Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Cork-Graphite Sensor
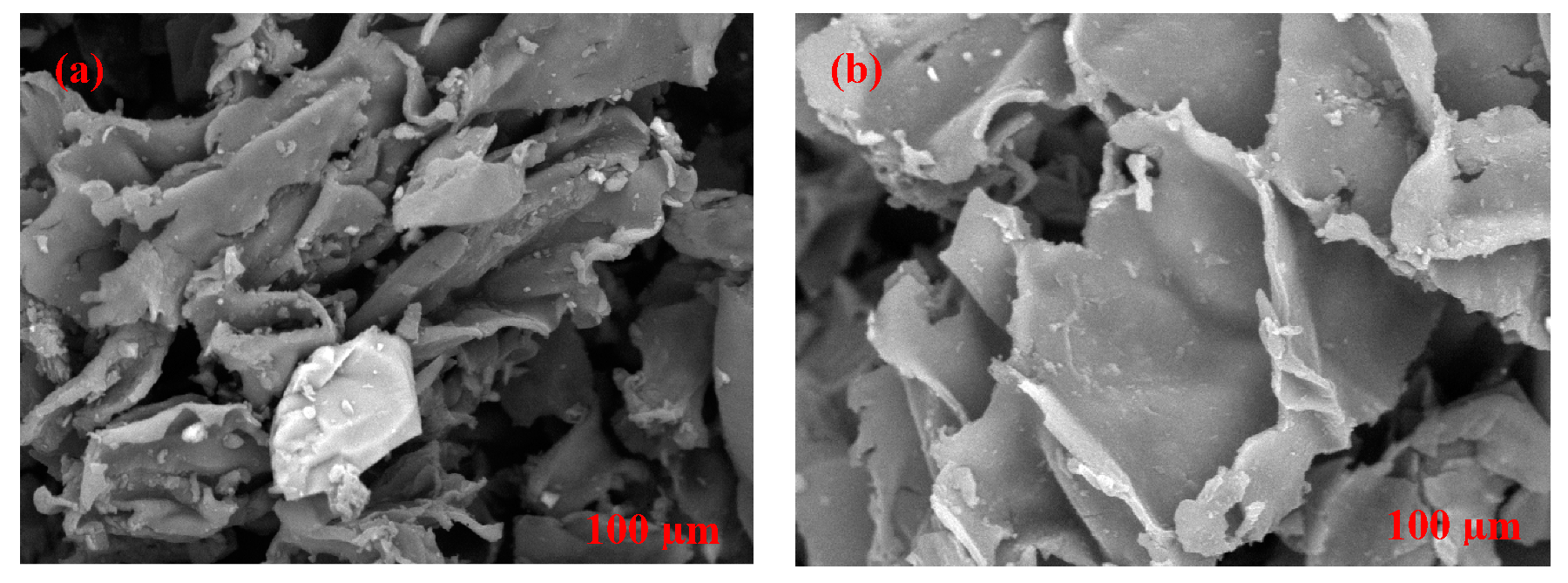
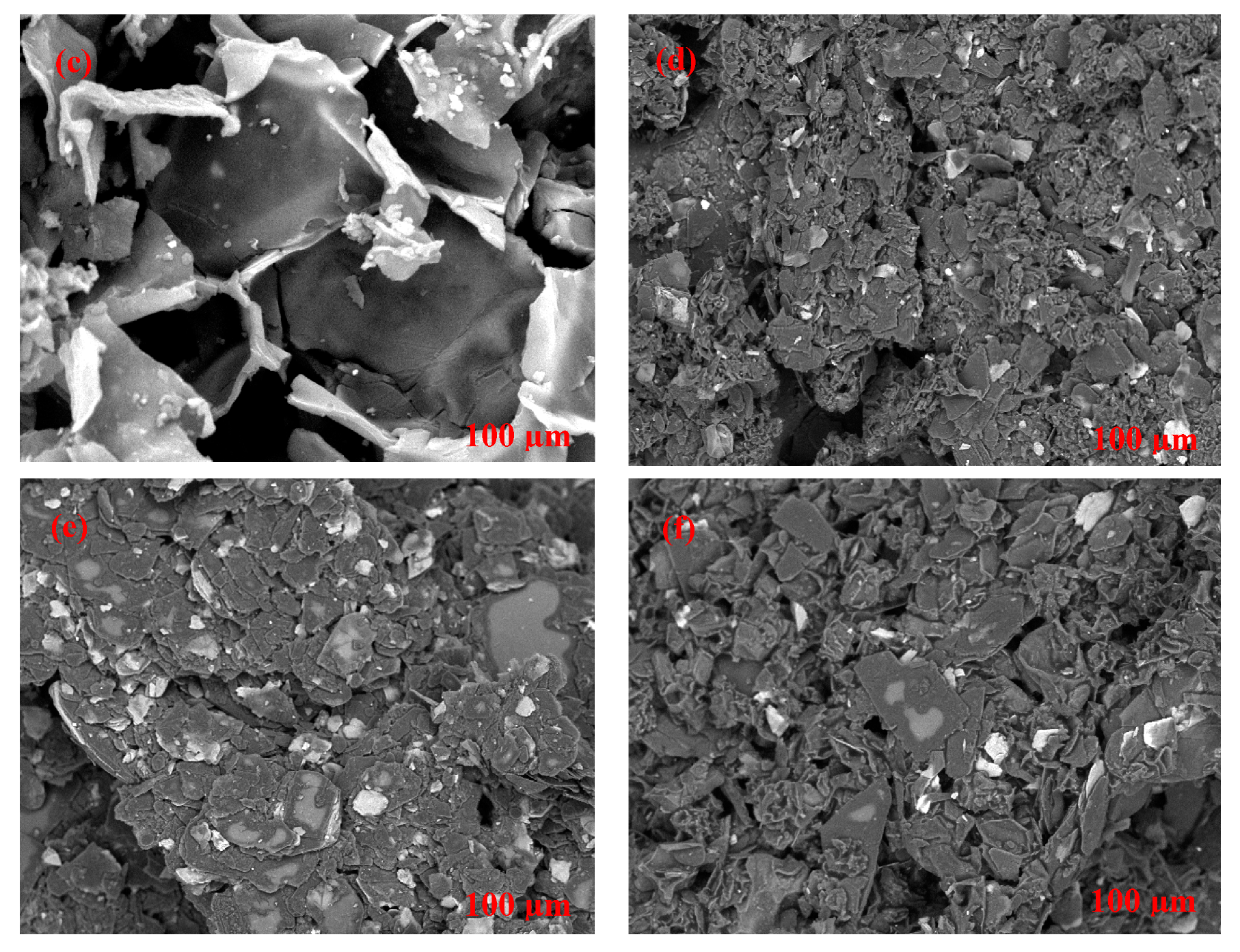
2.3. Morphological Characterization of Cork-Graphite Sensor
2.4. Analytical Techniques
2.5. Electrochemical Measurements
3. Results
3.1. Physical and Chemical Characterization of Cork
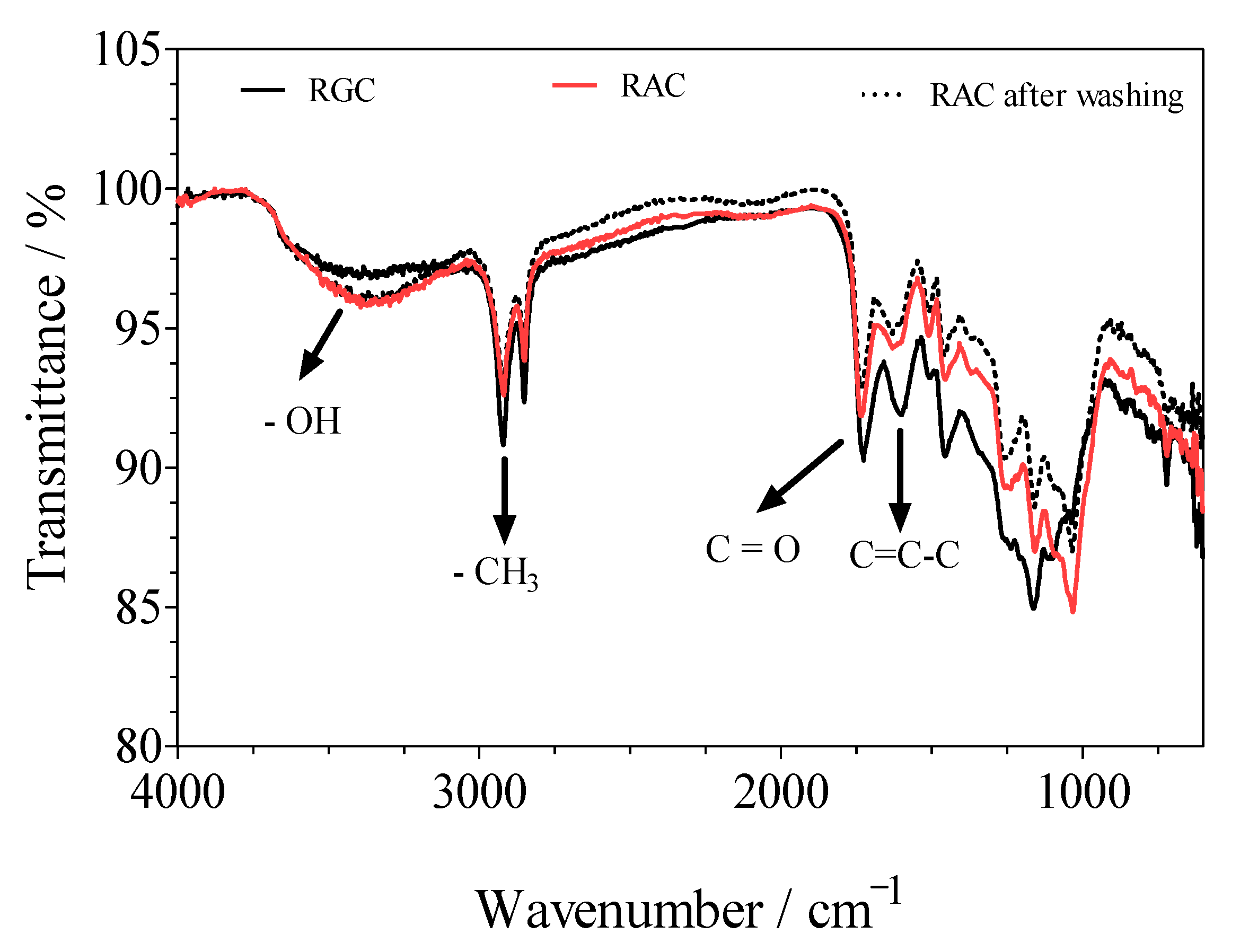
3.2. Fourier-Transform Infrared Spectroscopy–(FTIR)
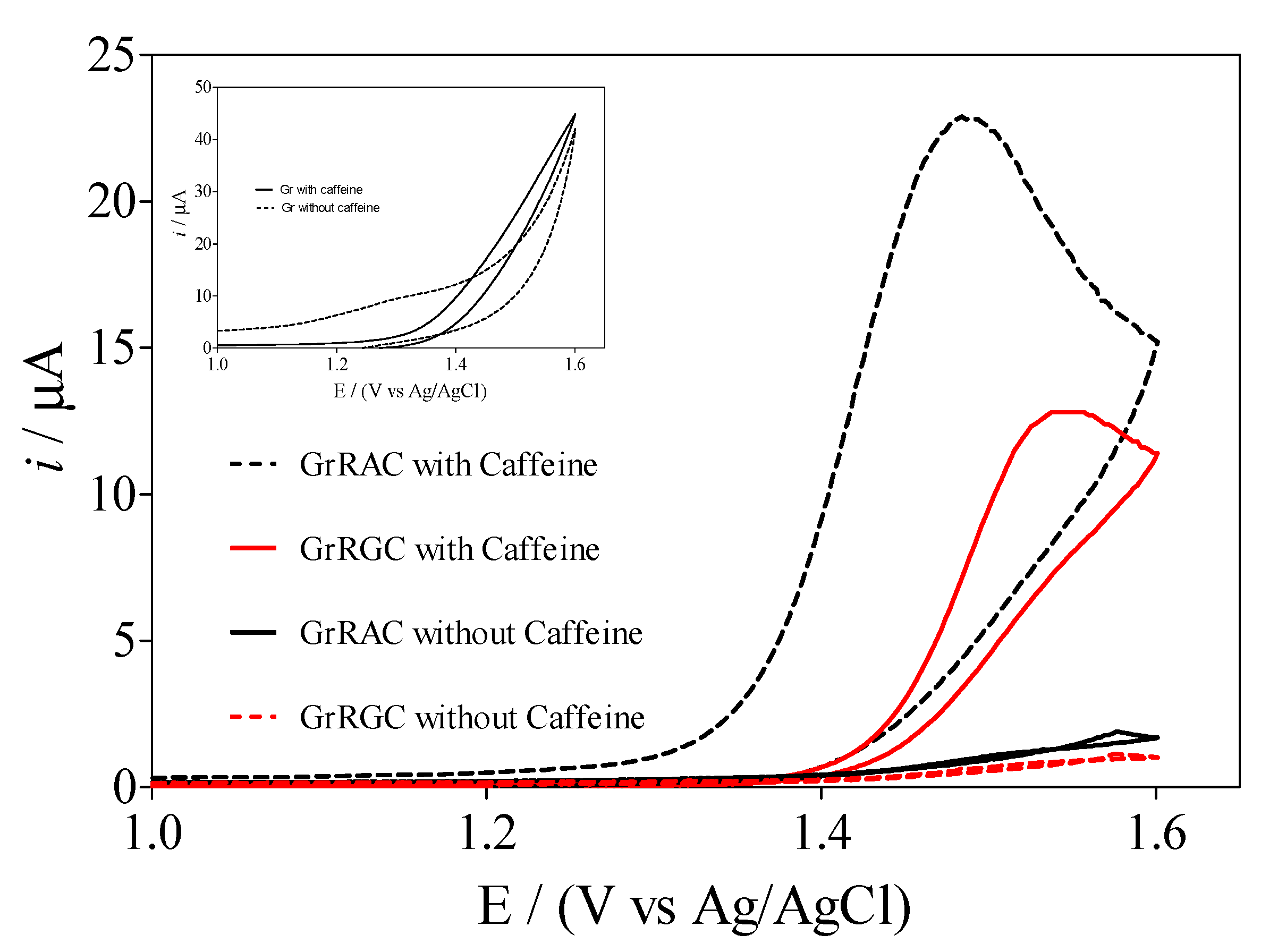
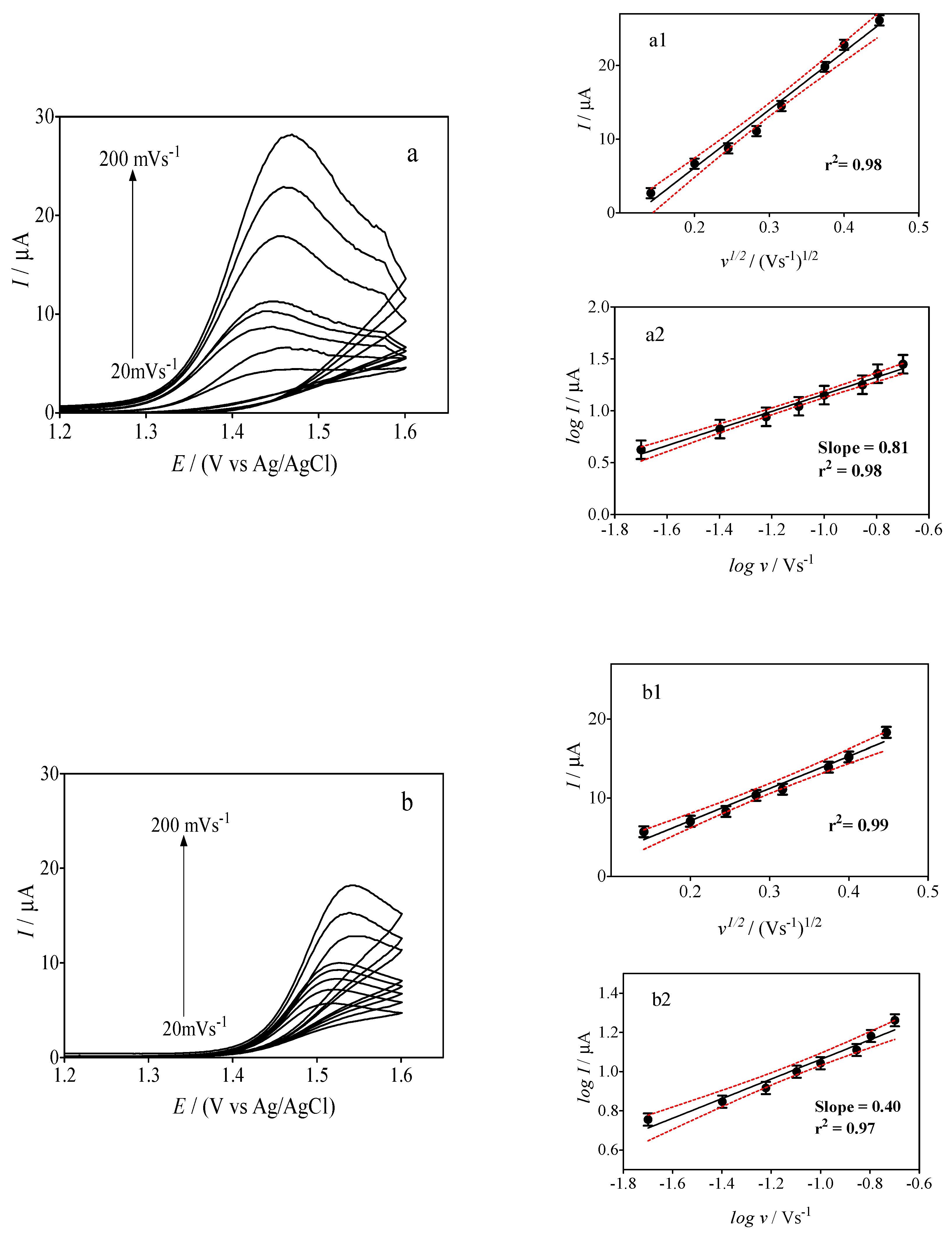
3.3. Electrochemical Behavior of Caffeine by Using Sensors
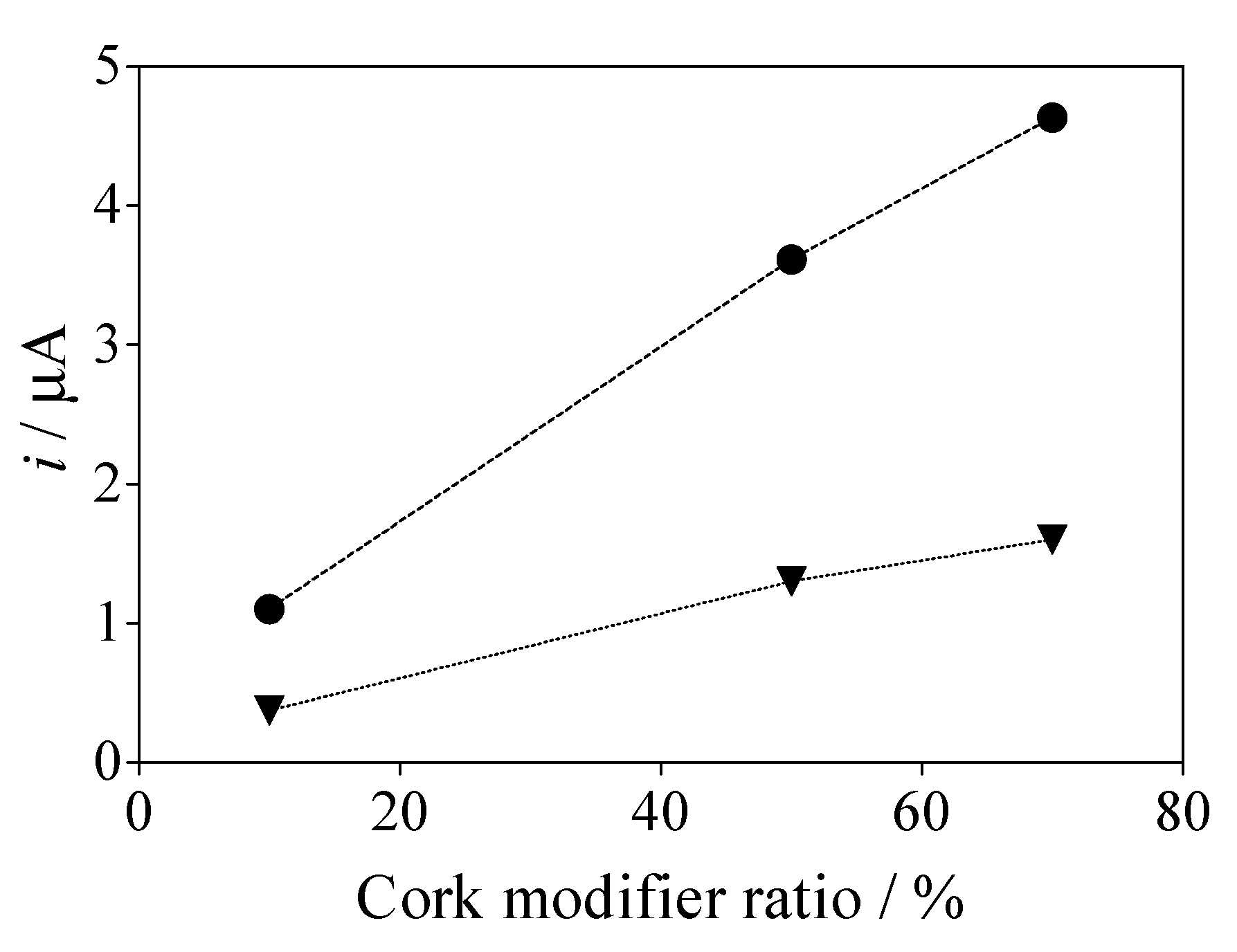
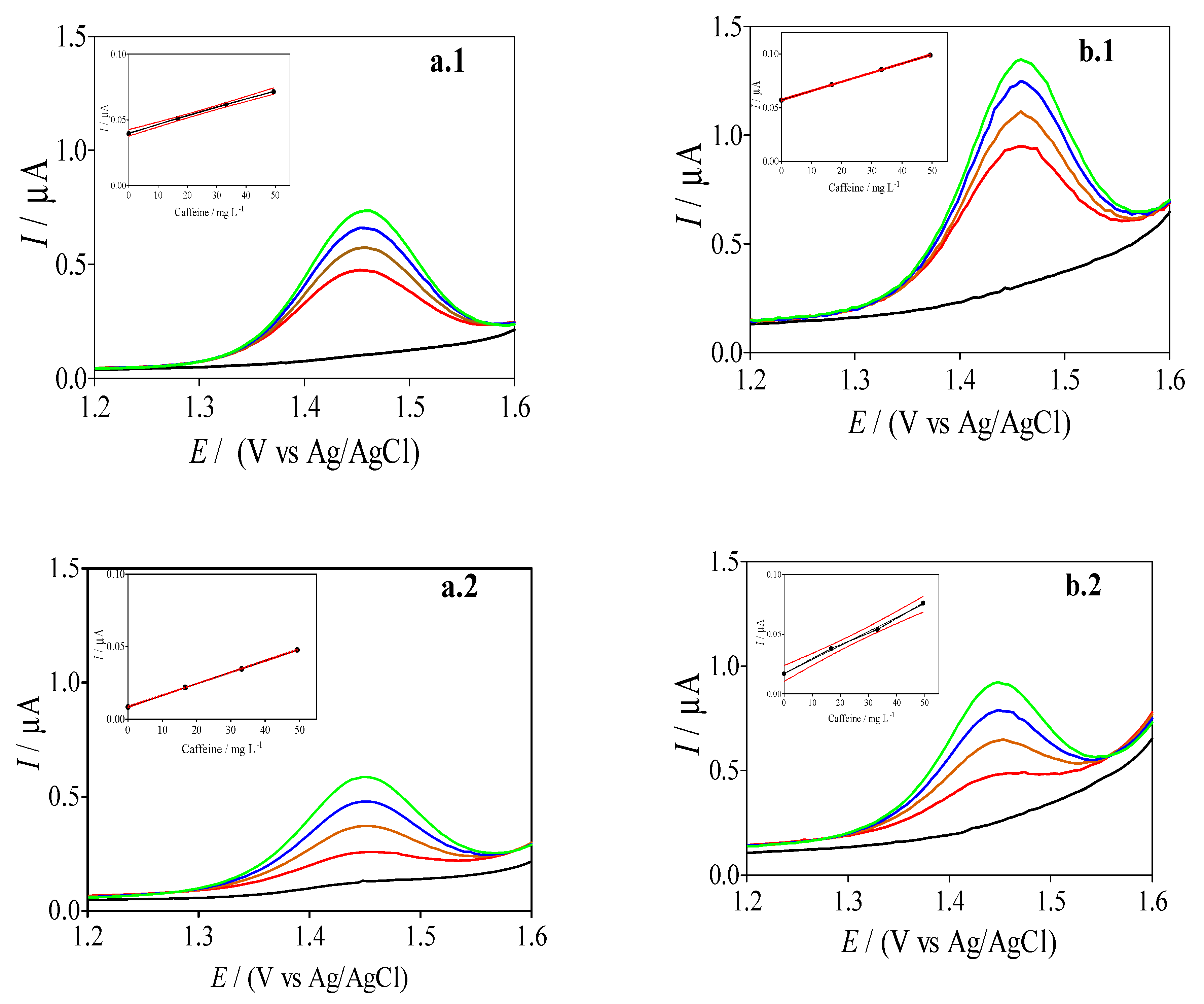
3.4. Effect of the Cork Ratio on the Electrochemical Response of Caffeine by Using Cork-Graphite Sensor
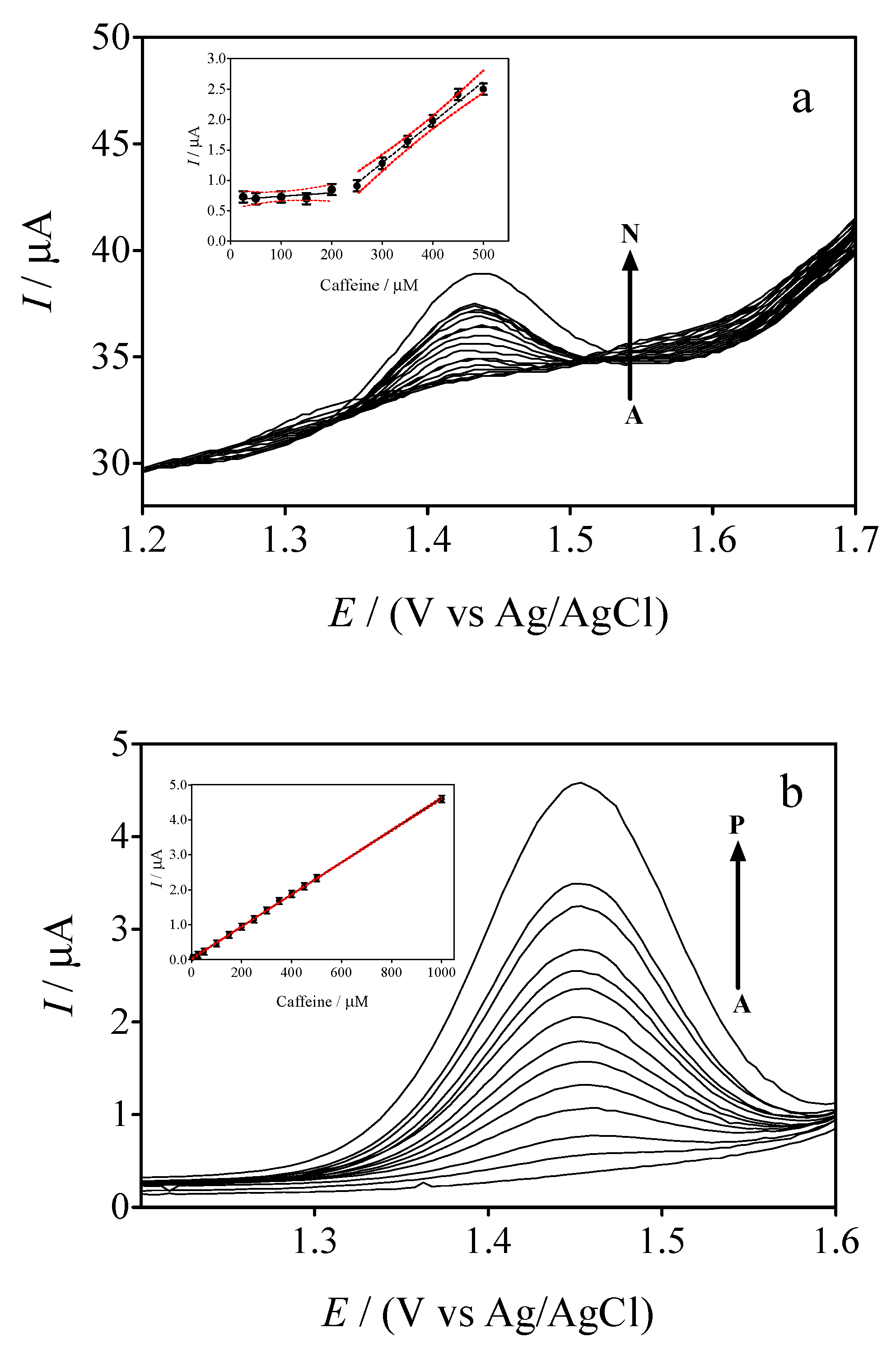
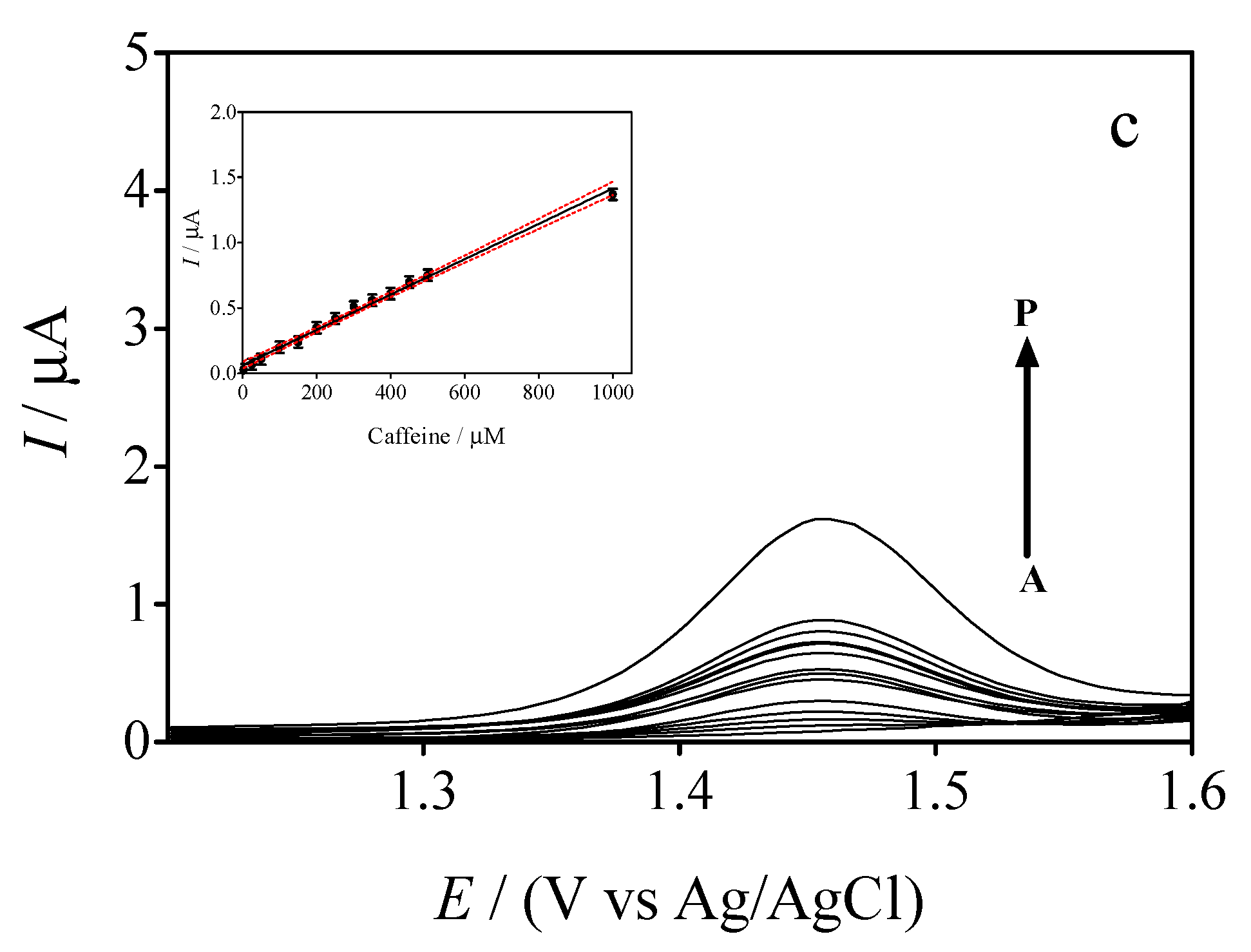
3.5. Differential Pulse Voltammetric (DPV) Experiments
3.6. Comparing the Electrochemical Performance of GrRAC and GrRGC Sensors with the Reports in the Literature
3.7. Stability of the Cork-Graphite Sensors
3.8. Applicability of Cork-Sensors for Determining Caffeine in Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, N.Y.; Bieder, A.; Raman, A.; Mileti, E.; Katayama, S.; Einarsdottir, E.; Fredholm, B.B.; Falk, A.; Tapia-Páez, I.; Daub, C.O.; et al. Acute doses of caffeine shift nervous system cell expression profiles toward promotion of neuronal projection growth. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Sheth, S.; Sheehan, K.; Dhukhwa, A.; Al Aameri, R.F.H.; Mamillapalli, C.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Oral Administration of Caffeine Exacerbates Cisplatin-Induced Hearing Loss. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Kamimori, G.H. Multiple caffeine doses maintain vigilance, attention, complex motor sequence expression, and manual dexterity during 77 hours of total sleep deprivation. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100051. [Google Scholar] [CrossRef]
- Ehlers, A.; Marakis, G.; Lampen, A.; Hirsch-Ernst, K.I. Risk assessment of energy drinks with focus on cardiovascular parameters and energy drink consumption in Europe. Food Chem. Toxicol. 2019, 130, 109–121. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous determination of salbutamol and propranolol in biological fluid samples using an electrochemical sensor based on functionalized-graphene, ionic liquid and silver nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8. [Google Scholar] [CrossRef]
- Arroyo-Gómez, J.J.; Villarroel-Rocha, D.; de Freitas-Araújo, K.C.; Martínez-Huitle, C.A.; Sapag, K. Applicability of activated carbon obtained from peach stone as an electrochemical sensor for detecting caffeine. J. Electroanal. Chem. 2018, 822, 171–176. [Google Scholar] [CrossRef]
- Mahanthappa, M.; Yellappa, S.; Kottam, N.; Srinivasa Rao Vusa, C. Sensitive determination of caffeine by copper sulphide nanoparticles modified carbon paste electrode. Sens. Actuators A Phys. 2016, 248, 104–113. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.M.; Chen, H.A. A voltammetric determination of caffeic acid in red wines based on the nitrogen doped carbon modified glassy carbon electrode. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Suely Fernandes, N.; Ferro, S.; De Battisti, A.; Quiroz, M.A. Fabrication and application of Nafion®-modified boron-doped diamond electrode as sensor for detecting caffeine. Diam. Relat. Mater. 2010, 19, 1188–1193. [Google Scholar] [CrossRef]
- Carolina Torres, A.; Barsan, M.M.; Brett, C.M.A. Simple electrochemical sensor for caffeine based on carbon and Nafion-modified carbon electrodes. Food Chem. 2014, 149, 215–220. [Google Scholar] [CrossRef]
- Goyal, R.N.; Bishnoi, S.; Agrawal, B. Electrochemical sensor for the simultaneous determination of caffeine and aspirin in human urine samples. J. Electroanal. Chem. 2011, 655, 97–102. [Google Scholar] [CrossRef]
- Brunetti, B.; Desimoni, E. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J. Food Compos. Anal. 2014, 33, 155–160. [Google Scholar] [CrossRef]
- Khoo, W.Y.H.; Pumera, M.; Bonanni, A. Graphene platforms for the detection of caffeine in real samples. Anal. Chim. Acta 2013, 804, 92–97. [Google Scholar] [CrossRef]
- Aklilu, M.; Tessema, M.; Redi-Abshiro, M. Indirect voltammetric determination of caffeine content in coffee using 1,4-benzoquinone modified carbon paste electrode. Talanta 2008, 76, 742–746. [Google Scholar] [CrossRef]
- Araújo, E.G.; Oliveira, G.R.; Santos, E.V.; Martínez-Huitle, C.A.; Panizza, M.; Fernandes, N.S. Applicability of electroanalysis for monitoring oxalic acid (OA)concentration during its electrochemical oxidation. J. Electroanal. Chem. 2013, 701, 32–35. [Google Scholar] [CrossRef]
- Yiǧit, A.; Yardim, Y.; Şentürk, Z. Voltammetric Sensor Based on Boron-Doped Diamond Electrode for Simultaneous Determination of Paracetamol, Caffeine, and Aspirin in Pharmaceutical Formulations. IEEE Sens. J. 2016, 16, 1674–1680. [Google Scholar] [CrossRef]
- Lourenção, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Mazo, L.H.; Fatibello-Filho, O. Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 2009, 78, 748–752. [Google Scholar] [CrossRef]
- Ali, H.S.; Abdullah, A.A.; Pınar, P.T.; Yardım, Y.; Şentürk, Z. Simultaneous voltammetric determination of vanillin and caffeine in food products using an anodically pretreated boron-doped diamond electrode: Its comparison with HPLC-DAD. Talanta 2017, 170, 384–391. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Paiva, S.S.M.; da Silva, D.R.; Vilar, V.J.P.; Martínez-Huitle, C.A.; dos Santos, E.V. Novel cork-graphite electrochemical sensor for voltammetric determination of caffeine. J. Electroanal. Chem. 2019, 839, 283–289. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Baldwin, R.P.; Buchanan, R.M. Chemically Modified Carbon Paste Electrodes. Electrochem. Soc. Ext. Abstr. 1984, 84-2, 933. [Google Scholar]
- González, P.; Cortínez, V.A.; Fontán, C.A. Determination of nickel by anodic adsorptive stripping voltammetry with a cation exchanger-modified carbon paste electrode. Talanta 2002, 58, 679–690. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Santos, E.C.M.M.; Silva, D.R.; Martínez-Huitle, C.A.; dos Santos, E.V. Simultaneous determination of paracetamol and caffeine in pharmaceutical formulations and synthetic urine using cork-modified graphite electrodes. J. Solid State Electrochem. 2020, 18–20. [Google Scholar] [CrossRef]
- Henrique, J.M.M.; Monteiro, M.K.S.; Cardozo, J.C.; Martínez-Huitle, C.A.; da Silva, D.R.; dos Santos, E.V. Integrated-electrochemical approaches powered by photovoltaic energy for detecting and treating paracetamol in water. J. Electroanal. Chem. 2020, 876, 114734. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Silvestre-Albero, A.M.; Ferreira, C.I.A.; Pereira, J.P.C.; Vilar, V.J.P.; Botelho, C.M.S.; Rodríguez-Reinoso, F.; Boaventura, R.A.R. Textural and surface characterization of cork-based sorbents for the removal of oil from water. Ind. Eng. Chem. Res. 2013, 52, 16427–16435. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandas, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Calas-Blanchard, C.; Comtat, M.; Marty, J.L.; Mauran, S. Textural characterisation of graphite matrices using electrochemical methods. Carbon N. Y. 2003, 41, 123–130. [Google Scholar] [CrossRef]
- Łukaszewski, M. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes—An Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Araujo, D.; Brito, C.; de Oliveira, S.D.; Silva, D.; Martinez-Huitle, C.; Aragao, C. Platinum Sensor for Quantifying Caffeine in Drug Formulations. Curr. Pharm. Anal. 2014, 10, 231–238. [Google Scholar] [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Anal. Chim. Acta 1999, 391, 105–126. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Martins, A.G.; Souza, R.S.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Treatment of vegetable oil refinery wastewater by sorption of oil and grease onto regranulated cork—A study in batch and continuous mode. Chem. Eng. J. 2015, 268, 92–101. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Kumara Swamy, B.E. Simultaneous electroanalysis of hydroquinone and catechol at poly(brilliant blue) modified carbon paste electrode: A voltammetric study. J. Electroanal. Chem. 2015, 756, 193–200. [Google Scholar] [CrossRef]
- AlAqad, K.M.; Suleiman, R.; Al Hamouz, O.C.S.; Saleh, T.A. Novel graphene modified carbon-paste electrode for promazine detection by square wave voltammetry. J. Mol. Liq. 2018, 252, 75–82. [Google Scholar] [CrossRef]
- Spãtaru, N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Anodic voltammetry of xanthine, theophylline, theobromine and caffeine at conductive diamond electrodes and its analytical application. Electroanalysis 2002, 14, 721–728. [Google Scholar] [CrossRef]
- Jakubowska, M. Signal Processing in Electrochemistry. Electroanalysis 2011, 23, 553–572. [Google Scholar] [CrossRef]
- Currie, L.A. International Union of Pure and Applied Chemistry Nomenclature in Evaluation of Analytical Methods Including Detection and Quantification Capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Danzer, K.; Currie, L.A. Guideline for calibration in analytical chemistry—Part 1. Fundamentals and single component calibration. Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. About estimating the limit of detection of heteroscedastic analytical systems. Anal. Chim. Acta 2009, 655, 30–37. [Google Scholar] [CrossRef]
- Brunetti, B.; Desimoni, E. Determination of theophylline at a cysteic acid modified glassy carbon electrode. Electroanalysis 2009, 21, 772–778. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Silva, T.A.; Fatibello-Filho, O. Simultaneous determination of isoproterenol, acetaminophen, folic acid, propranolol and caffeine using a sensor platform based on carbon black, graphene oxide, copper nanoparticles and PEDOT:PSS. Talanta 2018, 183, 329–338. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Bęczkowska, I. Nafion covered lead film electrode for the voltammetric determination of caffeine in beverage samples and pharmaceutical formulations. Food Chem. 2015, 172, 24–29. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Li, L.; Hu, P. Nitrogen-doped carbon nanotubes decorated poly (L-Cysteine) as a novel, ultrasensitive electrochemical sensor for simultaneous determination of theophylline and caffeine. Talanta 2018, 178, 449–457. [Google Scholar] [CrossRef]

| Electrode | CDL | Ageometric | Areal | RF (=Areal/Ageometric) |
|---|---|---|---|---|
| μF | mm2 | mm2 | ||
| Gr | 0.50 | 0.45 | 0.83 | 1.85 |
| GrRAC | 0.07 | 0.45 | 0.12 | 0.26 |
| GrRGC | 0.02 | 0.45 | 0.03 | 0.07 |
| Electrodes | Method | Electrolyte | Sensitivity/µA cm−2 mM−1 | LOD/µM | References |
|---|---|---|---|---|---|
| 1 CA-ZnFe modified glass carbon | DPV | 1 M H2SO4 | _ | 10 | [6] |
| 2 CuNPs-GO-CB-PEDOT:PSS/GCE | SWV | phosphate buffer solution (pH 7.0) | 0.028 | 3.4 | [42] |
| 3 Nafion covered lead film electrode | DPV | 0.1 M H2SO4 | _ | 7.98 | [43] |
| 4 EPPGE | SWV | phosphate buffer | 0.17 | 0.008 | [11] |
| 5 CuS NPs MCPE | DPV | acetate buffer (pH 7.0) | 545.12 | 0.018 | [7] |
| 6 MCPE | SWV | phosphate buffer, pH 6 | _ | 0.3 | [14] |
| 7 Nitrogen doped carbon/GCE | DPV | 0.01 M H2SO4-Na2SO4 (pH 1.70) | _ | 0.02 | [44] |
| GrRAC | DPV | 0.1 M H2SO4 | 637 | 2.94 | This work |
| GrRGC | DPV | 0.1 M H2SO4 | 158 | 6.05 | This work |
| Drugs | Labelled/mg | HPLC/mg | GrRAC/mg | GrRGC/mg | GrRAC/E1 a (%) | GrRGC/E2 a (%) |
|---|---|---|---|---|---|---|
| A | 65 | 61.4 | 61.9 | 55.5 | 0.96 | −3.06 |
| B | 30 | 27.6 | 29.7 | 28.6 | 7.64 | 3.55 |
| Beverages | Labelled/µM | HPLC/µM | GrRAC/µM | GrRGC/µM | E1a (%) | E2a (%) |
| Coca-Cola | 515 | 601 | 655 | 573 | −27.1 | −11.3 |
| Pepsi | 515 | 546 | 558 | 526 | −8.3 | −2.1 |
| Energetic | 1648 | 1542 | 1677 | 1771 | −1.7 | −7.5 |
Publisher‘s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.K.S.; Da Silva, D.R.; Quiroz, M.A.; Vilar, V.J.P.; Martínez-Huitle, C.A.; Dos Santos, E.V. Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination. Materials 2021, 14, 37. https://doi.org/10.3390/ma14010037
Monteiro MKS, Da Silva DR, Quiroz MA, Vilar VJP, Martínez-Huitle CA, Dos Santos EV. Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination. Materials. 2021; 14(1):37. https://doi.org/10.3390/ma14010037
Chicago/Turabian StyleMonteiro, Mayra K. S., Djalma R. Da Silva, Marco A. Quiroz, Vítor J. P. Vilar, Carlos A. Martínez-Huitle, and Elisama V. Dos Santos. 2021. "Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination" Materials 14, no. 1: 37. https://doi.org/10.3390/ma14010037
APA StyleMonteiro, M. K. S., Da Silva, D. R., Quiroz, M. A., Vilar, V. J. P., Martínez-Huitle, C. A., & Dos Santos, E. V. (2021). Applicability of Cork as Novel Modifiers to Develop Electrochemical Sensor for Caffeine Determination. Materials, 14(1), 37. https://doi.org/10.3390/ma14010037









