Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
- C45—C45 steel sample;
- PS—The C45 steel phosphate in zinc/iron-based solution sample;
- PPS—The C45 steel phosphate in zinc/iron-based solution sample and painted;
- BSW—Black Sea water, pH = 6.15.
2.3. Methods
3. Results and Discussion
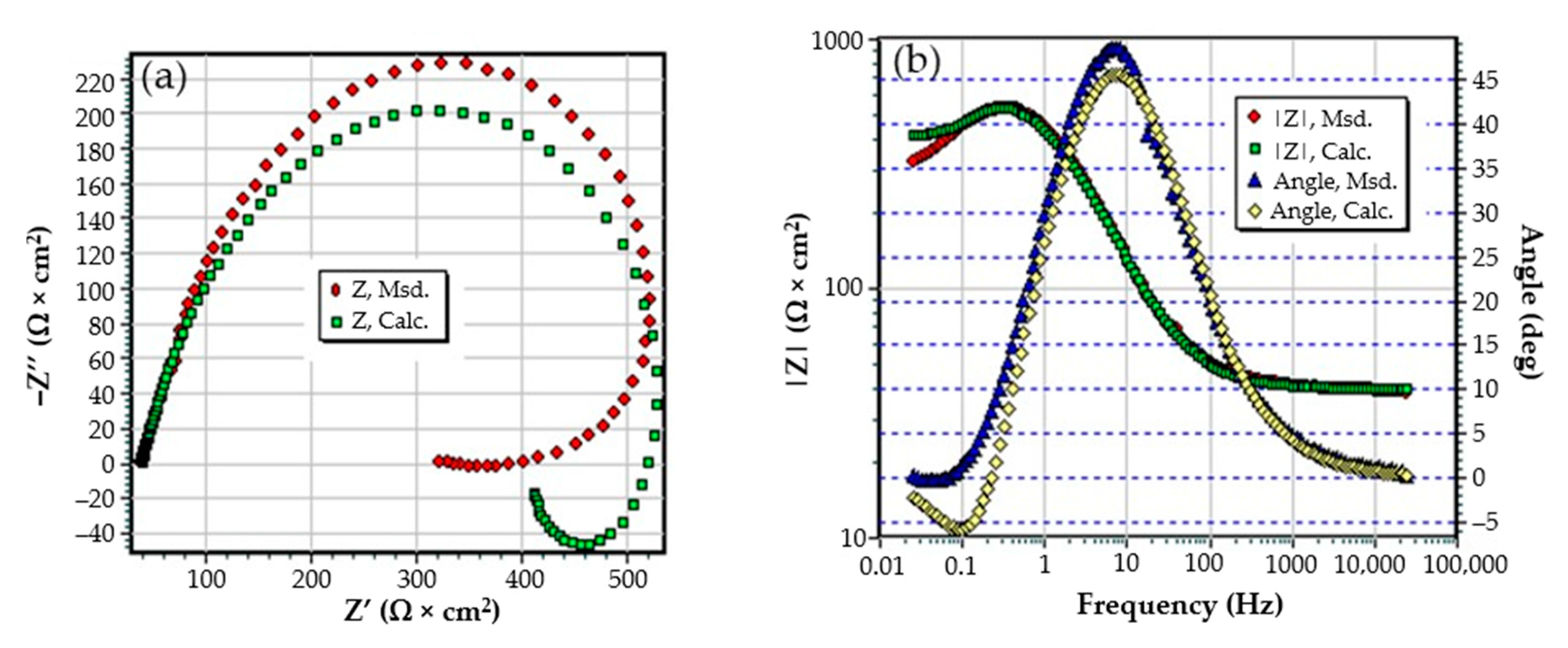
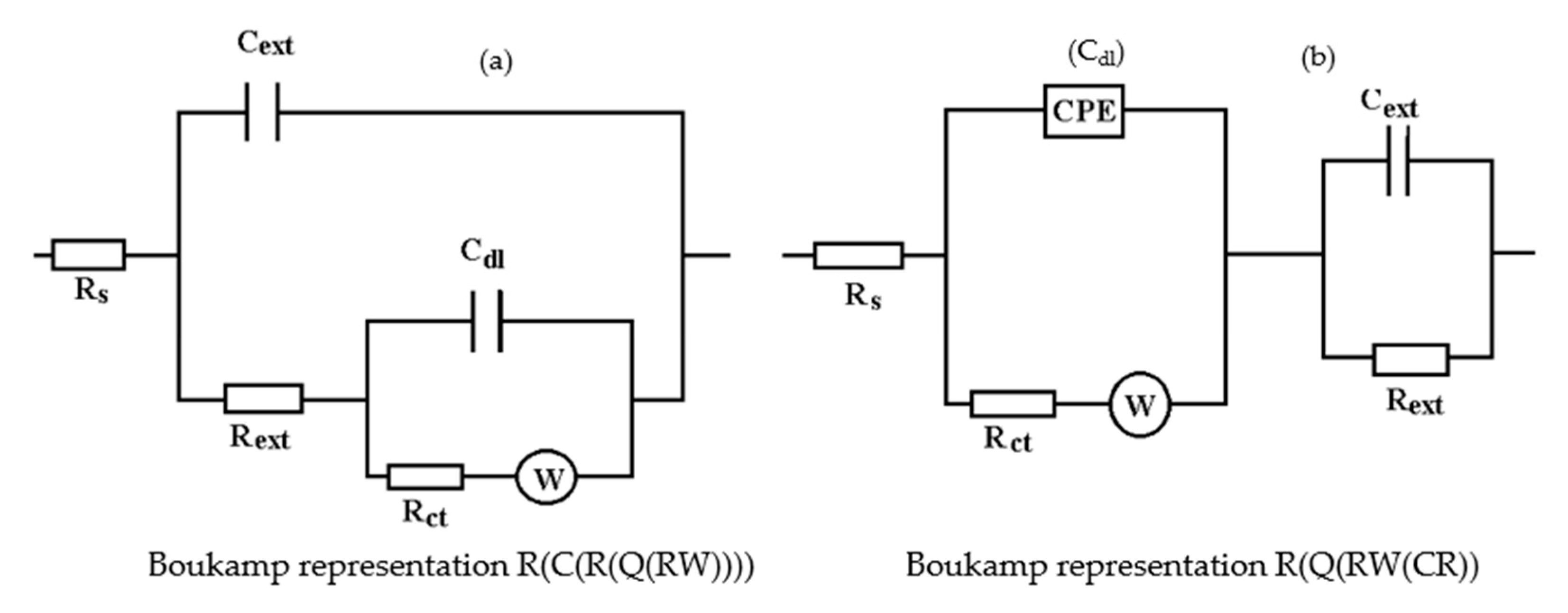
3.1. The Effects of Prolonged Immersion of C45 in Black Seawater
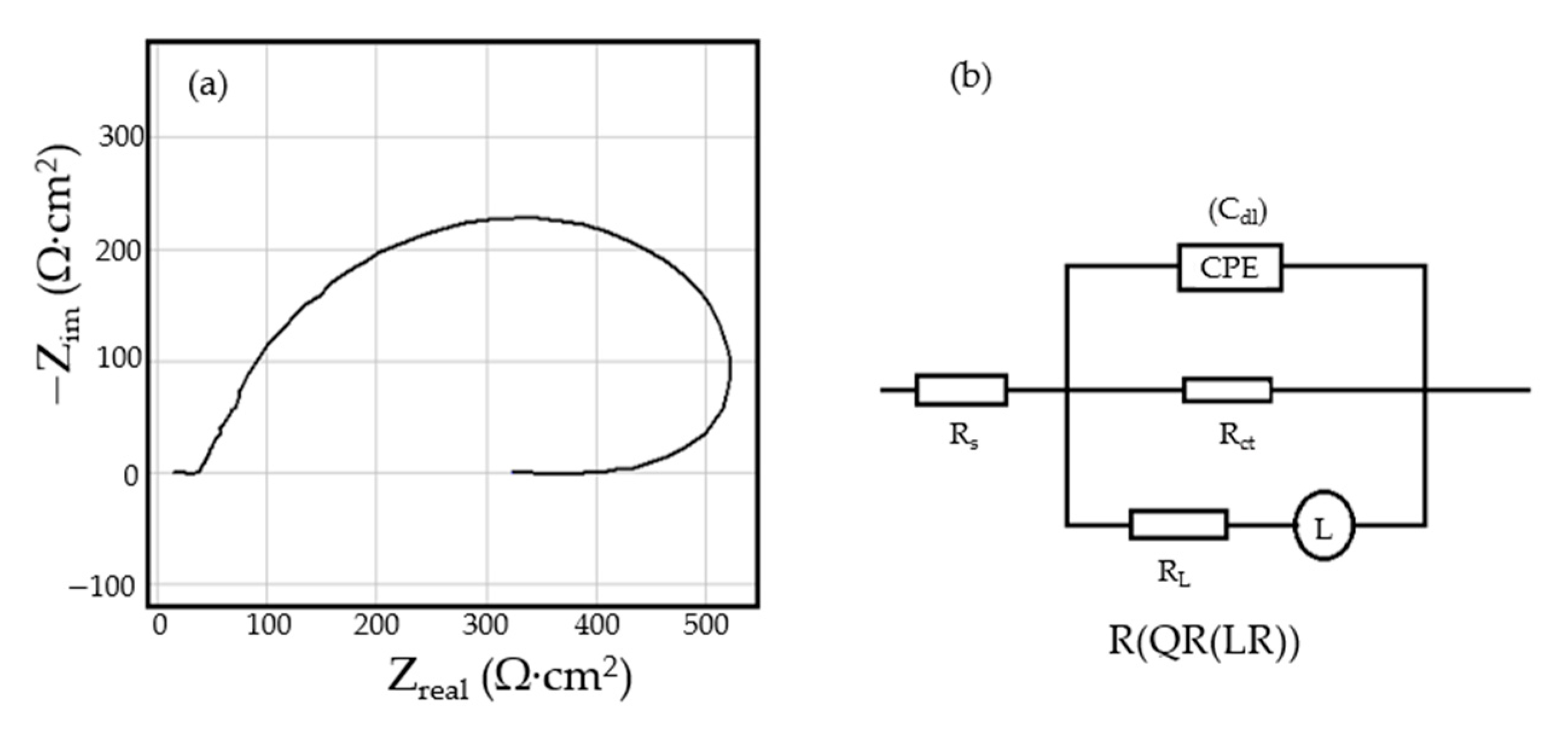
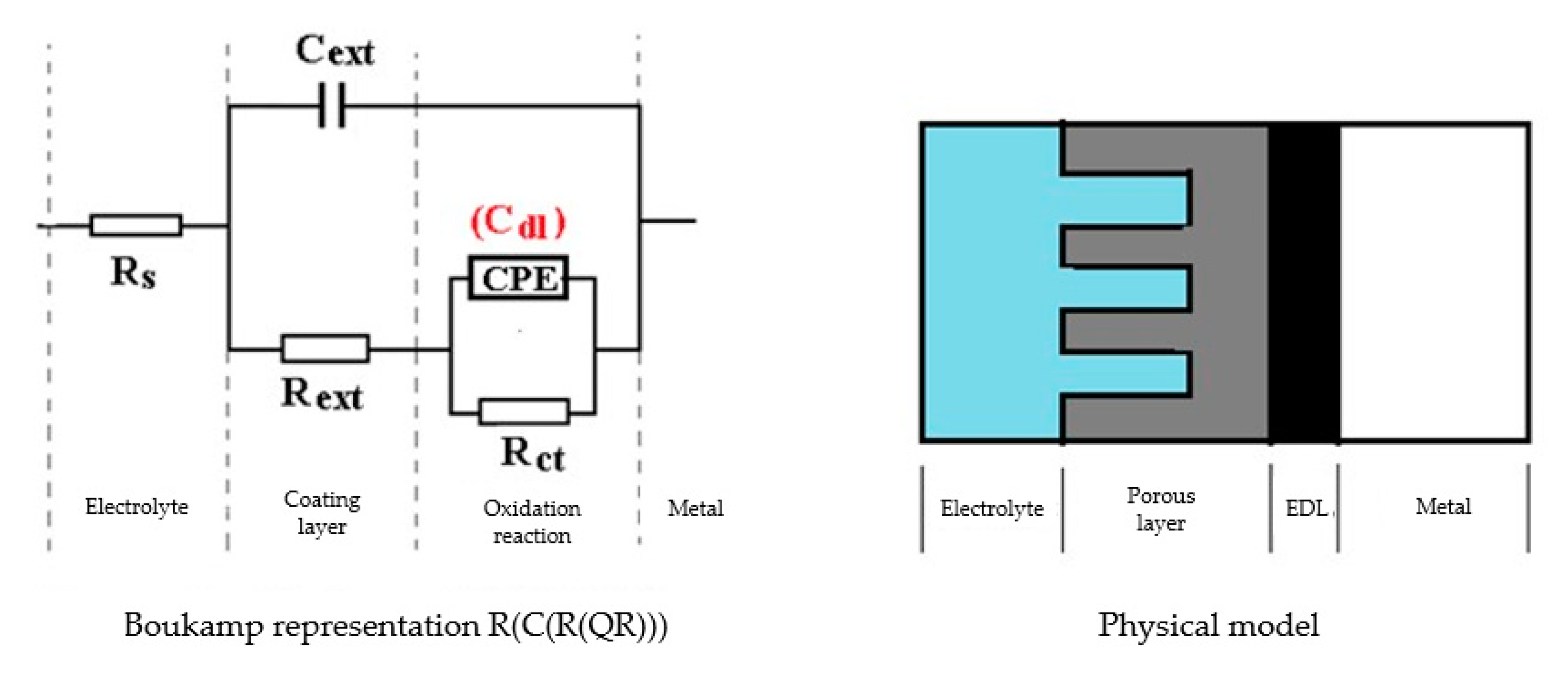
- The resistance of the solution (the electrolyte) increases slightly with the immersion time of the samples in seawater. This is because, in addition to the actual resistance of the liquid, there is also the physical resistance of the layer of corrosion products deposited on the surface. The strength of the product layer is very low because it is porous, non-adherent, and soaked in liquid.
- The resistance of the outer porous layer increases over time, due to the increase of its thickness, and accordingly decreases its electrical capacity.
- The constant Q, from the expression of the constant phase element that replaces the capacity of the double-electric layer, increases greatly with the immersion time (3.418·× 10−4—after 1 h, 6.92 ×·10−3—after 43 days and 1.25 ×·10−2—after 85 days). As a result, the impedance of this element increases greatly (according to the calculation relationship of ZCPE (CPE impedance)). The Cdl deviation from the idealist (expressed by the value of the exponent n) is large and is due to the increase in roughness due to corrosion.
- The layer of products, deposited on the surface, acts as a screen and causes the charge transfer resistance to decrease over time.
- The constant W, from the expression of the diffusion impedance, has a relatively low value and is manifested only in the case of the sample maintained for 85 days in seawater. As a result, the diffusion impedance is high and opposes the charge transfer along with Rct, thus reducing the corrosion rate over time.
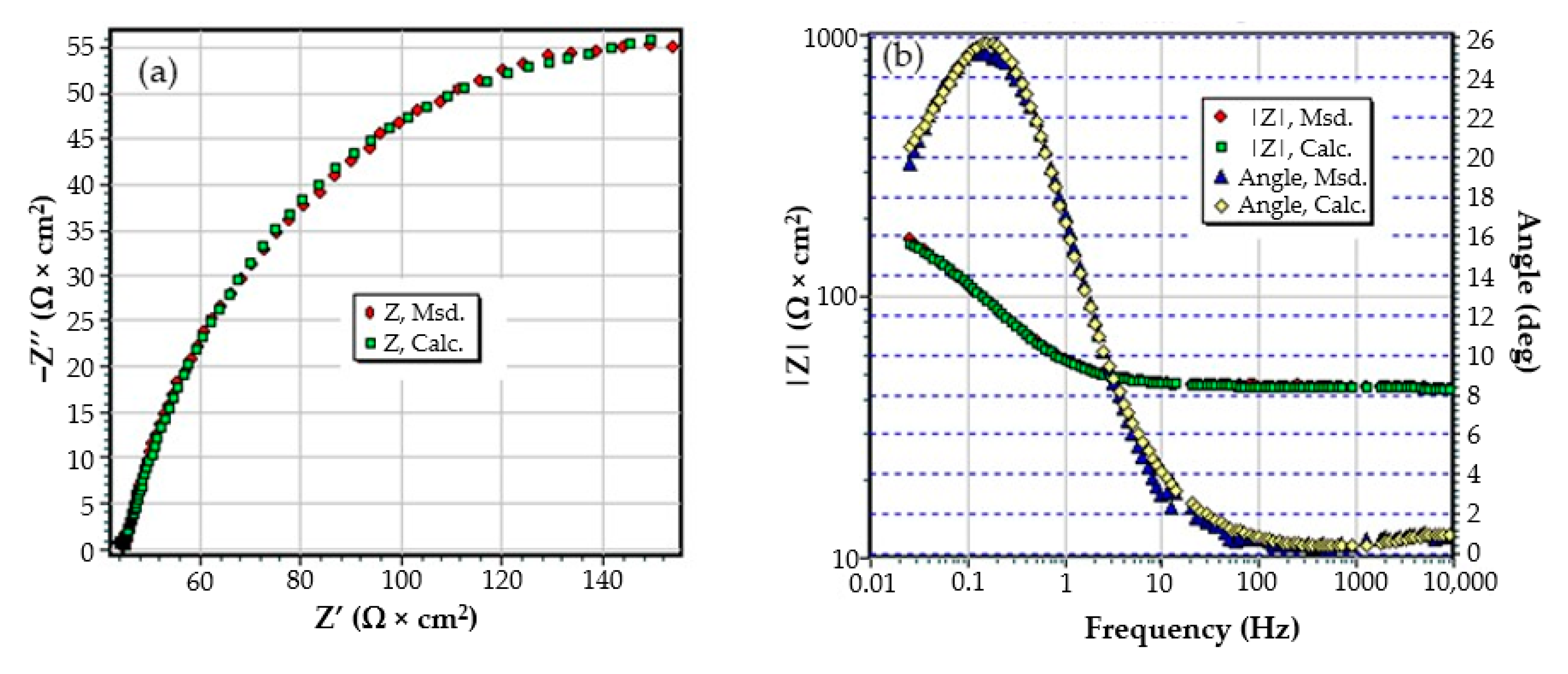
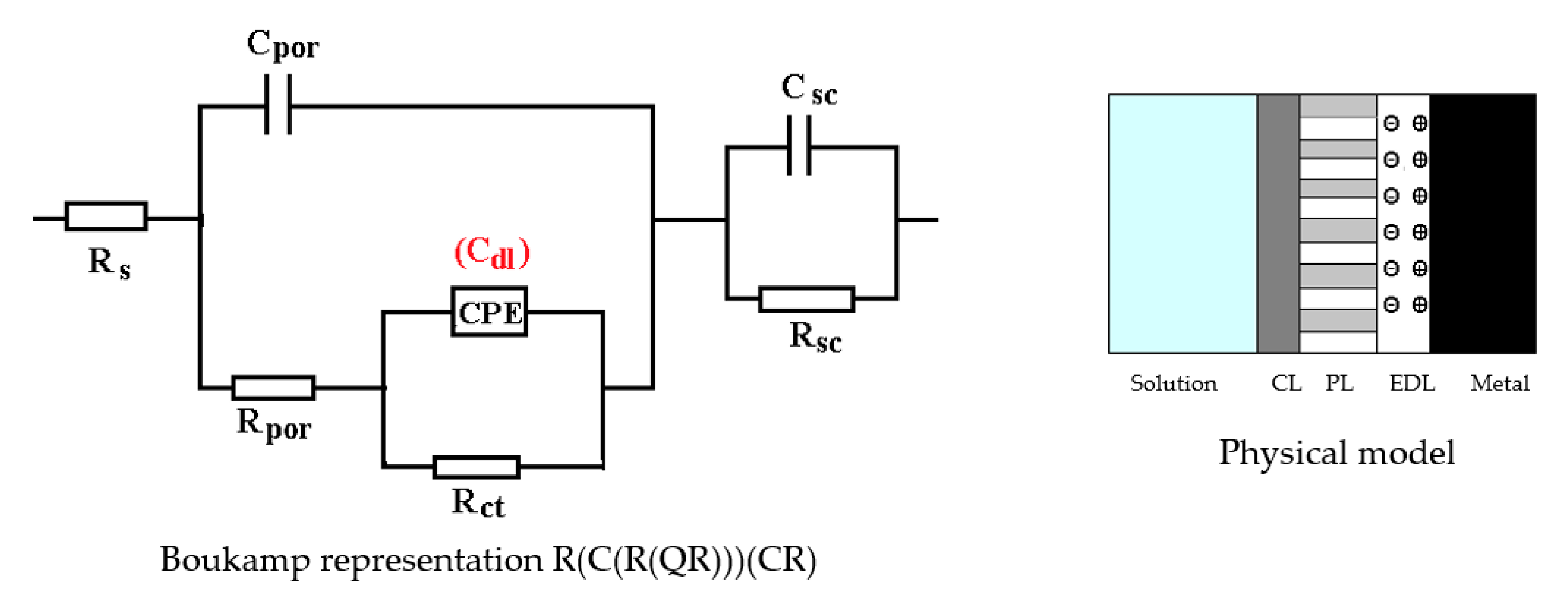
3.2. Effects of Immersing C45 Phosphate (PS) in Black Sea Water
- Although a layer (phosphate layer) is already deposited on the surface of the alloy, and after various periods another layer (corrosion products) is deposited on it, only a circuit with two constants was needed to correlate with the experimental data, considering a bi-layer structure of the surface (a passive layer-electric double layer and an outer layer). The outer layer consists of the phosphate layer and the corrosion products layer, very well intertwined, by occupying the pores of the phosphate layer with corrosion products;
- In this case, the resistance of the electrolyte (Rs) does not change with immersion time, the values obtained oscillating around the average value (40.58 Ω∙cm2), within the limit of experimental errors. This is because the outer layer is very porous (slightly permeable to solution) and has low resistance;
- After the first immersion period (43 days), the strength of the outer layer decreases instead of increasing. This could be due to the fact that the seawater partially damages the phosphate layer, widening its pores, and the corrosion products layer is not yet quite consistent. However, after 85 days of immersion, the resistance of the outer layer appreciably increases, due to the clogging of the pores of the phosphating layer and the thickening of the product layer;
- As in the case of the base alloy (C45) immersed in seawater, the resistance of the double-electric layer (charge transfer resistance) decreases with the immersion time in the corrosion environment, due to the screen effect of the outer layer;
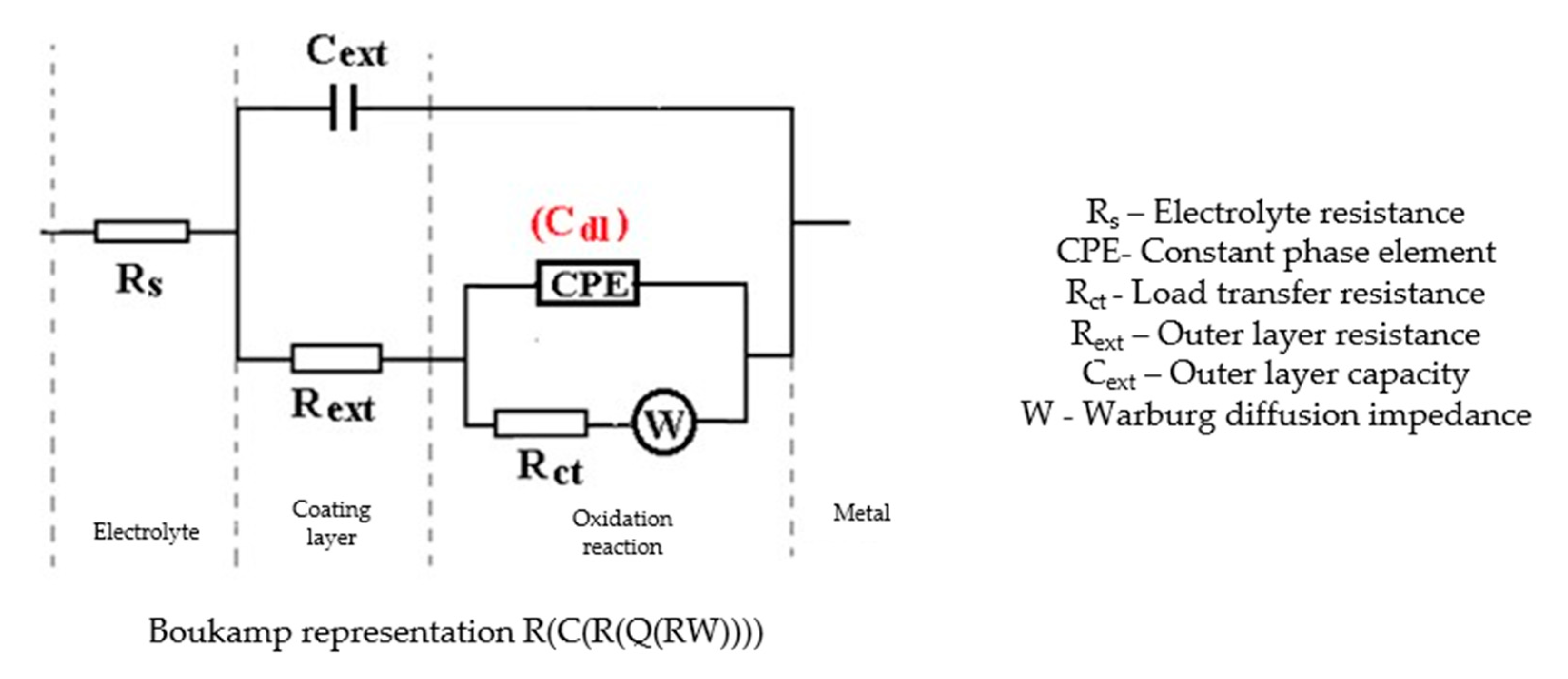
- In the initial moments and in the period of up to 43 days of immersion, the corrosion process is controlled only kinetically, while at long immersion periods, the corrosion is controlled both kinetically and by diffusion;
- The capacity of the double-electric layer (Cdl) is replaced here by a constant phase element, which takes into account the variation of the electrical capacity of the double layer with frequency. The values of the frequency exponent fact that in these cases the electric double layer behaves like a non-ideal capacitor.
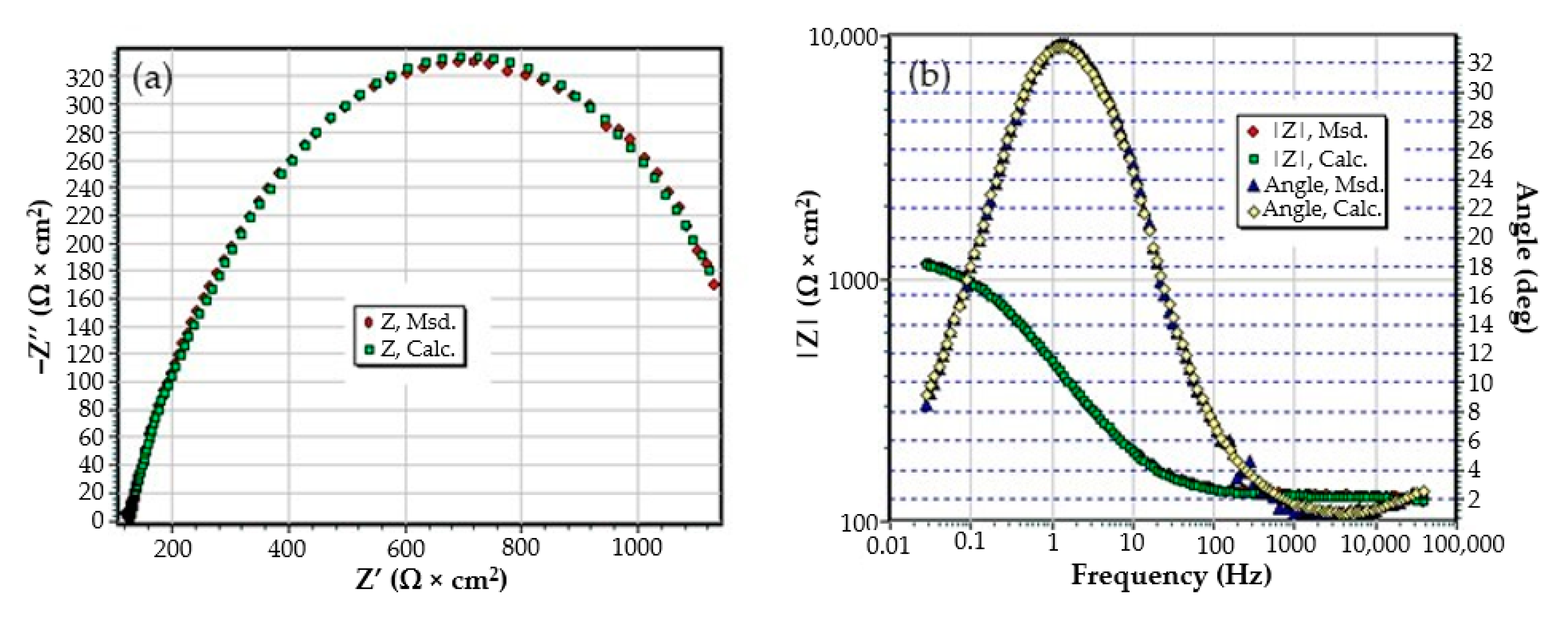
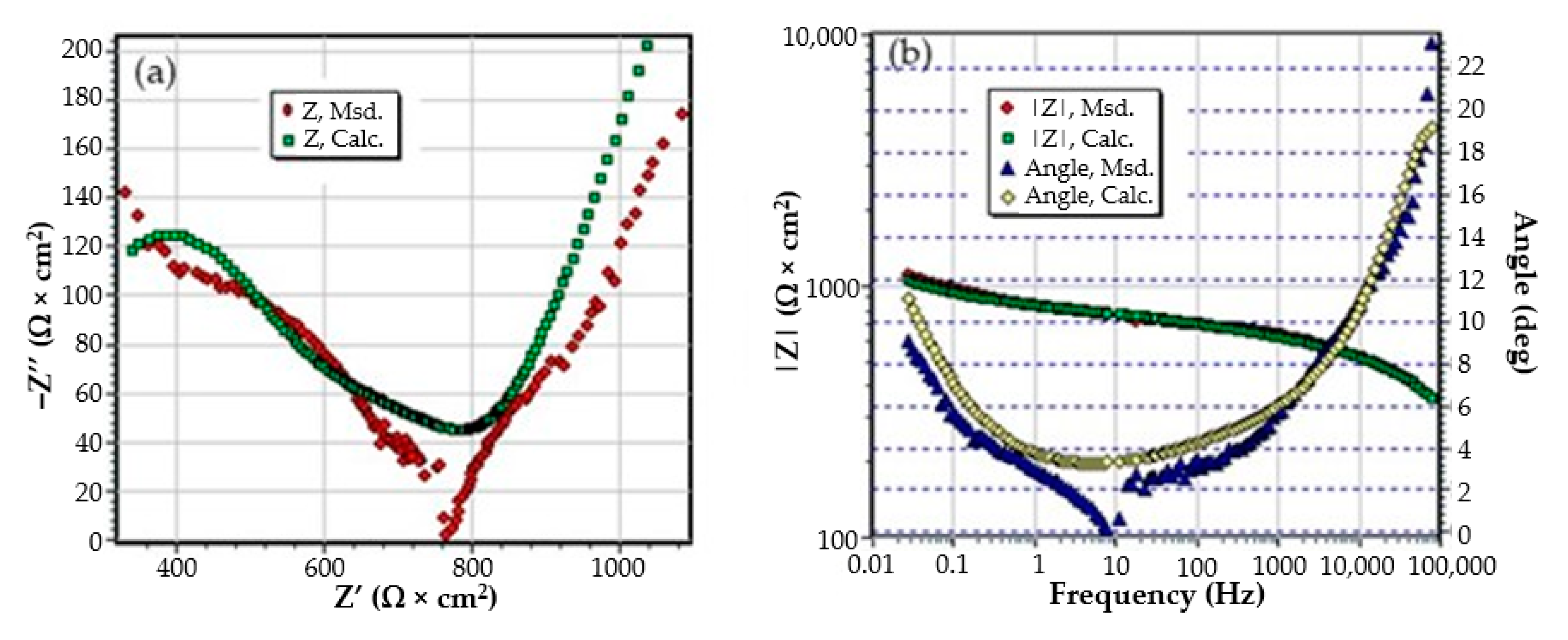
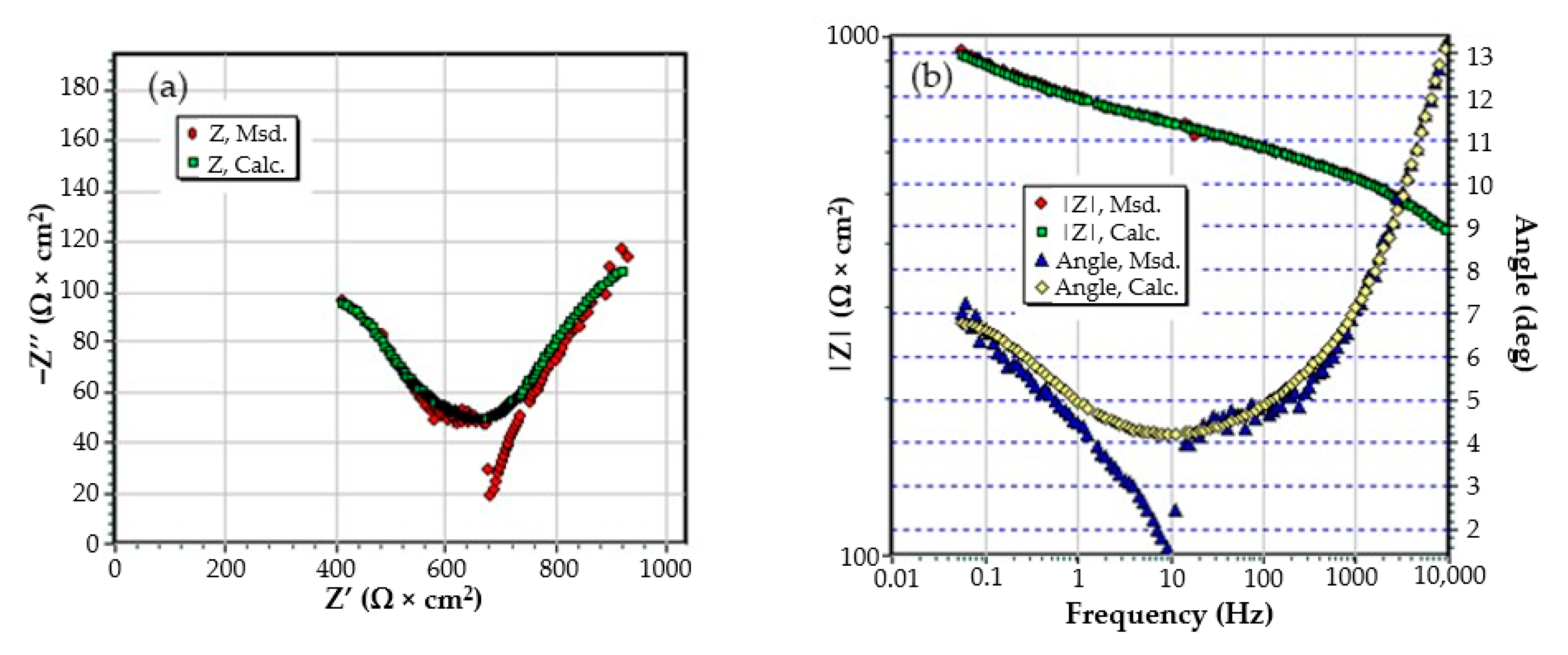
3.3. Effects of Immersing the Phosphate and Painted C45 (PPS) Sample in Seawater
- The chosen equivalent circuit describes very well the experimental data as indicated by the relative error values: χ2 = 7.2 × 10−5, impedance measurement error percentage εz = 0.846% and the relative percentage errors for each circuit element εec < 5%;
- Although the geometry of the measuring cell (distance between electrodes, sample surface) was the same as in the previous measurements, the resistance of the electrolyte (Rs) is approximately three times higher (38.67 for C45 and 0.41 for SP). This is because of the actual resistance of the liquid column in addition to the resistance of the phosphate layer is also added the resistance of the paint film, which is very slightly electrically conductive;
- The resistance of the porous layer (phosphating layer) is low and is practically equal to that found for the PS/BSW system, where only the phosphate layer is present (Rext ≡ Rpor = 11.04 Ω·cm2);
- Due to the shields produced by the phosphate layer and the paint layer, the charge transfer resistance (Rct) is very high, so the corrosion resistance is good.
4. Conclusions
- The immersion time increase results in a significant decrease of the Rct value, from 588 cm2 to 155 Ω∙cm2, respectively, 130 Ω∙cm2; this aspect indicates a decrease of the corrosion rate with the immersion periods.
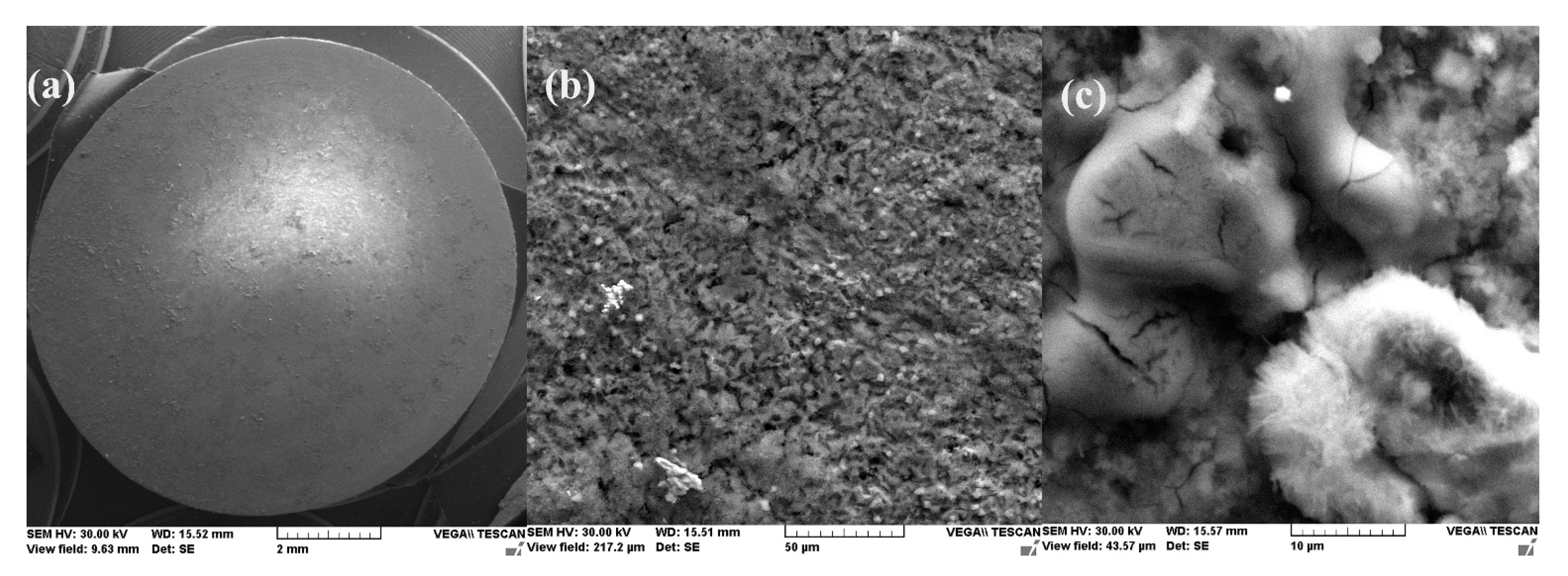
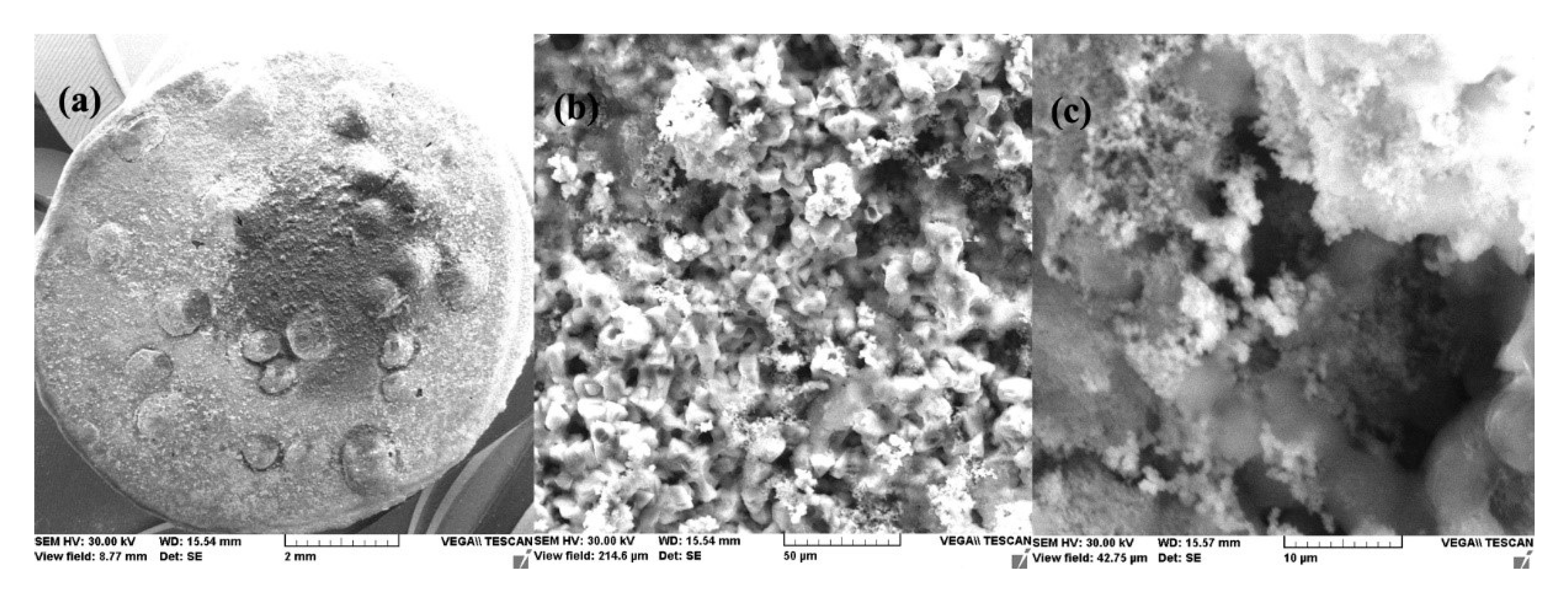
- After 1 h of immersion, on the surface of the C45 sample were absorbed ions from saltwater or insoluble corrosion products creating a discontinuous layer. Over time, the thickness of the products layer increase acting as a screen causing the charge transfer resistance decrease.
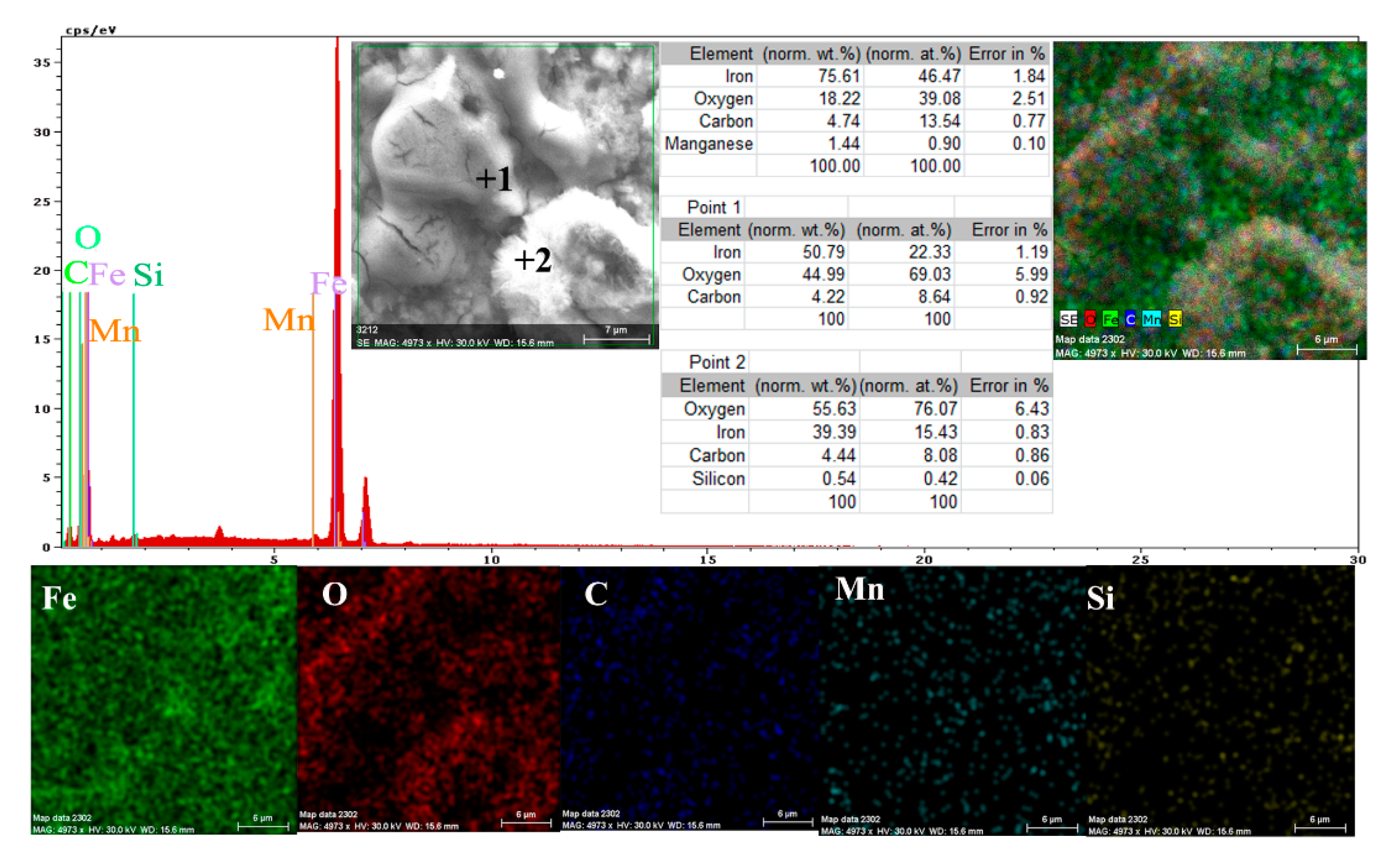
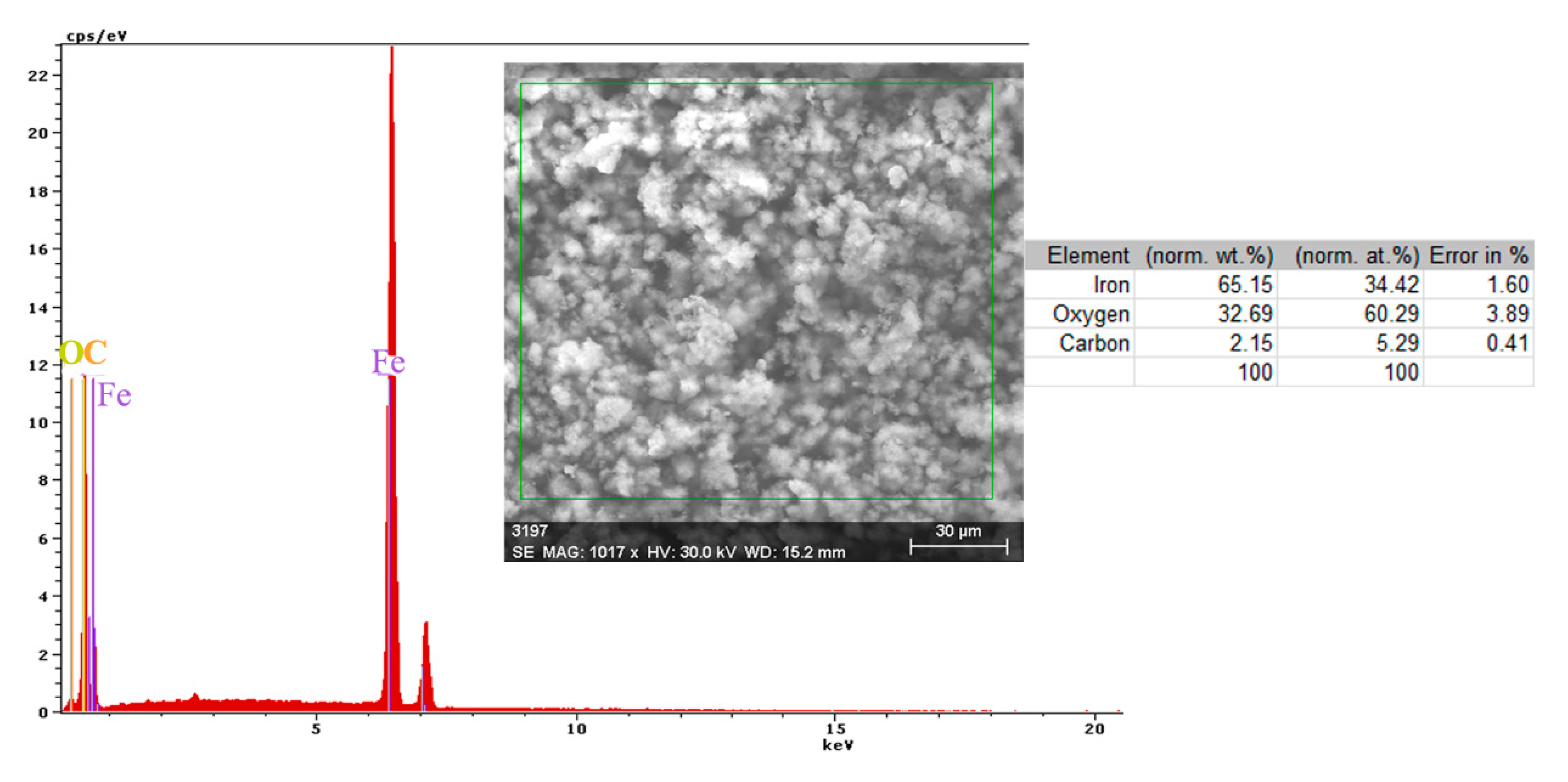
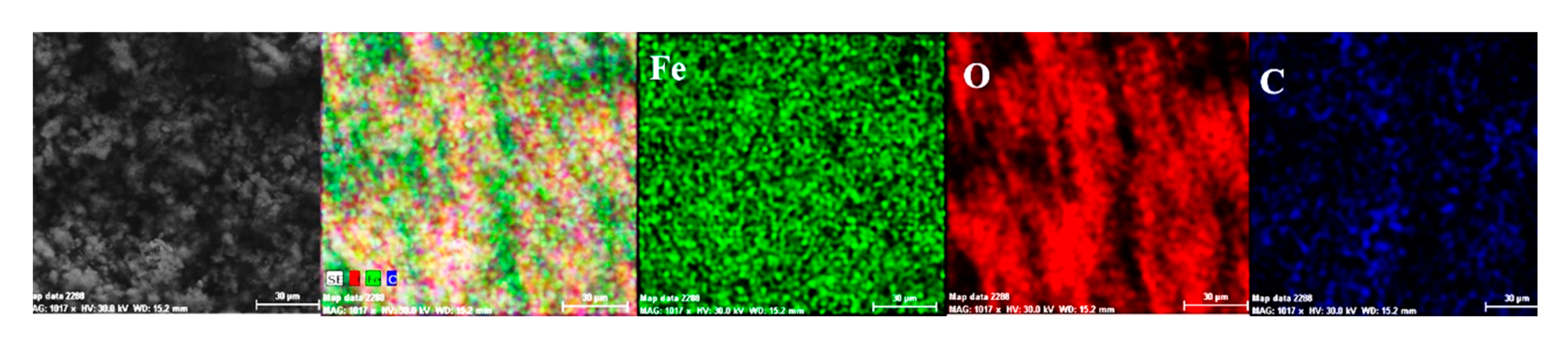
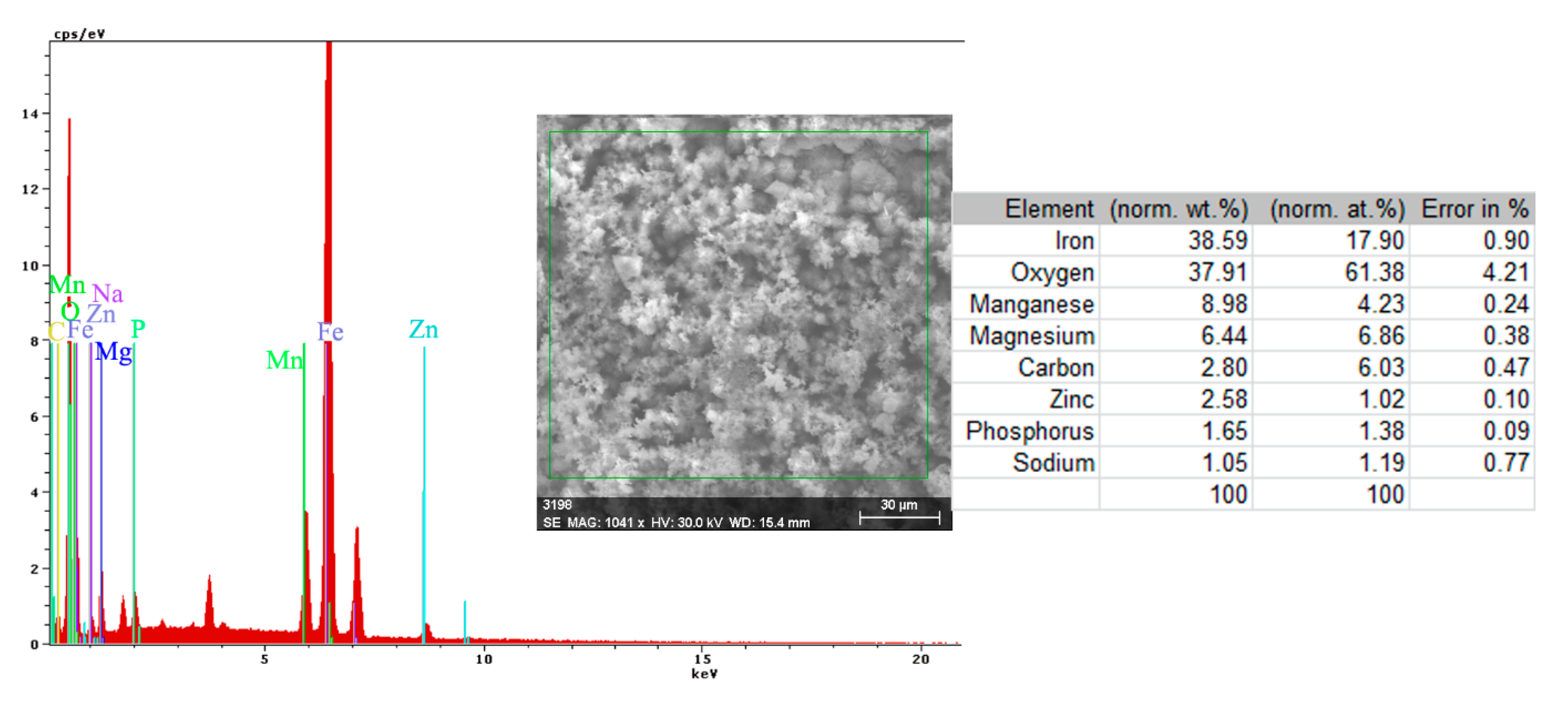
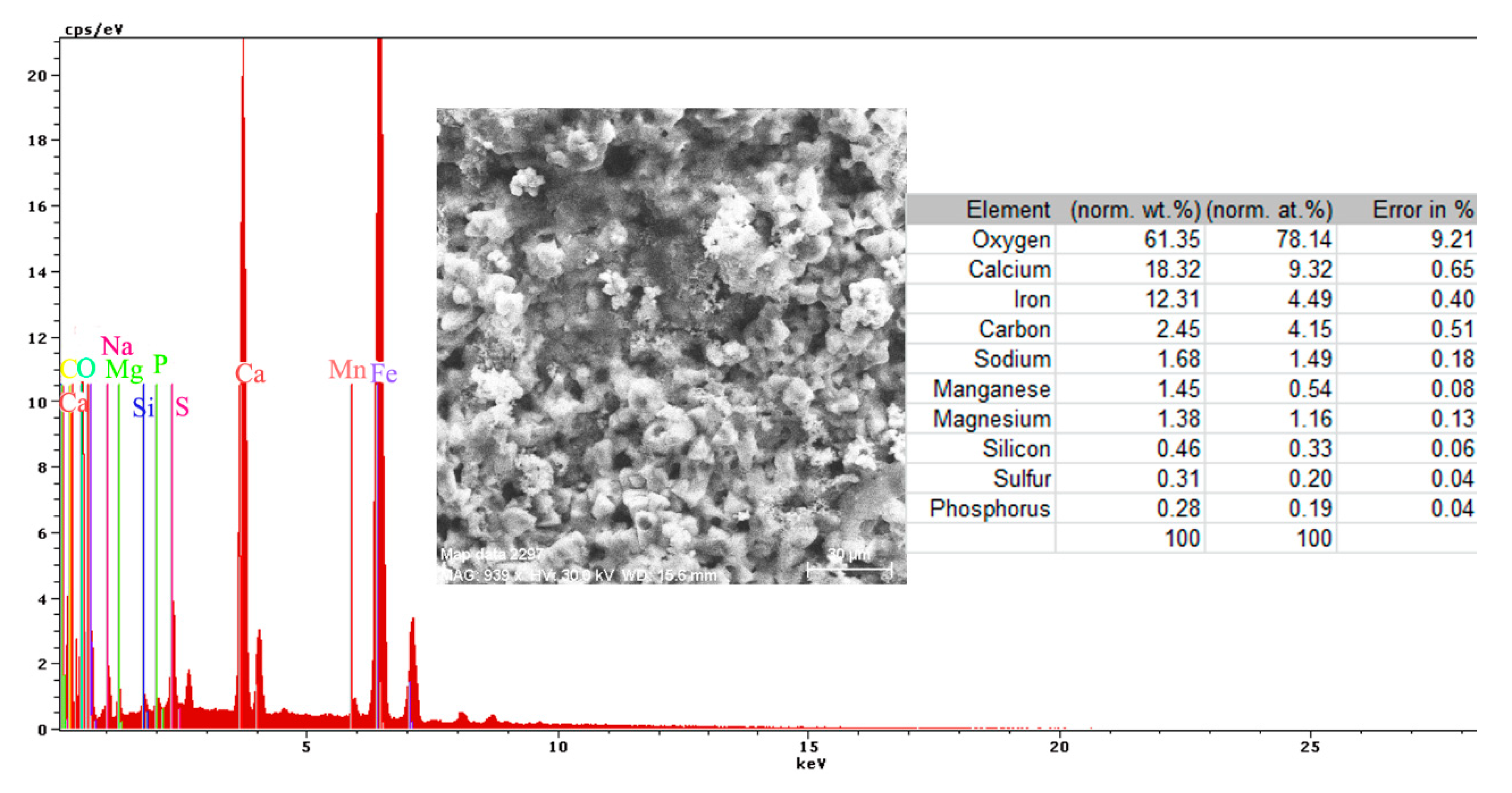
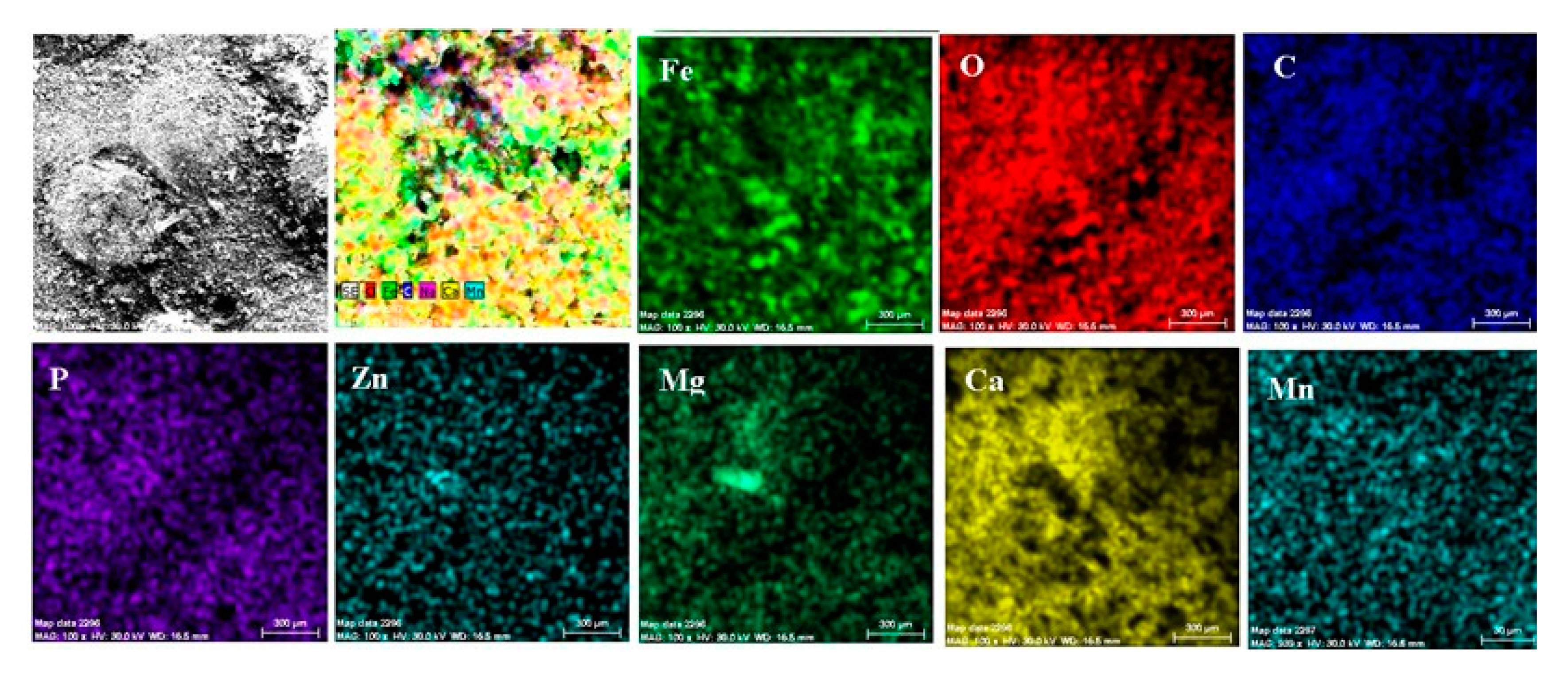
- The EDS spectra and quantitation data indicate the presence of the iron and oxygen on the C45 surface for all the immersion times. For the C45 sample, immersed for only 1 h, the EDS spectra show small amounts of manganese and silicon, which confirm the hypothesis above.
- For the other sample, the EDS data indicate only the presence of iron and oxygen along with small amounts of carbon. This layer has a protective role for a while and after that, the degradation of the material occurs based on a continuous process of corrosion.
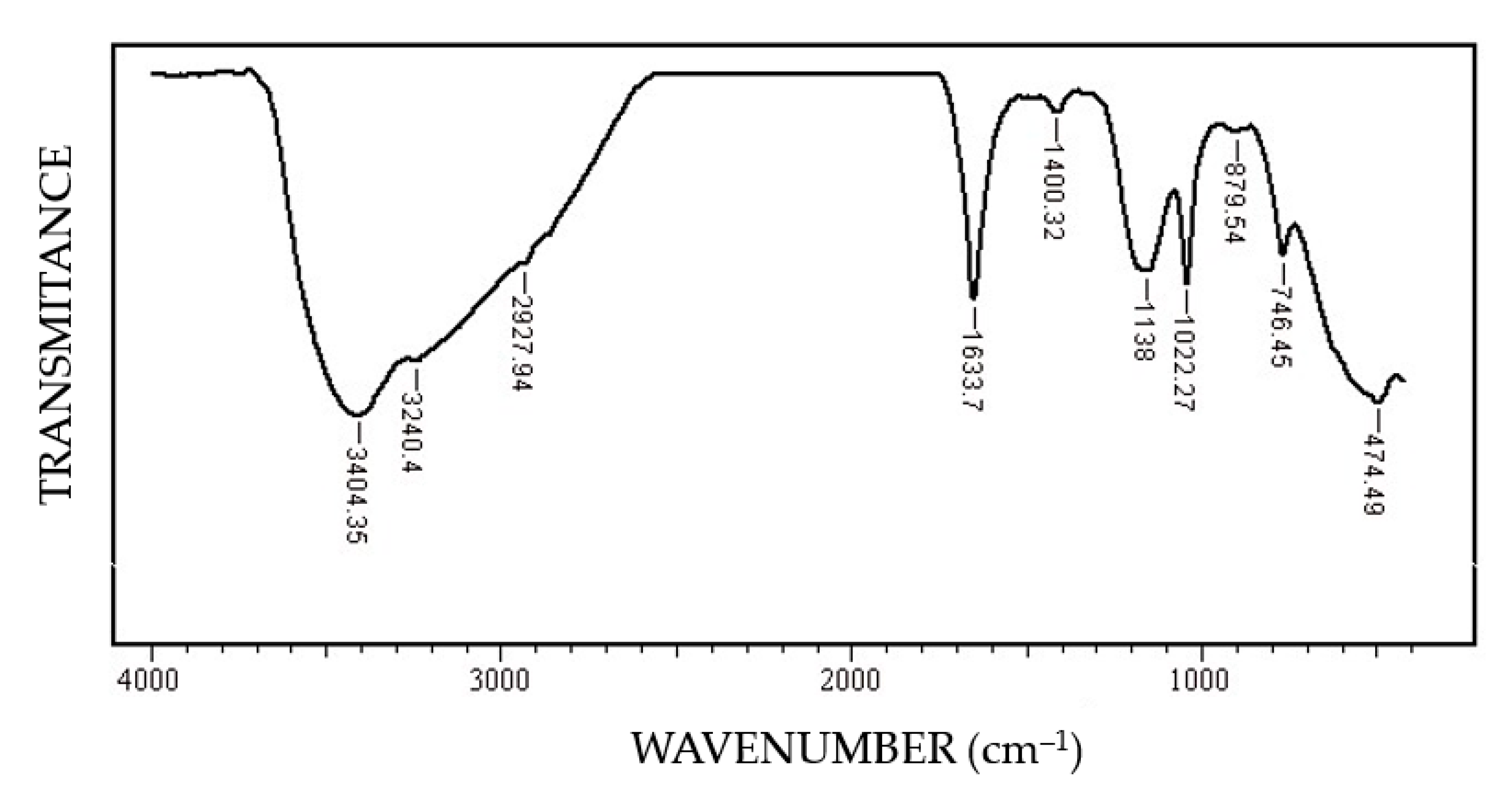
- The FT-IR spectrum confirms the presence of the iron oxyhydroxide on the C45 surface.
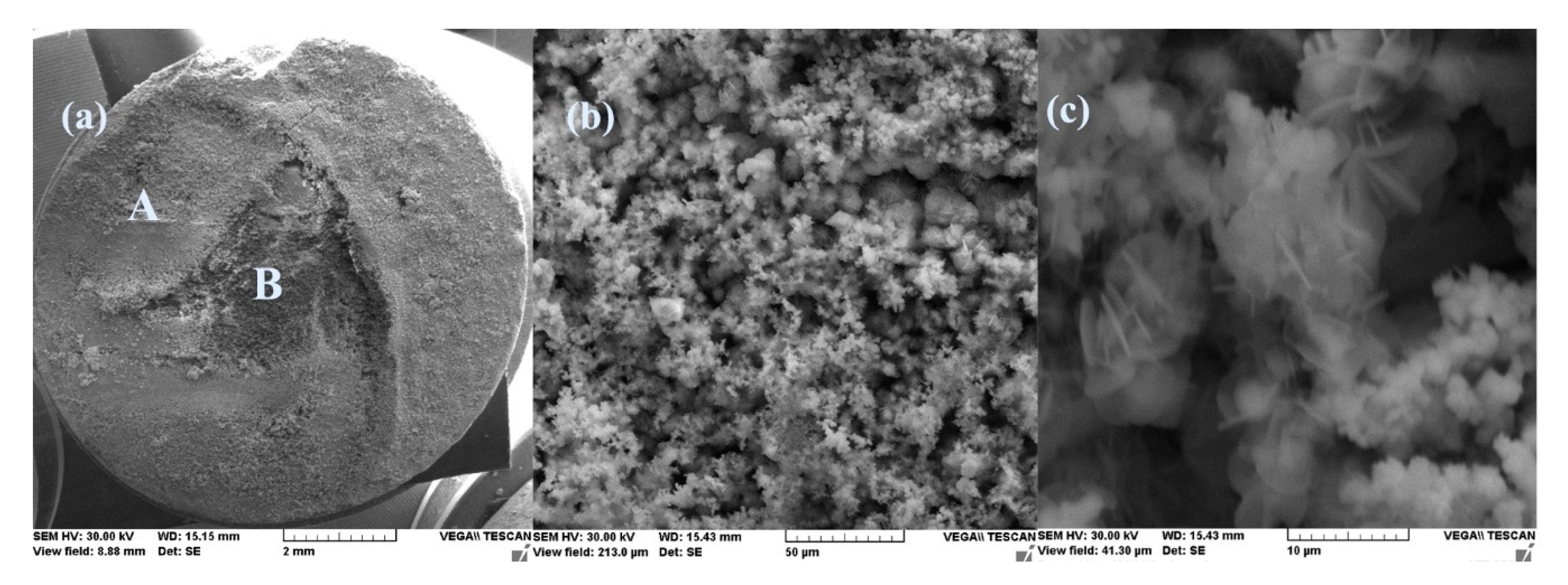
- After immersion, the surface of the samples indicated a bi-layer structure (the phosphate layer and the corrosion products layer).
- After 43 days immersion, the phosphate layer is partially damaged, widening its pores, but after 85 days, the resistance of the outer layer increases due to the thickness increase of the product layer.
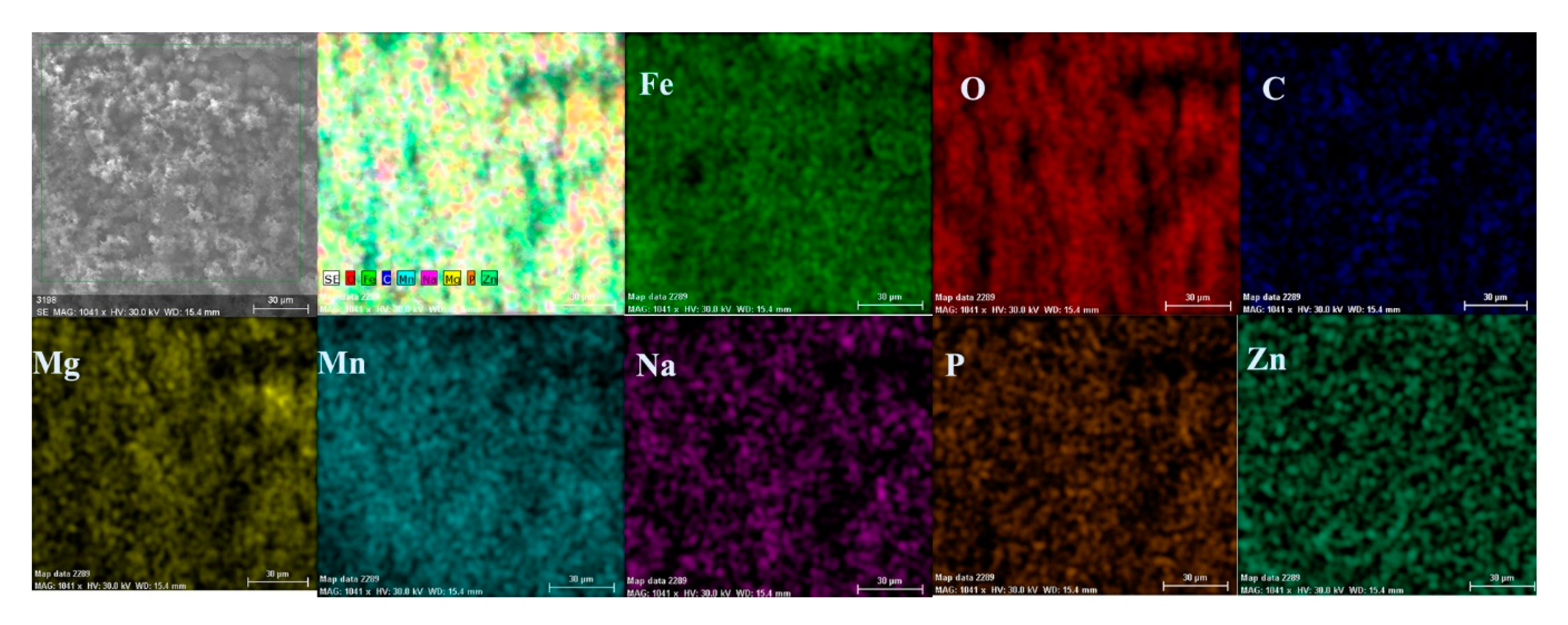
- The composition of the crust form on the surface sample present not just iron and oxygen, but also phosphorus and zinc from the phosphate layer and carbon, magnesium, and sodium from the seawater composition.
- The EIS data reveal that the phosphate layer resistance is equal compared with the value obtained for the PS sample.
- Even the saltwater damaged the paint layer; this sample has good corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Odusote, J.K.; Ajiboye, T.K.; Rabiu, A.B. Evaluation of Mechanical Properties of Medium Carbon Steel Quenched in Water and Oil. J. Miner. Mater. Charact. Eng. 2012, 11, 859–862. [Google Scholar] [CrossRef]
- Boumerzoug, Z.; Derfouf, C.; Baudin, T. Effect of Welding on Microstructure and Mechanical Properties of an Industrial Low Carbon Steel. Engineering 2010, 2, 502–506. [Google Scholar] [CrossRef]
- Panda, A.; Duplak, J.; Hatala, M.; Krenicky, T.; Vrabel, P. Research on the Durability of Selected Cutting Materials in the Process of Turning Carbon Steel. MM Sci. J. 2016, 2016, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Karavaeva, M.V.; Nurieva, S.K.; Zaripov, N.G.; Ganeev, A.V.; Valiev, R.Z. Microstructure and mechanical properties of medium-carbon steel subjected to severe plastic deformation. Met. Sci. Heat Treat. 2012, 54, 155–159. [Google Scholar] [CrossRef]
- Kimapong, K.; Poonayom, P.; Wattanajitsiri, V. Microstructure and wear resistance of hardfacing weld metal on JIS-S50C carbon steel in agricultural machine parts. Adv. Eng. Forum. 2016, 872, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Burduhos-Nergis, D.P.; Baciu, C.; Vizureanu, P.; Lohan, N.M.; Bejinariu, C. Materials types and selection for carabiners manufacturing: A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2019; Volume 572. [Google Scholar]
- Bejinariu, C.; Darabont, D.C.; Baciu, E.R.; Georgescu, I.S.; Bernevig-Sava, M.A.; Baciu, C. Considerations on applying the method for assessing the level of safety at work. Sustainability 2017, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Hamidinejad, S.M.; Kolahan, F.; Kokabi, A.H. The modeling and process analysis of resistance spot welding on galvanized steel sheets used in car body manufacturing. Mater. Des. 2012, 34, 759–767. [Google Scholar] [CrossRef]
- Sekban, D.M.; Aktarer, S.M.; Xue, P.; Ma, Z.Y.; Purcek, G. Impact toughness of friction stir processed low carbon steel used in shipbuilding. Mater. Sci. Eng. A 2016, 672, 40–48. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical review: Microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Nedeff, V.; Bejenariu, C.; Lazar, G.; Agop, M. Generalized lift force for complex fluid. Powder Technol. 2013, 235, 685–695. [Google Scholar] [CrossRef]
- Nica, P.E.; Agop, M.; Gurlui, S.; Bejinariu, C.; Focsa, C. Characterization of aluminum laser produced plasma by target current measurements. Jpn. J. Appl. Phys. 2012, 51, 106102. [Google Scholar] [CrossRef]
- Bacaita, E.S.; Bejinariu, C.; Zoltan, B.; Peptu, C.; Andrei, G.; Popa, M.; Magop, D.; Agop, M. Nonlinearities in drug release process from polymeric microparticles: Long-time-scale behaviour. J. Appl. Math. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Manna, M. Characterisation of phosphate coatings obtained using nitric acid free phosphate solution on three steel substrates: An option to simulate TMT rebars surfaces. Surf. Coat. Technol. 2009, 203, 1913–1918. [Google Scholar] [CrossRef]
- Abdalla, K.; Rahmat, A.; Azizan, A. Influence of activation treatment with nickel acetate on the zinc phosphate coating formation and corrosion resistance. Mater. Corros. 2014, 65, 977–981. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Phosphate Surface Treatment for Improving the Corrosion Resistance of the C45 Carbon Steel Used in Carabiners Manufacturing. Materials 2020, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Etteyeb, N.; Sanchez, M.; Dhouibi, L.; Alonso, C.; Andrade, C.; Triki, E. Corrosion protection of steel reinforcement by a pretreatment in phosphate solutions: Assessment of passivity by electrochemical techniques. Corros. Eng. Sci. Technol. 2006, 41, 336–341. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M. Electrochemical phosphate conversion coatings: A review. Surf. Rev. Lett. 2017, 24. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Bejinariu, C.; Toma, S.-L.; Tugui, A.-C.; Baciu, E.-R. Carbon steel carabiners improvements for use in potentially explosive atmospheres. MATEC Web Conf. 2020, 305, 00015. [Google Scholar] [CrossRef]
- Bejinariu, C.; Burduhos-Nergiș, D.P.; Cimpoeșu, N.; Bernevig-Sava, M.A.; Toma, Ș.L.; Darabont, D.C.; Baciu, C. Study on the anticorrosive phosphated steel carabiners used at personal protective equipment. Qual. Access Success 2019, 20, 71–76. [Google Scholar]
- Schmidt, D.P.; Shaw, B.A.; Sikora, E.; Shaw, W.W. Corrosion protection assessment of barrier properties of several zinc-containing coating systems on steel in artificial seawater. Corrosion 2006, 62, 323–339. [Google Scholar] [CrossRef]
- Guenbour, A.; Benbachir, A.; Kacemi, A. Evaluation of the corrosion performance of zinc-phosphate-painted carbon steel. Surf. Coat. Technol. 1999, 113, 36–43. [Google Scholar] [CrossRef]
- Asadi, V.; Danaee, I.; Eskandari, H. The effect of immersion time and immersion temperature on the corrosion behavior of Zinc phosphate conversion coatings on carbon steel. Mater. Res. 2015, 18, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Möller, H.; Boshoff, E.; Froneman, H. The corrosion behaviour of a low carbon steel in natural and synthetic seawaters. J. S. Afr. Inst. Min. Metall. 2006, 106, 585–592. [Google Scholar]
- Burduhos-Nergis, D.P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Evaluation of the corrosion resistance of phosphate coatings deposited on the surface of the carbon steel used for carabiners manufacturing. Appl. Sci. 2020, 10, 2753. [Google Scholar] [CrossRef]
- Díaz, B.; Freire, L.; Mojío, M.; Nóvoa, X.R. Optimization of conversion coatings based on zinc phosphate on high strength steels, with enhanced barrier properties. J. Electroanal. Chem. 2015, 737, 174–183. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.-P.; Sandu, A.-V.; Burduhos-Nergis, D.-D.; Darabont, D.-C.; Comaneci, R.-I.; Bejinariu, C. Shock Resistance Improvement of Carbon Steel Carabiners Used at PPE. MATEC Web Conf. 2019, 290, 12004. [Google Scholar] [CrossRef]
- Mobin, M.; Shabnam, H. Corrosion behavior of mild steel and SS 304L In presence of dissolved nickel under aerated and deaerated conditions. Mater. Res. 2011, 14, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Nagiub, A.M. Evaluation of Corrosion Behavior of Copper in Chloride Media Using Electrochemical Impedance Spectroscopy (EIS). Port. Electrochim. Acta 2005, 23, 301–314. [Google Scholar] [CrossRef]
- Chen, M.; Du, C.Y.; Yin, G.P.; Shi, P.F.; Zhao, T.S. Numerical analysis of the electrochemical impedance spectra of the cathode of direct methanol fuel cells. Int. J. Hydrog. Energy 2009, 34, 1522–1530. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, J. Introduction of Electrochemical Impedance Spectroscopy; Science Press of China: Beijing, China, 2002; ISBN 7-03-009854-4. [Google Scholar]
- Xu, X.; Liu, S.; Smith, K.; Cui, Y.; Wang, Z. An overview on corrosion of iron and steel components in reclaimed water supply systems and the mechanisms involved. J. Clean. Prod. 2020, 276. [Google Scholar] [CrossRef]
- Boily, J.F.; Felmy, A.R. On the protonation of oxo- and hydroxo-groups of the goethite (α-FeOOH) surface: A FTIR spectroscopic investigation of surface O-H stretching vibrations. Geochim. Cosmochim. Acta 2008, 72, 3338–3357. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. EIS study of corrosion behavior of metallic materials in ethanol blended gasoline containing water as a contaminant. Fuel 2011, 90, 1181–1187. [Google Scholar] [CrossRef]

| Element | C | Mn | P | Cu | Cr | Si | Fe |
|---|---|---|---|---|---|---|---|
| Percent (wt.%) | 0.45 | 0.98 | 0.02 | 0.15 | 0.17 | 0.22 | balance |
| Name of the Active Substance | Quantity |
|---|---|
| Sodium hydroxide (NaOH) | 0.75 g |
| Sodium azotite (NaNO2) | 0.45 g |
| Sodium tripolyphosphate (Na5P3O10) | 0.05 g |
| Phosphoric acid (H2PO4) | 10.00 mL |
| Nitric acid (HNO3) | 4.00 mL |
| Zinc (Zn) | 3.50 g |
| iron (Fe) | 0.038 g |
| Corrosion Environment | Immersion Time (Days) | C45 | PS | PPS |
|---|---|---|---|---|
| pH of BSW | Initial | 6.15 | 6.15 | 6.15 |
| 45 or 36 | 6.25 | 7.30 | 7.22 | |
| 85 or 78 | 6.71 | 7.57 | 7.76 |
| Immersion Time | Rs Ω·cm2 | CPE | Rct Ω·cm2 | Rext Ω·cm2 | W Ss1/2/cm2 | 103 × χ2 | εz | |
|---|---|---|---|---|---|---|---|---|
| Q Ssn/cm2 | n | |||||||
| 43 days | 39.470 | 6.92 × 10−3 | 0.749 | 155 | 5.01 | - | 2.439 | 4.94 |
| 85 days | 43.590 | 1.25 × 10−2 | 0.772 | 130 | 145.90 | 0.065 | 0.090 | 0.95 |
| Immersion Time | Rs Ω·cm2 | CPE | Rct Ω·cm2 | Rext Ω·cm2 | Cext µF/cm2 | W Ss1/2/cm2 | 103 × χ2 | εz | |
|---|---|---|---|---|---|---|---|---|---|
| Q Ssn/cm2 | n | ||||||||
| 1 h | 40.41 | 2.51 × 10−4 | 0.613 | 883.00 | 11.04 | 2.870 | - | 0.694 | 2.44 |
| 43 days | 39.47 | 5.10 × 10−3 | 0.866 | 334.20 | 12.35 | 2.667 | - | 0.155 | 1.24 |
| 85 days | 41.86 | 6.63 × 10−3 | 0.732 | 280.60 | 14.55 | 147 | 0.042 | 0.036 | 0.55 |
| - | Rs Ω·cm2 | Cpor µF/cm2 | CPE | Rct Ω·cm2 | RSC Ω·cm2 | CSC µF/cm2 | 103·× χ2 | εz | |
|---|---|---|---|---|---|---|---|---|---|
| Q Ssn/cm2 | n | ||||||||
| - | 115 | 0.307 | 6.81 × 10−4 | 0.68 | 1121 | 21.70 | 1200 | 0.072 | 0.846 |
| εEC | 1.36 | 3.010 | 0.66 | 0.38 | 334.20 | 4.61 | 5.15 | - | - |
| Immersion Time | Rs Ω·cm2 | CPE | Rct Ω·cm2 | Rext Ω·cm2 | Cext µF/cm2 | W Ss1/2/cm2 | 103·× χ2 | εz | |
|---|---|---|---|---|---|---|---|---|---|
| Q Ssn/cm2 | n | ||||||||
| 36 days | 315.40 | Cdl = 1.73 µF/cm2 | 176.50 | 266.90 | 0.022 | 5.96 10-3 | 4.13 | 6.43 | |
| 78 days | 385.70 | 6.63 × 10−3 | 0.732 | 145.80 | 184.60 | 0.267 | 6.42 10-3 | 1.75 | 2.64 |
| Corrosion Rate | Immersion Time | C45 | PS | PPS |
|---|---|---|---|---|
| CR (mpy) | 1 h | 0.027 | 0.011 | 0.008 |
| 45 (36) days | 0.062 | 0.029 | 0.022 | |
| 85 (78) days | 0.036 | 0.034 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejinariu, C.; Burduhos-Nergis, D.-P.; Cimpoesu, N. Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater. Materials 2021, 14, 188. https://doi.org/10.3390/ma14010188
Bejinariu C, Burduhos-Nergis D-P, Cimpoesu N. Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater. Materials. 2021; 14(1):188. https://doi.org/10.3390/ma14010188
Chicago/Turabian StyleBejinariu, Costica, Diana-Petronela Burduhos-Nergis, and Nicanor Cimpoesu. 2021. "Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater" Materials 14, no. 1: 188. https://doi.org/10.3390/ma14010188
APA StyleBejinariu, C., Burduhos-Nergis, D.-P., & Cimpoesu, N. (2021). Immersion Behavior of Carbon Steel, Phosphate Carbon Steel and Phosphate and Painted Carbon Steel in Saltwater. Materials, 14(1), 188. https://doi.org/10.3390/ma14010188







