Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties
Abstract
1. Introduction
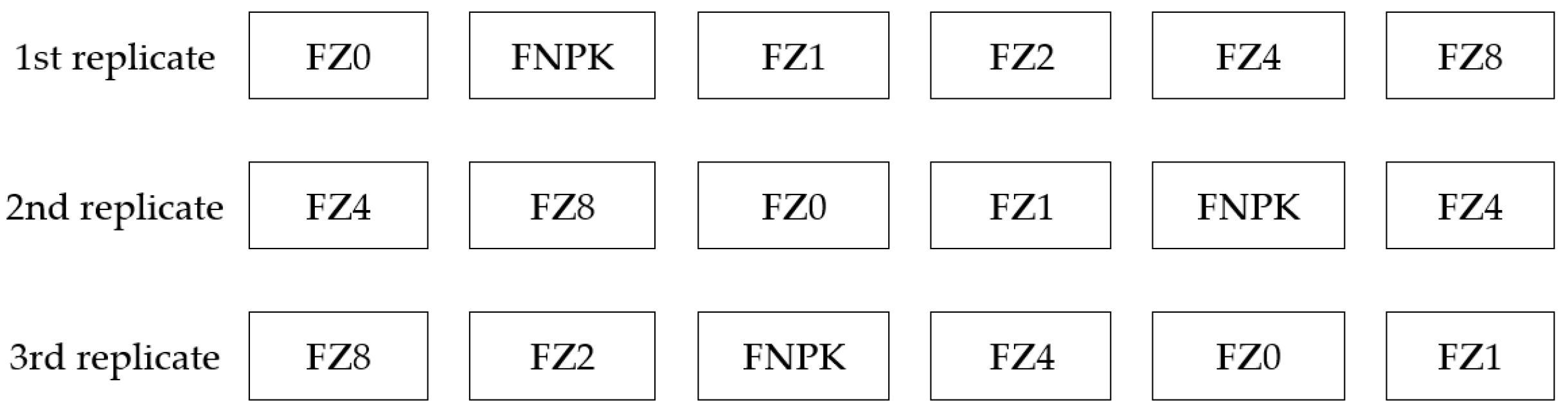
2. Materials and Methods
- -
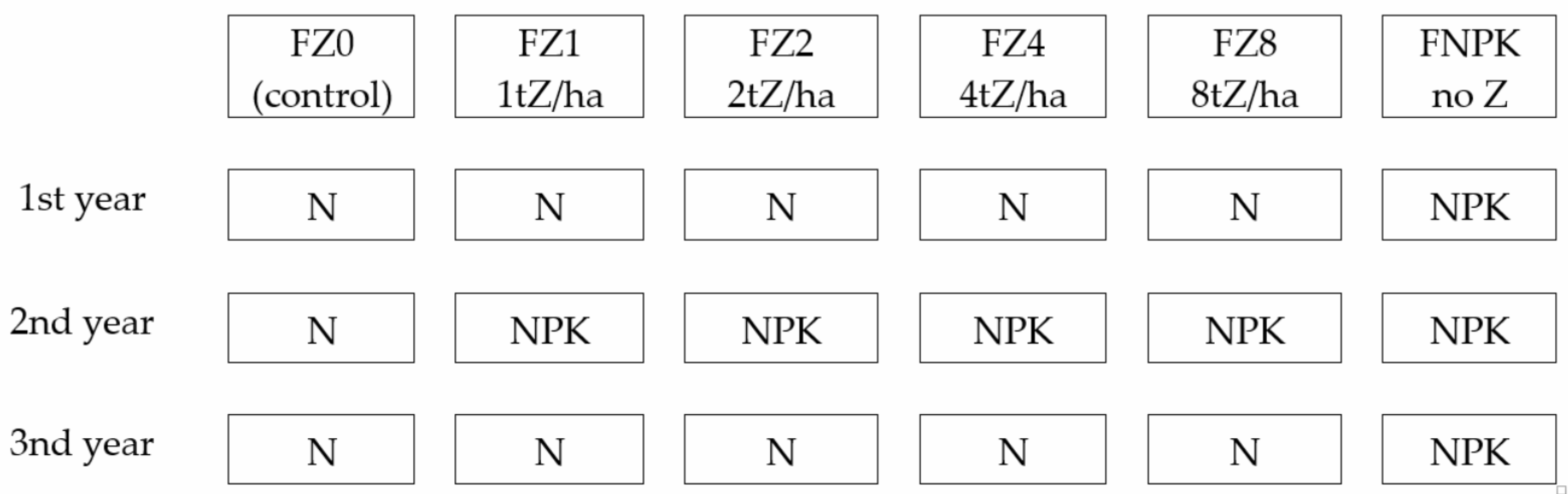
- Compare NPK and zeolite action on soil and plants (first year of the experiment);
- -
- Look for eventual advantages of zeolite plus NPK over standard NPK fertilization (second year of the experiment). In this year we decided to apply NPK on all but FZ0 plots to prevent exhaustion of soil nutrients;
- -
- Look for the eventual presence of the long-term zeolitization effects (third year of the experiment).
- pH in KCl (1:5 soil:solution ratio);
- Cation exchange capacity, CEC, at pH = 8.2 using Ba+2 as index cations (Mehlich method);
- Soil variable surface charge (called also pH-dependent charge), Qv (μmol g−1), measured from potentiometric titration curves registered under nitrogen atmosphere using an auto-titrator SM Titrino 702 (Metrohm, AG-Switzerland). The suspensions of the studied material in 1 mol·dm−3 NaCl solution were adjusted to pH = 2.95 (not changing within 5 min) and slowly titrated to pH = 10 with 0.1 mol·dm−3 NaOH. The amount (Mole) of the base consumed by the whole suspension, Nsusp, was used for neutralization of acidic groups of the solid surface, NS, and the acids present in the supernatant, Nsol. The NS value (NS = Nsusp-Nsol) measured between any two pH values is equivalent to variable surface charge developed by a soil in the given pH range. It is responsible for changes of soil CEC with changes in soil reaction and for a part of soil buffering capacity. More details on the method and calculations are given in Józefaciuk et al. [40];
- Amount of gravitational, GW, and plant available, PAW, water from selected points of water moisture versus water potential curves (called water retention or pF curves) were measured according to the procedure described in Richards [41] and Mullins et al. [42]. For water potential (pF) measurements, tensiometers placed in the soil were used and the soil moisture was measured with TDR hygrometer [43]. The pF is defined as a logarithm of a pressure necessary to remove water from soil pores (macropores). The pressure, understood as a water suction (F), is expressed as water height (in cm). The water retention curve provides the best characteristic of soil water storage at high moistures. Based on the above curve, one can distinguish different kinds of water stored in the studied medium. Gravitational water (GW) stored between pF = 0 and pF = 2.2 can easily flow down the soil profile under gravitation force and it is generally not used by plants. Water available for plants (PAW) occurs between soil water potential corresponding to pF = 2.2 (field water capacity, FWC) and pF = 4.2 (permanent wilting point);
- Mesopore volume, V (cm3 g−1), and average mesopore radius, R (μm), measured by mercury intrusion porosimetry using Micromeritics Autopore IV 9500 (Norcross, GA, USA) porosimeter. Pores detected by mercury intrusion porosimetry (MIP) belong to the range between 3 nm and 200 µm, roughly. The MIP measurements were carried out for 8 mm diameter and 8 mm height cylindrical aggregates prepared from the homogenized soil material and subjected to five wetting–drying cycles to stabilize the structure. The volume of mercury intruded into the aggregate at the maximum pressure was assumed to be equal to the mesopore volume. The average mesopore radius was calculated from pore size distribution functions obtained as the porosimeter reports. Details on porosimetric studies are described in Sridharan and Venkatappa Rao [44]. Mesopores play a crucial role in the formation of soil structure. They govern soil water, air and solute transport, soil compaction, aeration, root growth and many others;
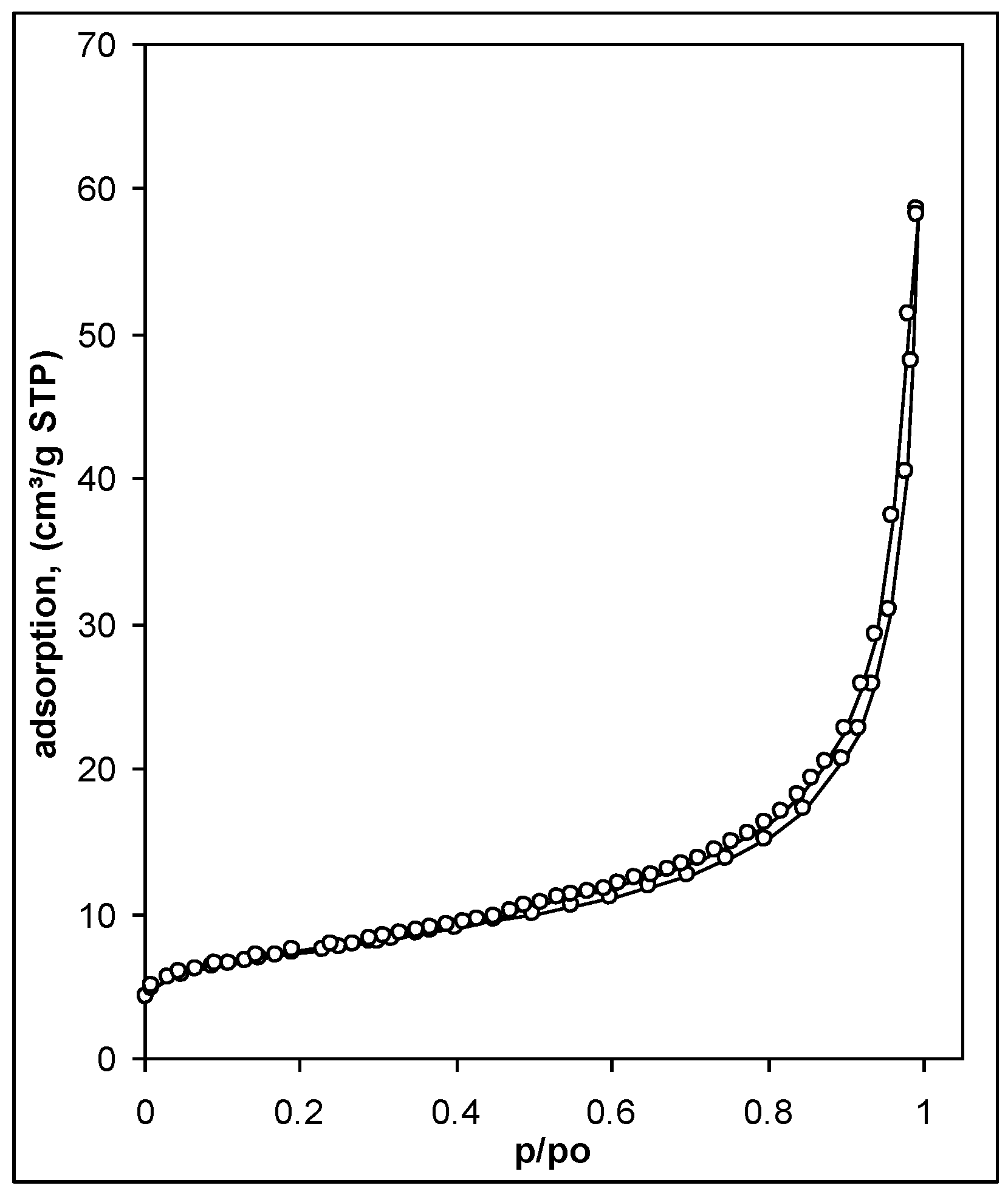
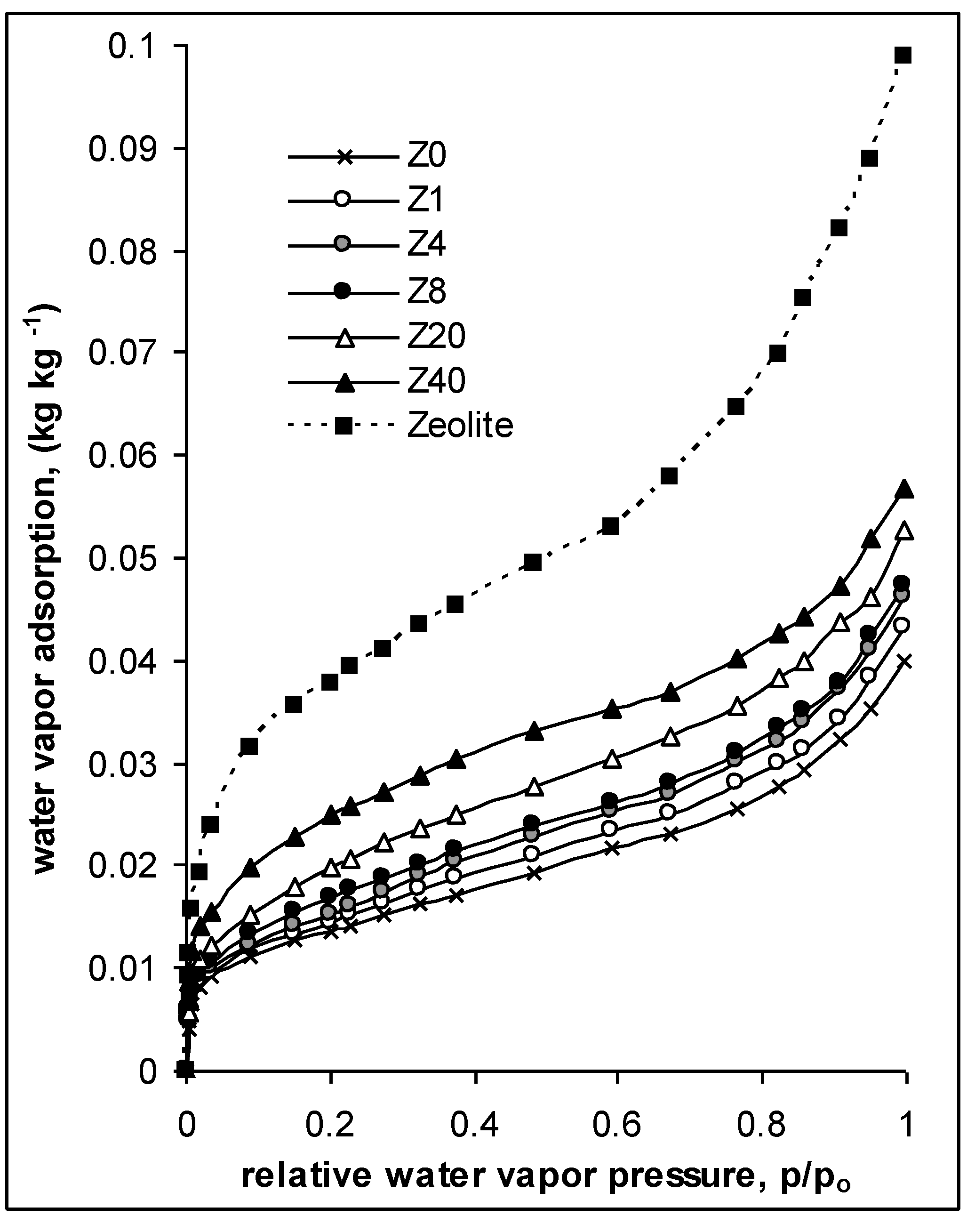
- Surface area, S (m2 g−1), and water vapor adsorption energy, E, estimated from water vapor adsorption/desorption isotherms for the soil aggregates (as in MIP). The isotherms were measured by weighing the samples after stepwise equilibration at different relative water vapor pressures, p/p0, at 20 °C. The surface area was calculated from the linear form of the standard BET equation [45]. The average adsorption energy, E, was calculated from the energy distribution function, f(E), derived from adsorption isotherms plotted in energy coordinates, assuming that adsorption energy at a given p/p0 equals to ln(p0/p). More details on the calculations are given in Józefaciuk et al. [46]. The water vapor adsorption isotherm provides the best characteristic of soil water content at low moistures that are most often met in upper soil layers at normal weather conditions. The surface area expresses the summary surface of all soil particles. Particularly high input to the surface area are clay minerals, amorphous phases and organic matter. Except for water binding, surface area is responsible for sorption of humic acids, pesticides and herbicides, immobilization of contaminants and soil catalytic properties. It frequently correlates with soil CEC. The water adsorption energy reflects water binding forces. Systems with higher adsorption energy may grasp water from systems with lower adsorption energy.
3. Results and Discussion
3.1. Weather Conditions
3.2. Effect of Zeolite on Plants
3.3. Effect of Zeolite on Soil
3.3.1. Field Soil
3.3.2. Laboratory Soil Studies
General Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yates, D.J.C. Studies on the surface area of zeolites, as determined by physical adsorption and X-ray crystallography. Can. J. Chem. 1968, 64, 1695–1701. [Google Scholar] [CrossRef]
- Torii, K. Utilization of natural zeolites in Japan. In Natural Zeolites: Occurrence, Properties, Use; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: Elmsford, NY, USA, 1978; pp. 441–450. [Google Scholar]
- Mumpton, F.A. La Roca Magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant. Res. Spec. 2004, 12, 183–189. [Google Scholar]
- Ramesh, K.; Reddy, D.D. Zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 219–240. [Google Scholar]
- Available online: https://www.mordorintelligence.com/industry-reports/zeolites-market (accessed on 30 December 2020).
- Available online: https://www.grandviewresearch.com/industry-analysis/zeolites-market (accessed on 30 December 2020).
- Available online: https://www.greatmining.com/zeolite-mining.html (accessed on 30 December 2020).
- Available online: https://www.fortunebusinessinsights.com/industry-reports/zeolite-market-101921 (accessed on 30 December 2020).
- Kralova, M.; Hrozinkova, A.; Ruzek, P.; Kovanda, F.; Kolousek, D. Synthetic and natural zeolites affecting the physical-chemical soil properties. Rostl. Vyrob. 1994, 40, 131–138. [Google Scholar]
- Jakkula, V.S.; Wani, S.P. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 119–126. [Google Scholar]
- Rehakova, M.; Cuvanova, S.; Dzivak, M.; Rimar, J.; Gavalova, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Xiubin, H.; Zhanbin, H. Zeolite applications for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar] [CrossRef]
- Azough, A.; Marashi, S.K.; Babaeinejad, T. Growth characteristics and response of wheat to cadmium, nickel and magnesium sorption affected by zeolite in soil polluted with armaments. J. Adv. Environ. Health Res. 2017, 5, 163–171. [Google Scholar]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Mohd, H.B.; Asima, J.; Arifin, A.; Hazandy, A.H.; Mohd, A.K. Elevation and variability of acidic sandy soil pH: Amended with conditioner, activator, organic and inorganic fertilizers. Afr. J. Agric. Res. 2013, 8, 4020–4024. [Google Scholar]
- Ahmed, O.H.; Sumalatha, G.; Majid, N.M.A. Use of zeolite in maize Zea mays. cultivation on nitrogen, potassium, and phosphorus uptake and use efficiency. Int. J. Phys. Sci. 2010, 5, 2393–2401. [Google Scholar]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Khan, H.; Khan, A.Z.; Khan, R.; Matsue, N.; Henmi, T. Soybean Leaf Area, Plant Height and Reproductive Development as Influenced by Zeolite Application and Allophanic Soil. J. Plant. Sci. 2008, 3, 277–286. [Google Scholar] [CrossRef]
- Moore, A.D.; Olsen, N.L.; Carey, A.M.; Leytem, A.B. Residual effects of fresh and composted dairy manure applications on potato production. Am. J. Potato Res. 2011, 88, 324–332. [Google Scholar] [CrossRef]
- Baninasab, B. Effects of the application of natural zeolite on the growth and nutrient status of radish Raphanus sativus L. J. Hortic. Sci. Biotechnol. 2009, 84, 13–16. [Google Scholar] [CrossRef]
- Kisic, I.; Mesic, M.; Basic, F.; Butorac, A.; Vadic, Z. The Effect of Liming and Fertilization on Yields of Maize and Winter Wheat. Agric. Conspec. Sci. 2004, 69, 51–57. [Google Scholar]
- Junrungreang, S.; Limtong, O.; Wattanaprapat, K.; Patsarayeangyong, T. Effect of zeolite and chemical fertilizer on the change of physical and chemical properties on Lat Ya soil series for sugar cane. In Proceedings of the 17th WCSS Symposium, Bangkok, Thailand, 12–14 August 2002. [Google Scholar]
- Litaor, M.I.; Katz, L.; Shenker, M. The influence of compost and zeolite co-addition on the nutrients status and plant growth in intensively cultivated Mediterranean Soils. Soil Use Manag. 2017, 33, 72–80. [Google Scholar] [CrossRef]
- Tallai, M.; Olah Zsuposne, A.; Sandor, Z.; Katai, J. The effect of using zeolite on some characteristics of sandy soil and on the Amount of the test plant biomass. Ann. Ser. Agric. Silvic. Vet. 2017, 6, 115–120. [Google Scholar]
- Aaina, H.N.; Ahmed, O.H.; Majid, N.M.A. Effects of clinoptilolite zeolite on phosphorus dynamics and yield of Zea Mays L cultivated on an acid soil. PLoS ONE 2018, 13, e0204401. [Google Scholar]
- Abdi, G.; Khosh-Khui, M.; Eshghi, S. Effects of Natural Zeolite on Growth and Flowering of Strawberry Fragariaxananassa Duch. Int. J. Agric. Res. 2006, 1, 384–389. [Google Scholar] [CrossRef]
- Cairo, P.C.; Machado de Armas, J.; Artiles, P.T.; Martin, B.D.; Carrazana, R.J.; Lopez, O.R. Effects of zeolite and organic fertilizers on soil quality and yield of sugarcane. Aust. J. Crop. Sci. 2017, 11, 733–738. [Google Scholar] [CrossRef]
- Jelic, M.; Dugalic, G.; Milivojevic, J.; Djekic, V. Effect of liming and fertilzation on yield and quality of oat Avena Sativa l on an acid luvisol soil. Rom. Agric. Res. 2013, 30, 249–258. [Google Scholar]
- Sepaskhah, A.R.; Barzegar, M. Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agric. Water Manag. 2010, 98, 38–44. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Ambrożewicz-Nita, A.; Franus, W.; Jozefaciuk, G. Early effect of clinoptilolite on yield and quality of oat Avena sativa L. Int. Agrophysics 2019, 33, 107–112. [Google Scholar] [CrossRef]
- Ozbahce, A.; Tari, A.F.; Gonulal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2015, 61, 615–626. [Google Scholar] [CrossRef]
- Kapusta, F. Cereals as a component of agriculture and Polish economy. Econ. XXI Century 2016, 2, 121–137. [Google Scholar]
- Eyde, T.H. Zeolites. Min. Eng. 2005, 57, 54–55. [Google Scholar]
- Ryzak, M.; Bieganowski, A. Determination of particle size distribution of soil using laser diffraction–Comparison with areometric method. Int. Agrophysics 2010, 24, 177–181. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Chapman, H.D. Cation-exchange capacity. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 891–901. [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants: BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Józefaciuk, G.; Szatanik-Kloc, A.; Lukowska, M.; Szerement, J. Pitfalls and Uncertainties of Using Potentiometric Titration for Estimation of Plant Roots Surface Charge and Acid-Base Properties. Am. J. Plant. Sci. 2014, 5, 1862–1876. [Google Scholar] [CrossRef]
- Richards, L.A. Methods for measuring soil moisture tension. Soil Sci. 1949, 68, 95–112. [Google Scholar] [CrossRef]
- Mullins, C.E.; Mandiringana, O.T.; Nisbet, T.R.; Aitken, M.N. The design, limitations, and use of a portable tensiometer. J. Soil Sci. 1986, 37, 691–700. [Google Scholar] [CrossRef]
- Skierucha, W. Design and performance of psychrometric soil water potential meter. Sens. Actuators A 2005, 118, 86–91. [Google Scholar] [CrossRef]
- Sridharan, A.; Venkatappa Rao, G. Pore size distribution of soils from mercury intrusion porosimetry data. Soil Sci. Soc. Am. Proc. 1972, 36, 980–981. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Lukowska, M.; Szerement, J. Determination of Energetic and Geometric Properties of Plant Roots Specific Surface from Adsorption/Desorption Isotherm. Am. J. Plant. Sci. 2013, 4, 1554–1561. [Google Scholar] [CrossRef][Green Version]
- Fotyma, M.; Fotyma, E. The structure of winter cereal yield depending on nitrogen fertilization. in Polish. Fragm. Agron. 1993, 10, 101–102. [Google Scholar]
- Nouri-Ganbalani, A.; Hassanpanah, D.; Nouri-Ganbalani, G. Effects of drought stress condition on the yield and yield components of advanced wheat genotypes in Ardabil. J. Food Agric. Environ. 2009, 7, 228–234. [Google Scholar]
- Brzozowska, I.; Brzozowski, J.; Cymes, I. Effect of weather conditions on spring triticale yield and content of macroelements in grain. J. Elem. 2018, 234, 1387–1397. [Google Scholar] [CrossRef]
- Hruskova, M.; Svec, I.; Kocourkova, Z. Interaction between wheat variety and harvest year analysed by statistical methods. Cereal Technol. 2011, 4, 152–159. [Google Scholar]
- Muste, S.; Modoran, C.; Man, S.; Muresan, V.; Birou, A. The influence of wheat genotype on its quality. J. Agroaliment. Process. Technol. 2010, 16, 99–103. [Google Scholar]
- Allen, E.; Ming, D.; Hossner, L.; Henninger, D.; Galindo, C. Growth and nutrient uptake of wheat in a clinoptilolite-phosphate rock substrate. Agron. J. 1995, 87, 1052–1059. [Google Scholar] [CrossRef]
- Helfferich, F.G.; Hwang, Y.L. Ion Exchange Kinetics. In Ion. Exchangers. Part. I; Dorfner, K., Ed.; De Gruyter: Basel, Switzerland, 1991; pp. 1277–1310. [Google Scholar]
- Ravali, C.; Rao, K.J.; Anjaiah, T.; Suresh, K. Effect of zeolite on soil physical and physico-chemical properties. Multilogic Sci. 2020, XXXIII, 776–781. [Google Scholar]
- Fudlel, A.Y.; Minardi, S.; Hartati, S.; Syamsiyah, J. Studying the residual effect of zeolite and manure on alfisols cation exchange capacity and green bean yield. J. Soil Sci. Agroclimatol. 2019, 16, 181–190. [Google Scholar] [CrossRef]
- Chomczynska, M.; Wasag, H.; Kujawska, J. Application of Spent Ion Exchange Sorbents for the Reclamation of Degraded Soils. J. Ecol. Eng. 2019, 20, 239–244. [Google Scholar]
- Filcheva, E.G.; Tsadilas, C.D. Influence of clinoptilolite and compost on soil properties. Commun. Soil Sci. Plant. Anal. 2002, 33, 595–607. [Google Scholar] [CrossRef]
- Lutzow, M.V.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Yasuda, H.; Tazuma, K.; Mizuta, N.; Nishide, H. Water retention variety of dune sand due to zeolite addition. Bull. Fac. Agric. Tottori Univ. 1995, 48, 27–34. [Google Scholar]
- Bernardi, A.C.C.; Oliveira, P.P.A.; Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 2013, 17, 16–21. [Google Scholar] [CrossRef]
- Lancellotti, I.; Toschi, T.; Passaglia, E.; Barbieri, L. Release of agronomical nutrient from zeolite substrate containing phosphatic waste. Environ. Sci. Pollut. Res. 2014, 21, 13237–13245. [Google Scholar] [CrossRef] [PubMed]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 464–473. [Google Scholar] [CrossRef]
- Liu, C.; Tong, F.; Yan, L.; Zhou, H.; Hao, S. Effect of Porosity on Soil-Water Retention Curves: Theoretical and Experimental Aspects. Geofluids 2020. [Google Scholar] [CrossRef]
- Hung, Z.T.; Petrovic, A.M. Clinoptilolite zeolite effect on evaporation rate and shoot growth rate of bentgrass on sand base grass. J. Turfgrass Manag. 1995, 25, 35–39. [Google Scholar]



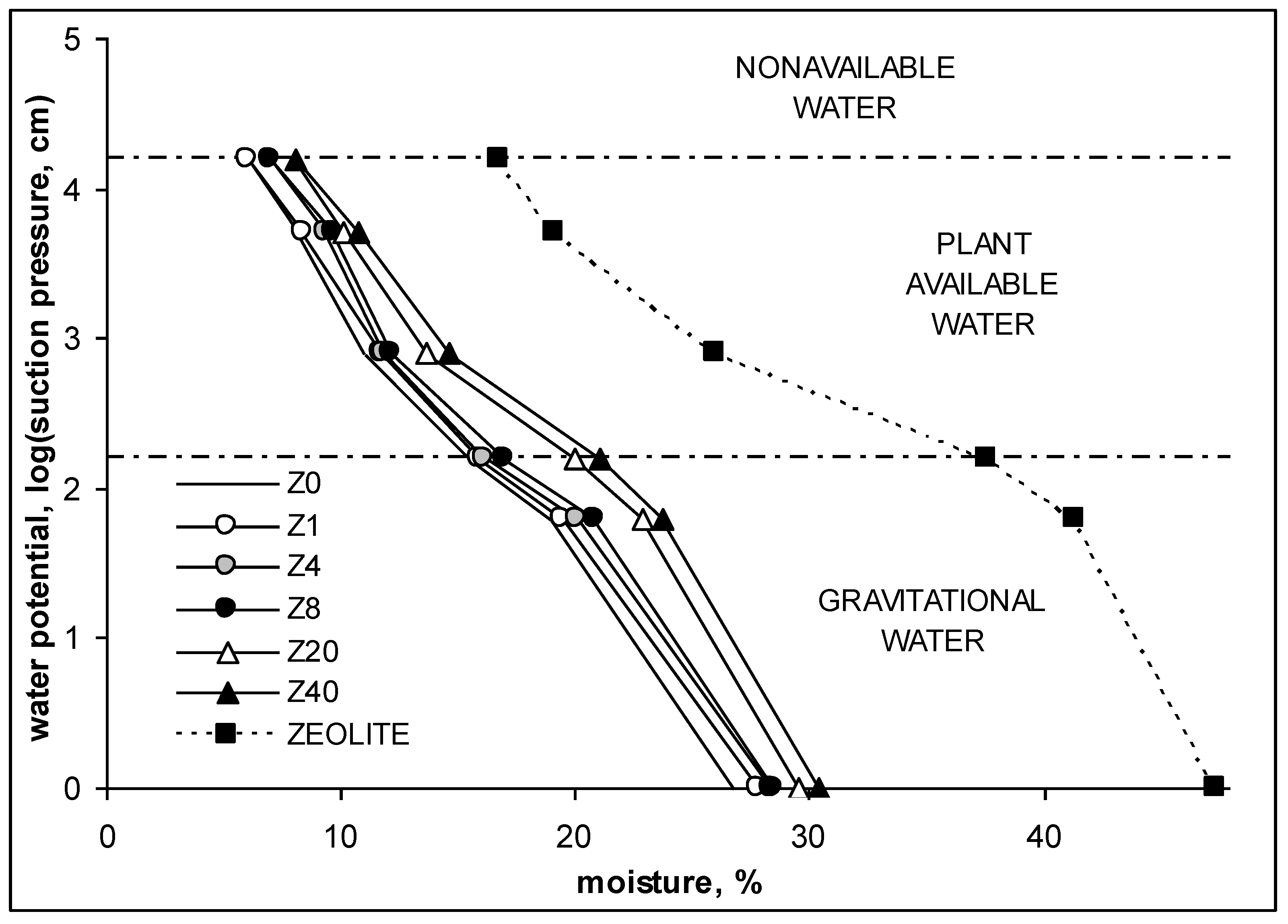
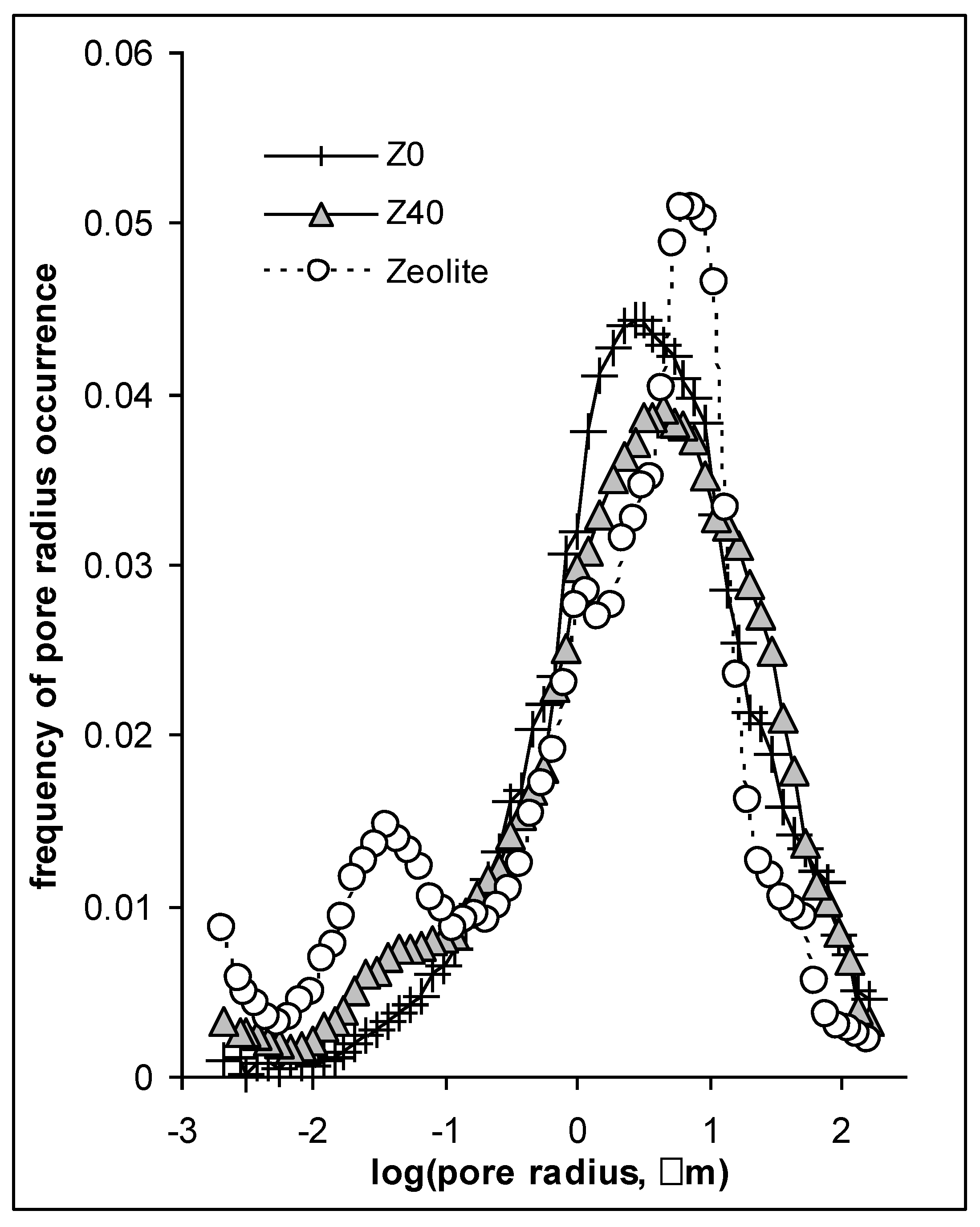
| Year | Climatic Data | April | May | June | July |
|---|---|---|---|---|---|
| 2014 | R, mm | 55 | 150 | 31 | 74 |
| T, °C | 9.0 | 12.6 | 14.9 | 17.8 | |
| 2015 | R, mm | 38 | 55 | 71.0 | 75 |
| T, °C | 9.1 | 14.0 | 19.0 | 21.0 | |
| 2016 | R, mm | 41 | 56 | 74 | 73 |
| T, °C | 9.5 | 15.0 | 19.0 | 20.0 | |
| Long-term data | R, mm | 39.8 | 59.9 | 66.5 | 80.6 |
| T, °C | 8.0 | 13.5 | 16.1 | 18.2 |
| First Year of the Experiment (2014) | ||||||
|---|---|---|---|---|---|---|
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| NE | 472.7 ± 12 ab | 474.7 ± 7 abc | 475 ± 12 abc | 483.7 ± 6 abcde | 495.3 ± 5 defg | 505.7 ± 9.2 efg |
| WHP | 74 ± 7 a | 92 ± 11 a | 96 ± 12 a | 101 ± 11 ab | 113 ± 17 b | 144 ± 21 c |
| WHS | 34 ± 3.3 a | 41 ± 7.8 a | 45 ± 5.4 a | 46 ± 5.0 a | 52 ± 8.7 ab | 64 ± 1.5 a |
| WHE | 41 ± 3.1 a | 51 ± 9.7 a | 51 ± 7.0 a | 55 ± 7.1 a | 60 ± 8.4 ab | 79 ± 14.9 b |
| LS | 58 ± 3.7 a | 61 ± 6.1 a | 65 ± 1.9 ab | 68 ± 2.5 ab | 68 ± 4.0 ab | 73 ± 7.2 b |
| LE | 4.3 ± 0.1 a | 4.9 ± 0.1 a | 5.1 ± 0.2 ab | 5.3 ± 0.4 bc | 5.3 ± 0.2 bc | 6.1 ± 0.5 c |
| NFE | 10 ± 0.9 | 13 ± 0.8 | 12 ± 0.8 | 12 ± 1.7 | 13 ± 1.3 | 16 ± 4.9 |
| NG | 17 ± 4.5 a | 17 ± 6.5 a | 23 ± 1.8 ab | 24 ± 1.5 ab | 26 ± 3.3 b | 30 ± 3.9 b |
| YG | 2.8 ± 0.1 a | 3.4 ± 0.1 b | 3.4 ± 0.1 b | 3.5 ± 0.1 b | 3.8 ± 0.1 c | 4.2 ± 0.2 d |
| Second year of the experiment (2015) | ||||||
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| NE | 475 ± 9 a | 505.3 ± 12 cefg | 501.8 ± 4 defg | 502 ± 7 efg | 501 ± 8 efg | 511 ± 10 g |
| WHP | 76 ± 5.5 ab | 132 ± 23 bc | 136 ± 35 bc | 130 ± 29 bc | 138 ± 35 bc | 141 ± 26 c |
| WHS | 37 ± 3.9 a | 43 ± 7.ab1 | 42 ± 8.3 ab | 48 ± 2.8 ab | 48 ± 10.1 ab | 70 ± 21 b |
| WHE | 44 ± 6.5 a | 51 ± 3.7 ab | 64 ± 10.7 abc | 57 ± 8.5 abc | 54 ± 14 abc | 84 ± 19 c |
| LS | 59 ± 6.4 ab | 69 ± 2.9 abc | 64 ± 11 abc | 71 ± 4.6 abc | 67 ± 7.9 abc | 77 ± 2.7 c |
| LE | 4.5 ± 0.3 a | 5.3 ± 0.3 ab | 5.2 ± 0.6 abcd | 5.5 ± 0.5 bcde | 5.8 ± 0.5 bcde | 6.5 ± 0.2 e |
| NFE | 14 ± 3.1 | 13 ± 1.5 | 14 ± 2.5 | 15 ± 1.5 | 13 ± 5.7 | 14 ± 1.5 |
| NG | 19 ± 3.6 abc | 29 ± 4.9 d | 27 ± 2.0 d | 31 ± 1.0 d | 32 ± 2.1 d | 32 ± 2.5 d |
| YG | 2.86 ± 0.4 a | 4.3 ± 1.2 bcd | 4.3 ± 1.2 bcd | 4.1 ± 0.3 bcd | 4.5 ± 0.64 cd | 4.71 ± 0.37 d |
| Third year of the experiment (2016) | ||||||
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| NE | 471 ± 10 abc | 473 ± 4 abcd | 471 ± 9 abc | 494 ± 4 bcd | 481 ± 13 abcde | 508 ± 10 fg |
| WHP | 74 ± 6.2 a | 91 ± 20.4 abc | 110 ± 18 bc | 96 ± 15 abc | 97 ± 11 bc | 139 ± 28 bc |
| WHS | 35 ± 5.1 a | 41 ± 1.4 ab | 45 ± 5.1 ab | 41 ± 2.4 ab | 48 ± 10.8 ab | 63 ± 18 b |
| WHE | 41 ± 8.1 a | 47 ± 6.8 a | 56 ± 14 abc | 62 ± 15.1 abc | 53 ± 7.3 abc | 81 ± 15.1 bc |
| LS | 57 ± 3.9 a | 64 ± 8.1 abc | 62 ± 3.8 abc | 61 ± 9 abc | 67 ± 8.4 abc | 75 ± 4.8 abc |
| LE | 4.3 ± 0.3 a | 4.8 ± 0.4 ab | 5.1 ± 0.3 abc | 4.4 ± 0.4 a | 5.3 ± 0.9 bcde | 6.3 ± 0.4 cde |
| NFE | 11 ± 1.5 a | 11 ± 1.5 a | 14 ± 2.5 a | 12 ± 1.0 a | 12 ± 2.5 a | 14 ± 2.0 a |
| NG | 17 ± 5.6 a | 28 ± 3.6 abcd | 23 ± 1.5 abcd | 24 ± 2.1 abcd | 18 ± 7.4 ab | 30 ± 3.1 cd |
| YG | 2.8 ± 0.3 a | 3.0 ± 0.2 ab | 3.1 ± 0.9 abc | 3.1 ± 0.6 abc | 2.9 ± 0.8 abc | 4.5 ± 0.2 d |
| First Year of the Experiment (2014) | ||||||
|---|---|---|---|---|---|---|
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| WTG | 32.8 ± 1.9 a | 33.0 ± 2.2 a | 32.0 ± 1.3 a | 31.6 ± 1.1 a | 32.1 ± 2.1 a | 35.6 ± 3.3 a |
| MG | 12 ± 0.6 a | 12 ± 0.4 a | 11 ± 0.1 a | 12 ± 0.1 a | 12 ± 0.4 a | 12 ± 0.7 a |
| DV | 76.9 ± 1.5 a | 74.4 ± 1.4 a | 74.5 ± 0.9 a | 73.0 ± 2.2 a | 73.0 ± 1.5 a | 73.4 ± 0.9 a |
| FN | 227 ± 8.2 a | 262 ± 44 ab | 274 ± 7 ab | 288 ± 8.1 ab | 307 ± 15 b | 309 ± 6.8 b |
| TPC | 11.0 ± 0.5 abcd | 11.2 ± 0.2 abcd | 11.3 ± 0.8 abcd | 11.4 ± 0.1 abcd | 12.2 ± 0.5 abcd | 12.4 ± 0.7 bcd |
| GW | 23 ± 1 a | 24.4 ± 1 ab | 25.1 ± 0.7 abc | 26.5 ± 1.4 bcd | 28.2 ± 0.9 cde | 29.7 ± 1 de |
| GI | 73 ± 1 a | 78 ± 1 ab | 82 ± 1 bc | 87 ± 1 cd | 91 ± 1.5 d | 85 ± 0.8 cd |
| Second year of the experiment (2015) | ||||||
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| WTG | 30.6 ± 1.3 a | 34.9 ± 2.3 ab | 36.2 ± 4.5 ab | 36.3 ± 3.7 b | 35.9 ± 3.9 ab | 37.6 ± 2.8 b |
| MG | 12 ± 0.2 a | 12 ± 0.5 a | 12 ± 0.3 a | 12 ± 0.7 a | 12 ± 1.1 a | 12 ± 0.3 a |
| DV | 76.7 ± 2.3 a | 74.0 ± 3.4 a | 74.6 ± 3.2 a | 73.0 ± 1.7 a | 72.8 ± 1.5 a | 71.8 ± 1.6 a |
| FN | 228 ± 3.2 a | 306 ± 34 b | 301 ± 8.8 b | 271 ± 31 ab | 277 ± 18 ab | 310 ± 9.6 b |
| TPC | 12.0 ± 0.8 abcd | 12.2 ± 0.3 abcd | 12.3 ± 1.2 bcd | 12.1 ± 0.6 abcd | 12.5 ± 1 cd | 12.5 ± 0.5 d |
| GW | 22.8 ± 0.8 a | 30.1 ± 1.5 de | 29.2 ± 1.3 de | 29.3 ± 0.5 de | 30.4 ± 1.9 de | 30.1 ± 0.2 d |
| GI | 73 ± 1.7 a | 85 ± 2.4 c | 84.0 ± 2.4 c | 86 ± 1.6 cd | 84 ± 3.5 cd | 85 ± 0.8 cd |
| Third year of the experiment (2016) | ||||||
| Parameter * | FZ0 | FZ1 | FZ2 | FZ4 | FZ8 | FNPK |
| WTG | 30.7 ± 1.9 a | 30.8 ± 1.2 a | 31.9 ± 1.6 a | 30.9 ± 2.9 a | 31.8 ± 2 a | 36.6 ± 2.7 a |
| MG | 12.5 ± 0.02 a | 12.5 ± 0.5 a | 12.0 ± 0.3 a | 12.3 ± 0.7 a | 12.4 ± 1.1 a | 12.0 ± 0.3 a |
| DV | 76.4 ± 2.3 a | 74.5 ± 4.8 a | 73.3 ± 2.9 a | 72.6 ± 2.7 a | 72.6 ± 2.6 a | 73.1 ± 0.6 a |
| FN | 228 ± 2.1 a | 261 ± 26 ab | 288 ± 17 b | 272 ± 41 ab | 278 ± 37 ab | 309 ± 4.1 b |
| TPC | 10.2 ± 0.5 a | 10.3 ± 0.6 ab | 10.4 ± 1 abcd | 10.5 ± 0.9 abcd | 10.4 ± 0.5 abc | 12.4 ± 1 bcd |
| GW | 23.0 ± 0.6 a | 23.2 ± 1.2 a | 23.5 ± 0.5 ab | 23.2 ± 1.1 a | 23.3 ± 1.1 ab | 29.7 ± 1 de |
| GI | 73.4 ± 2.9 a | 78 ± 4.4 bc | 72 ± 1 a | 77 ± 1 b | 73 ± 1.4 a | 86 ± 1 cd |
| Year of the Experiment | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|
| Parameter * | Zeolite | FZ0 | FZ8 | FZ0 | FZ8 | FZ0 | FZ8 |
| pH | 7.4 ± 0.11 a | 4.7 ± 0.35 b | 4.9 ± 0.42 b | 4.8 ± 0.25 b | 5.0 ± 0.33 b | 4.8 ± 0.36 b | 5.0 ± 0.42 b |
| S, m2 g−1 | 125 ± 6.3 a | 51 ± 3.7 b | 52 ± 3.8 b | 52 ± 5.0 b | 53 ± 1.5 b | 51 ± 3.6 b | 53 ± 4.3 b |
| E, RT | 4.9 ± 0.33 a | 5.0 ± 0.42 b | 5.1 ± 0.27 b | 5.0 ± 0.44 b | 5.1 ± 0.38 b | 5.0 ± 0.33 b | 5.1 ± 0.36 b |
| Qv. cMol kg−1 | 1.5 ± 0.11 a | 1.7 ± 038 b | 1.7 ± 0.42 b | 1.7 ± 0.21 b | 1.6 ± 031 b | 1.8 ± 0.44 b | 1.7 ± 047 b |
| CEC, cMol kg−1 | 127 ± 5.3 a | 6.6 ± 0.49 b | 6.8 ± 0.43 b | 6.7 ± 0.37 b | 6.8 ± 0.36 b | 6.6 ± 0.41 b | 6.9 ± 0.28 b |
| Vpor, cm3 g−1 | 0.5 ± 0.17 a | 0.4 ± 0.2 b | 0.4 ± 0.12 b | 0.4 ± 0.16 b | 0.4 ± 0.09 b | 0.4 ± 0.13 b | 0.5 ± 0.17 b |
| Rav, µm | 1.4 ± 0.19 a | 3.0 ± 0.24 b | 2.9 ± 0.33 b | 3.0 ± 0.14 b | 2.9 ± 0.10 b | 3.1 ± 0.21 b | 3.0 ± 0.11 b |
| GW% | 10 ± 0.4 a | 11 ± 1.6 a | 11 ± 0.8 a | 10 ± 0.9 a | 12 ± 1.1 a | 11 ± 1.8 a | 12 ± 1.3 a |
| PAW% | 21 ± 0.2 a | 10 ± 1.4 b | 10 ± 1.3 b | 9 ± 1.2 b | 10 ± 1.7 b | 9 ± 1.1 b | 10 ± 1.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties. Materials 2021, 14, 2617. https://doi.org/10.3390/ma14102617
Szatanik-Kloc A, Szerement J, Adamczuk A, Józefaciuk G. Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties. Materials. 2021; 14(10):2617. https://doi.org/10.3390/ma14102617
Chicago/Turabian StyleSzatanik-Kloc, Alicja, Justyna Szerement, Agnieszka Adamczuk, and Grzegorz Józefaciuk. 2021. "Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties" Materials 14, no. 10: 2617. https://doi.org/10.3390/ma14102617
APA StyleSzatanik-Kloc, A., Szerement, J., Adamczuk, A., & Józefaciuk, G. (2021). Effect of Low Zeolite Doses on Plants and Soil Physicochemical Properties. Materials, 14(10), 2617. https://doi.org/10.3390/ma14102617






