Corrosion Behavior of Surface-Treated Metallic Implant Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Treatments
2.2. X-ray Residual Stress Analysis
2.3. Electrochemical Testing
2.4. Surface Roughness Measurements
3. Results
3.1. Mechanical Surface Treatment
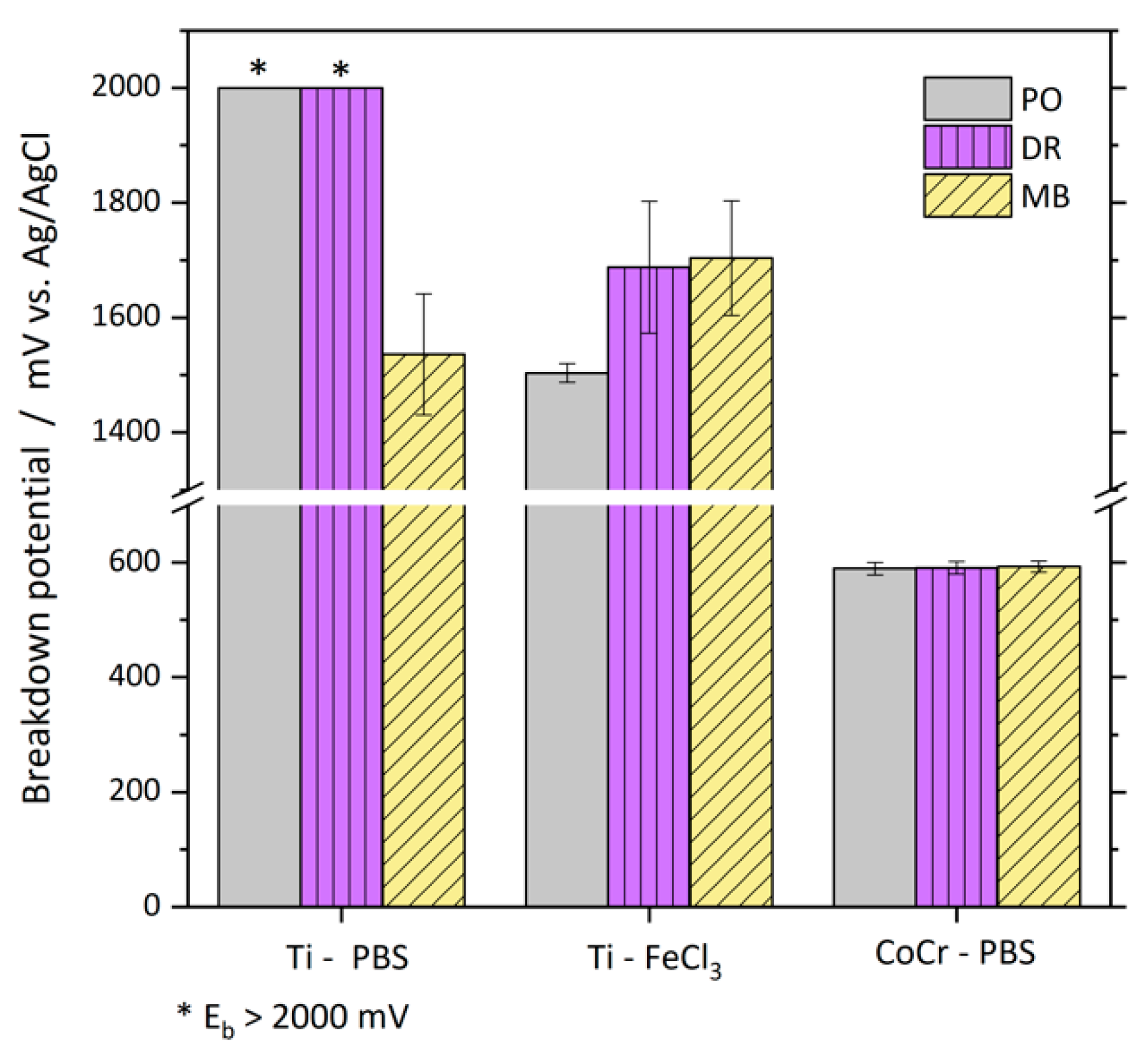
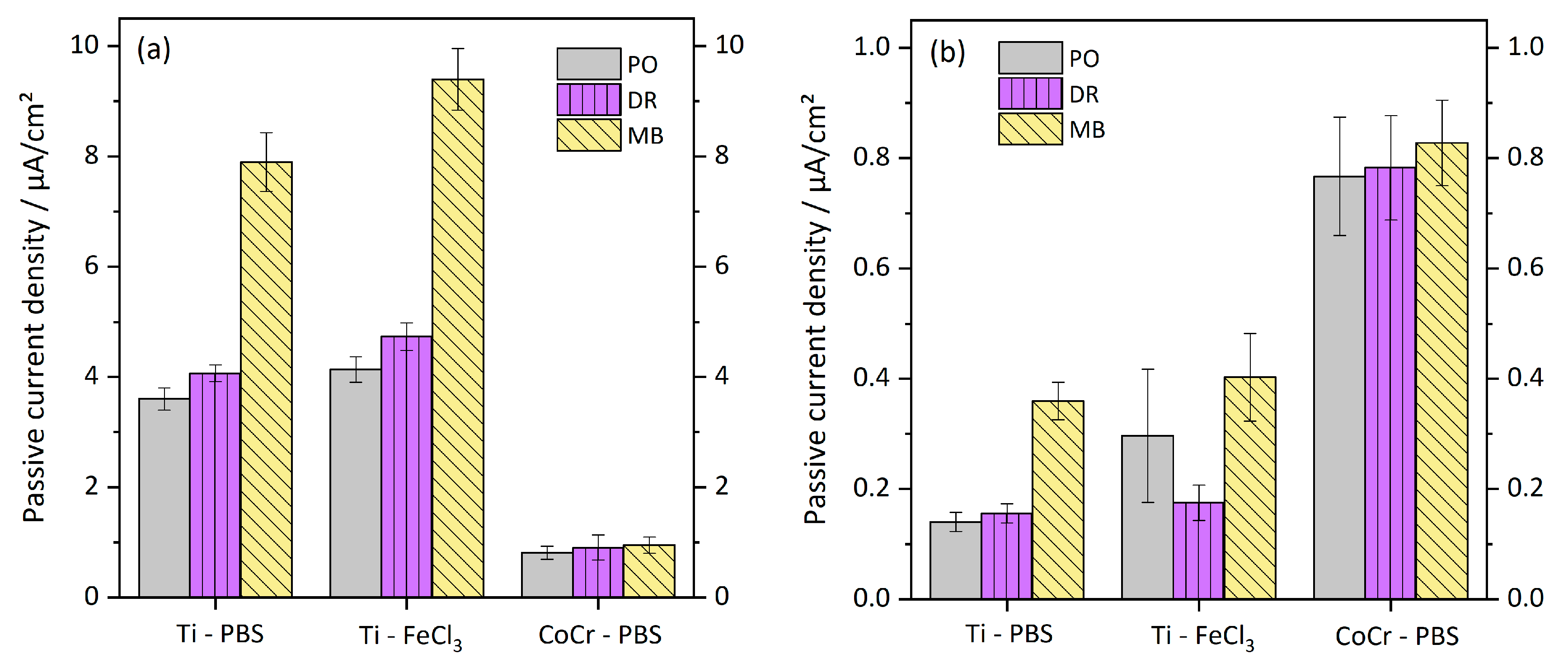
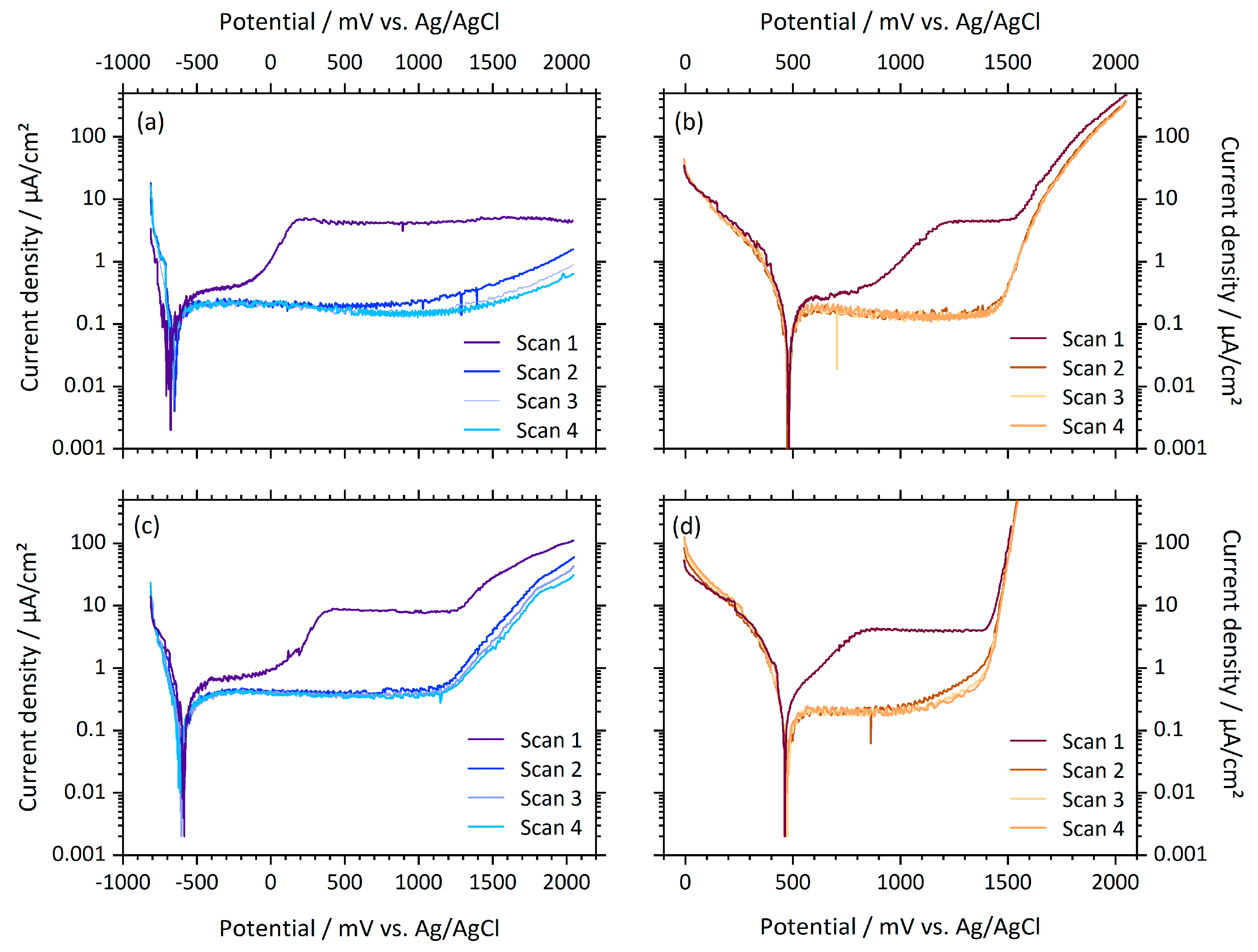
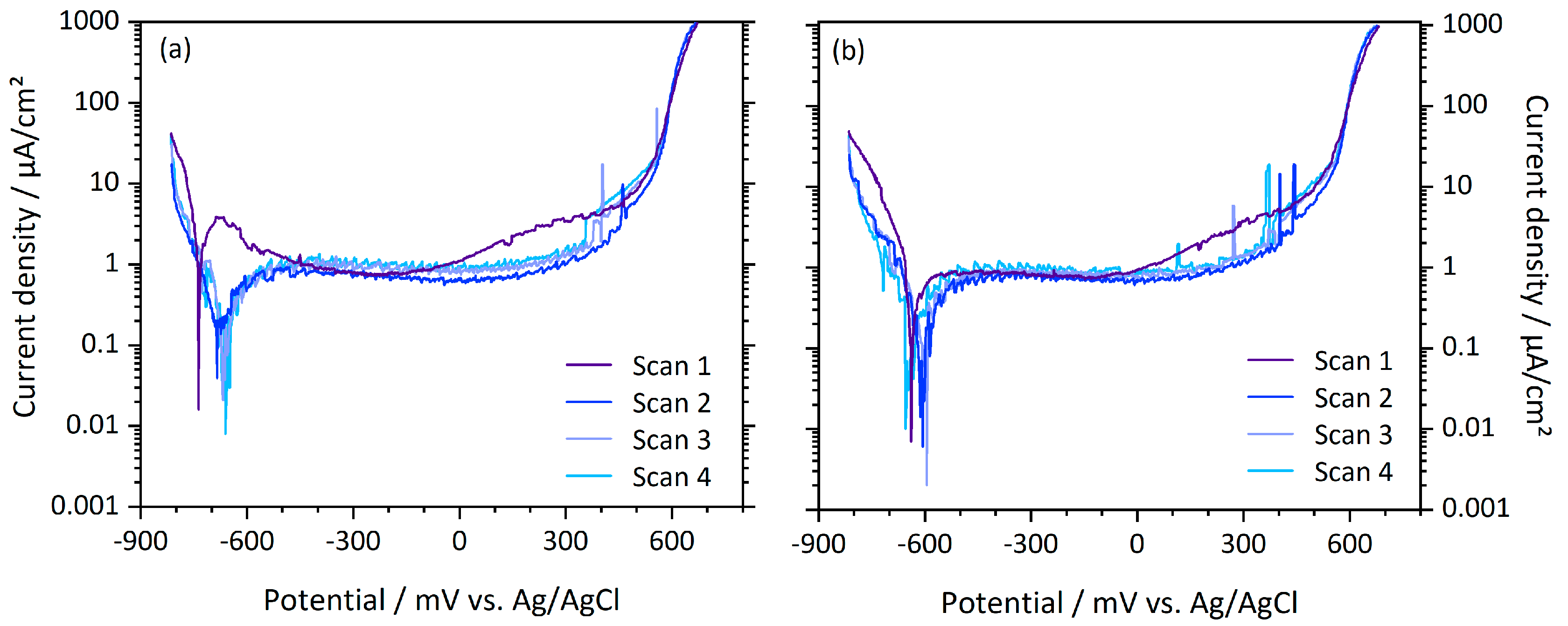
3.2. Electrochemical Tests
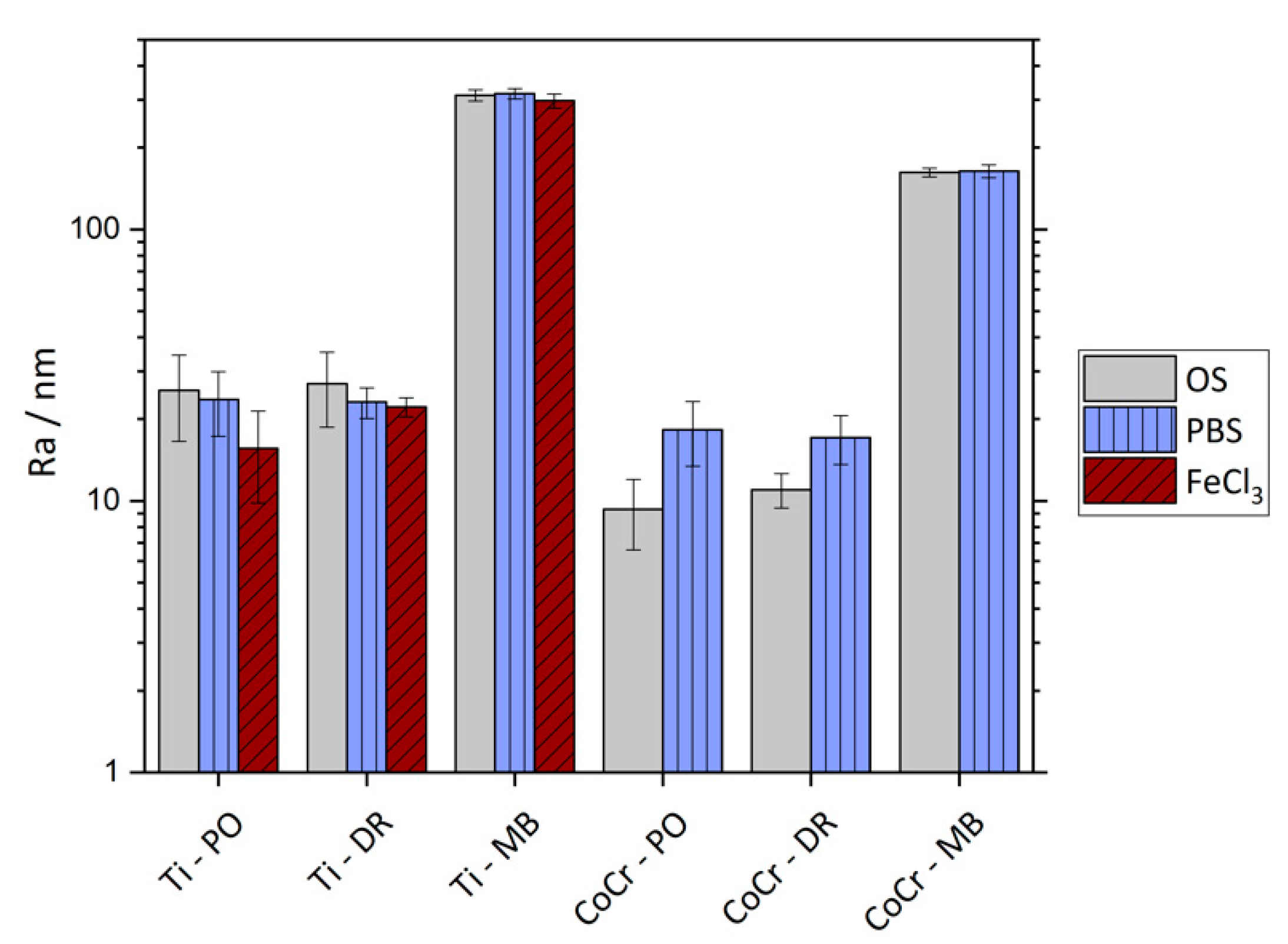
3.3. Surface Roughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussey, D.K.; McGrory, B.J. Ten-Year Cross-Sectional Study of Mechanically Assisted Crevice Corrosion in 1352 Consecutive Patients With Metal-on-Polyethylene Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.J.; Pourzal, R.; Jacobs, J.J. What Surgeons Need to Know About Adverse Local Tissue Reaction in Total Hip Arthroplasty. J. Arthroplast. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.L.; Buckley, C.A.; Jacobs, J.J. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J. Biomed. Mater. Res. 1993, 27, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Alzahrani, M.; Langohr, D.; Teeter, M.G.; Howard, J.L.; Schemitsch, E.H.; Lanting, B.A. Catastrophic femoral head trunnion dissociation: A case series with surface wear analysis. HIP Int. 2019, 29, NP1–NP5. [Google Scholar] [CrossRef]
- Hunter, A.M.; Hsu, A.; Shah, A.; Naranje, S.M. A case example and literature review of catastrophic wear before catastrophic failure: Identification of trunnionosis and metallosis in metal-on-polyethylene hip arthroplasty prior to frank failure or fracture. Eur. J. Orthop. Surg.Traumatol. 2019, 29, 711–715. [Google Scholar] [CrossRef]
- Martin, A.J.; Jenkins, D.R.; Van Citters, D.W. Role of corrosion in taper failure and head disassociation in total hip arthroplasty of a single design. J. Orthop. Res. 2018, 36, 2996–3003. [Google Scholar] [CrossRef]
- Gascoyne, T.C.; Turgeon, T.R.; Burnell, C.D. Retrieval Analysis of Large-Head Modular Metal-on-Metal Hip Replacements of a Single Design. J. Arthroplast. 2018, 33, 1945–1952. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Kocagöz, S.B.; Hanzlik, J.A.; Underwood, R.J.; Gilbert, J.L.; MacDonald, D.W.; Lee, G.-C.; Mont, M.A.; Kraay, M.J.; Klein, G.R.; et al. Do Ceramic Femoral Heads Reduce Taper Fretting Corrosion in Hip Arthroplasty? A Retrieval Study. Clin. Orthop. Relat. Res. 2013, 471, 3270–3282. [Google Scholar] [CrossRef]
- Rehmer, A.; Bishop, N.E.; Morlock, M.M. Influence of assembly procedure and material combination on the strength of the taper connection at the head–neck junction of modular hip endoprostheses. Clin. Biomech. 2012, 27, 77–83. [Google Scholar] [CrossRef]
- Falkenberg, A.; Biller, S.; Morlock, M.M.; Huber, G. Micromotion at the head-stem taper junction of total hip prostheses is influenced by prosthesis design-, patient- and surgeon-related factors. J. Biomech. 2020, 98, 109424. [Google Scholar] [CrossRef]
- Panagiotidou, A.; Cobb, T.; Meswania, J.; Skinner, J.; Hart, A.; Haddad, F.; Blunn, G. Effect of impact assembly on the interface deformation and fretting corrosion of modular hip tapers: An in vitro study. J. Orthop. Res. 2018, 36, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Ramoutar, D.N.; Crosnier, E.A.; Shivji, F.; Miles, A.W.; Gill, H.S. Assessment of Head Displacement and Disassembly Force With Increasing Assembly Load at the Head/Trunnion Junction of a Total Hip Arthroplasty Prosthesis. J. Arthroplast. 2017, 32, 1675–1678. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majzoobi, G.H.; Abbasi, F. On the effect of shot-peening on fretting fatigue of Al7075-T6 under cyclic normal contact loading. Surf. Coat. Technol. 2017, 328, 292–303. [Google Scholar] [CrossRef]
- Liu, K.K.; Hill, M.R. The effects of laser peening and shot peening on fretting fatigue in Ti–6Al–4V coupons. Tribol. Int. 2009, 42, 1250–1262. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Liu, Y. Effect of shot peening on the fatigue properties of nickel-based superalloy GH4169 at high temperature. Results Phys. 2018, 11, 452–460. [Google Scholar] [CrossRef]
- Sonntag, R.; Reinders, J.; Gibmeier, J.; Kretzer, J.P. Fatigue performance of medical Ti6Al4V alloy after mechanical surface treatments. PLoS ONE 2015, 10, e0121963. [Google Scholar] [CrossRef] [PubMed]
- Altenberger, I.; Nalla, R.K.; Sano, Y.; Wagner, L.; Ritchie, R.O. On the effect of deep-rolling and laser-peening on the stress-controlled low- and high-cycle fatigue behavior of Ti–6Al–4V at elevated temperatures up to 550 °C. Int. J. Fatigue 2012, 44, 292–302. [Google Scholar] [CrossRef]
- Eto, S.; Miura, Y.; Tani, J.; Fujii, T. Effect of residual stress induced by pulsed-laser irradiation on initiation of chloride stress corrosion cracking in stainless steel. Mater. Sci. Eng. A 2014, 590, 433–439. [Google Scholar] [CrossRef]
- Wu, S.-X.; Wang, S.-R.; Wang, G.-Q.; Yu, X.-C.; Liu, W.-T.; Chang, Z.-Q.; Wen, D.-S. Microstructure, mechanical and corrosion properties of magnesium alloy bone plate treated by high-energy shot peening. Trans. Nonferrous Met. Soc. China 2019, 29, 1641–1652. [Google Scholar] [CrossRef]
- Trdan, U.; Sano, T.; Klobčar, D.; Sano, Y.; Grum, J.; Šturm, R. Improvement of corrosion resistance of AA2024-T3 using femtosecond laser peening without protective and confining medium. Corros. Sci. 2018, 143, 46–55. [Google Scholar] [CrossRef]
- Lee, H.; Mall, S.; Allen, W.Y. Fretting fatigue behavior of shot-peened Ti–6Al–4V under seawater environment. Mater. Sci. Eng. A 2006, 420, 72–78. [Google Scholar] [CrossRef]
- Weingärtner, R.; Hoffmeister, J.; Schulze, V. Mechanical Surface Treatment by Micro Peening. HTM J. Heat Treat. Mater. 2015, 70, 59–65. [Google Scholar] [CrossRef]
- Pypen, C.M.J.M.; Plenk, H., Jr.; Ebel, M.F.; Svagera, R.; Wernisch, J. Characterization of microblasted and reactive ion etched surfaces on the commercially pure metals niobium, tantalum and titanium. J. Mater. Sci. Mater. Med. 1997, 8, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, B.; Macherauch, E. Röntgenographische Untersuchung von Spannungszuständen in Werkstoffen. Materialwissenschaft Werkst. 1995, 26, 148–160. [Google Scholar] [CrossRef]
- ASTM. F2129-15, Standard Test Method for Conducting Cyclic Poentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Baxmann, M.; Pfaff, A.M.; Schilling, C.; Grupp, T.M.; Morlock, M.M. Biomechanical Evaluation of the Fatigue Performance, the Taper Corrosion and the Metal Ion Release of a Dual Taper Hip Prosthesis under Physiological Environmental Conditions. Biotribology 2017, 12, 1–7. [Google Scholar] [CrossRef]
- ASTM. G48-03, Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103. [Google Scholar] [CrossRef]
- Sivakumar, B.; Pathak, L.C.; Singh, R. Role of surface roughness on corrosion and fretting corrosion behaviour of commercially pure titanium in Ringer’s solution for bio-implant application. Appl. Surf. Sci. 2017, 401, 385–398. [Google Scholar] [CrossRef]
- Karimi, S.; Nickchi, T.; Alfantazi, A. Effects of bovine serum albumin on the corrosion behaviour of AISI 316L, Co–28Cr–6Mo, and Ti–6Al–4V alloys in phosphate buffered saline solutions. Corros. Sci. 2011, 53, 3262–3272. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B. The corrosion resistance of Nitinol alloy in simulated physiological solutions: Part 1: The effect of surface preparation. Mater. Sci. Eng. C 2012, 32, 1087–1096. [Google Scholar] [CrossRef]
- Hunt, N.P.; Cunningham, S.J.; Golden, C.G.; Sheriff, M. An investigation into the effects of polishing on surface hardness and corrosion of orthodontic archwires. Angle Orthod. 1999, 69, 433–440. [Google Scholar] [PubMed]
- Chi, G.; Yi, D.; Liu, H. Effect of roughness on electrochemical and pitting corrosion of Ti-6Al-4V alloy in 12 wt.% HCl solution at 35 °C. J. Mater. Res. Technol. 2019. [Google Scholar] [CrossRef]
- Azar, V.; Hashemi, B.; Rezaee Yazdi, M. The effect of shot peening on fatigue and corrosion behavior of 316L stainless steel in Ringer’s solution. Surf. Coat. Technol. 2010, 204, 3546–3551. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, I.J.; Lee, D.Y.; Kim, J.G. Influence of surface roughness on the electrochemical behavior of carbon steel. J. Appl. Electrochem. 2013, 43, 507–514. [Google Scholar] [CrossRef]
- Goldberg, J.R.; Gilbert, J.L.; Jacobs, J.J.; Bauer, T.W.; Paprosky, W.; Leurgans, S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin. Orthop. Relat. Res. 2002, 401, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.; Panagiotidou, A.P.; Khan, M.; Blunn, G.; Haddad, F.S. Corrosion at the head-neck interface of current designs of modular femoral components. Bone Joint J. 2016, 98, 579–584. [Google Scholar] [CrossRef]
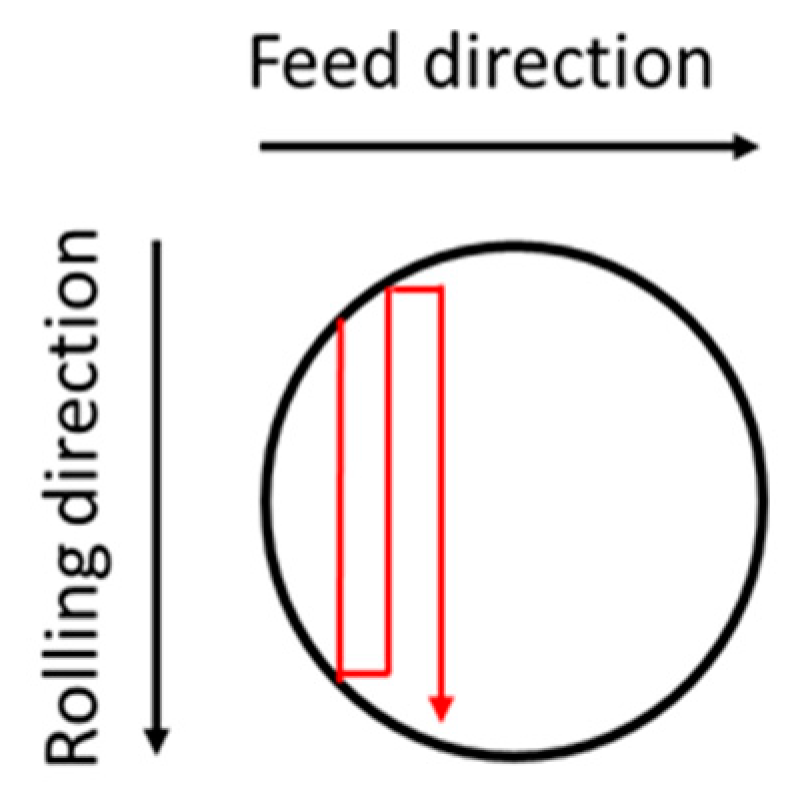
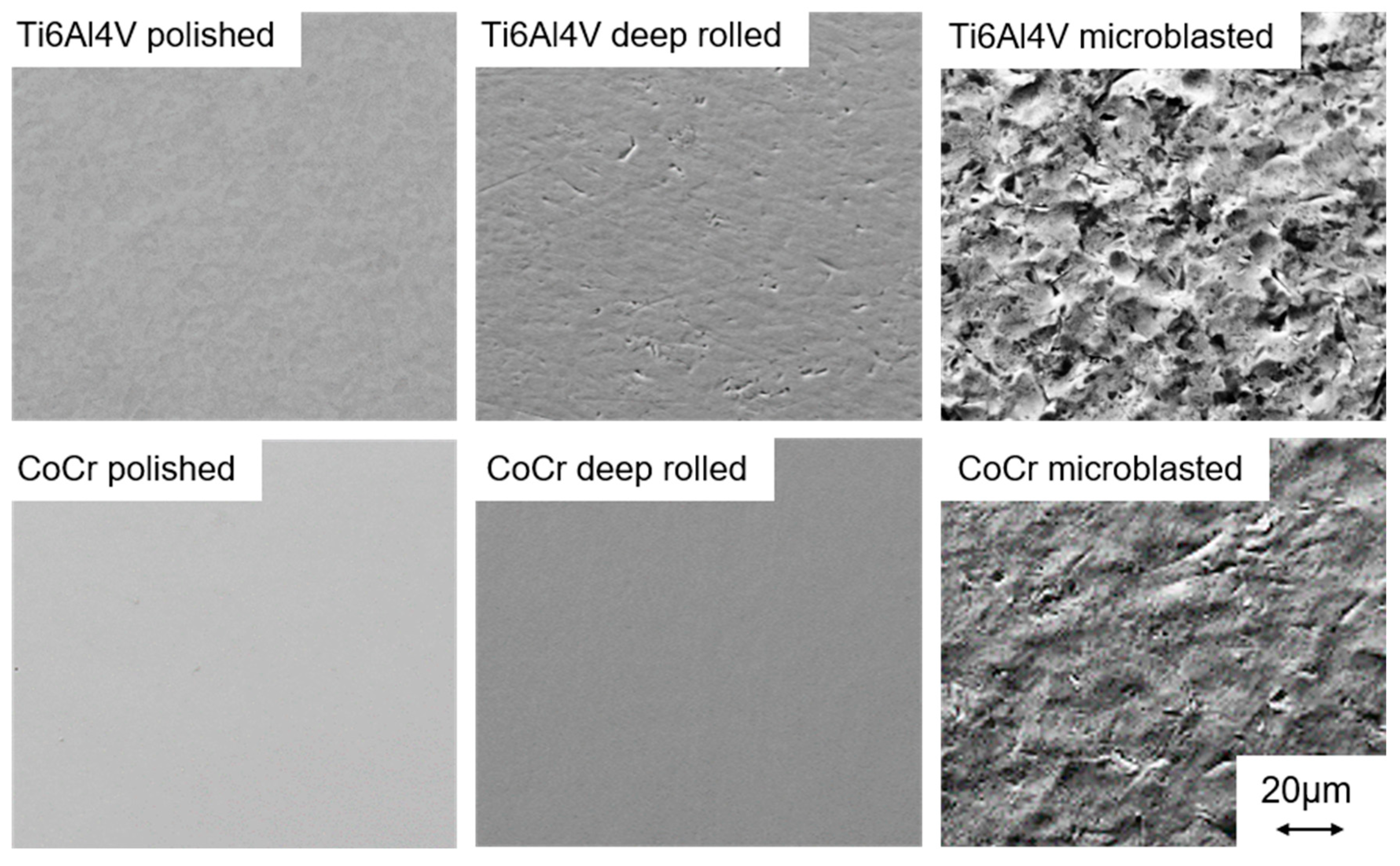
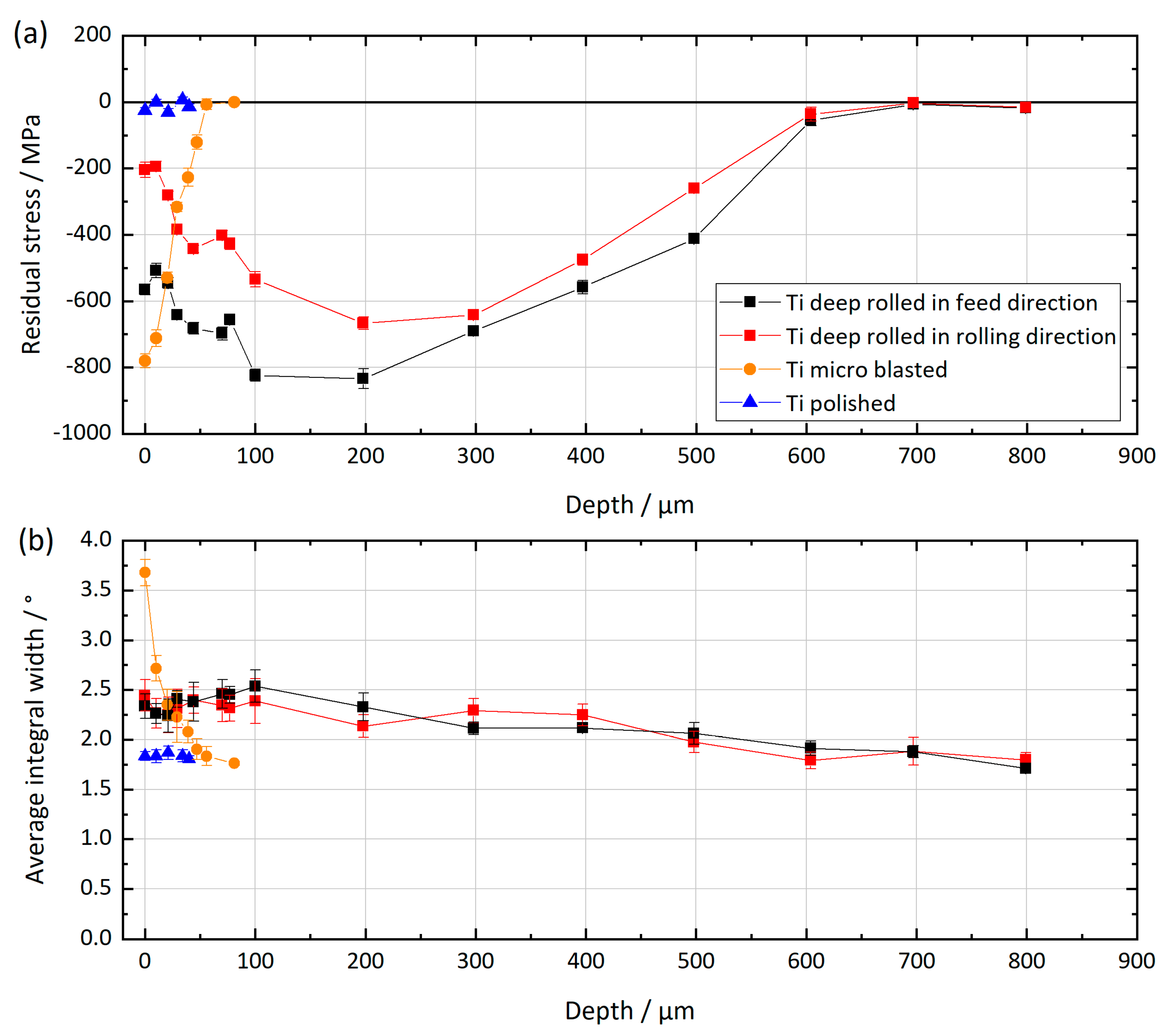
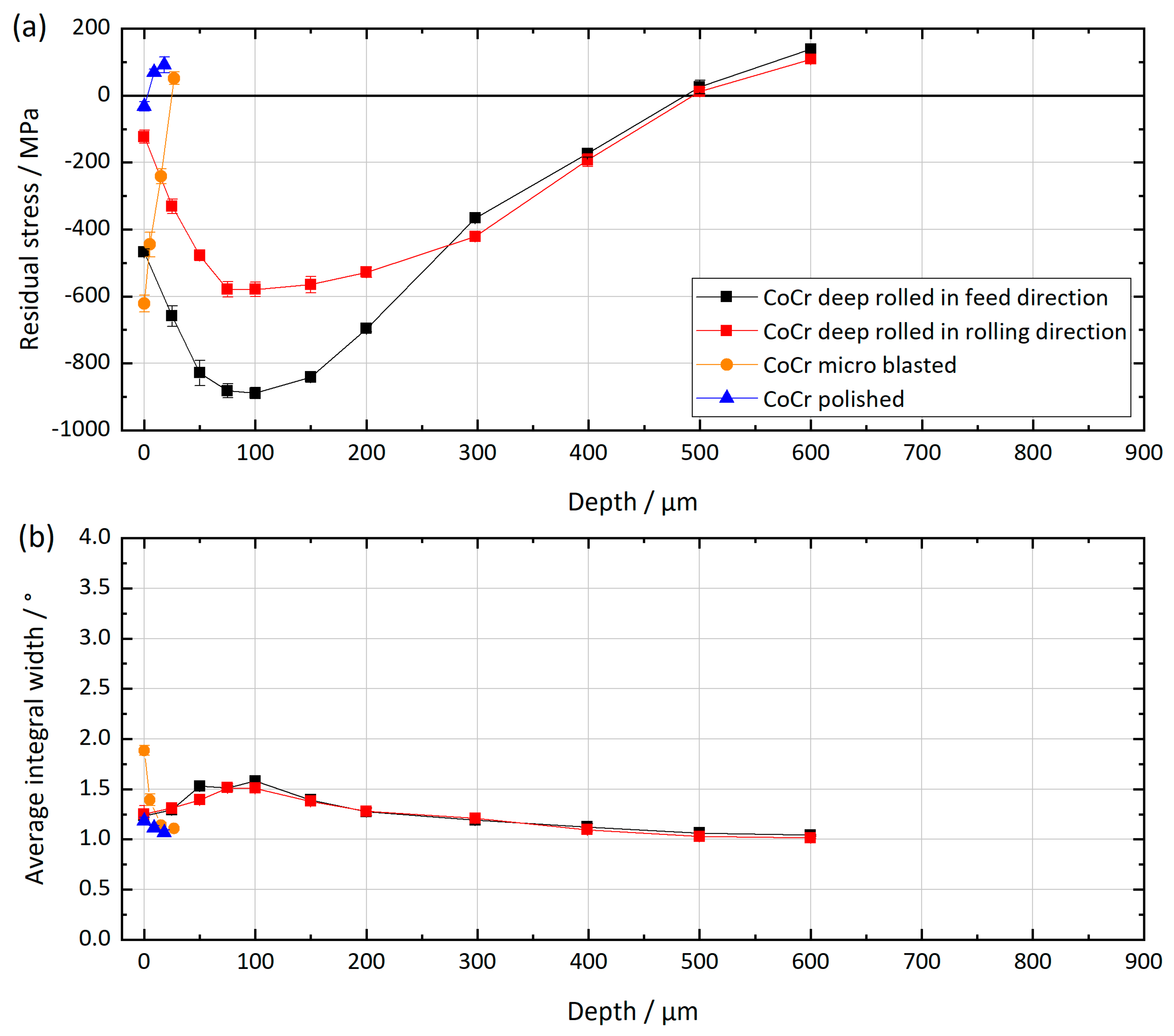
| Parameters Deep Rolling | Parameters Microblasting | |
|---|---|---|
| Ball material / diameter | Ceramic / 6 mm | Glass beads MS 550B / 20–30 µm |
| Pressure | 180 bar | 3 bar |
| Feed rate | 2000 mm/min | 8 mm/s |
| Line spacing between traces | 0.04 mm | 1 mm |
| Initial distance to surface | – | 10 mm |
| Coverage ratio | – | 100% |
| Flow rate | – | 1.5 kg/min |
| Cycle 1 / nAcm−2 | Cycles no. 2–4 / nAcm−2 | |||||
|---|---|---|---|---|---|---|
| PO | DR | MB | PO | DR | MB | |
| Ti-PBS | 3600 (200) | 4067 (153) | 7900 (529) | 140 (17) | 156 (17) | 359 (34) |
| Ti-FeCl3 | 4133 (231) | 4733 (252) | 9400 (557) | 297 (11) | 175 (6) | 403 (79) |
| CoCr-PBS | 813 (118) | 908 (225) | 950 (145) | 767 (107) | 783 (95) | 828 (78) |
| PO / mV | DR / mV | MB / mV | |
|---|---|---|---|
| PBS | −615 (94) | −642 (71) | −566 (30) |
| FeCl3 | 498 (27) | 469 (25) | 461 (31) |
| Ti6Al4V / nm | CoCrMo / nm | |||||
|---|---|---|---|---|---|---|
| PO | DR | MB | PO | DR | MB | |
| Ra / OS | 25.5 (8.9) | 27.0 (8.3) | 311.9 (15.1) | 9.3 (2.7) | 11.0 (1.6) | 162.2 (6.0) |
| Ra / PBS | 23.6 (6.3) | 23.1 (3.0) | 315.9 (14.2) | 18.3 (4.9) | 17.1 (3.5) | 163.7 (8.9) |
| Ra / FeCl3 | 15.6 (5.8) | 22.2 (1.8) | 297.5 (17.6) | – | – | – |
| Rz / OS | 177.9 (63.3) | 210.1 (53.4) | 2004 (131) | 68.5 (20.4) | 69.1 (17.5) | 1077 (91) |
| Rz / PBS | 178.3 (61.6) | 200.5 (28.7) | 2010 (160) | 122.7 (23.9) | 106.4 (15.4) | 1097 (89) |
| Rz / FeCl3 | 109.5 (32.4) | 192.8 (22.5) | 1883 (115) | – | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bormann, T.; Mai, P.T.; Gibmeier, J.; Sonntag, R.; Müller, U.; Kretzer, J.P. Corrosion Behavior of Surface-Treated Metallic Implant Materials. Materials 2020, 13, 2011. https://doi.org/10.3390/ma13092011
Bormann T, Mai PT, Gibmeier J, Sonntag R, Müller U, Kretzer JP. Corrosion Behavior of Surface-Treated Metallic Implant Materials. Materials. 2020; 13(9):2011. https://doi.org/10.3390/ma13092011
Chicago/Turabian StyleBormann, Therese, Phuong Thao Mai, Jens Gibmeier, Robert Sonntag, Ulrike Müller, and J. Philippe Kretzer. 2020. "Corrosion Behavior of Surface-Treated Metallic Implant Materials" Materials 13, no. 9: 2011. https://doi.org/10.3390/ma13092011
APA StyleBormann, T., Mai, P. T., Gibmeier, J., Sonntag, R., Müller, U., & Kretzer, J. P. (2020). Corrosion Behavior of Surface-Treated Metallic Implant Materials. Materials, 13(9), 2011. https://doi.org/10.3390/ma13092011





