DC and AC Conductivity, Biosolubility and Thermal Properties of Mg-Doped Na2O–CaO–P2O5 Glasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Preparation
2.2. Glass Characterization
2.3. Impedance Spectroscopy Measurements
2.4. Solubility in PBS
2.5. Thermal Analysis Measurements
3. Results and Discussion
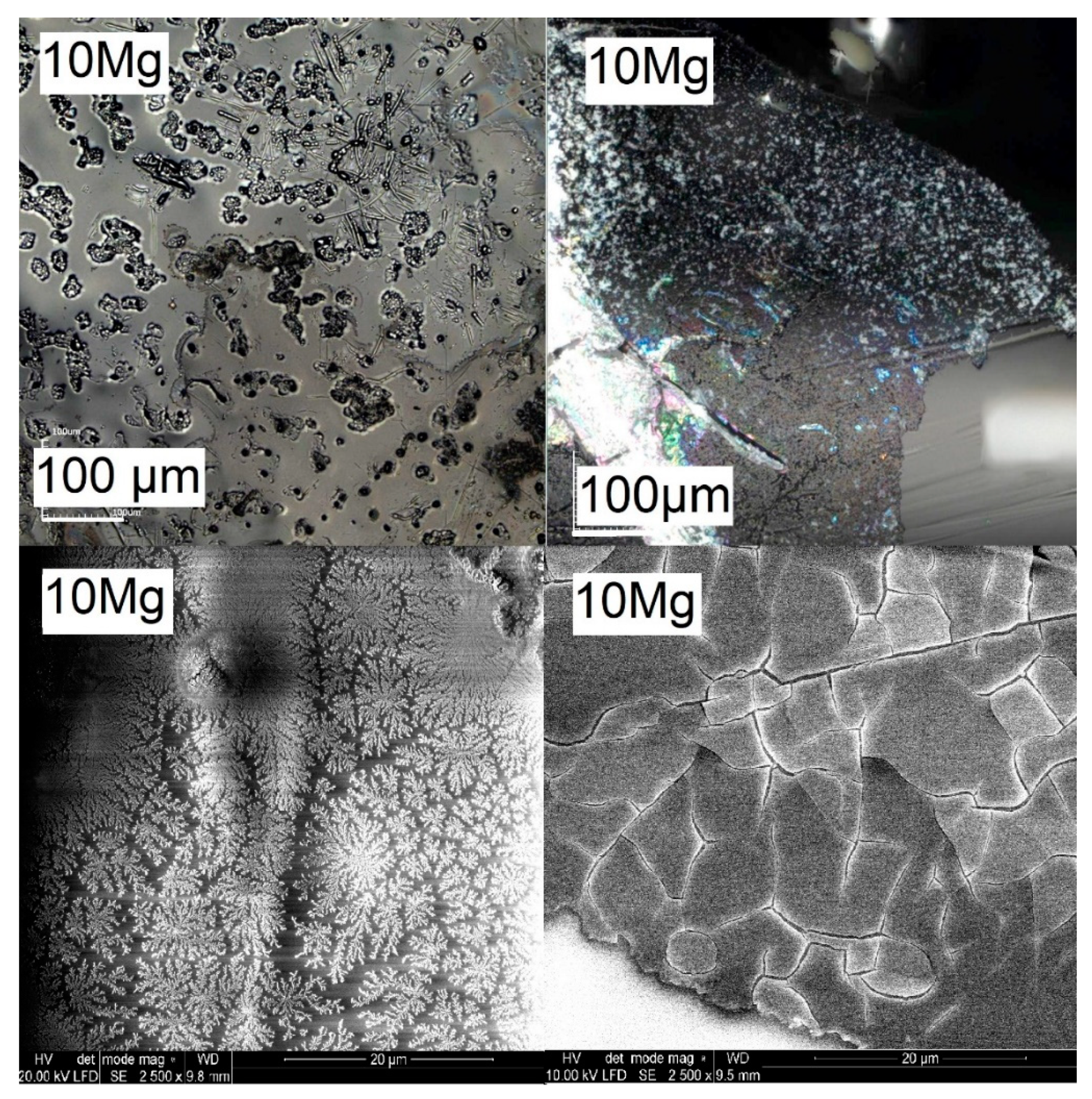
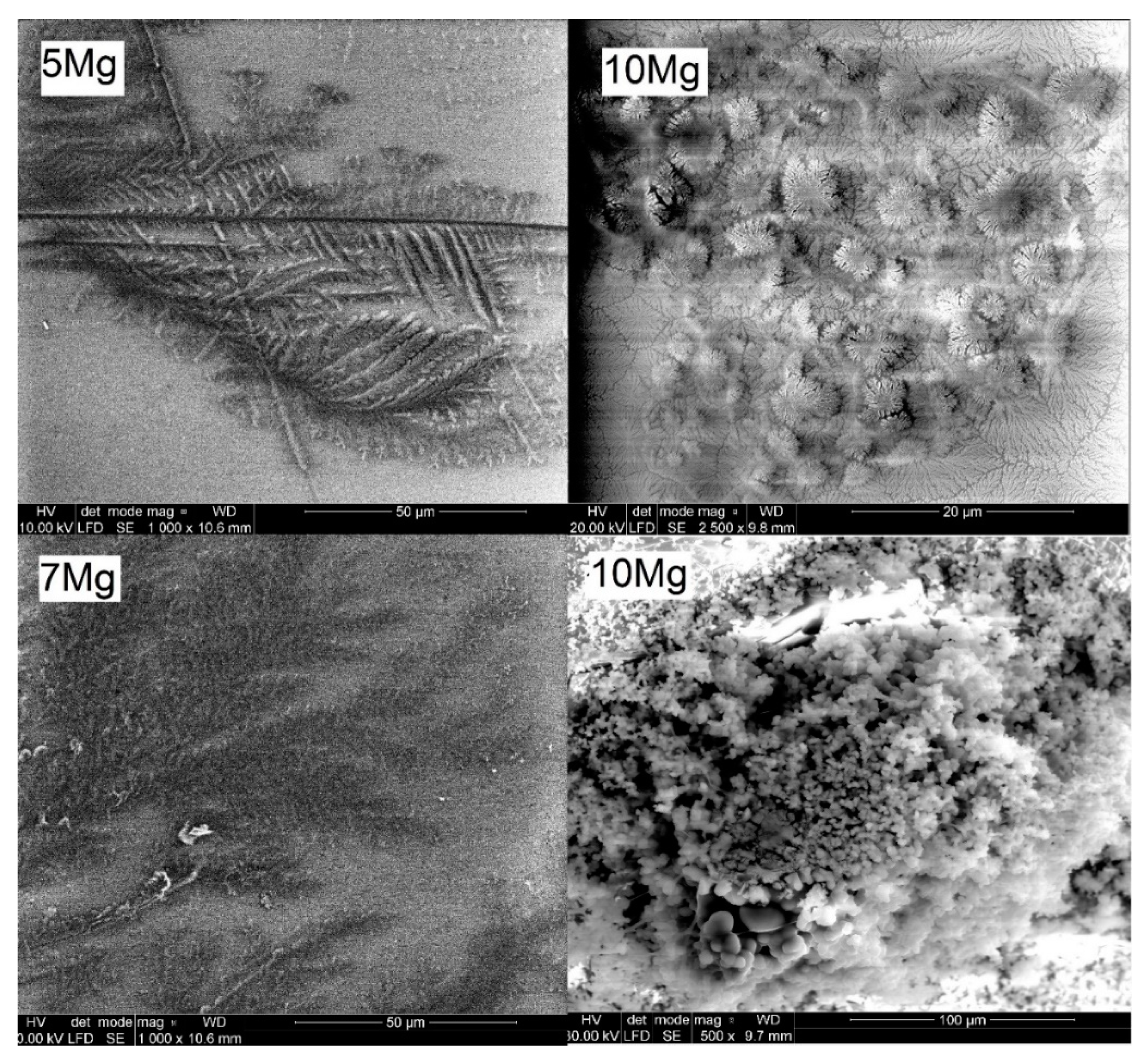
3.1. Confocal Microscopy and Compositional Analysis
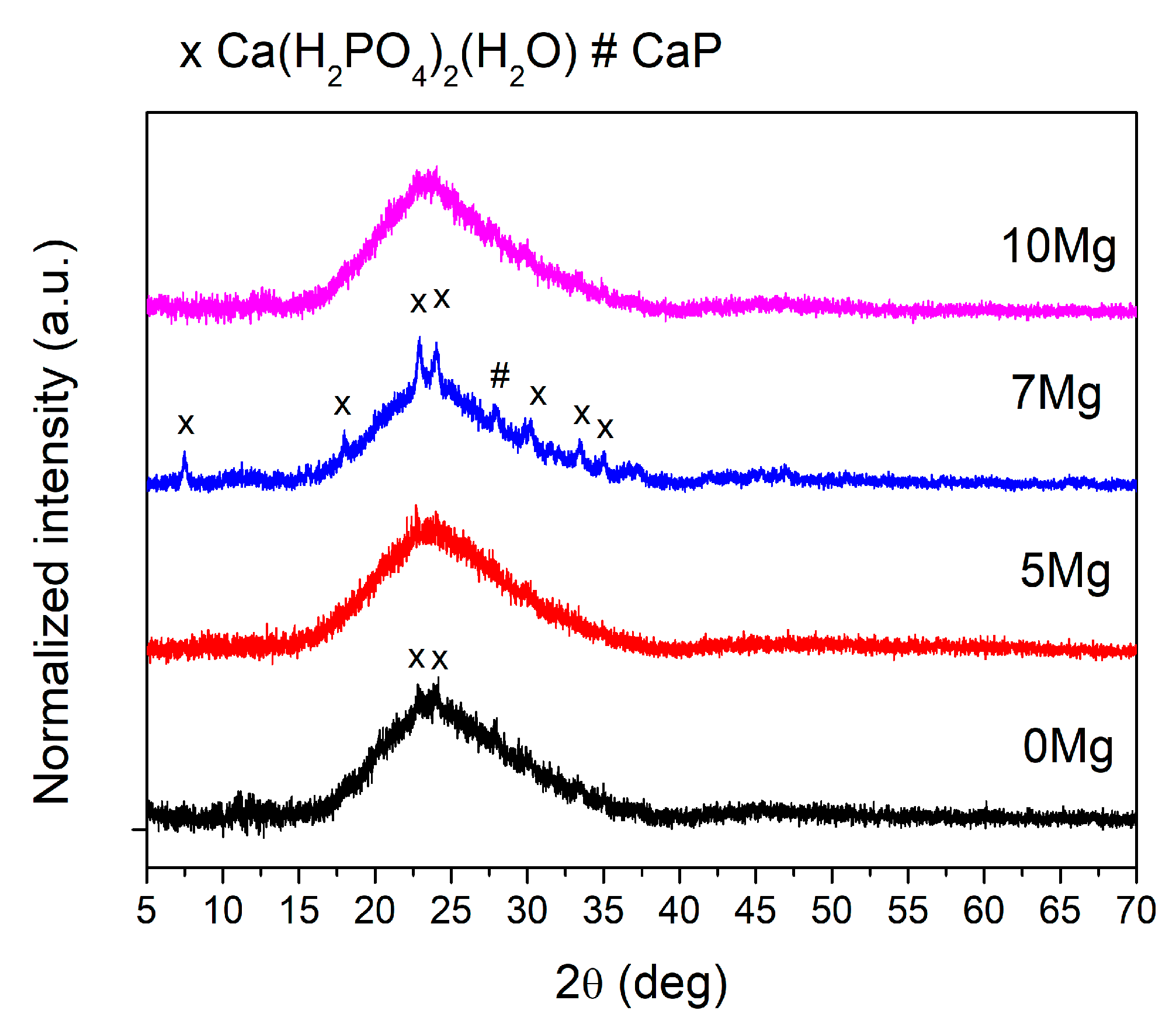
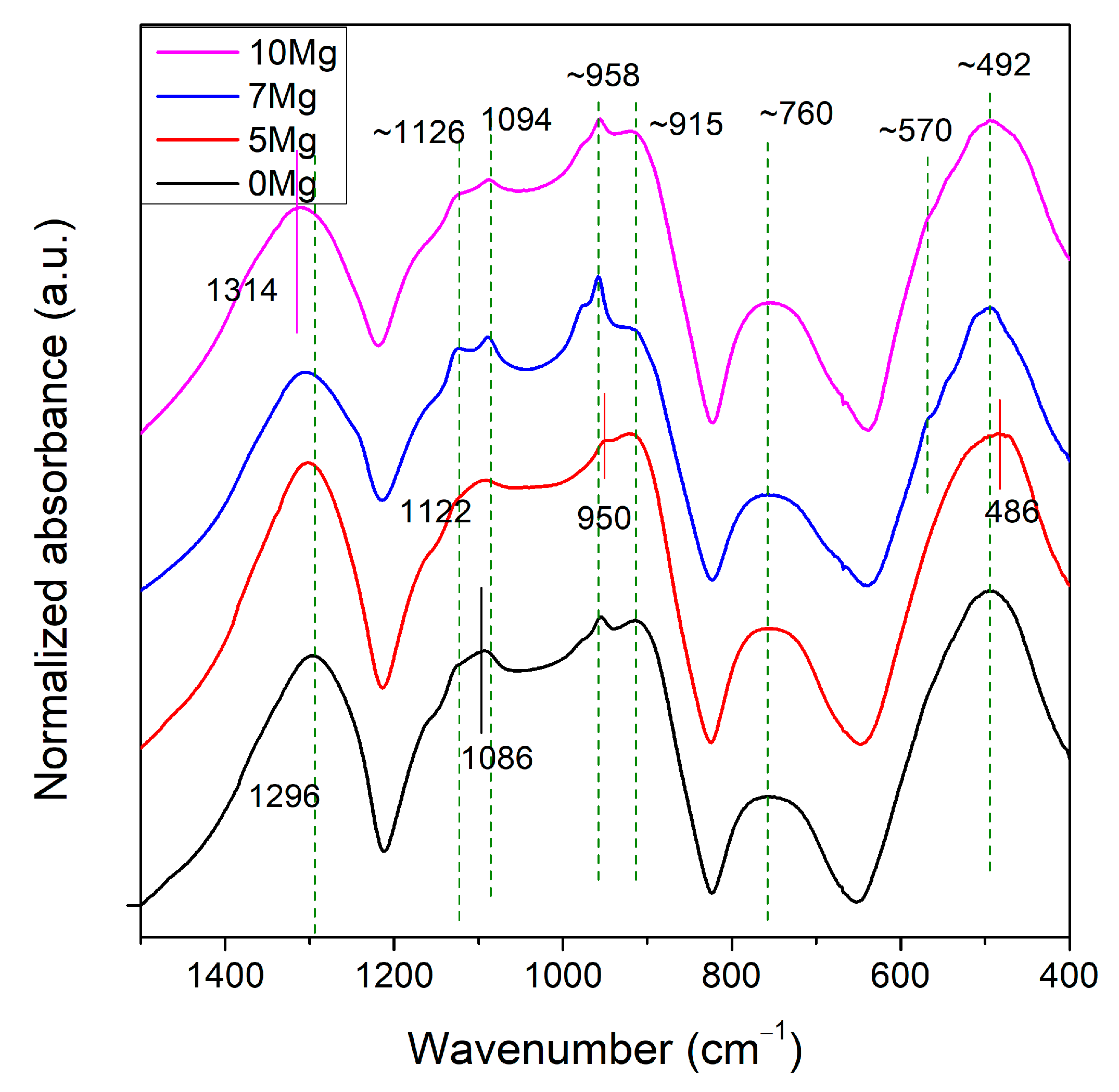
3.2. Structural Analysis
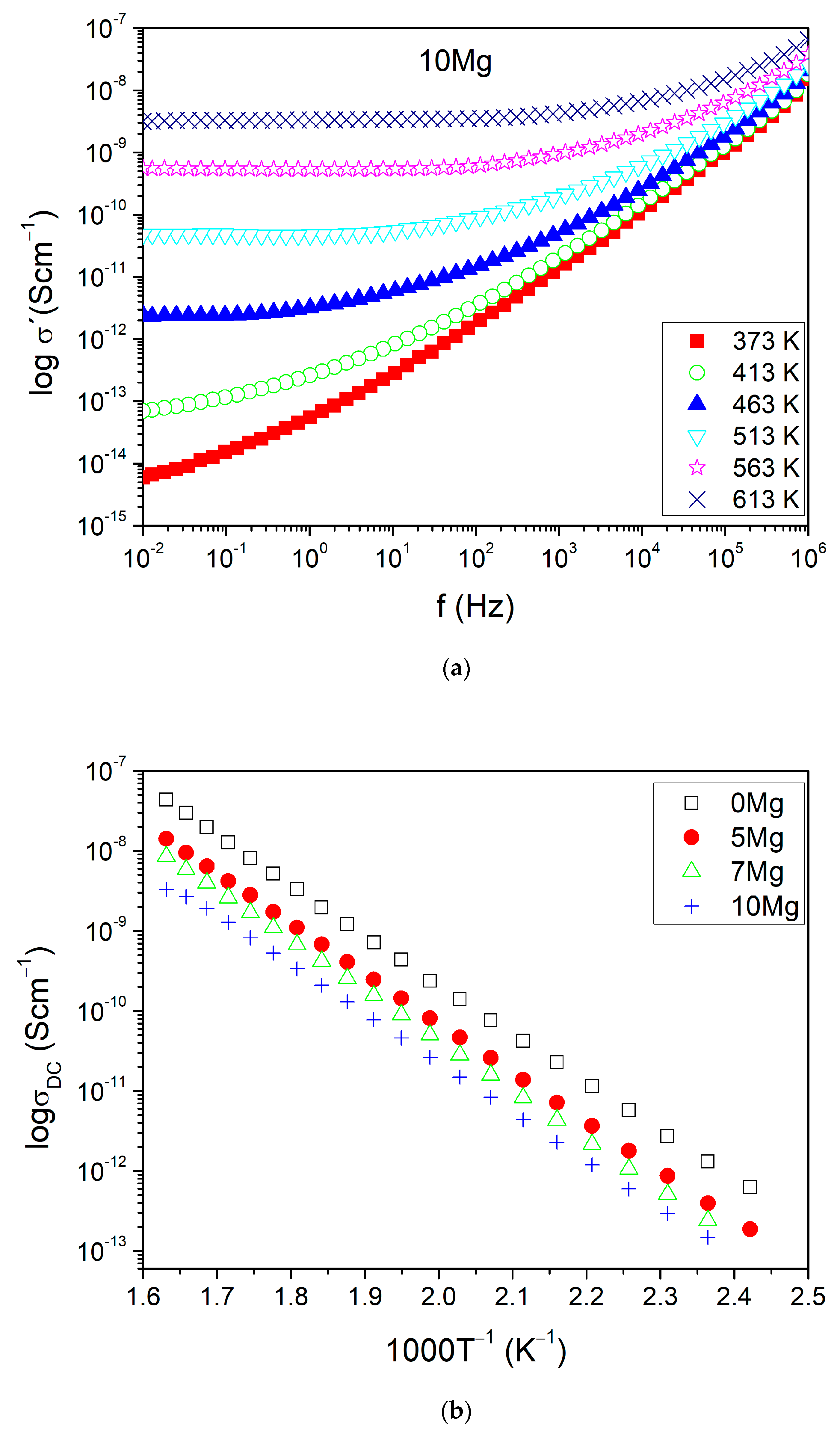
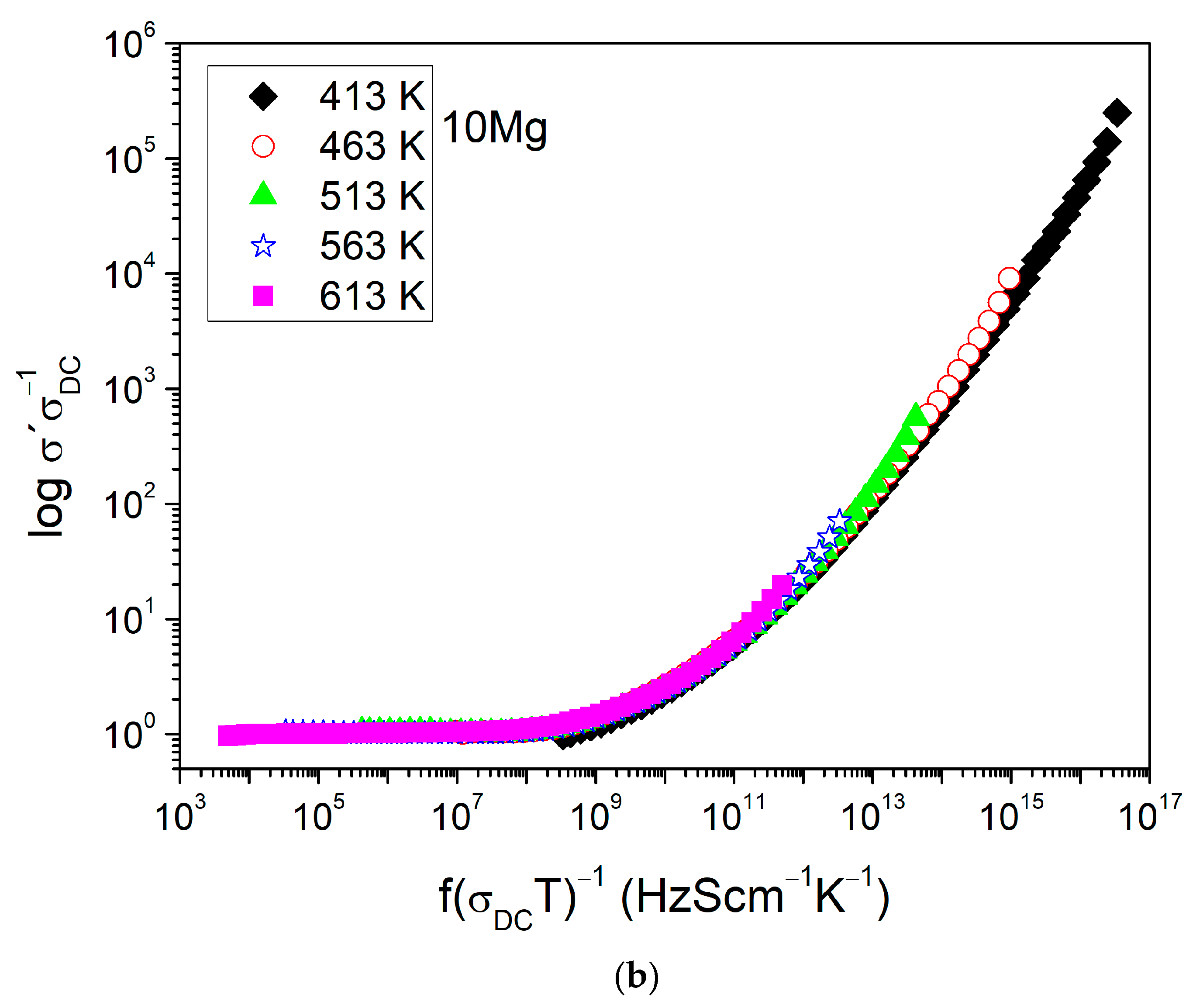
3.3. Electrical Properties
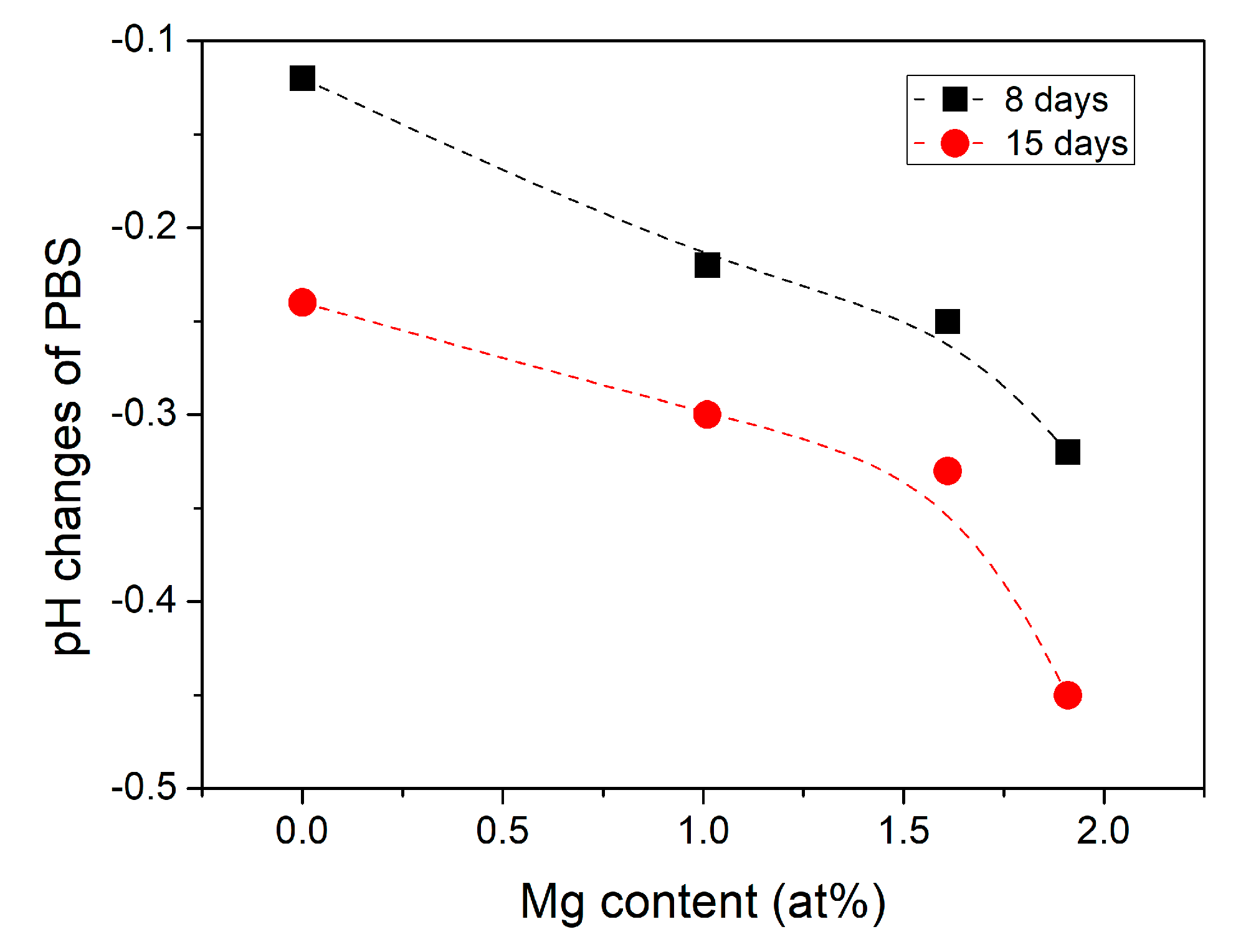
3.4. Biosolubility in PBS
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neel, E.A.A.; Pickup, D.M.; Valappil, S.P.; Newport, R.J.; Knowles, J.C. Bioactive functional materials: A perspective on phosphate-based glasses. J. Mater. Chem. 2009, 19, 690–701. [Google Scholar] [CrossRef]
- Marzouk, M.A.; ElBatal, F.H.; Abdelghany, A.M. Ultraviolet and infrared absorption spectra of Cr2O3 doped–Sodium metaphosphate, lead metaphosphate and zinc metaphosphate glasses and effects of gamma irradiation: A comparative study. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2013, 114, 658–667. [Google Scholar] [CrossRef]
- Ali, S.; Wójcik, N.A.; Jonson, B.; Kamitsos, E.I.; Li, X.; Luo, J.; Möncke, D. Synthesis, structural characterization, and thermal properties of Ca- and La-doped soda-lime glasses by laser melting. Int. J. Appl. Glas. Sci. 2020, 11, 699–706. [Google Scholar] [CrossRef]
- Lee, I.-H.; Shin, S.-H.; Foroutan, F.; Lakhkar, N.J.; Gong, M.-S.; Knowles, J.C. Effects of magnesium content on the physical, chemical and degradation properties in a MgO−CaO−Na2O−P2O5 glass system. J. Non-Cryst. Solids 2013, 363, 57–63. [Google Scholar] [CrossRef]
- Wójcik, N.A.; Jonson, B.; Möncke, D.; Palles, D.; Kamitsos, E.I.; Ghassemali, E.; Seifeddine, S.; Eriksson, M.; Ali, S. Influence of synthesis conditions on glass formation, structure and thermal properties in the Na2O-CaO-P2O5 system doped with Si3N4 and Mg. J. Non-Cryst. Solids 2018, 494, 66–77. [Google Scholar] [CrossRef]
- Möncke, D.; Kamitsos, E.I.; Palles, D.; Limbach, R.; Winterstein-Beckmann, A.; Honma, T.; Yao, Z.; Rouxel, T.; Wondraczek, L. Transition and post-transition metal ions in borate glasses: Borate ligand speciation, cluster formation, and their effect on glass transition and mechanical properties. J. Chem. Phys. 2016, 145, 124501. [Google Scholar] [CrossRef] [PubMed]
- Thieme, A.; Möncke, D.; Limbach, R.; Fuhrmann, S.; Kamitsos, E.I.; Wondraczek, L. Structure and properties of alkali and silver sulfophosphate glasses. J. Non-Cryst. Solids 2015, 410, 142–150. [Google Scholar] [CrossRef]
- Griebenow, K.; Bragatto, C.B.; Kamitsos, E.I.; Wondraczek, L. Mixed-modifier effect in alkaline earth metaphosphate glasses. J. Non-Cryst. Solids 2018, 481, 447–456. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Properties of high nitrogen content mixed alkali earth oxynitride glasses (AExCa1−x)1.2(1)SiO1.9(1)N0.86(6), AE=Mg, Sr, Ba. J. Non-Cryst. Solids 2009, 355, 1259–1263. [Google Scholar] [CrossRef]
- Ali, S.; Jonson, B. Compositional effects on the properties of high nitrogen content alkaline-earth silicon oxynitride glasses, AE=Mg, Ca, Sr, Ba. J. Eur. Ceram. Soc. 2011, 31, 611–618. [Google Scholar] [CrossRef]
- Ali, S.; Jonson, B.; Pomeroy, M.J.; Hampshire, S. Issues associated with the development of transparent oxynitride glasses. Ceram. Int. 2015, 41, 3345–3354. [Google Scholar] [CrossRef]
- Šantić, A.; Skoko, Ž.; Gajović, A.; Reis, S.T.; Day, D.E.; Moguš-Milanković, A. Physical properties of lead iron phosphate glasses containing Cr2O3. J. Non-Cryst. Solids 2011, 357, 3578–3584. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Salinas, A.J.; Roman, J.; Gil, M. Effect of magnesium content on the in vitro bioactivity of CaO-MgO-SiO2-P2O5 sol-gel glasses. J. Mater. Chem. 1999, 9, 515–518. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Poggetto, G.D.; Milazzo, M.; Blanco, I.; Ciprioti, S.V. Structure, drug absorption, bioactive and antibacterial properties of sol-gel SiO2/ZrO2 materials. Ceram. Int. 2020, 46, 29459–29465. [Google Scholar] [CrossRef]
- Murawski, L.; Barczynski, R.J.; Samatowicz, D. Electronic conductivity in Na2O-FeO-P2O5 glasses. Solid State Ion. 2003, 157, 293–298. [Google Scholar] [CrossRef]
- Bih, L.; Abbas, L.; Mohdachi, S.; Nadiri, A. Thermal and electrical properties of mixed alkali in Li2O–Na2O–WO3–P2O5 glasses. J. Mol. Struct 2008, 891, 173–177. [Google Scholar] [CrossRef]
- Wójcik, N.A.; Jonson, B.; Barczyński, R.J.; Kupracz, P.; Möncke, D.; Ali, S. Electrical properties of Na2O-CaO-P2O5 glasses doped with SiO2 and Si3N4. Solid State Ion. 2018, 325, 157–162. [Google Scholar] [CrossRef]
- Maheswaran, A.; Hirankumar, G.; Heller, N.; Karthickprabhu, S.; Kawamura, J. Structure, dielectric and bioactivity of P2O5–CaO–Na2O–B2O3 bioactive glass. Appl. Phys. A 2014, 117, 1323–1327. [Google Scholar] [CrossRef]
- Al-Ani, S.K.J.; Al-Hassany, I.H.O.; Al-Dahan, Z.T. The optical properties and a.c. conductivity of magnesium phosphate glasses. J. Mater. Sci. 1995, 30, 3720–3729. [Google Scholar] [CrossRef]
- Higazy, A.A. Electrical conductivity and dielectric constant of magnesium phosphate glasses. Mater. Lett. 1995, 22, 289–296. [Google Scholar] [CrossRef]
- Khor, S.F.; Talib, Z.A.; Daud, W.M.; Sidek, H.A.A.; Ng, B.H. Effects of MgO on dielectric properties and electrical conductivity of ternary zinc magnesium phosphate glasses. J. Non-Cryst. Solids 2009, 355, 2533–2539. [Google Scholar] [CrossRef]
- Turk, M.; Deliormanli, A.M. Electrically conductive borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater Appl 2017, 32, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Porwal, H.; Grasso, S.; Cordero-Arias, L.; Li, C.C.; Boccaccini, A.R.; Reece, M.J. Processing and bioactivity of 45S5 Bioglass(A (R))-graphene nanoplatelets composites. J. Mater. Sci. Mater. Med. 2014, 25, 1403–1413. [Google Scholar] [CrossRef]
- Tigunta, S.; Pisitpipathsin, N.; Kantha, P.; Eitssayeam, S.; Rujijanagul, G.; Tunkasiri, T.; Pengpat, K. Electrical Properties of Calcium Phosphate/BZT Bioglass-Ceramics Prepared by Incorporation Method. Ferroelectrics 2014, 459, 188–194. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakamura, S.; Yamashita, K. Enhanced osteobonding by negative surface charges of electrically polarized hydroxyapatite. J. Biomed. Mater. Res. 2001, 57, 477–484. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kokubo, T.; Yamauro, T. Compositional dependence of bioactivity of glasses in the system CaO-SiO2-Al2O3: Itsin vitro evaluation. J. Mater. Sci. Mater. Med. 1992, 3, 119–125. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Konidakis, I.; Varsamis, C.P.E.; Kamitsos, E.I.; Moncke, D.; Ehrt, D. Structure and Properties of Mixed Strontium-Manganese Metaphosphate Glasses. J. Phys. Chem. C 2010, 114, 9125–9138. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Neel, E.A.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial gallium-doped phosphate-based glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Moustafa, Y.; El-Egili, K. Infrared Studies on the Structure of Sodium Phosphate Glasses. J. Non-Cryst. Solids 1998, 240, 144–153. [Google Scholar] [CrossRef]
- Carta, D.; Pickup, D.M.; Knowles, J.C.; Ahmed, I.; Smith, M.E.; Newport, R.J. A structural study of sol-gel and melt-quenched phosphate-based glasses. J. Non-Cryst. Solids 2007, 353, 1759–1765. [Google Scholar] [CrossRef]
- Shih, P.Y. Properties and FTIR spectra of lead phosphate glasses for nuclear waste immobilization. Mater. Chem. Phys. 2003, 80, 299–304. [Google Scholar] [CrossRef]
- Scholz, R.; Frost, R.L.; Xi, Y.F.; Graca, L.M.; Lagoeiro, L.; Lopez, A. Vibrational spectroscopic characterization of the phosphate mineral phosphophyllite-Zn2Fe(PO4)(2)center dot 4H(2)O, from Hagendorf Sud, Germany and in comparison with other zinc phosphates. J. Mol. Struct. 2013, 1039, 22–27. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Chrzanowski, W.; Valappil, S.P.; O’Dell, L.A.; Pickup, D.M.; Smith, M.E.; Newport, R.J.; Knowles, J.C. Doping of a high calcium oxide metaphosphate glass with titanium dioxide. J. Non-Cryst. Solids 2009, 355, 991–1000. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Lebugle, A.; Sfihi, H.; Barroug, A. Nanocrystalline apatites in biological systems: Characterisation, structure and properties. Mater. Werkst. 2007, 38, 996–1002. [Google Scholar] [CrossRef]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef]
- Walter, G.; Vogel, J.; Hoppe, U.; Hartmann, P. The structure of CaO–Na2O–MgO–P2O5 invert glass. J. Non-Cryst. Solids 2001, 296, 212–223. [Google Scholar] [CrossRef]
- Jonscher, A.K. Universal Dielectric Response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Anderson, O.L.; Stuart, D.A. Calculation of Activation Energy of Ionic Conductivity in Silica Glasses by Classical Methods. J. Am. Ceram. Soc. 1954, 37, 573–580. [Google Scholar] [CrossRef]
- Nascimento, M.; Nascimento, E.; Watanabe, S. Test of Anderson-Stuart model and the "universal" conductivity in rubidium and cesium silicate glasses. Braz. J. Phys. 2005, 35, 626–631. [Google Scholar] [CrossRef]
- Nascimento, M.L.F. Test of the Anderson-Stuart model and correlation between free volume and the ’universal’ conductivity in sodium silicate glasses. J. Mater. Sci. 2007, 42, 3841–3850. [Google Scholar] [CrossRef]
- Kurkjian, C.R. Mechanical properties of phosphate glasses. J. Non-Cryst. Solids 2000, 263–264, 207–212. [Google Scholar] [CrossRef]
- Nascimento, M. Anderson-Stuart Model of Ionic Conductors in Na2O-SiO2 Glasses. Cienc. Eng. Sci. Eng. J. 2003, 12, 7. [Google Scholar]
- Dyre, J.C.; Schroder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 2000, 72, 873–892. [Google Scholar] [CrossRef]
- Cramer, C.; Brunklaus, S.; Gao, Y.; Funke, K. Dynamics of mobile ions in single- and mixed-cation glasses. J. Phys. Condens. Matter 2003, 15, S2309–S2321. [Google Scholar] [CrossRef]
- Wójcik, N.A.; Kupracz, P.; Barczyński, R.J.; Jonson, B.; Ali, S. Ion conduction in beryllium-alumino-silicate glasses doped with sodium or sodium and lithium ions. Solid State Ion. 2019, 341, 115055. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Farag, M.M.; Knowles, J.C. Dissolution and drug release profiles of phosphate glasses doped with high valency oxides. J. Mater. Sci. Mater. Med. 2016, 27, 108. [Google Scholar] [CrossRef]
- Hanifi, A.R.; Crowley, C.M.; Pomeroy, M.J.; Hampshire, S. Bioactivity potential of calcium alumino-silicate glasses and glass–ceramics containing nitrogen and fluorine. J. Mater. Sci. 2014, 49, 4590–4594. [Google Scholar] [CrossRef]
- Schuhladen, K.; Wang, X.; Hupa, L.; Boccaccini, A.R. Dissolution of borate and borosilicate bioactive glasses and the influence of ion (Zn, Cu) doping in different solutions. J. Non-Cryst. Solids 2018, 502, 22–34. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Hupa, L.; Boccaccini, A.R. Bioactivity and dissolution behavior of boron-containing bioactive glasses under static and dynamic conditions in different media. Biomed. Glasses 2019, 5, 124–139. [Google Scholar] [CrossRef]
- Lee, S.; Maçon, A.L.B.; Kasuga, T. Structure and dissolution behavior of orthophosphate MgO–CaO–P2O5–Nb2O5 glass and glass-ceramic. Mater. Lett. 2016, 175, 135–138. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Correia, R.N.; Fernandes, M.H. Surface modifications of a glass and a glass-ceramic of the MgO-3CaO · P2O5-SiO2 system in a simulated body fluid. Biomaterials 1995, 16, 849–854. [Google Scholar] [CrossRef]
- Wójcik, N.A.; Ali, S.; Mielewczyk-Gryń, A.; Jonson, B. Two-step synthesis of niobium doped Na–Ca–(Mg)–P–Si–O glasses. J. Mater. Sci. 2021, 56, 7613–7625. [Google Scholar] [CrossRef]
- Watts, S.J.; Hill, R.G.; O’Donnell, M.D.; Law, R.V. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 2010, 356, 517–524. [Google Scholar] [CrossRef]
- Konidakis, I.; Varsamis, C.P.E.; Kamitsos, E.I. Effect of synthesis method on the structure and properties of AgPO3-based glasses. J. Non-Cryst. Solids 2011, 357, 2684–2689. [Google Scholar] [CrossRef]
- Palles, D.; Konidakis, I.; Varsamis, C.P.E.; Kamitsos, E.I. Vibrational spectroscopic and bond valence study of structure and bonding in Al2O3-containing AgI-AgPO3 glasses. Rsc. Adv. 2016, 6, 16697–16710. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Brauer, D.S.; Hupa, L. Bioactive Glasses: Fundamentals, Technology and Applications; Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Salman, S.M.; Salama, S.N.; Darwish, H.; Mahdy, E. The role of MgO on the structural properties of CaO–Na2O(MgO)–P2O5–CaF2–SiO2 derived glass ceramics. Ceram. Int. 2010, 36, 55–61. [Google Scholar] [CrossRef]



| ID | Starting Composition (mol%) | Final Composition (mol%) |
|---|---|---|
| 0Mg | 10Na2O–60P2O5–30CaO | 7.7Na2O–56.9P2O5–32.6CaO–2.7Al2O3 |
| 5Mg | 5Na2O–5MgO–60P2O5–30CaO | 6Na2O–5MgO–55.5P2O5–29.8CaO–3.7Al2O3 |
| 7Mg | 3Na2O–7MgO–60P2O5–30CaO | 4.9Na2O–7.8MgO–55.8P2O5–29CaO–2.5Al2O3 |
| 10Mg | 10MgO–60P2O5–30CaO | 9.4MgO–57.7P2O5–31.2CaO–1.7Al2O3 |
| Sample ID | νas(PO2)− | νs(PO2)− | νas(PO3)2− End Groups | vas(P–O–P) in Large Rings | vas(P–O–P) in Chains | vs(P–O–P) | v(O–P–O) in (PO2)− Modes |
|---|---|---|---|---|---|---|---|
| 0Mg | 1296 | 1126 | 1094 | 956 | 910 | 758 | 492 |
| 5Mg | 1304 | 1122 | 1094 | 950 | 918 | 758 | 486 |
| 7Mg | 1312 | 1126 | 1088 | 960 | 914 | 764 | 494 |
| 10Mg | 1314 | 1128 | 1086 | 958 | 916 | 760 | 492 |
| References | [28,29] | [28,29] | [28,29] | [28] | [28,30,31,32] | [30,31,32] | [30] |
| IDs | σDC at 473 K (Scm−1) | EA (eV) ±0.001 | lnσ0 (KScm−1) ±0.01 |
|---|---|---|---|
| 0Mg | 4.21 × 10−11 | 1.267 | 13.38 |
| 5Mg | 1.38 × 10−11 | 1.273 | 12.41 |
| 7Mg | 8.22 × 10−12 | 1.276 | 11.99 |
| 10Mg | 4.34 × 10−12 | 1.256 | 10.86 |
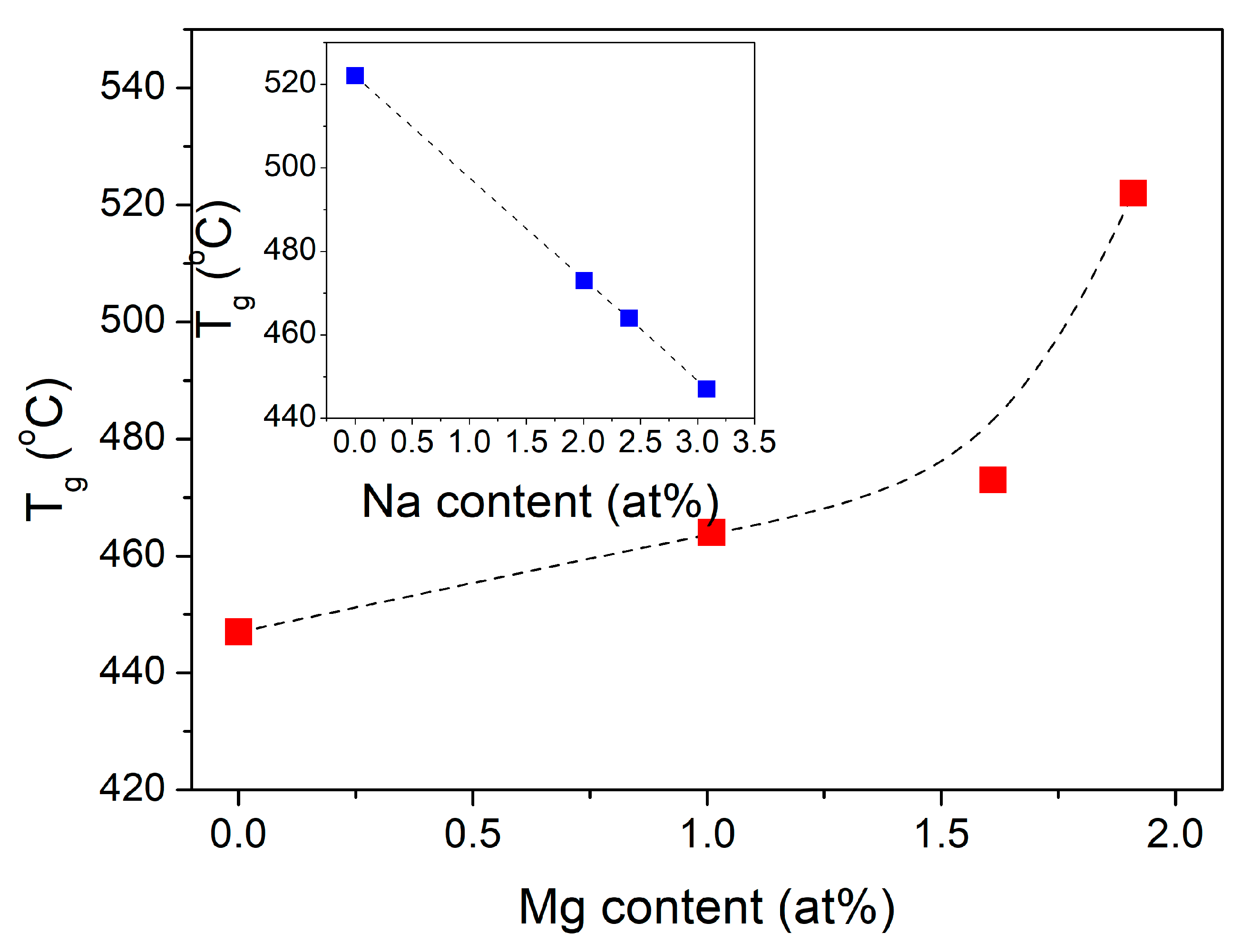
| Sample ID | Tg (°C) | Tcr onset (°C) | Tcr middle (°C) | S (°C) |
|---|---|---|---|---|
| 0Mg | 447 | 646 | 708 | 199 |
| 5Mg | 464 | 655 | 735 | 191 |
| 7Mg | 473 | 662 | 779 | 189 |
| 10Mg | 522 | 697 | 748 | 175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, N.A.; Ali, S.; Karczewski, J.L.; Jonson, B.; Bartmański, M.; Barczyński, R.J. DC and AC Conductivity, Biosolubility and Thermal Properties of Mg-Doped Na2O–CaO–P2O5 Glasses. Materials 2021, 14, 2626. https://doi.org/10.3390/ma14102626
Wójcik NA, Ali S, Karczewski JL, Jonson B, Bartmański M, Barczyński RJ. DC and AC Conductivity, Biosolubility and Thermal Properties of Mg-Doped Na2O–CaO–P2O5 Glasses. Materials. 2021; 14(10):2626. https://doi.org/10.3390/ma14102626
Chicago/Turabian StyleWójcik, Natalia Anna, Sharafat Ali, Jakub Lech Karczewski, Bo Jonson, Michał Bartmański, and Ryszard Jan Barczyński. 2021. "DC and AC Conductivity, Biosolubility and Thermal Properties of Mg-Doped Na2O–CaO–P2O5 Glasses" Materials 14, no. 10: 2626. https://doi.org/10.3390/ma14102626
APA StyleWójcik, N. A., Ali, S., Karczewski, J. L., Jonson, B., Bartmański, M., & Barczyński, R. J. (2021). DC and AC Conductivity, Biosolubility and Thermal Properties of Mg-Doped Na2O–CaO–P2O5 Glasses. Materials, 14(10), 2626. https://doi.org/10.3390/ma14102626







