Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
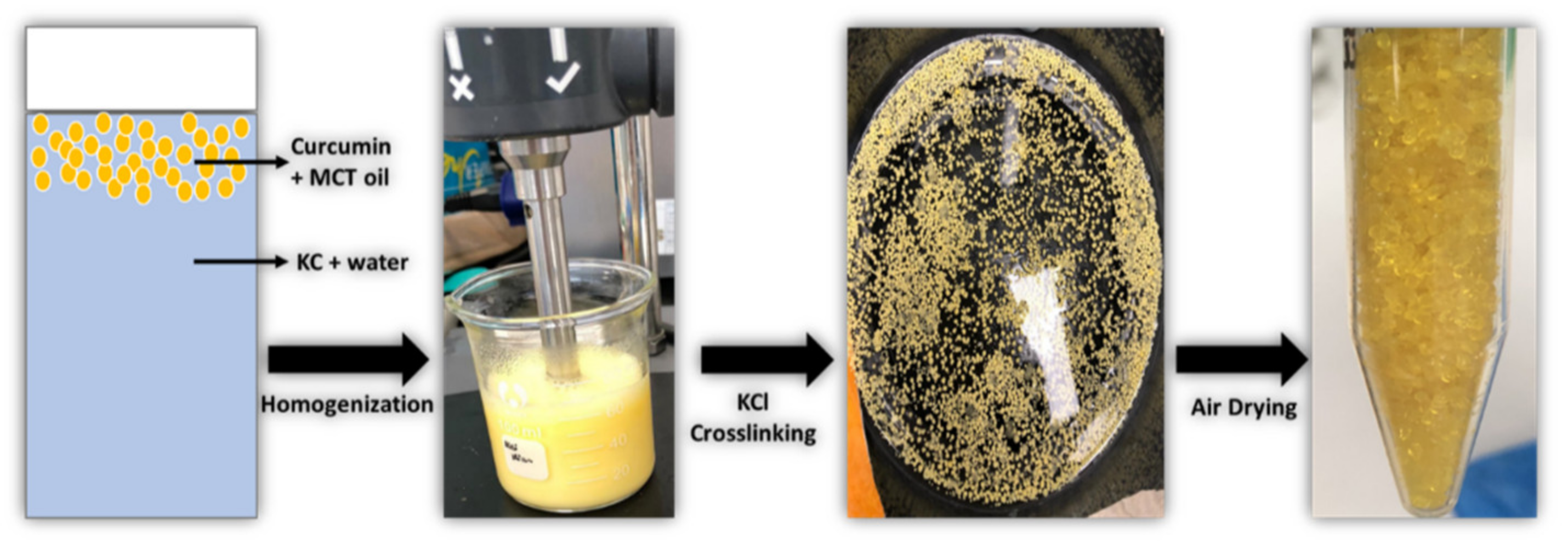
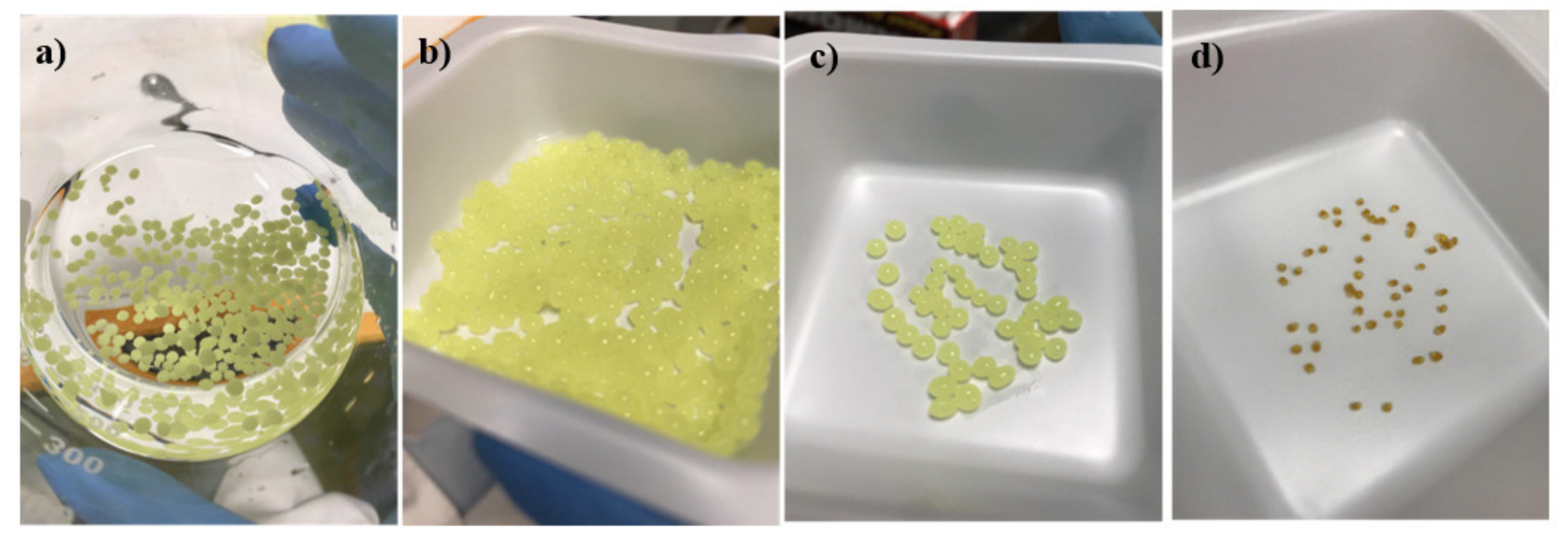
2.2. Preparation of Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Bead Formulation
2.3. Biophysical Characterization of CUR-MCT-KC Formulation
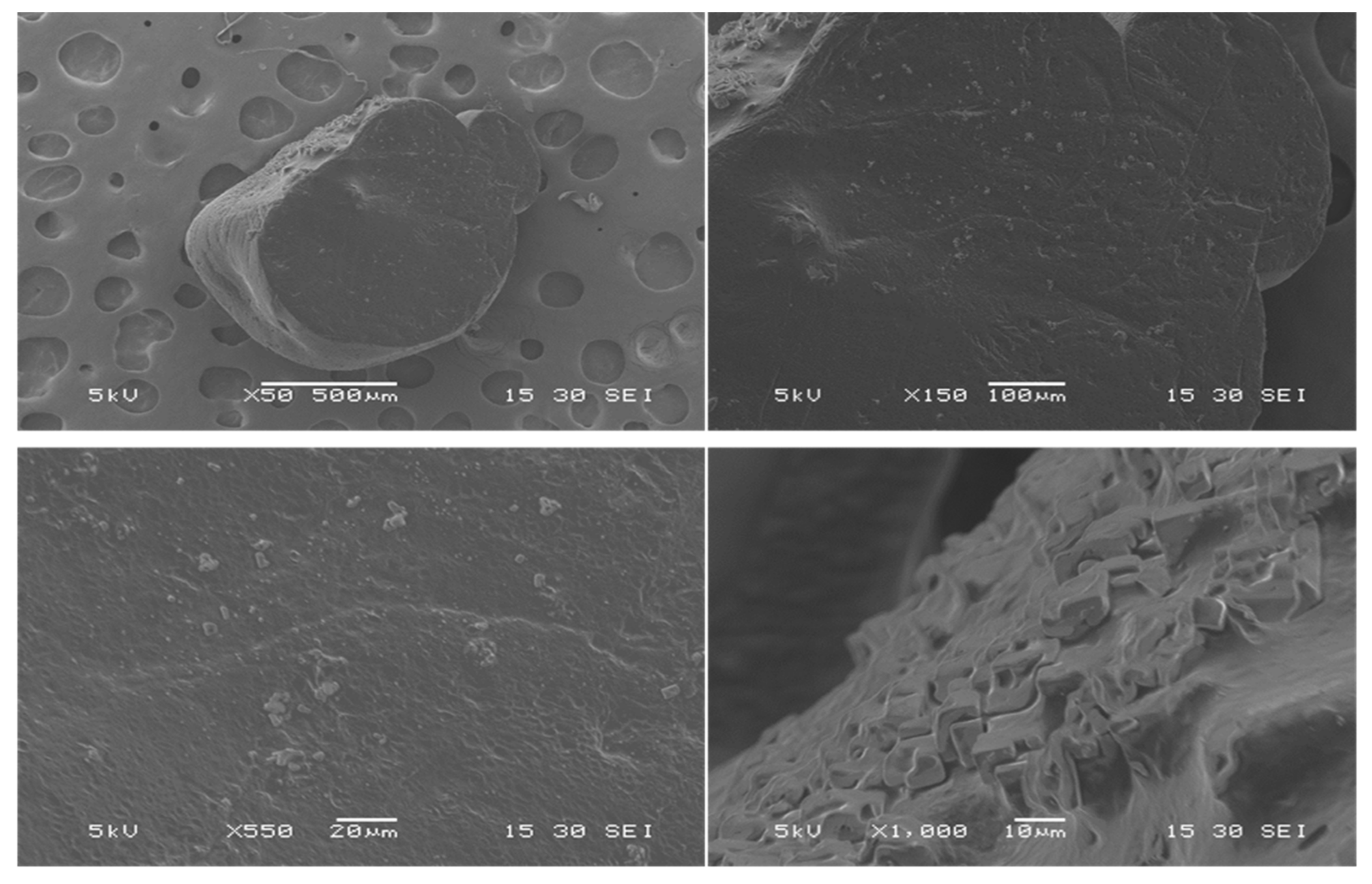
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. UV-Visible Spectrophotometric Analysis
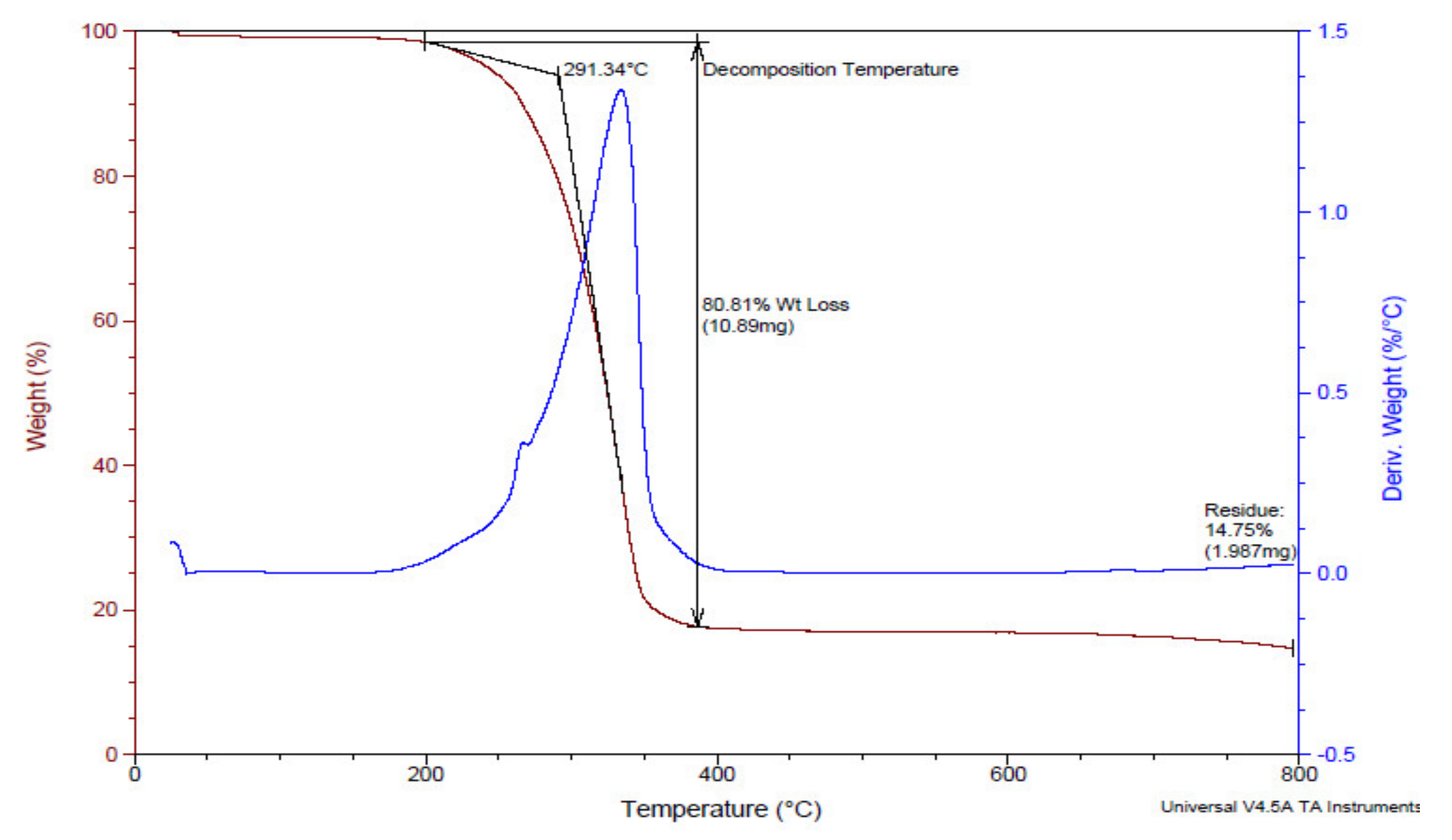
2.3.3. Thermogravimetric Analysis (TGA)
2.4. Chemical Analysis of CUR-MCT-KC Formulation
2.5. Determination of CUR Encapsulation Efficiency (EE)
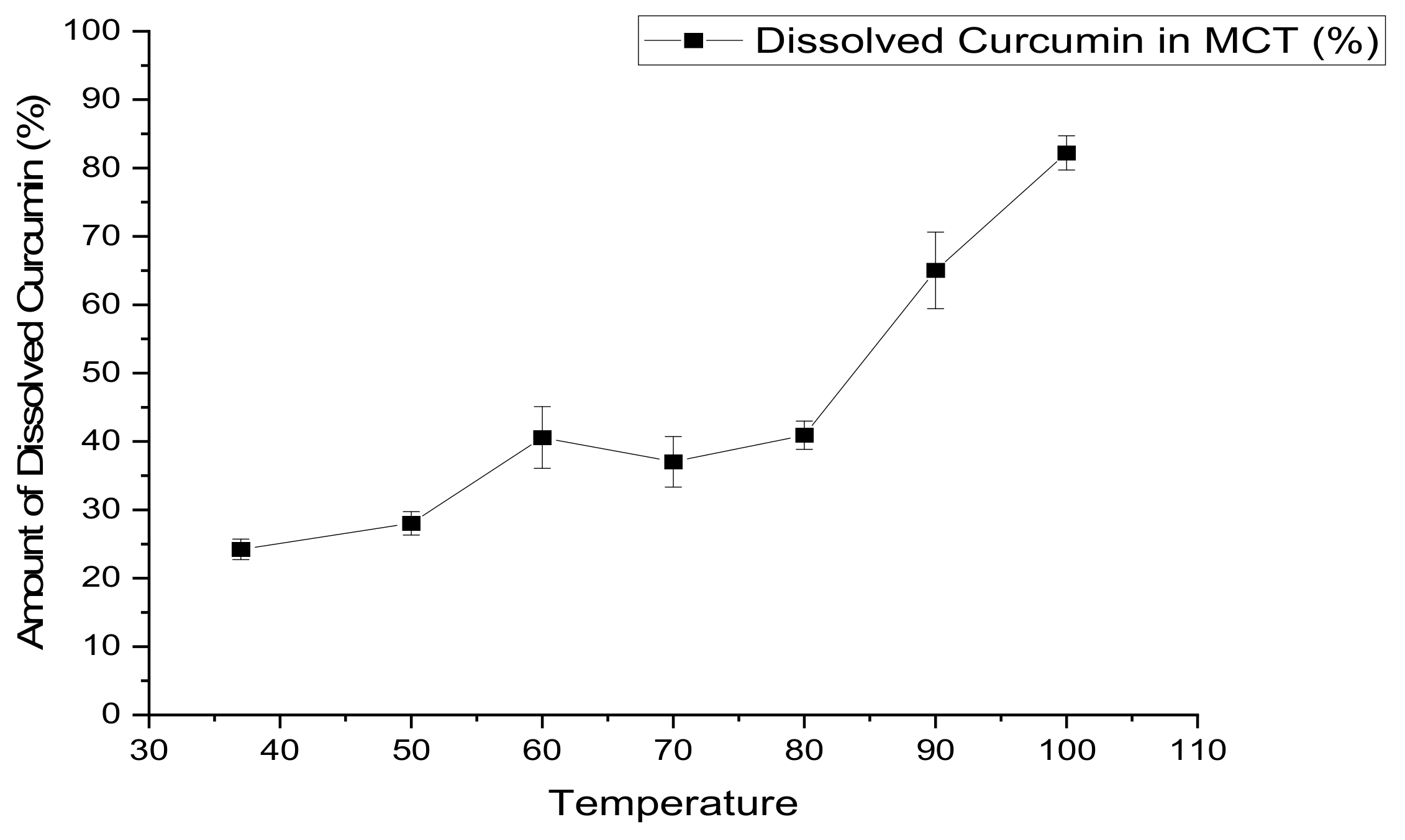
2.6. Measurement of CUR Solubility in MCT Oil
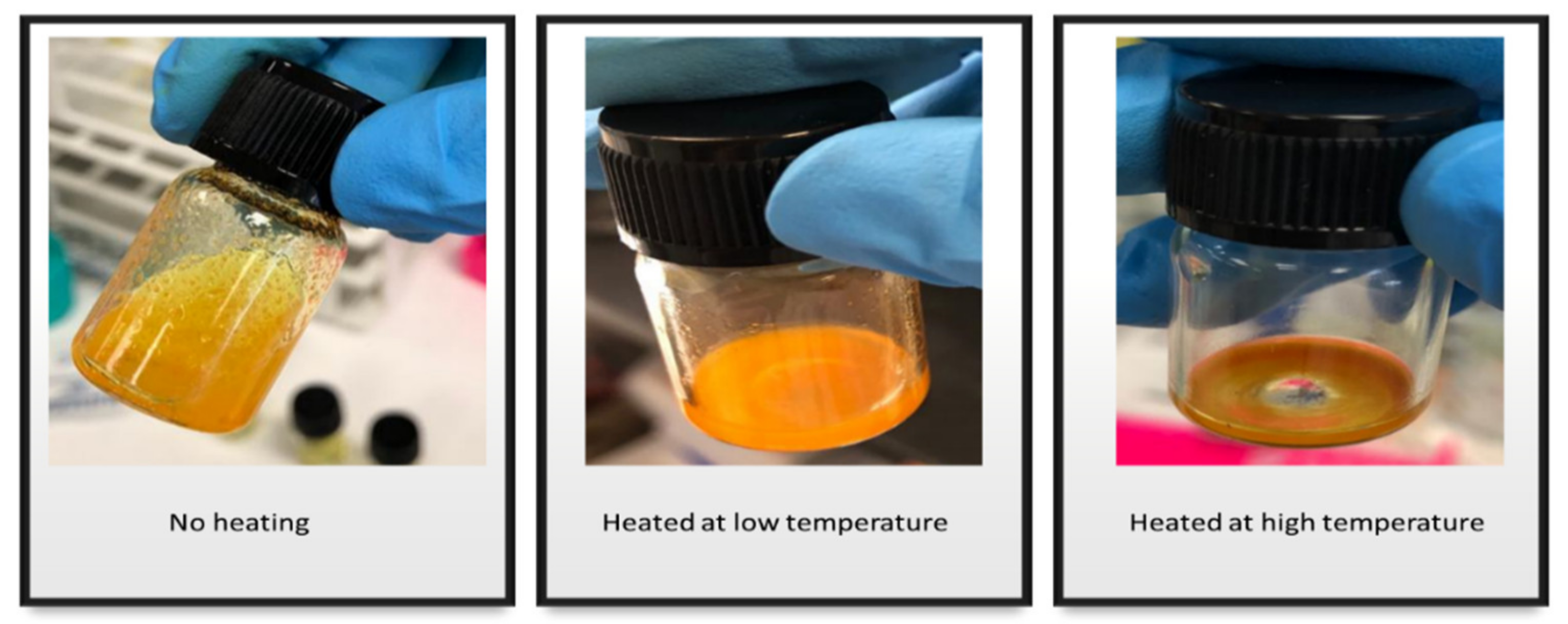
The Effect of Heat on the Solubility of CUR in MCT Oil
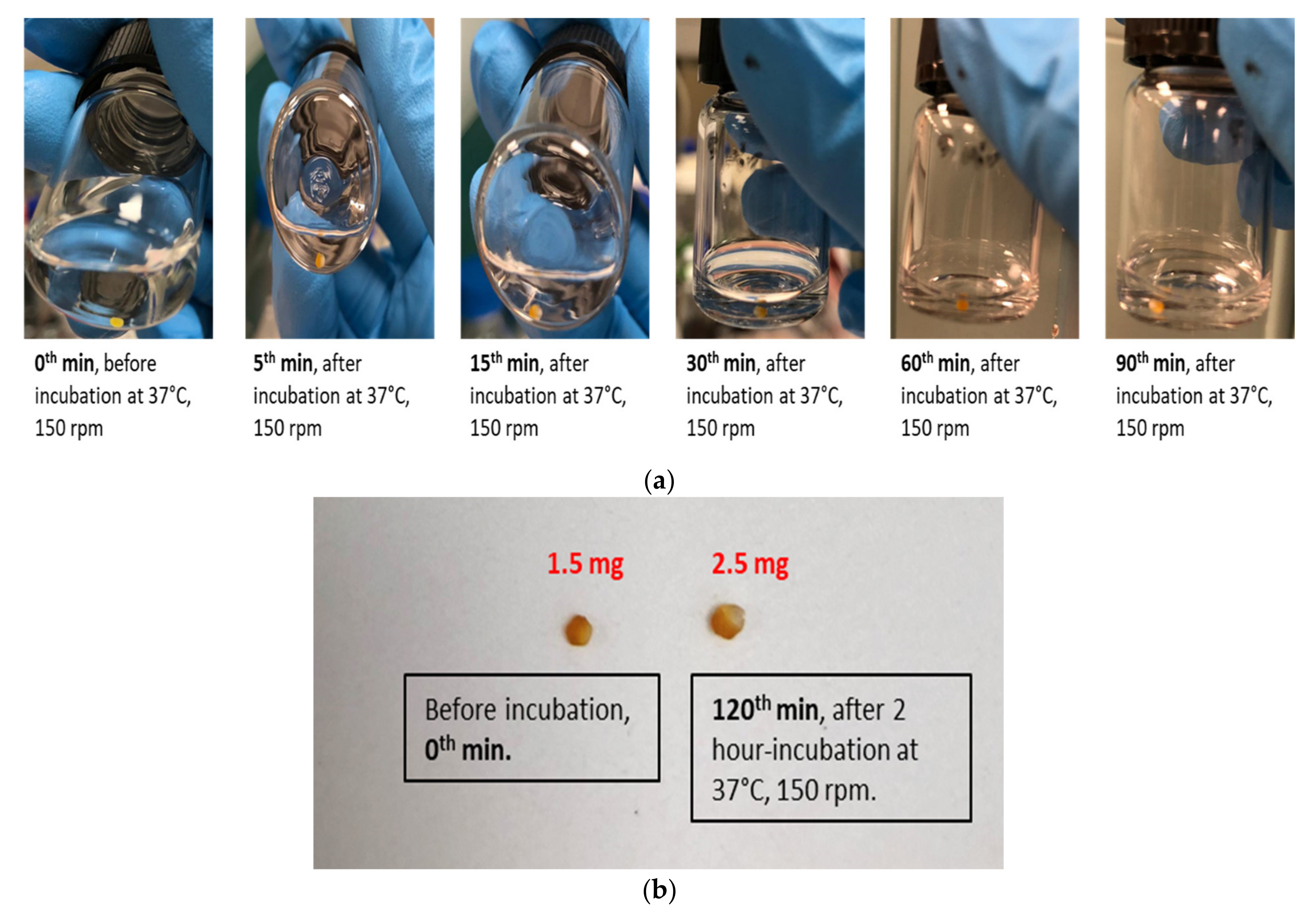
2.7. In Vitro Dissolution Study of CUR-MCT Formulation
2.8. In Vitro Release Study of CUR-MCT-KC Formulation
2.8.1. In Vitro Release Profile at Different pH
2.8.2. In Vitro Release Profile in Simulated GI Conditions
2.9. Experimental Analysis
3. Results and Discussion
3.1. Fabrication and Biophysical Characterization of CUR-MCT-KC Oral Delivery System
3.1.1. CUR-MCT-KC Design Concept and Optimization
3.1.2. Biophysical Characterization of CUR-MCT-KC Formulation
SEM
TGA
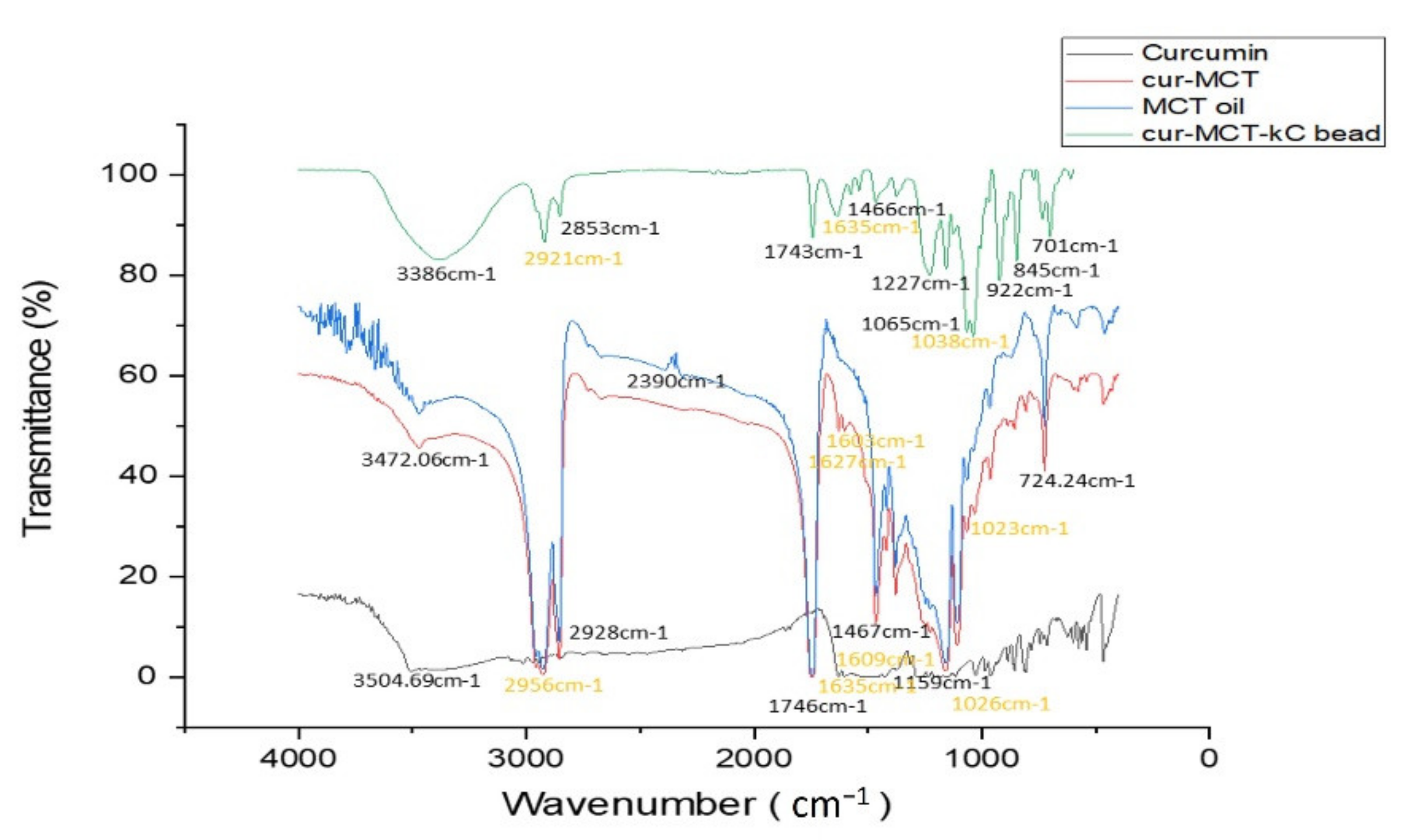
FTIR
3.1.3. Encapsulation Efficiency (EE) of CUR-MCT-KC Formulation
3.2. Interactions of Hydrophobic CUR with MCT Oil
3.2.1. Solubility of CUR in MCT Oil
3.2.2. The Effect of Heat on the Solubility of CUR in MCT
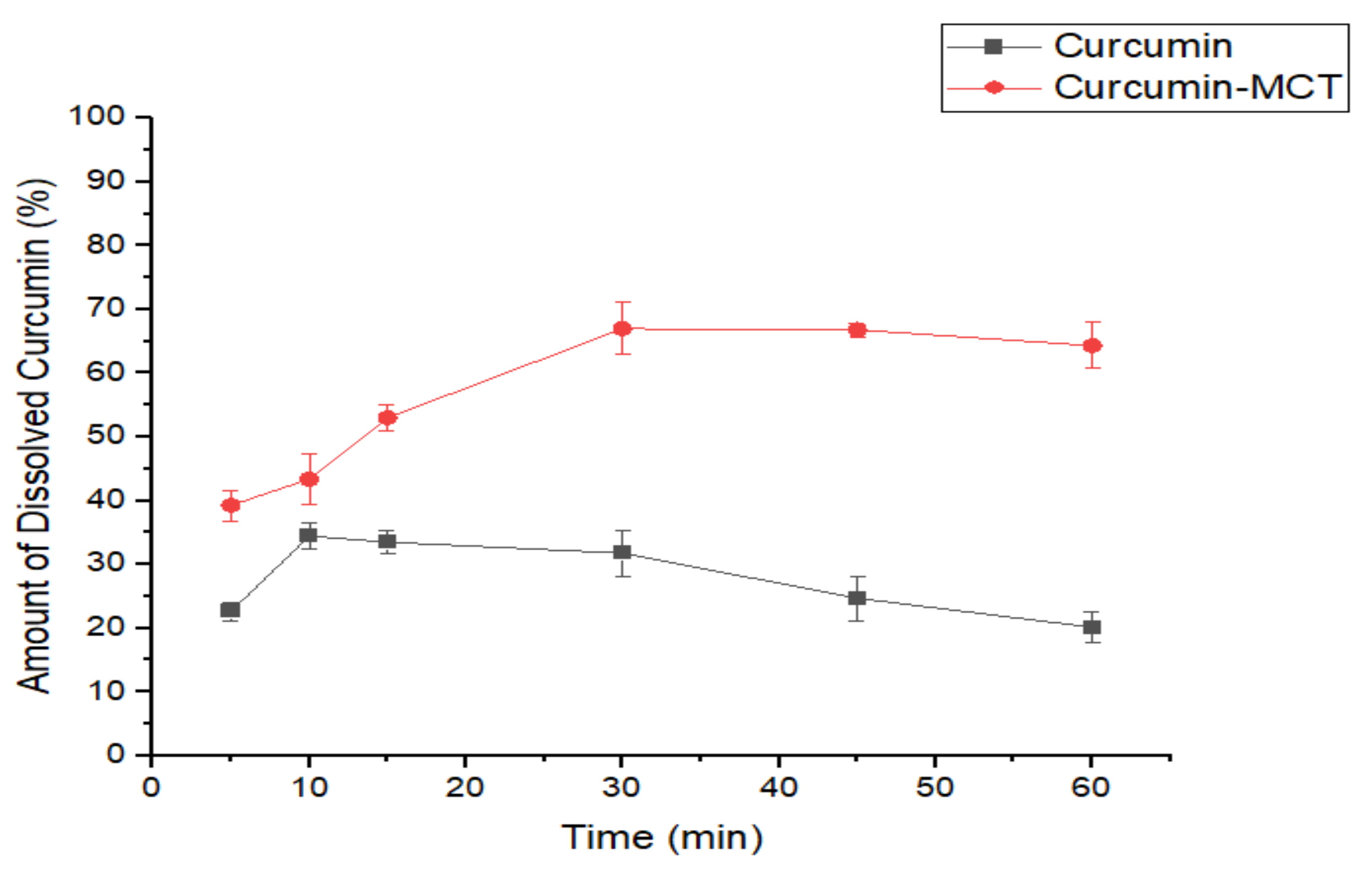
3.2.3. In Vitro Dissolution Study of CUR-MCT Formulation
3.3. In Vitro Release Study of CUR-MCT-KC Formulation in Simulated GI Conditions
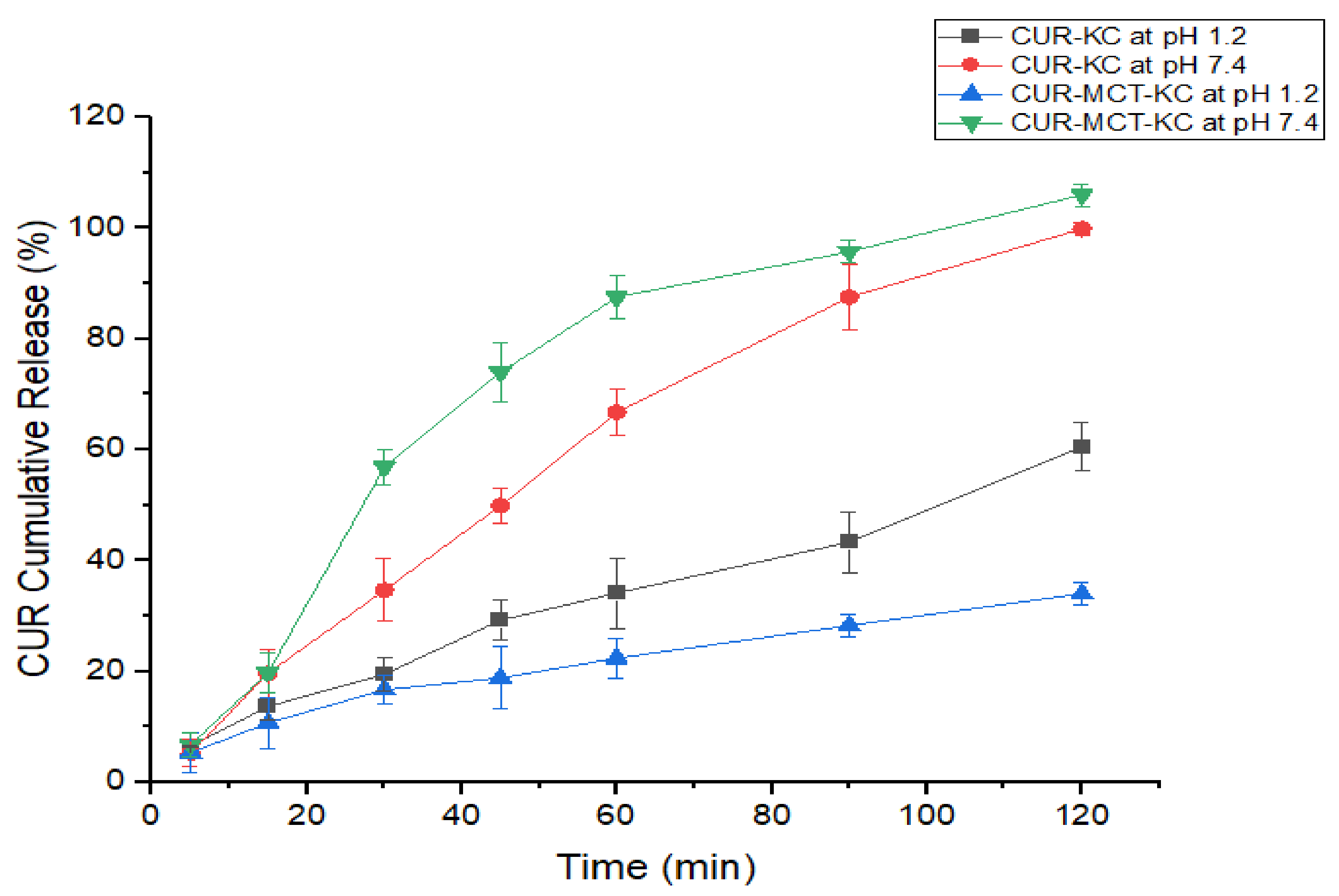
3.3.1. CUR-KC vs. CUR-MCT-KC at Different pH
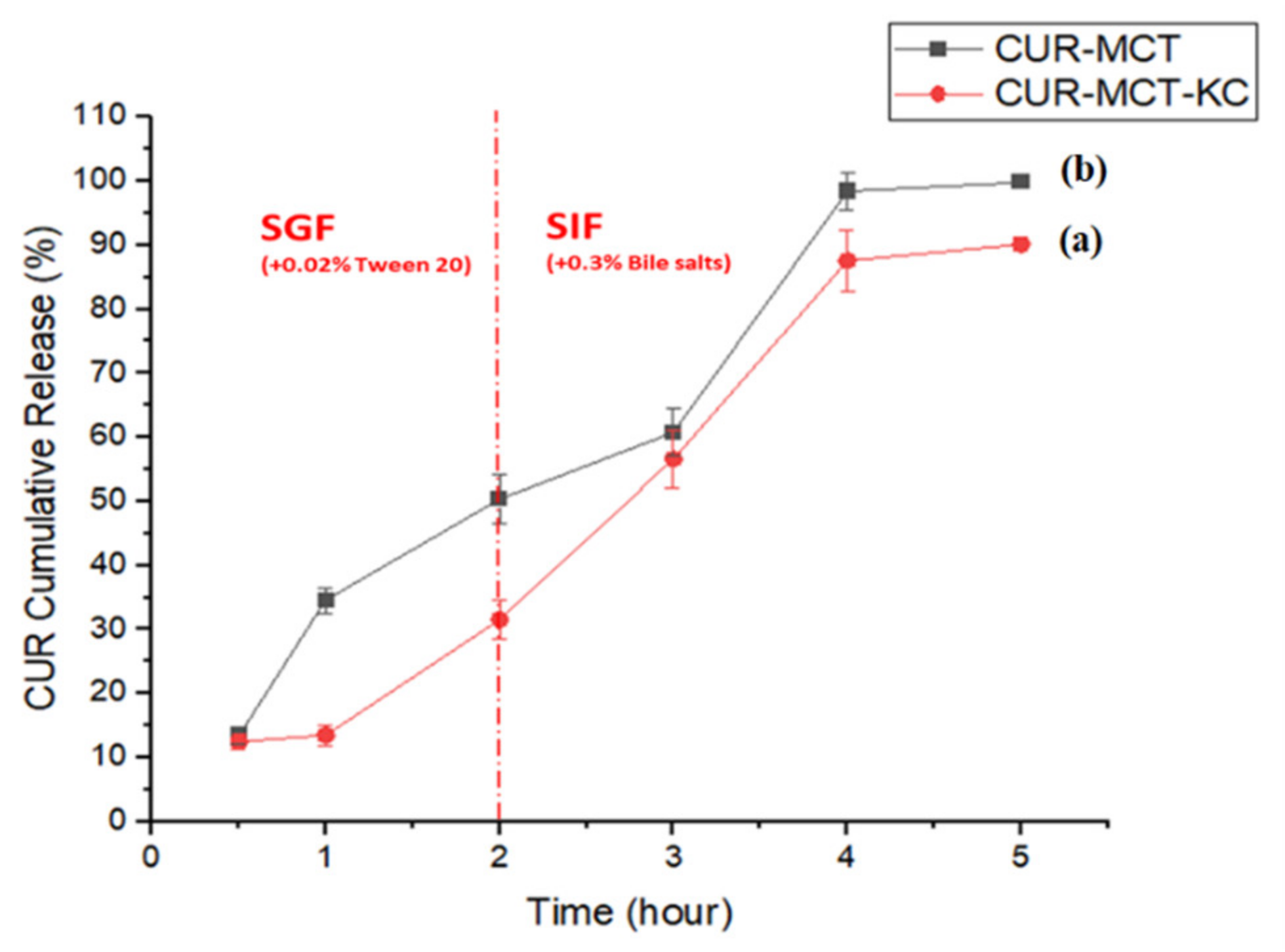
3.3.2. In Vitro Release Profile of CUR-MCT-KC
In SGF
CUR-MCT Emulsion vs. CUR-MCT-KC Beads
Stomach pH of Fasted- and Fed-Stated
In SIF
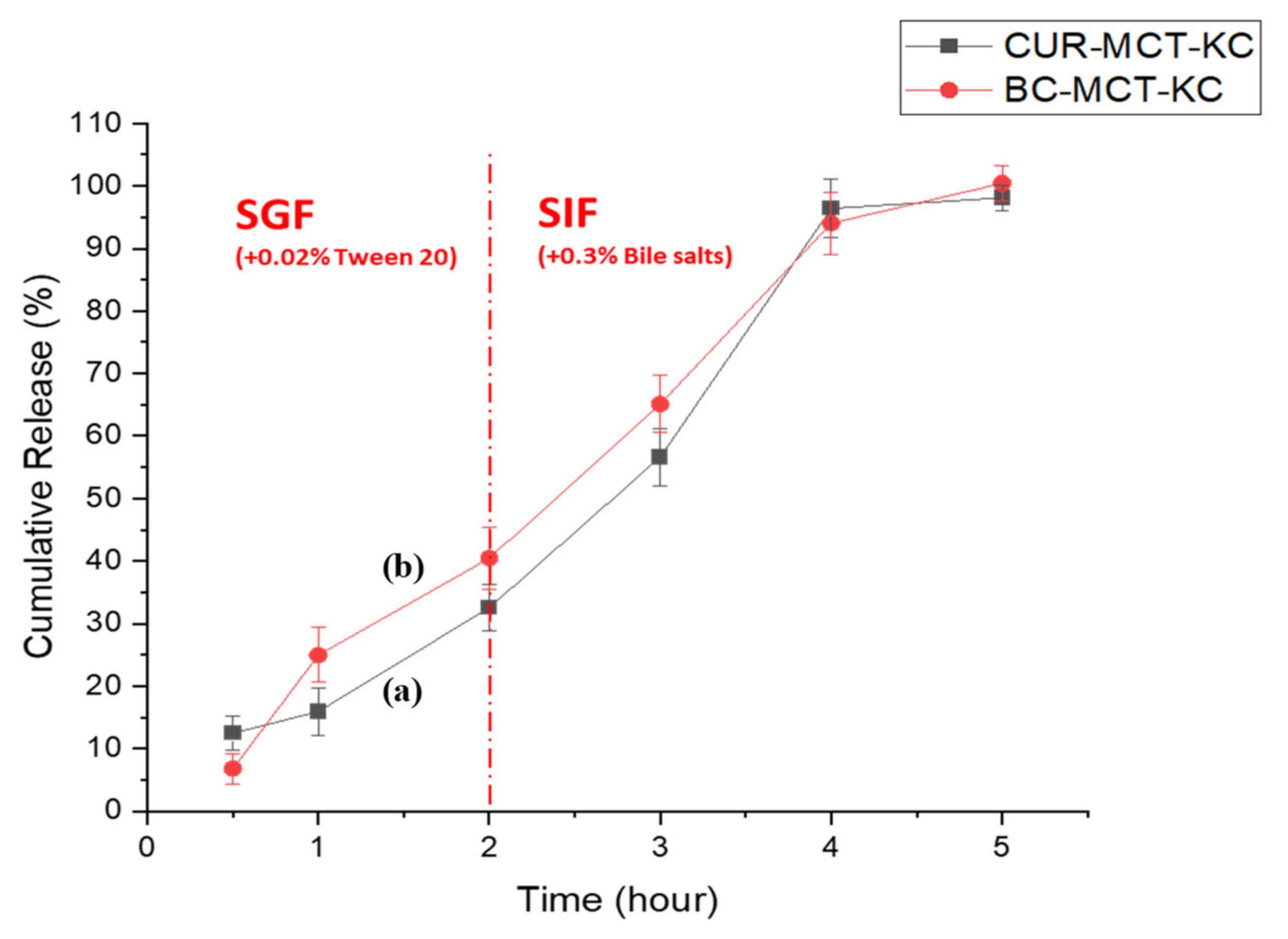
3.3.3. In Vitro Release Profile of Beta Carotene (BC)-Loaded MCT-KC Formulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | FTIR Absorption Band | Descriptions—Characteristics of Different Functional Group | References |
|---|---|---|---|
| CUR | 3504.69 cm−1 | Assigned to the stretching vibrations of phenolic hydroxyl (O–H) group | [1,47] |
| 1628 cm−1 | Related to the overlapping stretching vibrations of carbonyl C=O and alkenes C=C vibrations | ||
| 1605 cm−1 | Indicated the stretching vibrations of benzene ring | ||
| 1026/856 cm−1 | Attributed to the C–O–C stretching vibrations | ||
| 724.24 cm−1 | Due to CH2 stretching vibrations of alkene group | ||
| MCT | 3472.06 cm−1 | Ascribed to the C=O of ester | |
| 2928 cm−1 | Due to the CH2 and CH3 vibration. (asymmetric stretching vibrations of C–H of aliphatic CH2 group; asymmetric stretching vibration of CH of aliphatic CH3 groups, which attributed to the alkyl of triglycerides found in large amounts in vegetable oils) | ||
| 1746.89 cm−1 | Attributed to the C=O stretching vibration (ester carbonyl functional groups of the triglycerides, C=O) | ||
| 1467.49 cm−1 | Indicated the stretching of CH2. (bending vibration of C–H of CH2 and CH3 aliphatic groups) | ||
| 1159.58 cm−1 | Assigned to C–O vibration (stretching vibration of C–O ester groups) | ||
| KC | 1223.64 cm−1, 1243.07 cm−1 | Assigned to the S=O of sulfate esters | |
| 1035.17 cm−1, 1035.62 cm−1 | Ascribed to the glycosidic linkage, C–O–C of 3,6 anhydro-D-galactose | ||
| 921.94 cm−1, 921.04 cm−1 | Due to C–O of 3,6-anhydro-D-galactose | ||
| 845.61 cm−1, 844.76 cm−1 | Attributed to the C–O–SO3 of D-galactose-4-sulfate |
References
- Moghaddasi, F.; Housaindokht, M.R.; Darroudi, M.; Bozorgmehr, M.R.; Sadeghi, A. Synthesis of nano curcumin using black pepper oil by O/W Nanoemulsion Technique and investigation of their biological activities. LWT 2018, 92, 92–100. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential applications of curcumin and curcumin nanoparticles: From traditional therapeutics to modern nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Designing excipient emulsions to increase nutraceutical bioavailability: Emulsifier type influences curcumin stability and bioaccessibility by altering gastrointestinal fate. Food Funct. 2015, 6, 2475–2486. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Nagatsu, A.; Akiyama, T.; Sugimoto, N.; Yamazaki, T.; Maitani, T.; Mizukami, H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003, 555, 311–316. [Google Scholar] [CrossRef]
- Pan, M.-H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Lao, C.D.; Ruffin, M.T., IV; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Ngan, C.L.; Basri, M.; Tripathy, M.; Karjiban, R.A.; Abdul-Malek, E. Physicochemical Characterization and Thermodynamic Studies of Nanoemulsion-Based Transdermal Delivery System for Fullerene. Sci. World J. 2014, 2014, 219035. [Google Scholar] [CrossRef]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Traul, K.; Driedger, A.; Ingle, D.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Shah, N.D.; Limketkai, B.N. The Use of Medium-Chain Triglycerides in Gastrointestinal Disorders. Pract. Gastroenterol. 2017, 41, 20–28. [Google Scholar]
- Evingür, A.G.; Pekcan, Ö. Drying of Polyacrylamide Composite Gels Formed with Various Kappa-Carrageenan Content. J. Fluoresc. 2011, 21, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of kappa-carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Avci, E.N. Radiation synthesis of poly(N-vinyl-2-pyrrolidone)-kappa-carrageenan hydrogels and their use in wound dressing applications. I. Prelim. Lab. Tests. J. Biomed. Mater. Res. A 2005, 74, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.; Ghannam, A.; Al-Ktaifani, M.; Abbas, A.; Hawat, M. Algal sulfated carrageenan inhibits proliferation of MDA-MB-231 cells via apoptosis regulatory genes. Mol. Med. Rep. 2015, 11, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- World Health Organization; Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives/Prepared by the Seventy-Ninth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series; World Health Organization: Geneva, Switzerland, 2015; Volume 70. [Google Scholar]
- Scientific Committee for Food. Reports of the Scientific Committee for Food; Thirty Fifth Series; European Commission: Brussels, Belgium; Luxembourg, 1996. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some Food Additives, Feed Additives and Naturally Occurring Substances; IARC Monographs; WHO: Geneva, Switzerland, 1983; pp. 79–94. [Google Scholar]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Sathuvan, M.; Thangam, R.; Gajendiran, M.; Vivek, R.; Balasubramanian, S.; Nagaraj, S.; Gunasekaran, P.; Madhan, B.; Rengasamy, R. kappa-Carrageenan: An effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr. Polym. 2017, 160, 184–193. [Google Scholar] [CrossRef]
- Joung, H.J.; Choi, M.J.; Kim, J.T.; Park, S.H.; Park, H.J.; Shin, G.H. Development of Food-Grade Curcumin Nanoemulsion and its Potential Application to Food Beverage System: Antioxidant Property and In Vitro Digestion. J. Food Sci. 2016, 81, N745–N753. [Google Scholar] [CrossRef]
- Jiang, Y. Micro- and Nano-Encapsulation and Controlled-Release of Phenolic Compounds and Other Food Ingredients; Graduate School-New Brunswick, The State University of New Jersey: New Brunswick, NJ, USA, 2009. [Google Scholar]
- Takenaka, M.; Ohkubo, T.; Okadome, H.; Sotome, I.; Itoh, T.; Isobe, S. Effective Extraction of Curcuminoids by Grinding Turmeric (Curcuma longa) with Medium-chain Triacylglycerols. Food Sci. Technol. Res. 2013, 19, 655–659. [Google Scholar] [CrossRef]
- Sari, T.; Mann, B.; Kumar, R.; Singh, R.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Dutta, A.; Boruah, B.; Manna, A.K.; Gohain, B.; Saikia, P.M.; Dutta, R.K. Stabilization of diketo tautomer of curcumin by premicellar anionic surfactants: UV-Visible, fluorescence, tensiometric and TD-DFT evidences. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Soukoulis, C.; Tsevdou, M.; Andre, C.M.; Cambier, S.; Yonekura, L.; Taoukis, P.S.; Hoffmann, L. Modulation of chemical stability and in vitro bioaccessibility of beta-carotene loaded in kappa-carrageenan oil-in-gel emulsions. Food Chem. 2017, 220, 208–218. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Wang, Y.; Kapronezai, K.; Lorente, L.R.; Zhang, R.; Pyrgiotakis, G.; Konduru, N.V.; Ericsson, M.; White, J.C.; De La Torre-Roche, R.; et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. [Google Scholar] [CrossRef]
- Kashif, A. Encapsulation of Curcumin in O/w Nanoemulsions and Its Bioaccessibility after In Vitro Digestion; Department of Food Science, University of Massachusetts Amherst: Amherst, MA, USA, 2010. [Google Scholar]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Improving the Oral Bioavailability of Curcumin Using Novel Organogel-Based Nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.M.; Al Bishri, W.; Balamash, K.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef]
- Richa, R.; Choudhury, A.R. Exploration of polysaccharide based nanoemulsions for stabilization and entrapment of curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef]
- Daniel-Da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef]
- Stone, A.K.; Nickerson, M.T. Formation and functionality of whey protein isolate–(kappa-, iota-, and lambda-type) carrageenan electrostatic complexes. Food Hydrocoll. 2012, 27, 271–277. [Google Scholar] [CrossRef]
- Ghanam, D.; Kleinebudde, P. Suitability of κ-carrageenan pellets for the formulation of multiparticulate tablets with modified release. Int. J. Pharm. 2011, 409, 9–18. [Google Scholar] [CrossRef]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.K.; Sreelakshmi, G.; Muraleedharan, C.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Li, J.; Lee, I.W.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-Eudragit(R) E PO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Yu, H.; Kiew, T.Y.; Hadinoto, K. Cost-effective alternative to nano-encapsulation: Amorphous curcumin–chitosan nanoparticle complex exhibiting high payload and supersaturation generation. Eur. J. Pharm. Biopharm. 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Lesmes, U.; McClements, D.J. Structure–function relationships to guide rational design and fabrication of particulate food delivery systems. Trends Food Sci. Technol. 2009, 20, 448–457. [Google Scholar] [CrossRef]
- Chen, C.; Johnston, T.D.; Jeon, H.; Gedaly, R.; McHugh, P.P.; Burke, T.G.; Ranjan, D. An in vitro study of liposomal curcumin: Stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int. J. Pharm. 2009, 366, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.; Kagan, D. Bioavailability of a Sustained Release Formulation of Curcumin. Integr. Med. 2014, 13, 24–30. [Google Scholar]
- Kumar, A.; Ahuja, A.; Ali, J.; Baboota, S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 279–312. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Molecular basis of the Cotton effects induced by the binding of curcumin to human serum albumin. Tetrahedron Asymmetry 2003, 14, 2433–2444. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef]
- Zhou, M.-H.; Ma, J.-S.; Li, J.; Ye, H.-R.; Huang, K.-X.; Zhao, X.-W. A κ-carrageenase from a newly isolated pseudoalteromonas-like bacterium, WZUC10. Biotechnol. Bioprocess Eng. 2008, 13, 545–551. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Jha, B. Purification and characterization of κ-carrageenase from a novel γ-proteobacterium, Pseudomonas elongata (MTCC 5261) syn. Microbulbifer elongatus comb. Nov. Biotechnol. Bioprocess Eng. 2007, 12, 668–675. [Google Scholar] [CrossRef]
- Rosario, N.L.; Ghaly, E.S. Matrices of water-soluble drug using natural polymer and direct compression method. Drug Dev. Ind. Pharm. 2002, 28, 975–988. [Google Scholar] [CrossRef]
- Capron, I.; Yvon, M.; Müller, G. In-vitro gastric stability of carrageenan. Food Hydrocoll. 1996, 10, 239–244. [Google Scholar] [CrossRef]
- Gu, S.Y.; Decker, E.A.; McClements, D.J. Influence of pH and iota-carrageenan concentration on physicochemical properties and stability of beta-lactoglobulin-stabilized oil-in-water emulsions. J. Agric. Food Chem. 2004, 52, 3626–3632. [Google Scholar] [CrossRef]
- Kara, S.; Arda, E.; Kavzak, B.; Pekcan, M.Ö. Phase transitions of κ-carrageenan gels in various types of salts. J. Appl. Polym. Sci. 2006, 102, 3008–3016. [Google Scholar] [CrossRef]
- Dai, W.-G.; Dong, L.C.; Song, Y.-Q. Nanosizing of a drug/carrageenan complex to increase solubility and dissolution rate. Int. J. Pharm. 2007, 342, 201–207. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I.; Noshadi, I.; Shu Fen, L.; Ngadi, N. Swelling behaviour and controlled drug release from cross-linked κ-carrageenan/NaCMC hydrogel by diffusion mechanism. J. Microencapsul. 2012, 29, 368–379. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Biofilm-Like Lactobacillus rhamnosus Probiotics Encapsulated in Alginate and Carrageenan Microcapsules Exhibiting Enhanced Thermotolerance and Freeze-Drying Resistance. Biomacromolecules 2013, 14, 3214–3222. [Google Scholar] [CrossRef]
- Thommes, M.; Baert, L.; Klooster, G.V.; Geldof, M.; Schueller, L.; Rosier, J.; Kleinebudde, P. Improved bioavailability of darunavir by use of κ-carrageenan versus microcrystalline cellulose as pelletisation aid. Eur. J. Pharm. Biopharm. 2009, 72, 614–620. [Google Scholar] [CrossRef]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper Gastrointestinal pH in Seventy-Nine Healthy, Elderly, North American Men and Women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef]
- McKim, J.M.; Willoughby, J.A.; Blakemore, W.R.; Weiner, M.L. Gastrointestinal Tract Digestion and Carrageenan: How Misconceptions have influenced the Understanding of Carrageenan Safety. J. Nutr. Biol. 2019, 5, 364–376. [Google Scholar]
- Wu, S.J. Degradation of kappa-carrageenan by hydrolysis with commercial alpha-amylase. Carbohydr. Polym. 2012, 89, 394–396. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, K.-X.; Ng, L.-L.E.; Loo, S.C.J. Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation. Materials 2021, 14, 2783. https://doi.org/10.3390/ma14112783
Tan K-X, Ng L-LE, Loo SCJ. Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation. Materials. 2021; 14(11):2783. https://doi.org/10.3390/ma14112783
Chicago/Turabian StyleTan, Kei-Xian, Ling-Ling Evelyn Ng, and Say Chye Joachim Loo. 2021. "Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation" Materials 14, no. 11: 2783. https://doi.org/10.3390/ma14112783
APA StyleTan, K.-X., Ng, L.-L. E., & Loo, S. C. J. (2021). Formulation Development of a Food-Graded Curcumin-Loaded Medium Chain Triglycerides-Encapsulated Kappa Carrageenan (CUR-MCT-KC) Gel Bead Based Oral Delivery Formulation. Materials, 14(11), 2783. https://doi.org/10.3390/ma14112783








