New-Generation Liquid Crystal Materials for Application in Infrared Region
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
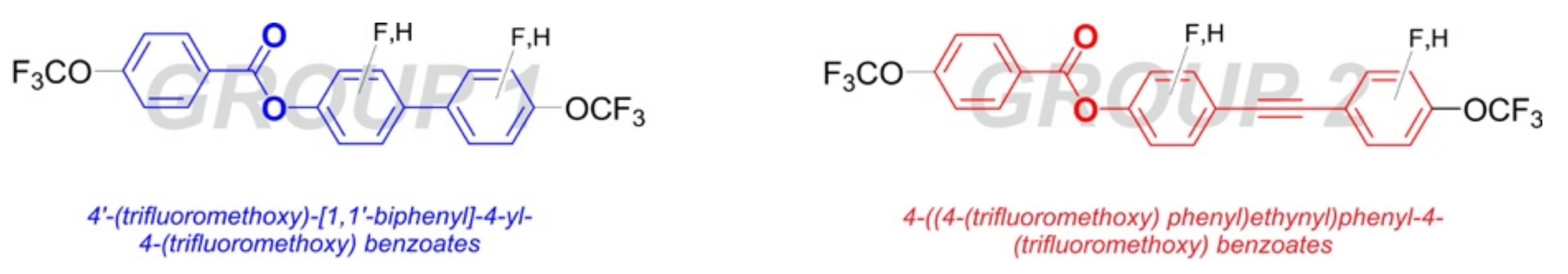
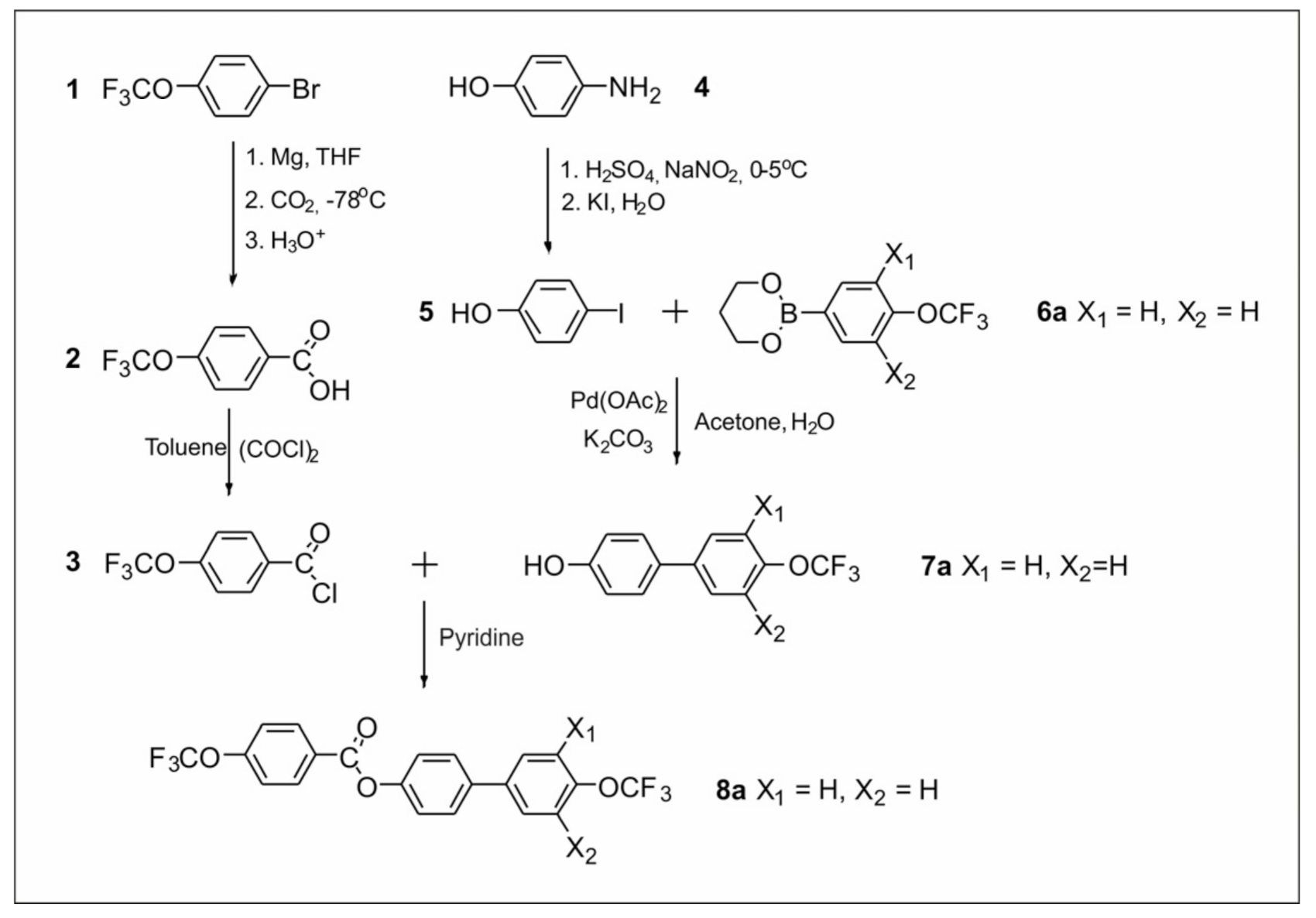
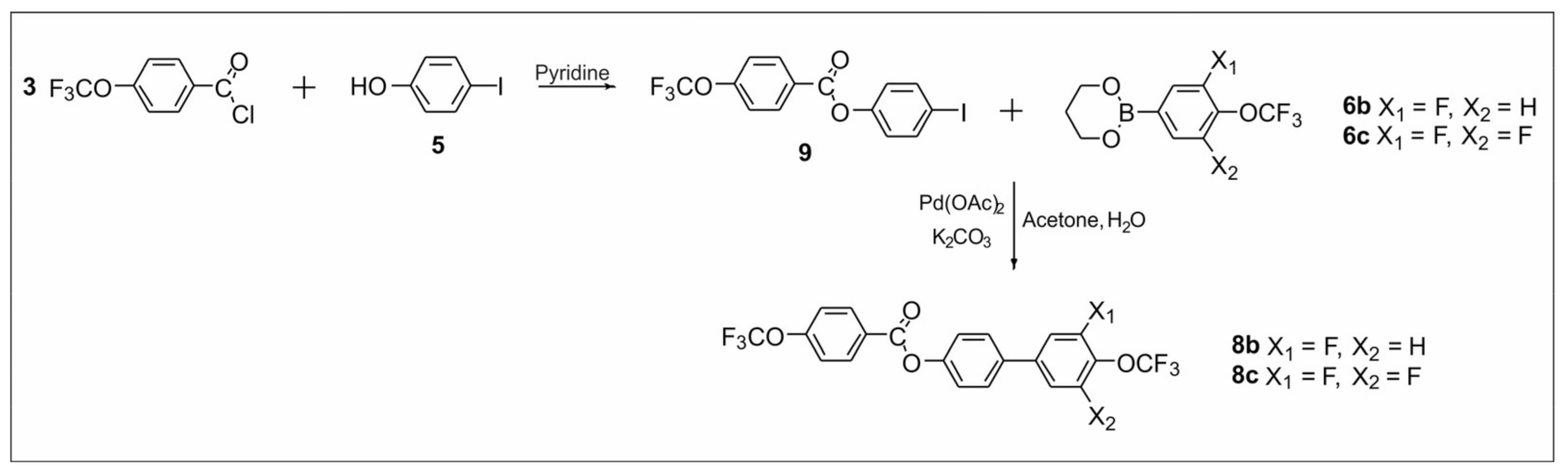
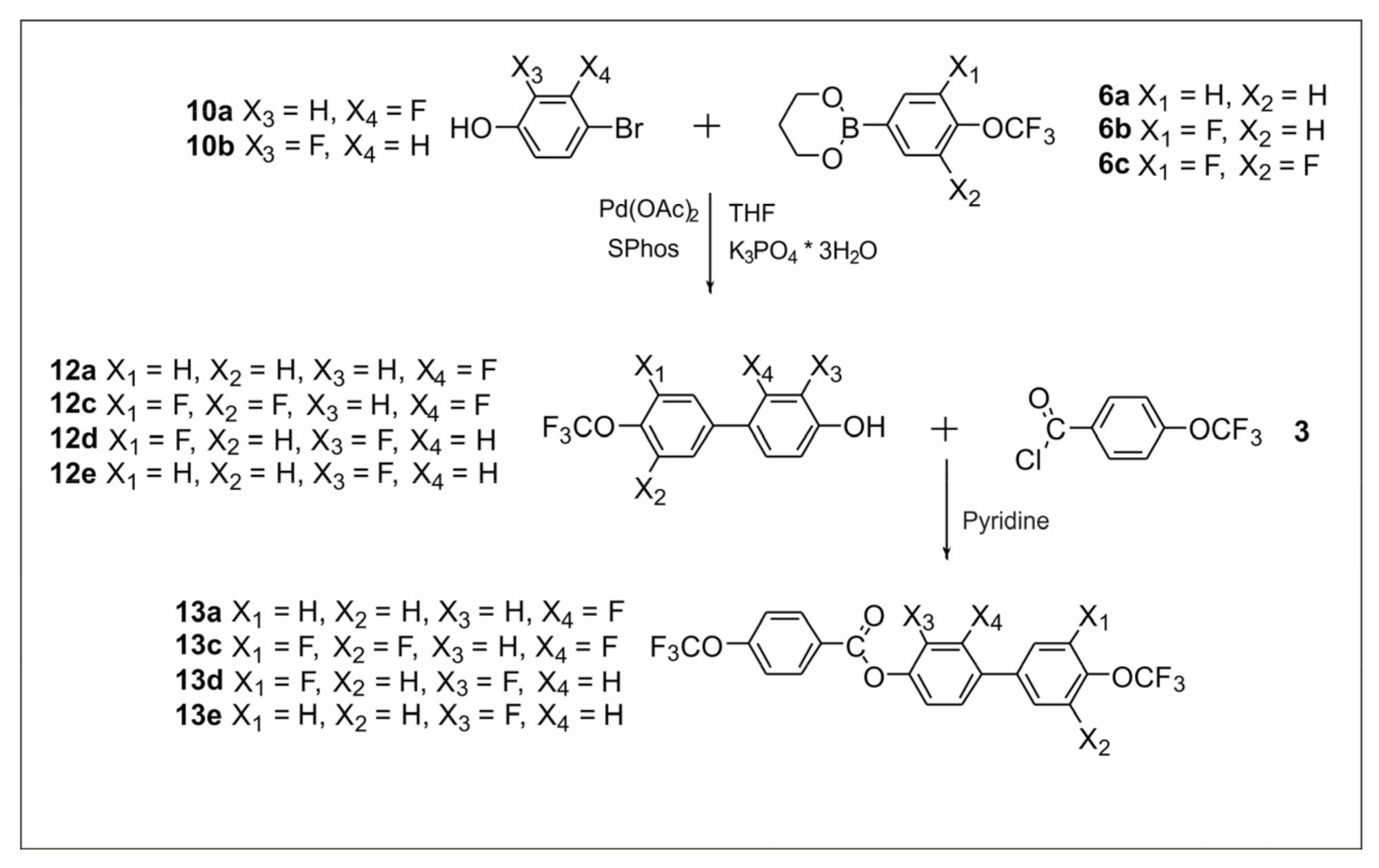
2.2.1. Synthesis of 4′-(Trifluoromethoxy)-[1,1′-biphenyl]-4-yl-4-(trifluoromethoxy) Benzoate Derivatives
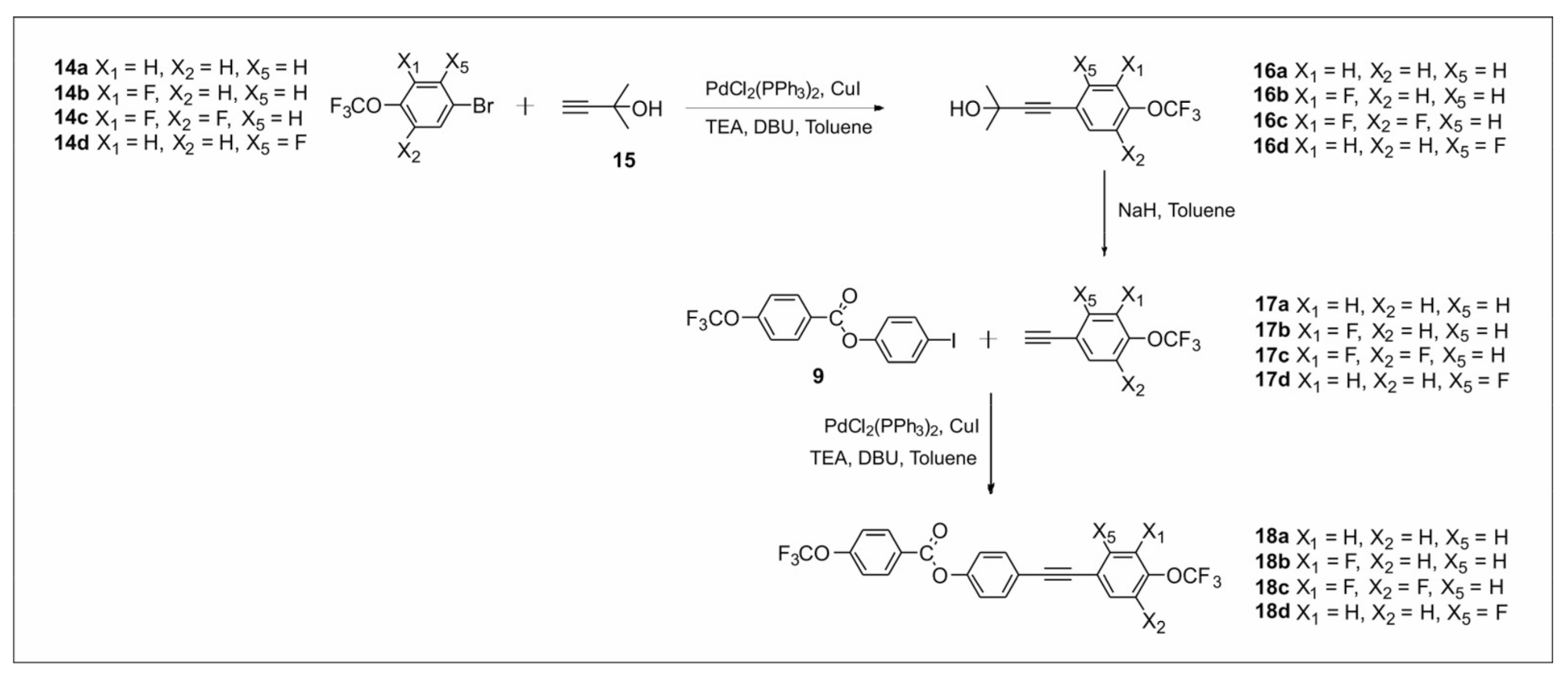
2.2.2. Synthesis of 4-((4-(Trifluoromethoxy)phenyl)ethynyl)phenyl-4-(trifluoromethoxy) Benzoate Derivatives
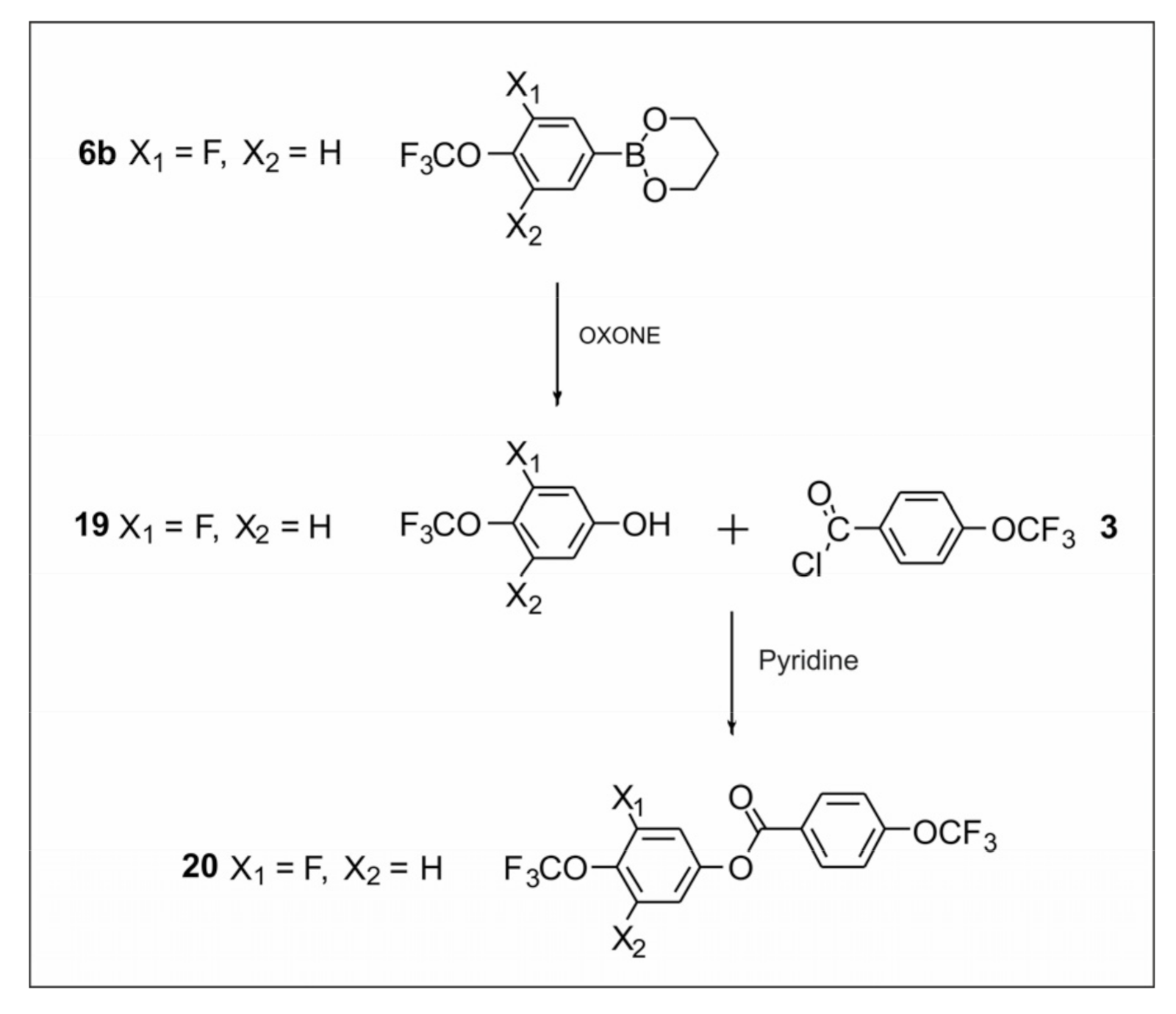
2.2.3. Synthesis of 4-(Trifluoromethoxy)phenyl-4-(trifluoromethoxy) Benzoate
2.3. Characterization and Measurements—Analytical Instrumentation
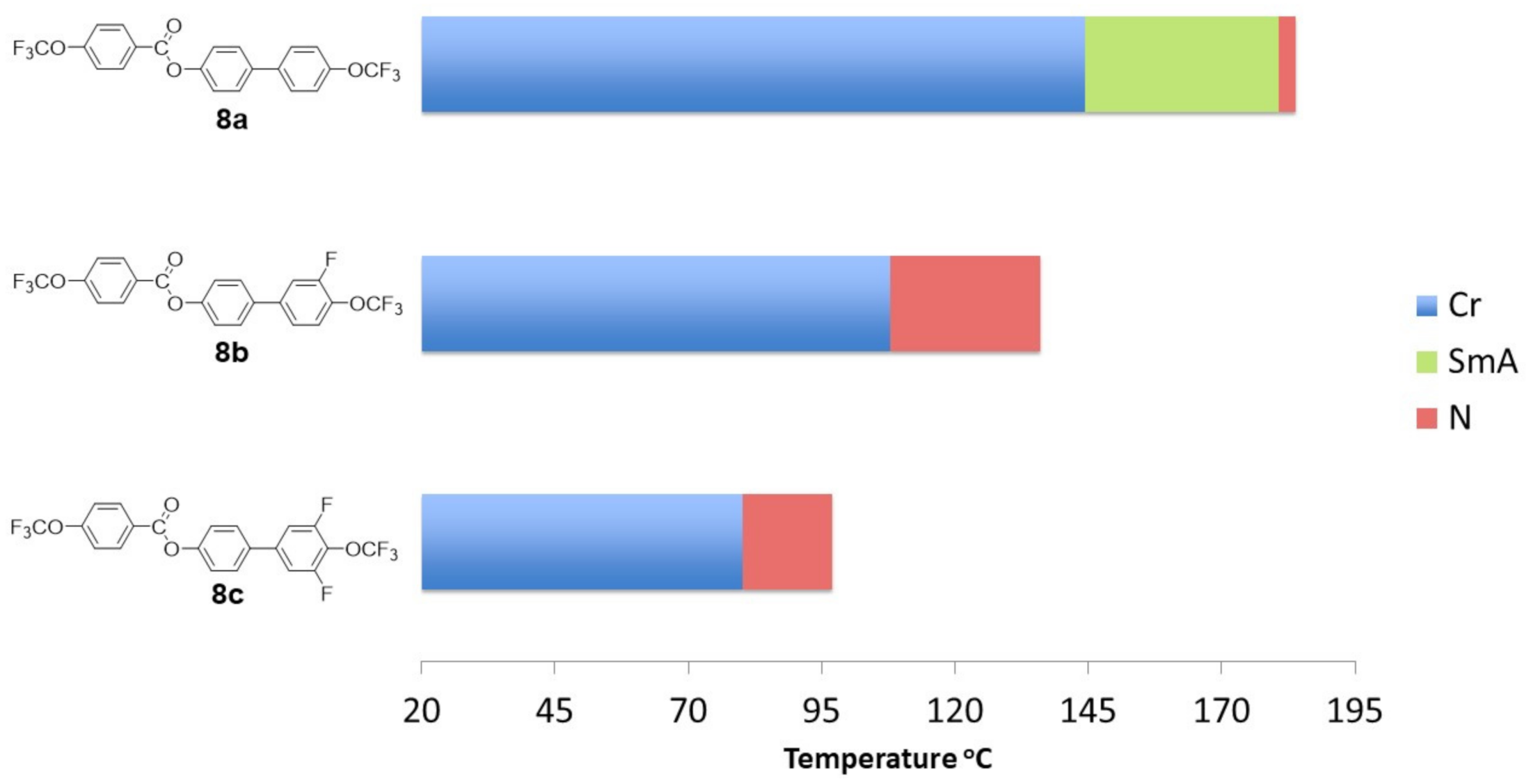
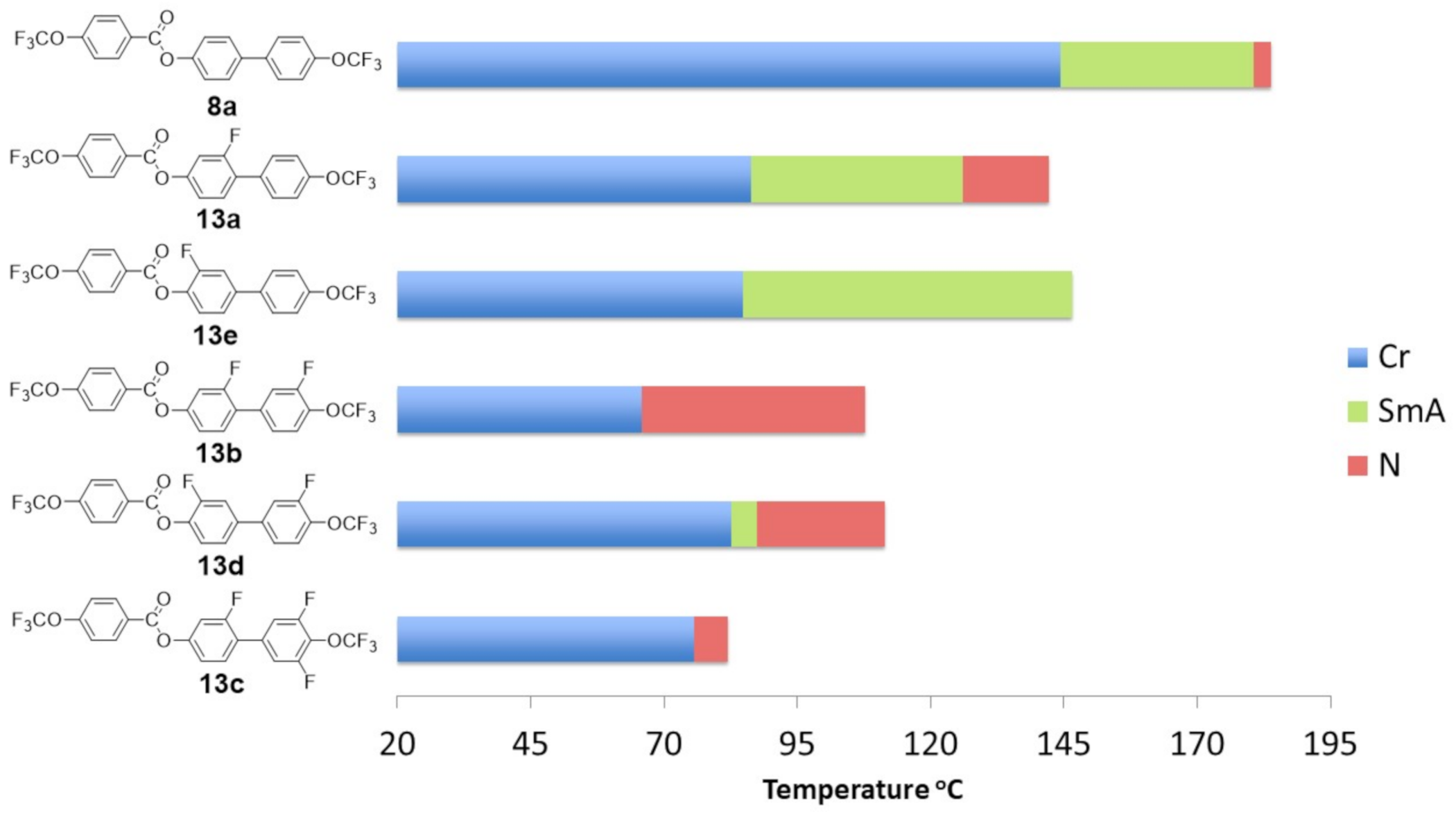
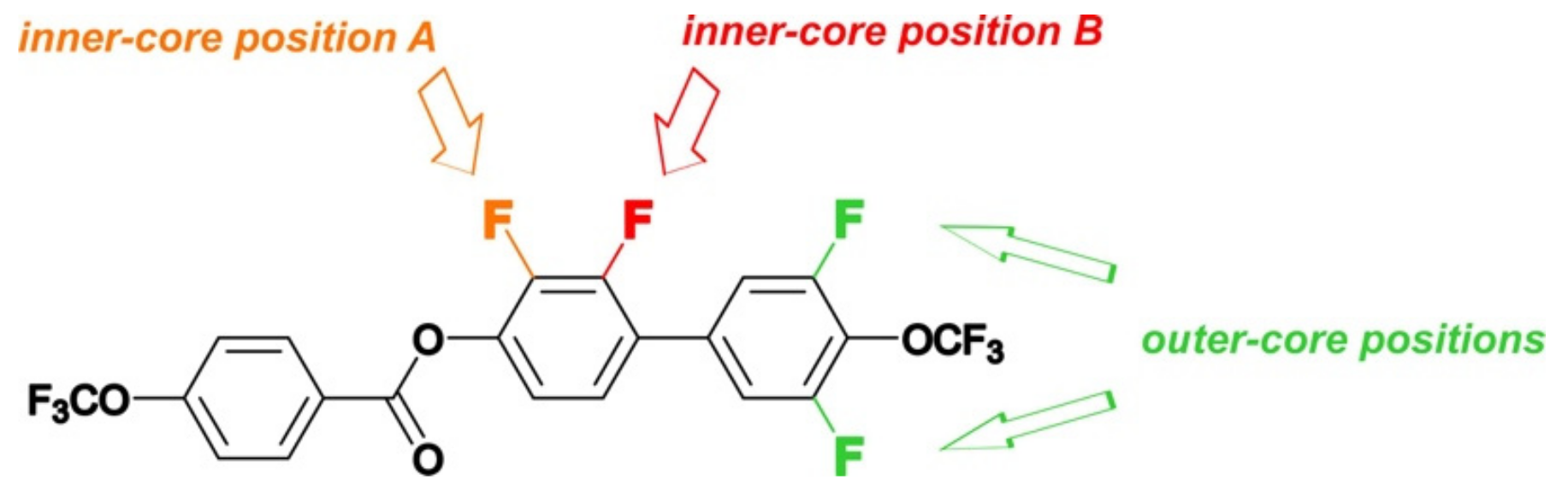
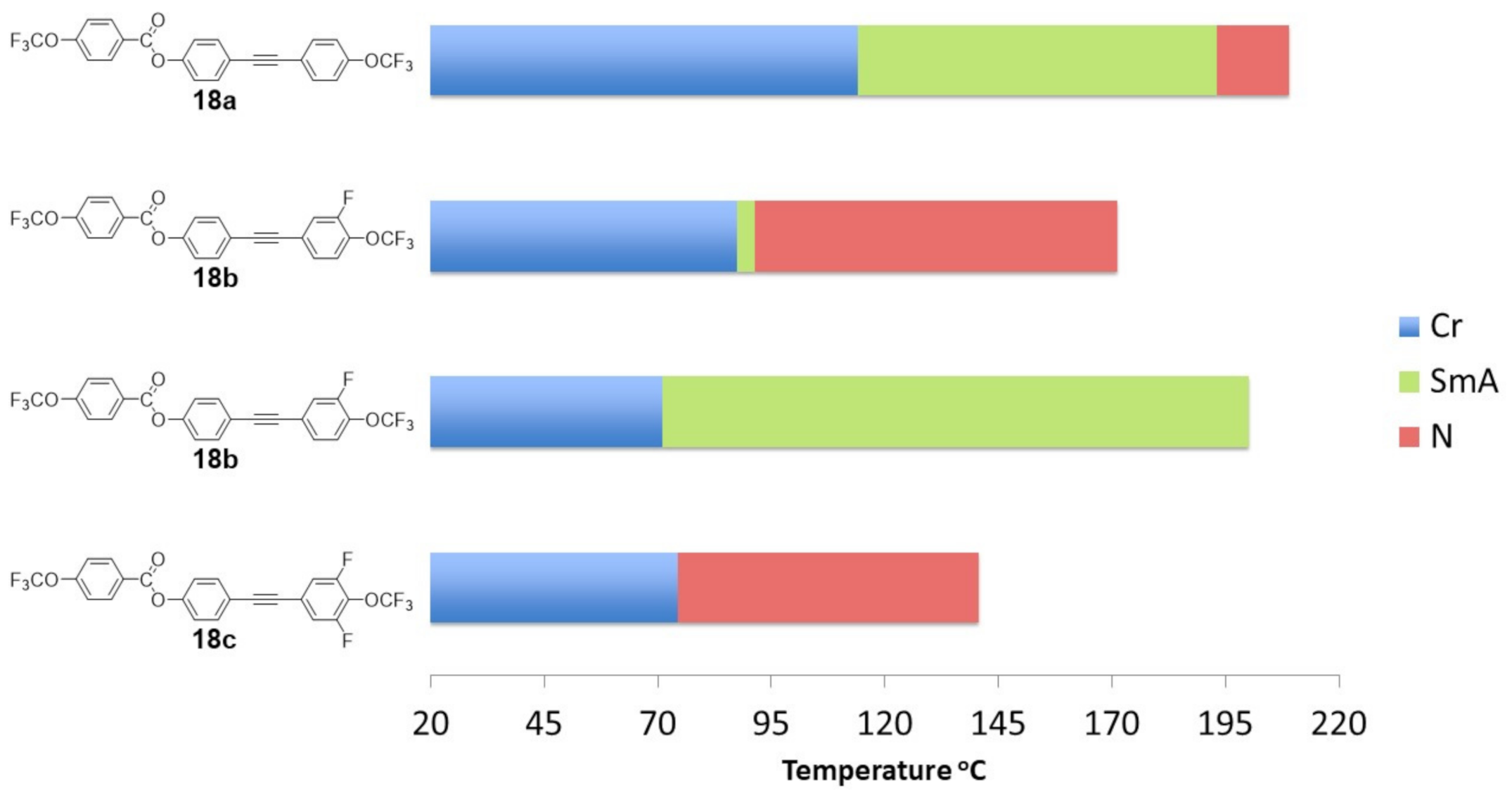
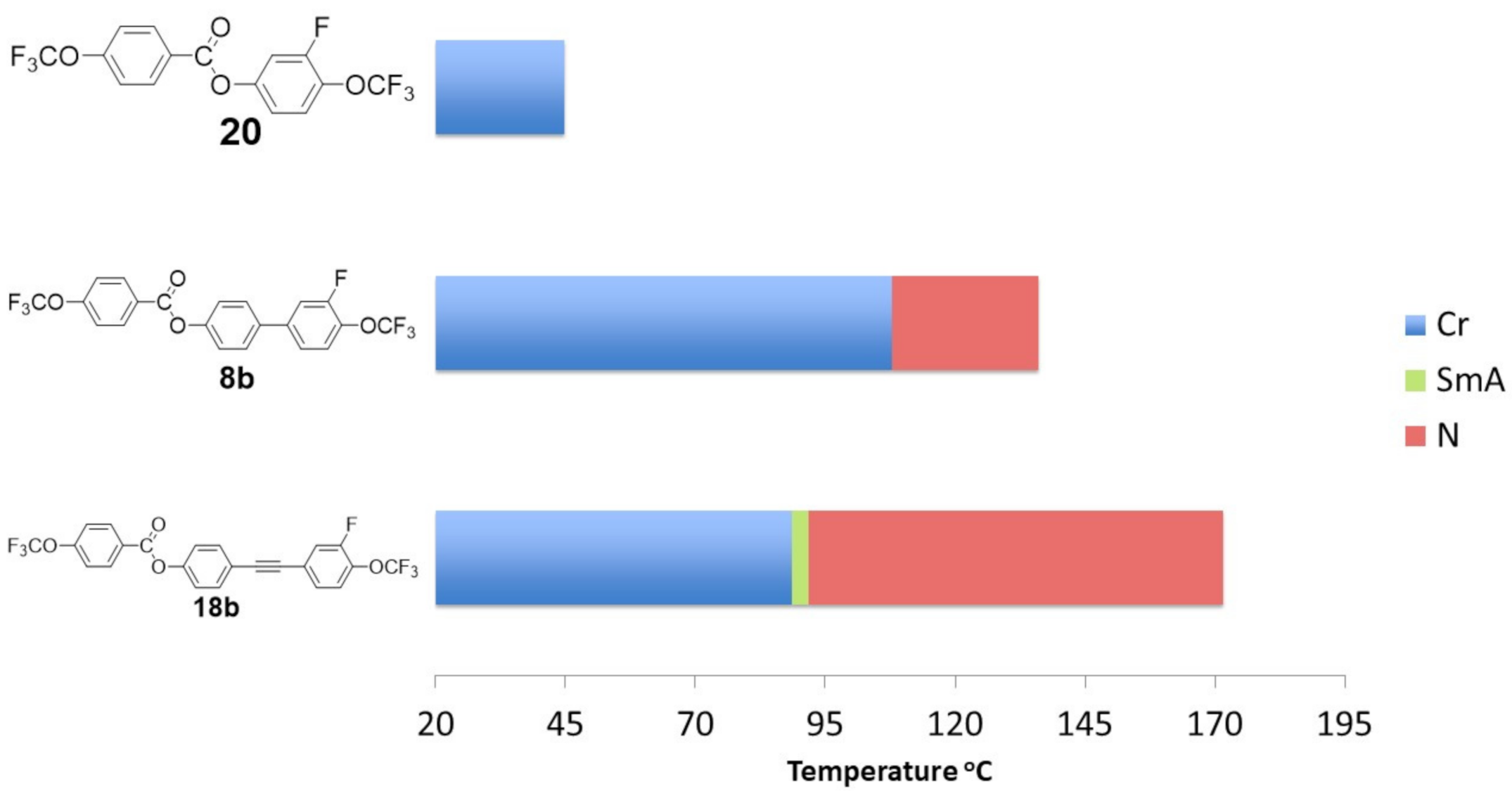
2.4. Mesomorphic Properties and Discussion
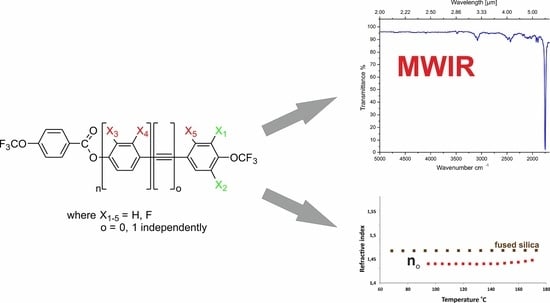
2.5. IR (2–6 μm) Absorption Properties
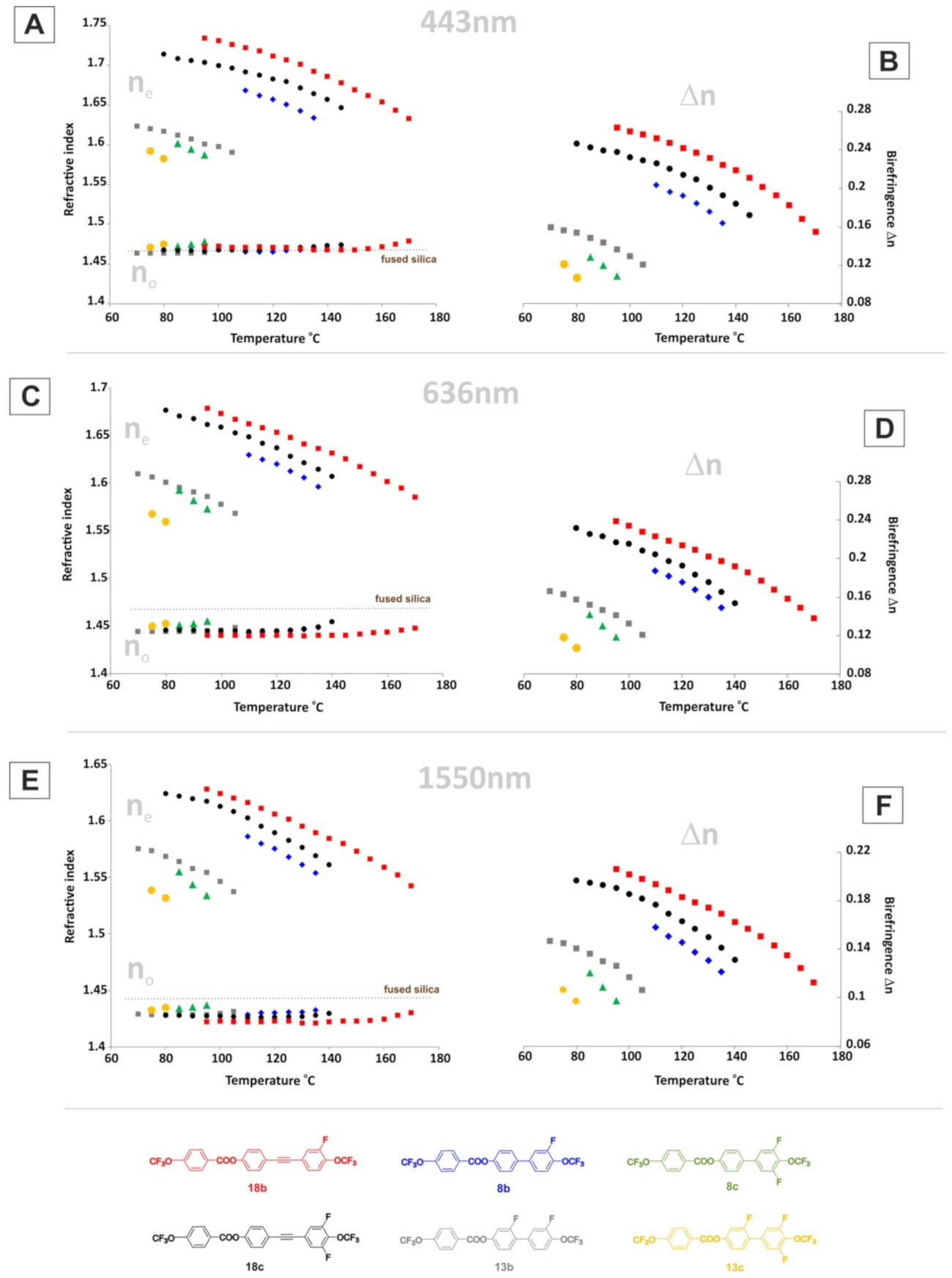
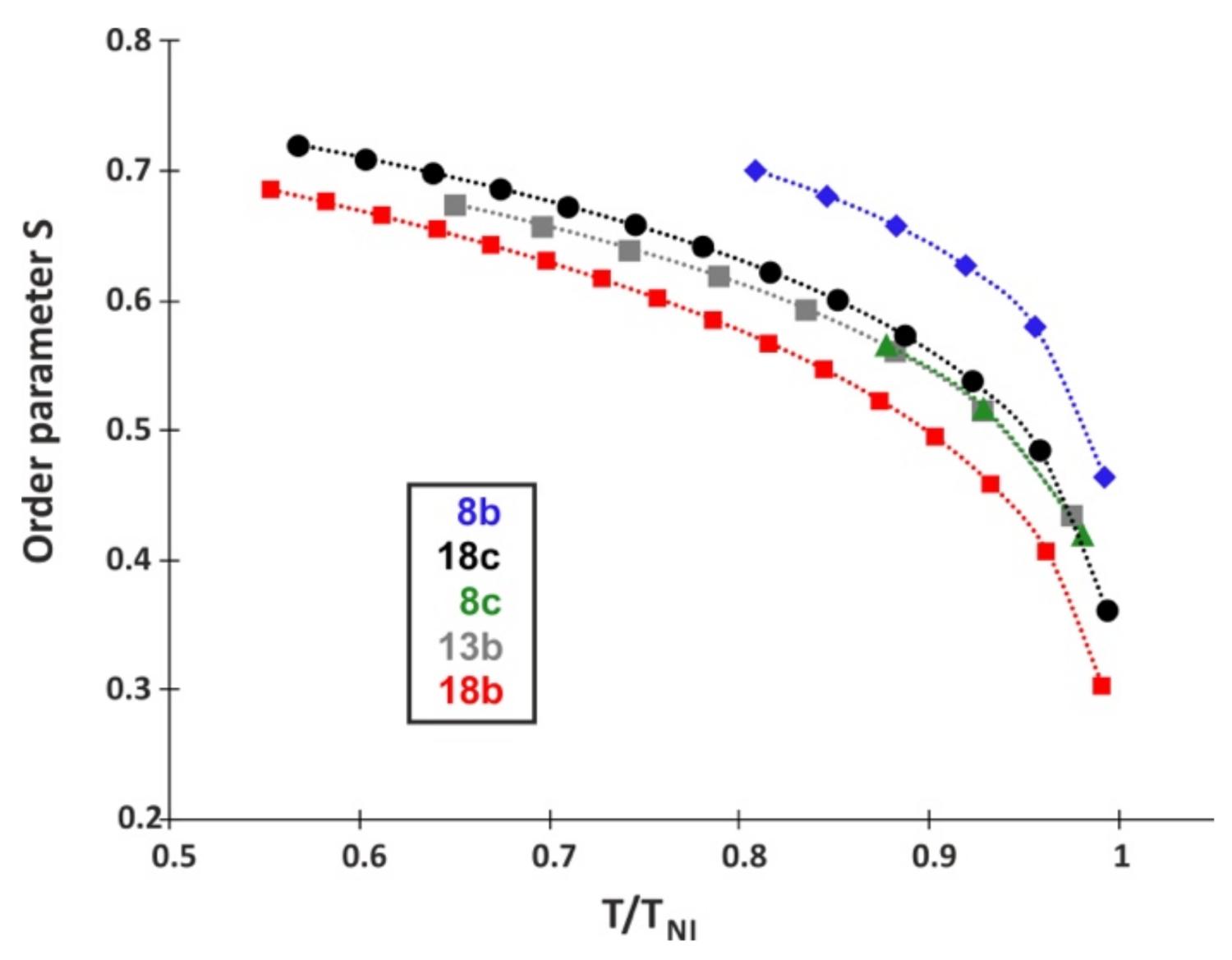
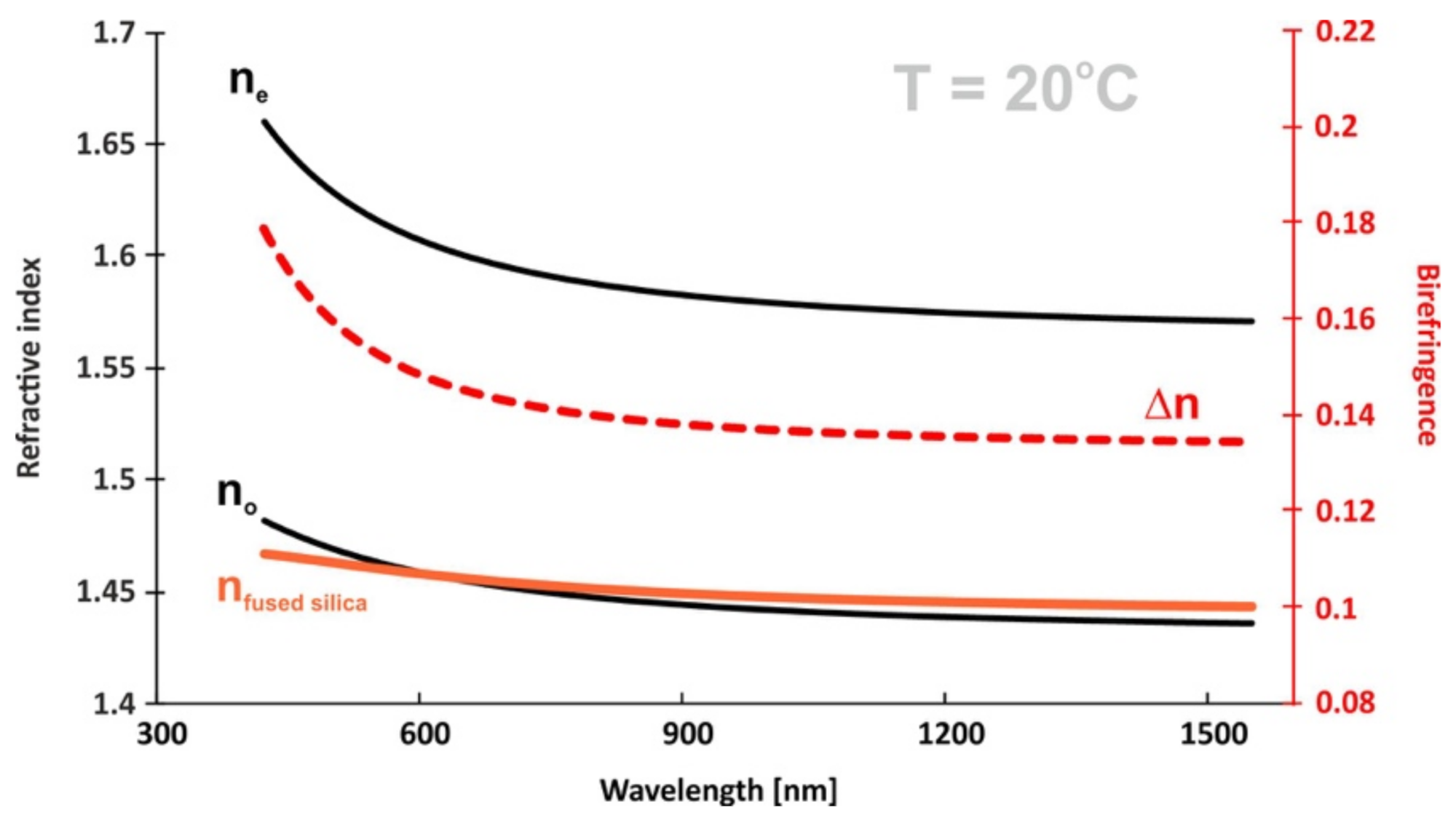
2.6. Optical Properties
3. Conclusions
- -
- Of the synthesized compounds, 12/13 had enantiotropic liquid crystalline phases.
- -
- Compounds with inner core lateral substitution tended to form temperature-stable smectic phases. The situation was different in the case of outer core, where nematic phases were observed.
- -
- From the viewpoint of the nematic phase, the optimal number of fluorine atoms in a rigid core should not be greater than 2.
- -
- The presence of absorption bands in the range of 2–6 µm is inevitable if a compound is to exhibit mesomorphic properties. Most often, these bands come from the vibrations of the C–H bonds from the rigid core or the C–O and C–F bonds related to the functionalization in the terminal and lateral positions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Tan, N.; Pivnenko, M.; Sibik, J.; Zeitler, J.A.; Chu, D. High-birefringence nematic liquid crystal for broadband THz applications. Liq. Cryst. 2016, 43, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Pauluth, D.; Tarumi, K. Advanced liquid crystals for television. J. Mater. Chem. 2004, 14, 1219–1227. [Google Scholar] [CrossRef]
- Pauluth, D.; Tarumi, K. Optimization of liquid crystals for television. J. Soc. Inf. Disp. 2005, 13, 693–702. [Google Scholar] [CrossRef]
- Chigrinov, V.G.; Yakovlev, D.A. Optimization and modeling of liquid crystal displays. Mol. Cryst. Liq. Cryst. 2006, 453, 107–121. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H. NIR–Vis–UV light-responsive actuator films of polymer-dispersed liquid crystal/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef]
- Yaghmaee, P.; Karabey, O.H.; Bates, B.; Fumeaux, C.; Jakoby, R. Electrically tuned microwave devices using liquid crystal technology. Int. J. Antennas Propag. 2013, 2013, 824214. [Google Scholar] [CrossRef] [Green Version]
- Mittra, R.; Nasri, A.; Donia, O.; Rmili, H. Novel Low-cost Phase Shifters for Millimeter Wave Applications; IEEE: Piscataway, NJ, USA, 2018; pp. 2127–2128. [Google Scholar]
- Nose, T.; Ito, R.; Honma, M. Potential of liquid-crystal materials for millimeter-wave application. Appl. Sci. Basel 2018, 8, 2544. [Google Scholar] [CrossRef] [Green Version]
- Polat, E.; Tesmer, H.; Reese, R.; Nickel, M.; Wang, D.; Schumacher, P.; Jakoby, R.; Maune, H. Reconfigurable millimeter-wave components based on liquid crystal technology for smart applications. Crystals 2020, 10, 346. [Google Scholar] [CrossRef]
- Kowerdziej, R.; Parka, J.; Krupka, J.; Olifierczuk, M.; Nowinowski-Kruszelnicki, E.; Jaroszewicz, L.; Chojnowska, O. Dielectric properties of highly anisotropic nematic liquid crystals for tunable microwave components. Appl. Phys. Lett. 2013, 103, 172902. [Google Scholar] [CrossRef]
- Reuter, M.; Garbat, K.; Vieweg, N.; Fischer, B.M.; Dabrowski, R.; Koch, M.; Dziaduszek, J.; Urban, S. Terahertz and optical properties of nematic mixtures composed of liquid crystal isothiocyanates, fluorides and cyanides. J. Mater. Chem. C 2013, 1, 4457–4463. [Google Scholar] [CrossRef]
- Reuter, M.; Vieweg, N.; Fischer, B.M.; Mikulicz, M.; Koch, M.; Garbat, K.; Dabrowski, R. Highly birefringent, low-loss liquid crystals for terahertz applications. APL Mater. 2013, 1, 012107. [Google Scholar] [CrossRef]
- Chodorow, U.; Parka, J.; Kula, P.; Herman, J.; Chojnowska, O.; Dabrowski, R.; Chigrinov, V.G. Terahertz properties of fluorinated liquid crystals. Liq. Cryst. 2013, 40, 1586–1590. [Google Scholar] [CrossRef]
- Chodorow, U.; Chojnowska, O.; Garbat, K.; Herman, J.; Parka, J. Liquid crystal materials with high birefringence for THz applications. In Proceedings of the 2014 39th International Conference on Infrared, Millimeter, and Terahertz Waves (Irmmw-Thz), Tucson, AZ, USA, 14–19 September 2014. [Google Scholar]
- Bulja, S.; Mirshekar-Syahkal, D.; Yazdanpanahi, M.; James, R.; Day, S.E.; Fernández, F.A. Liquid crystal based phase shifters in 60 GHz band. In Proceedings of the 3rd European Wireless Technology Conference, Paris, France, 27–28 September 2010; pp. 37–40. [Google Scholar]
- Li, J.; Chu, D. Liquid crystal-based enclosed coplanar waveguide phase shifter for 54–66 ghz applications. Crystals 2019, 9, 650. [Google Scholar] [CrossRef] [Green Version]
- Lapanik, V.; Sasnouski, G.; Timofeev, S.; Shepeleva, E.; Evtyushkin, G.; Haase, W. New highly anisotropic liquid crystal materials for high-frequency applications. Liq. Cryst. 2018, 45, 1242–1249. [Google Scholar] [CrossRef]
- Economou, E.C.; Lovejoy, J.; Harward, I.; Nobles, J.E.; Kula, P.; Herman, J.; Glushchenko, A.; Celinski, Z. Liquid crystal based tunable spurline filters with notch frequencies at 50 and 85 GHz. Microw. Opt. Technol. Lett. 2018, 60, 672–679. [Google Scholar] [CrossRef]
- Lane, S.; Brown, J.; Tremer, M.; Uber, C.; Gallagher, E.; Collins, S.; Benoit, M.; Miniscalco, W. Radiation testing of liquid crystal optical devices for space laser communication. Opt. Eng. 2009, 48, 114002. [Google Scholar] [CrossRef]
- Kuyken, B.; Liu, X.P.; Osgood, R.M.; Baets, R.; Roelkens, G.; Green, W.M.J. Mid-infrared to telecom-band supercontinuum generation in highly nonlinear silicon-on-insulator wire waveguides. Opt. Express 2011, 19, 20172–20181. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Chakravarty, S.; Chung, C.-J.; Xu, X.; Chen, R. Mid-infrared silicon photonic waveguides and devices [Invited]. Photonics Res. 2018, 6, 254. [Google Scholar] [CrossRef]
- Soref, R. Mid-infrared photonics in silicon and germanium. Nat. Photonics 2010, 4, 495–497. [Google Scholar] [CrossRef]
- Fang, Y.; Ge, Y.; Wang, C.; Zhang, H. Mid-Infrared Photonics using 2D materials: Status and challenges. Laser Photonics Rev. 2020, 14, 1900098. [Google Scholar] [CrossRef]
- Rogalski, A. Infrared detectors: An overview. Infrared Phys. Technol. 2002, 43, 187–210. [Google Scholar] [CrossRef] [Green Version]
- Brugioni, S.; Meucci, R. Liquid crystals in the mid-infrared region and their applications. Infrared Phys. Technol. 2004, 46, 17–21. [Google Scholar] [CrossRef]
- Alves, F.D.P.; Karunasiri, G.; Hanson, N.; Byloos, M.; Liu, H.C.; Bezinger, A.; Buchanan, M. NIR, MWIR and LWIR quantum well infrared photodetector using interband and intersubband transitions. Infrared Phys. Technol. 2007, 50, 182–186. [Google Scholar] [CrossRef]
- Saito, M.; Hayashi, K. Integration of liquid crystal elements for creating an infrared Lyot filter. Opt. Express 2013, 21, 11984–11993. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, P.; Xia, W.; Wang, J.; Zhou, C.; Zhang, H. A low-loss and high birefringence fluoride photonic crystal fiber in near infrared band. Optik 2019, 185, 772–776. [Google Scholar] [CrossRef]
- Rave, E.; Roodenko, K.; Katzir, A. Infrared photonic crystal fiber. Appl. Phys. Lett. 2003, 83, 1912–1914. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Liu, Y.; Wang, F.; Luo, D. Electrically switchable bistable dual frequency liquid crystal light shutter with hyper-reflection in near infrared. Liq. Cryst. 2019, 46, 1727–1733. [Google Scholar] [CrossRef]
- Choi, G.J.; Jung, H.M.; Lee, S.H.; Gwag, J.S. Infrared shutter using cholesteric liquid crystal. Appl. Opt. 2016, 55, 4436–4440. [Google Scholar] [CrossRef]
- Piecek, W.; Jaroszewicz, L.; Miszczyk, E.; Raszewski, Z.; Mrukiewicz, M.; Kula, P.; Jasek, K.; Perkowski, P.; Nowinowski-Kruszelnicki, E.; Zieliński, J.; et al. Mid-wave infrared liquid crystal shutter for breathalyzer applications. Opto-Electron. Rev. 2017, 25, 103–109. [Google Scholar] [CrossRef]
- Xu, S.; Ren, H.; Wu, S.-T. Adaptive liquid lens actuated by liquid crystal pistons. Opt. Express 2012, 20, 28518–28523. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.; Rommel, S.; Johnson, S.; Farca, G.; Rebolledo, N.; Selwyn, S.; Anderson, M. Electro-optic steering of a laser beam. SPIE Newsroom 2011, 13. [Google Scholar] [CrossRef]
- Frantz, J.; Myers, J.; Bekele, R.; Spillmann, C.; Naciri, J.; Kołacz, J.; Gotjen, H.; Shaw, L.; Sanghera, J.; Sodergren, B.; et al. Non-Mechanical Beam Steering in the Mid-Wave Infrared. In Proceedings of the SPIE Defense + Commercial Sensing 2017 Conference, Anaheim, CA, USA, 9–13 April 2017; p. 101810X. [Google Scholar]
- McManamon, P.F.; Dorschner, T.A.; Corkum, D.L.; Friedman, L.J.; Hobbs, D.S.; Holz, M.; Liberman, S.; Nguyen, H.Q.; Resler, D.P.; Sharp, R.C.; et al. Optical phased array technology. Proc. IEEE 1996, 84, 268–298. [Google Scholar] [CrossRef]
- Ren, H.; Wu, S.-T. Introduction to Adaptive Lenses; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rogalski, A.; Chrzanowski, K. Infrared devices and techniques. Opto-Electron. Rev. 2002, 10, 111–136. [Google Scholar]
- Chodorow, U.; Mazur, R.; Morawiak, P.; Herman, J.; Harmata, P.; Martyniuk, P. Switchable Fabry–Perot filter for mid-infrared radiation. Liq. Cryst. 2019, 46, 1877–1880. [Google Scholar] [CrossRef]
- Węgłowska, D.; Czerwiński, M.; Kula, P.; Mrukiewicz, M.; Mazur, R.; Herman, J. Fast-response halogenated 4-alkyl-4″-cyano-p-terphenyls as dual frequency addressing nematics. Fluid Phase Equilibria 2020, 522, 112770. [Google Scholar] [CrossRef]
- Wąchała, A.; Pytlarczyk, M.; Kula, P. On the balance between nematic and smectic phases in 2′,3′-difluoro-4,4″-dialkyl-p-terphenyls. Liq. Cryst. 2019, 46, 1558–1567. [Google Scholar] [CrossRef]
- Kula, P.; Herman, J.; Pluczyk, S.; Harmata, P.; Mangelinckx, G.; Beeckman, J. Synthesis and mesomorphic properties of laterally substituted 4,4′′′-dialkyl-p-quaterphenyls. Liq. Cryst. 2014, 41, 503–513. [Google Scholar] [CrossRef]
- Herman, J.; Harmata, P.; Strzeżysz, O.; Czerwiński, M.; Urban, S.; Kula, P. Synthesis and properties of chosen 4-butyl-phenyltolane derivatives—On the influence of core substitution on birefringence, mesomorphic and dielectric properties. J. Mol. Liq. 2018, 267, 511–519. [Google Scholar] [CrossRef]
- Dziaduszek, J.; Kula, P.; Dabrowski, R.; Drzewiński, W.; Garbat, K.; Urban, S.; Gauza, S. General synthesis method of alkyl-alkoxy multi-fluorotolanes for negative high birefringence nematic mixtures. Liq. Cryst. 2012, 39, 239–247. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Dziaduszek, J.; Garbat, K.; Urban, S.; Filipowicz, M.; Herman, J.; Czerwiński, M.; Harmata, P. Nematic compounds and mixtures with high negative dielectric anisotropy. Liq. Cryst. 2017, 44, 1534–1548. [Google Scholar] [CrossRef]
- Ziobro, D.; Dziaduszek, J.; Filipowicz, M.; Dąbrowski, R.; Czub, J.; Urban, S. Synthesis of fluorosubstituted three ring esters and their dielectric properties. Mol. Cryst. Liq. Cryst. 2009, 502, 258–271. [Google Scholar] [CrossRef]
- Węgłowska, D.; Kula, P.; Herman, J. High birefringence bistolane liquid crystals: Synthesis and properties. RSC Adv. 2015, 6, 403–408. [Google Scholar] [CrossRef]
- Urbańska, M.; Perkowski, P.; Szala, M. Synthesis and properties of antiferroelectric and/or ferroelectric compounds with the –CH2O group close to chirality centre. Liq. Cryst. 2019, 46, 2245–2255. [Google Scholar] [CrossRef]
- Herman, J.; Dmochowska, E.; Pytlarczyk, M.; Harmata, P. Synthesis and mesomorphism of tetrafluoro substituted 4-cyano oligophenyls. Liq. Cryst. 2019, 46, 1666–1671. [Google Scholar] [CrossRef]
- Herman, J.; Kula, P. Design of new super-high birefringent isothiocyanato bistolanes—synthesis and properties. Liq. Cryst. 2017, 44, 1462–1467. [Google Scholar] [CrossRef]
- Szczuciński, Ł.; Dąbrowski, R.; Urban, S.; Garbat, K.; Filipowicz, M.; Dziaduszek, J.; Czerwiński, M. Synthesis, mesogenic and dielectric properties of fluorosubstituted isothiocyanatoterphenyls. Liq. Cryst. 2015, 42, 1706–1729. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Kula, P.; Spadło, A.; Dziaduszek, J.; Filipowicz, M.; Dąbrowski, R.; Czub, J.; Urban, S. Mesomorphic, dielectric, and optical properties of fluorosubstituted biphenyls, terphenyls, and quaterphenyls. Opto-Electron. Rev. 2008, 16, 379–385. [Google Scholar] [CrossRef]
- Mrukiewicz, M.; Perkowski, P.; Urbańska, M.; Węgłowska, D.; Piecek, W. Electrical conductivity of ion-doped fluoro substituted liquid crystal compounds for application in the dynamic light scattering effect. J. Mol. Liq. 2020, 317, 113810. [Google Scholar] [CrossRef]
- Pytlarczyk, M.; Dmochowska, E.; Czerwiński, M.; Herman, J. Effect of lateral substitution by chlorine and fluorine atoms of 4-alkyl-p-terphenyls on mesomorphic behaviour. J. Mol. Liq. 2019, 292, 111379. [Google Scholar] [CrossRef]
- Wu, S.-T.; Cox, R.J. Potential infrared liquid crystals. Liq. Cryst. 1989, 5, 1415–1424. [Google Scholar] [CrossRef]
- Harmata, P.; Herman, J.; Kula, P. Liquid crystals for IR: Part II synthesis and properties of perfluoroalkyl- or perfluoroalkoxy-terminated tolanes. Liq. Cryst. 2019, 47, 2144–2160. [Google Scholar] [CrossRef]
- Harmata, P.; Herman, J.; Kula, P. Liquid crystals for IR: Part I—synthesis and properties of perfluoroalkyl or perfluoroalkoxy terminated oligophenyls. Liq. Cryst. 2019, 47, 2122–2143. [Google Scholar] [CrossRef]
- Kula, P.; Czerwinski, M.; Herman, J.; Harmata, P. Liquid crystals for IR: Part III-Bi- and multicomponent mixtures based on perfluoroalkyl or perfluoroalkoxy terminated oligophenyls and tolanes. Liq. Cryst. 2020, 47, 2161–2170. [Google Scholar] [CrossRef]
- Hu, M.; An, Z.; Li, J.; Chen, H.; Peng, F.; Wu, S.-T.; Wang, X.; Li, M. Low mid-infrared absorption tolane liquid crystals terminated by 2,2-difluorovinyloxyl: Synthesis, characterization and properties. J. Mater. Chem. C 2016, 4, 4939–4945. [Google Scholar] [CrossRef]
- Peng, F.; Chen, Y.; Wu, S.-T.; Tripathi, S.; Twieg, R. Low loss liquid crystals for infrared applications. Liq. Cryst. 2014, 41, 1545–1552. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, H.; Sun, J.; Kula, P.; Dabrowski, R.; Tripathi, S.; Twieg, R.; Wu, S.-T. Low absorption liquid crystals for mid-wave infrared applications. Opt. Express 2011, 19, 10843–10848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; An, Z.; Li, J.; Mo, L.; Yang, Z.; Li, J.; Che, Z.; Yang, X. Tolane liquid crystals bearing fluorinated terminal group and their mid-wave infrared properties. Liq. Cryst. 2014, 41, 1696–1702. [Google Scholar] [CrossRef]
- Szaniawska, K.; Nasilowski, T.; Woliński, T.R.; Thienpont, H. Tunable properties of light propagation in photonic liquid crystal fibers. Opto-Electron. Rev. 2006, 14, 339–343. [Google Scholar] [CrossRef]
- Wolinski, T.R.; Ertman, S.; Lesiak, P.; Domanski, A.W.; Czapla, A.; Dabrowski, R.; Nowinowski-Kruszelnicki, E.; Wojcik, J. Photonic liquid crystal fibers—a new challenge for fiber optics and liquid crystals photonics. Opto-Electron. Rev. 2006, 14, 329–334. [Google Scholar] [CrossRef]
- Wolinski, T.R.; Szaniawska, K.; Bondarczuk, K.; Lesiak, P.; Domanski, A.W.; Dabrowski, R.; Nowinowski-Kruszelnicki, E.; Wojcik, J. Propagation properties of photonic crystal fibers filled with nematic liquid crystals. Opto-Electron. Rev. 2005, 13, 177–182. [Google Scholar]
- Kedzierski, J.; Garbat, K.; Raszewski, Z.; Kojdecki, M.A.; Kowiorski, K.; Jaroszewicz, L.R.; Miszczyk, E.; Dabrowski, R.; Zielinski, J.; Piecek, W. Optical properties of a liquid crystal with small ordinary and extraordinary refractive indices and small optical anisotropy. Opto-Electron. Rev. 2014, 22, 162–165. [Google Scholar] [CrossRef]
- Sage, I.; Chaplin, D. Low ri liquid-crystals for integrated-optics. Electron. Lett. 1987, 23, 1192–1193. [Google Scholar] [CrossRef]
- Haller, I. Thermodynamic and static properties of liquid crystals. Prog. Solid State Chem. 1975, 10, 103–118. [Google Scholar] [CrossRef]
- Zywucki, B.J.; Kuczynski, W. The orientational order in nematic liquid crystals from birefringence measurements. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 512–515. [Google Scholar] [CrossRef]
- Malitson, I.H. Interspecimen comparison of the refractive index of fused silica. J. Opt. Soc. Am. 1965, 55, 1205–1209. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Qiu, Q.; Shi, S.-J. Temperature dependence of the refractive index of optical fibers. Chin. Phys. B 2014, 23, 034201. [Google Scholar] [CrossRef]
- Rodney, W.S.; Spindler, R.J. Index of refraction of fused quartz glass for ultraviolet, visible, and infrared wavelengths. J. Opt. Soc. Am. 1954, 44, 677–679. [Google Scholar] [CrossRef]
- Wray, J.H.; Neu, J.T. Refractive index of several glasses as a function of wavelength and temperature. J. Opt. Soc. Am. 1969, 59, 774–776. [Google Scholar] [CrossRef]


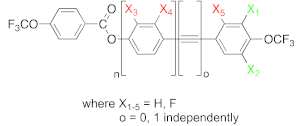 | ||||||||||||||
| Compound Number | n | o | X1 | X2 | X3 | X4 | X5 | Temperatures and Enthalpies of Phase Transitions | ||||||
| Cr | (°C) (kJ/mol) | SmA | (°C) (kJ/mol) | N | (°C) (kJ/mol) | Iso | ||||||||
| 8a | 1 | 0 | H | H | H | H | H | * | 144.4 22.66 | * | 180.6 2.88 | * | 183.9 1.33 | * |
| 8b | 1 | 0 | F | H | H | H | H | * | 107.8 27.55 | – | * | 136.0 0.60 | * | |
| 8c | 1 | 0 | F | F | H | H | H | * | 80.2 24.91 | – | * | 96.9 0.42 | * | |
| 13a | 1 | 0 | H | H | H | F | H | * | 86.4 18.25 | * | 126.1 1.16 | * | 142.1 0.87 | * |
| 13b | 1 | 0 | F | H | H | F | H | * | 65.8 17.19 | – | * | 107.8 0.51 | * | |
| 13c | 1 | 0 | F | F | H | F | H | * | 75.7 29.23 | – | * | 81.9 0.35 | * | |
| 13d | 1 | 0 | F | H | F | H | H | * | 82.7 24.67 | * | 87.4 0.17 | * | 111.4 0.85 | * |
| 13e | 1 | 0 | H | H | F | H | H | * | 84.8 21.43 | * | 146.5 3.92 | – | * | |
| 18a | 1 | 1 | H | H | H | H | H | * | 114.1 28.14 | * | 193.1 1.23 | * | 209.5 0.92 | * |
| 18b | 1 | 1 | F | H | H | H | H | * | 87.5 27.84 | * | 91.3 0.19 | * | 171.1 0.69 | * |
| 18c | 1 | 1 | F | F | H | H | H | * | 74.3 24.13 | – | * | 140.6 0.54 | * | |
| 18d | 1 | 1 | H | H | H | H | F | * | 71.1 20.03 | * | 200.2 4.65 | – | * | |
| 20 | 0 | 0 | F | H | H | H | H | * | 44.8 23.19 | – | – | * | ||
| Temperature [°C] | no | ne | ||||
|---|---|---|---|---|---|---|
| Ao | Bo | Co | Ae | Be | Ce | |
| 20 | 1.4323 | 1.0331 × 104 | −2.7761 × 108 | 1.5654 | 1.3305 × 104 | 6.4903 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmata, P.; Herman, J. New-Generation Liquid Crystal Materials for Application in Infrared Region. Materials 2021, 14, 2616. https://doi.org/10.3390/ma14102616
Harmata P, Herman J. New-Generation Liquid Crystal Materials for Application in Infrared Region. Materials. 2021; 14(10):2616. https://doi.org/10.3390/ma14102616
Chicago/Turabian StyleHarmata, Piotr, and Jakub Herman. 2021. "New-Generation Liquid Crystal Materials for Application in Infrared Region" Materials 14, no. 10: 2616. https://doi.org/10.3390/ma14102616