) | Water Solubility (%) |
|---|---|---|
| Control | 2.15 ± 0.09 a | 1.33 ± 0.03 a |
| 10%HMDI-6SH/TTT | 2.05 ± 0.08 a,b | 1.11 ± 0.09 a |
| 20%HMDI-6SH/TTT | 1.93 ± 0.04 b,c | 1.04 ± 0.11 a |
| 30%HMDI-6SH/TTT | 1.60 ± 0.09 d | 1.29 ± 0.10 a |
| 40%HMDI-6SH/TTT | 1.41 ± 0.12 d | 1.79 ± 0.12 b |
| Resins | Tg (°C) |
|---|---|
| Control | 100 |
| 10% HMDI-6SH/TTT | 89 |
| 20% HMDI-6SH/TTT | 63 |
| 30% HMDI-6SH/TTT | 57 |
| 40% HMDI-6SH/TTT | 55 |
| Resins | Before Water Immersion | After Water Immersion | ||||
|---|---|---|---|---|---|---|
| FS (MPa) | FM (GPa) | TS (KJ/m2) | FS (MPa) | FM (GPa) | TS (KJ/m2) | |
| Control | 92.5 ± 5.9 a,A | 2.53 ± 0.23 a,A | 4.76 ± 0.92 a,A | 76.7 ± 8.7 a, B | 2.44 ± 0.02 a,A | 3.40 ± 0.41 a,B |
| 10%HMDI-6SH/TTT | 93.4 ± 4.0 a,A | 2.52 ± 0.25 a,A | 5.46 ± 0.31 a,A | 88.8 ± 2.9 b,A | 2.54 ± 0.16 a,A | 5.07 ± 0.59 b,A |
| 20%HMD-6SH/TTT | 91.4 ± 2.0 a,A | 2.20 ± 0.05 b,A | 9.75 ± 0.97 b,A | 76.0 ± 5.3 a,B | 1.90 ± 0.25 b, B | 8.09 ± 1.32 c,A |
| 30%HMDI-6SH/TTT | 79.3 ± 5.9 b,A | 1.95 ± 0.18 c,A | 10.52 ± 1.93 b,A | 58.9 ± 3.5 c,B | 1.35 ± 0.13 c,B | 7.96 ± 1.68 c,A |
| 40%HMDI-6SH/TTT | 21.4 ± 1.2 c,A | 0.39 ± 0.06 d,A | 8.22 ± 1.29 b,A | 17.7 ± 4.0 d,A | 0.26 ± 0.09 d,A | 5.90 ± 1.24 b,B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; He, J.; Garoushi, S.; Vallittu, P.K.; Lassila, L. Enhancing Toughness and Reducing Volumetric Shrinkage for Bis-GMA/TEGDMA Resin Systems by Using Hyperbranched Thiol Oligomer HMDI-6SH. Materials 2021, 14, 2817. https://doi.org/10.3390/ma14112817
Yu B, He J, Garoushi S, Vallittu PK, Lassila L. Enhancing Toughness and Reducing Volumetric Shrinkage for Bis-GMA/TEGDMA Resin Systems by Using Hyperbranched Thiol Oligomer HMDI-6SH. Materials. 2021; 14(11):2817. https://doi.org/10.3390/ma14112817
Chicago/Turabian StyleYu, Biao, Jingwei He, Sufyan Garoushi, Pekka K. Vallittu, and Lippo Lassila. 2021. "Enhancing Toughness and Reducing Volumetric Shrinkage for Bis-GMA/TEGDMA Resin Systems by Using Hyperbranched Thiol Oligomer HMDI-6SH" Materials 14, no. 11: 2817. https://doi.org/10.3390/ma14112817
APA StyleYu, B., He, J., Garoushi, S., Vallittu, P. K., & Lassila, L. (2021). Enhancing Toughness and Reducing Volumetric Shrinkage for Bis-GMA/TEGDMA Resin Systems by Using Hyperbranched Thiol Oligomer HMDI-6SH. Materials, 14(11), 2817. https://doi.org/10.3390/ma14112817









