Novel Graphene/In2O3 Nanocubes Preparation and Selective Electrochemical Detection for L-Lysine of Camellia nitidissima Chi
Abstract
1. Introduction
2. Results and Discussion
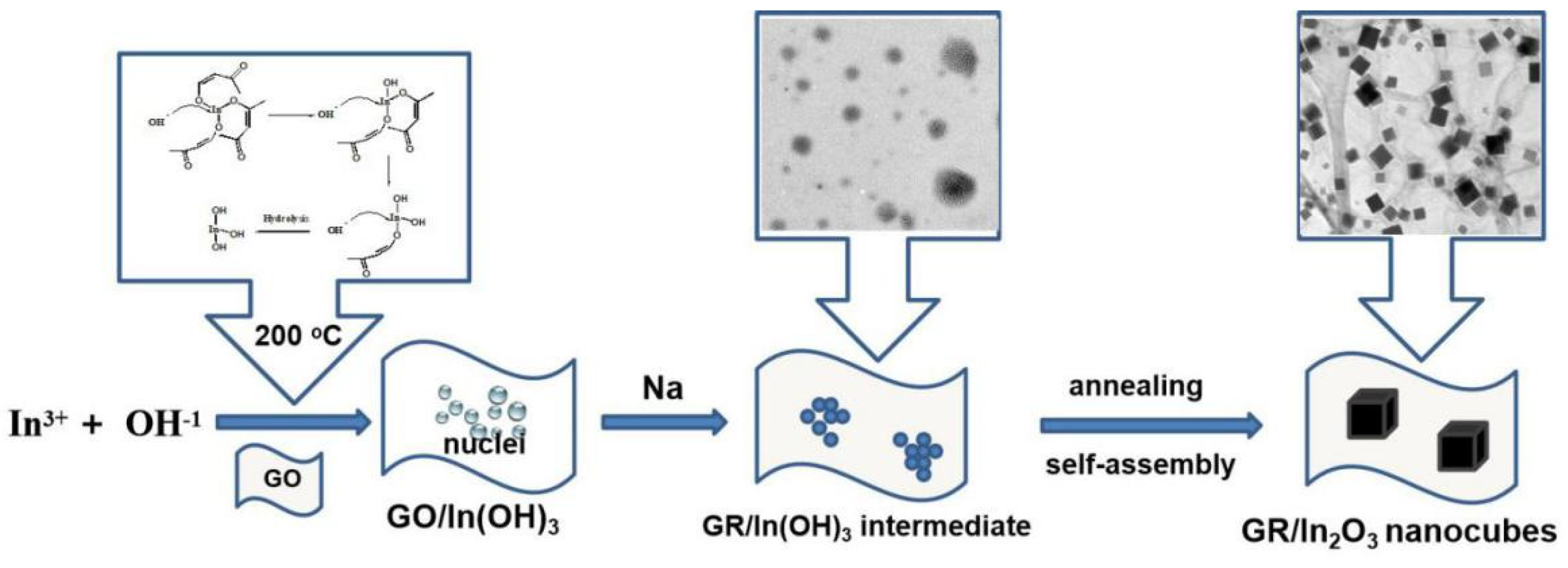
2.1. Preparation and Characterization of GR/In2O3 Nanocubes
2.2. The Mechanism of the Prepared GR/In2O3 Nanocubes
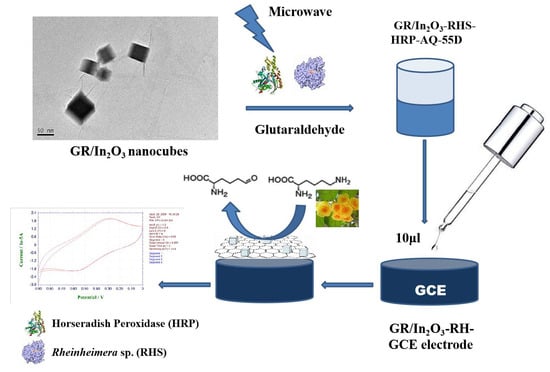
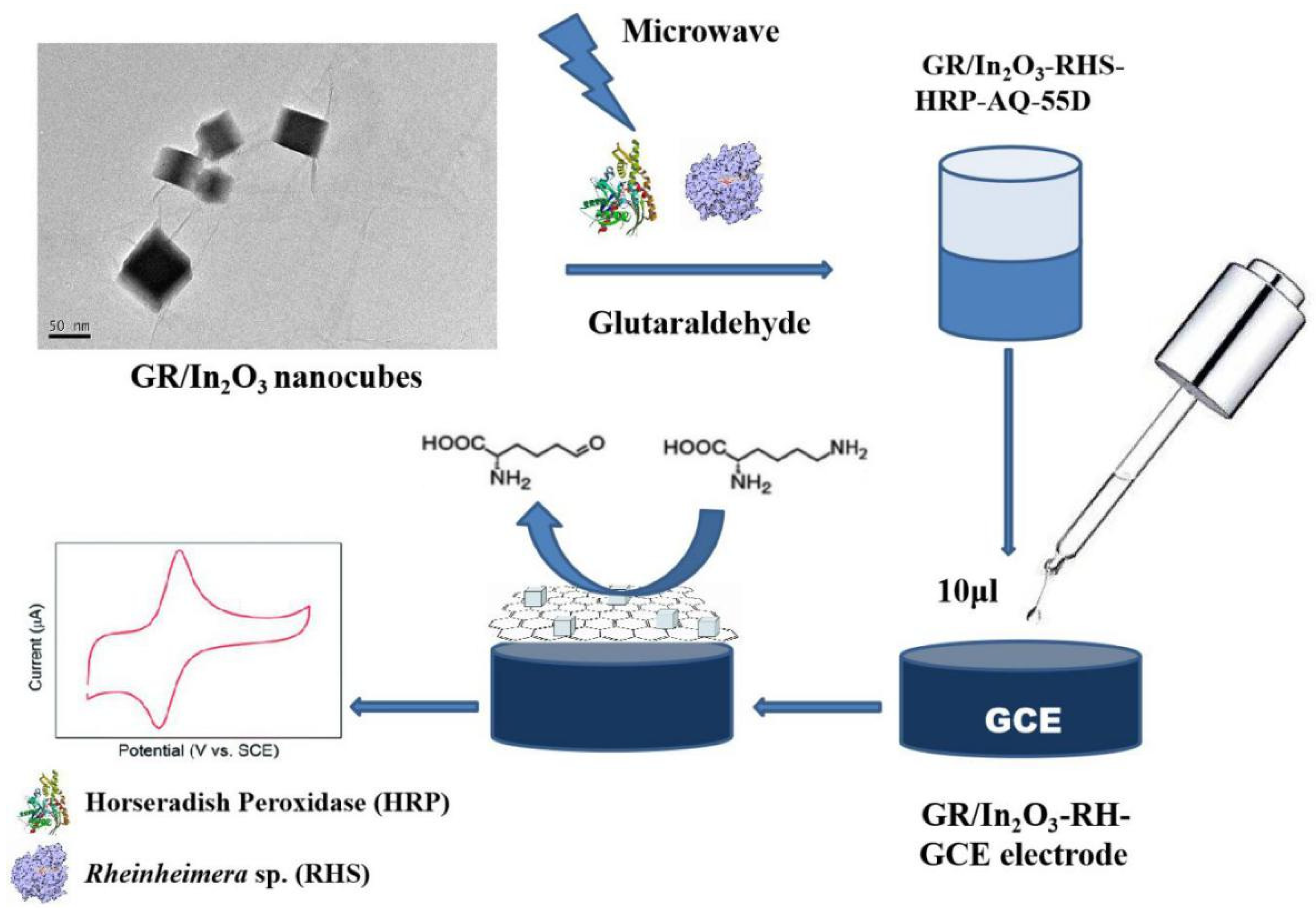
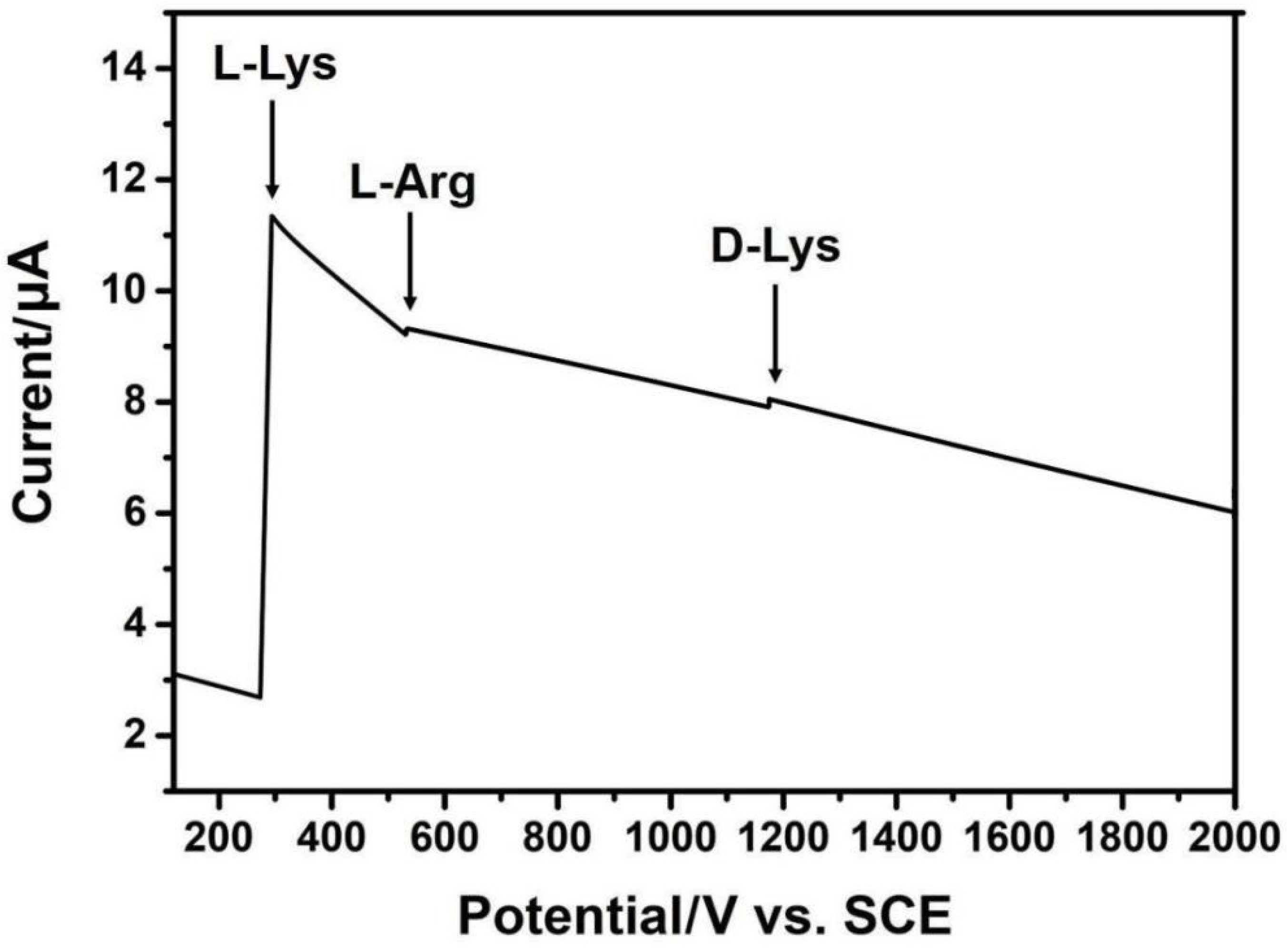
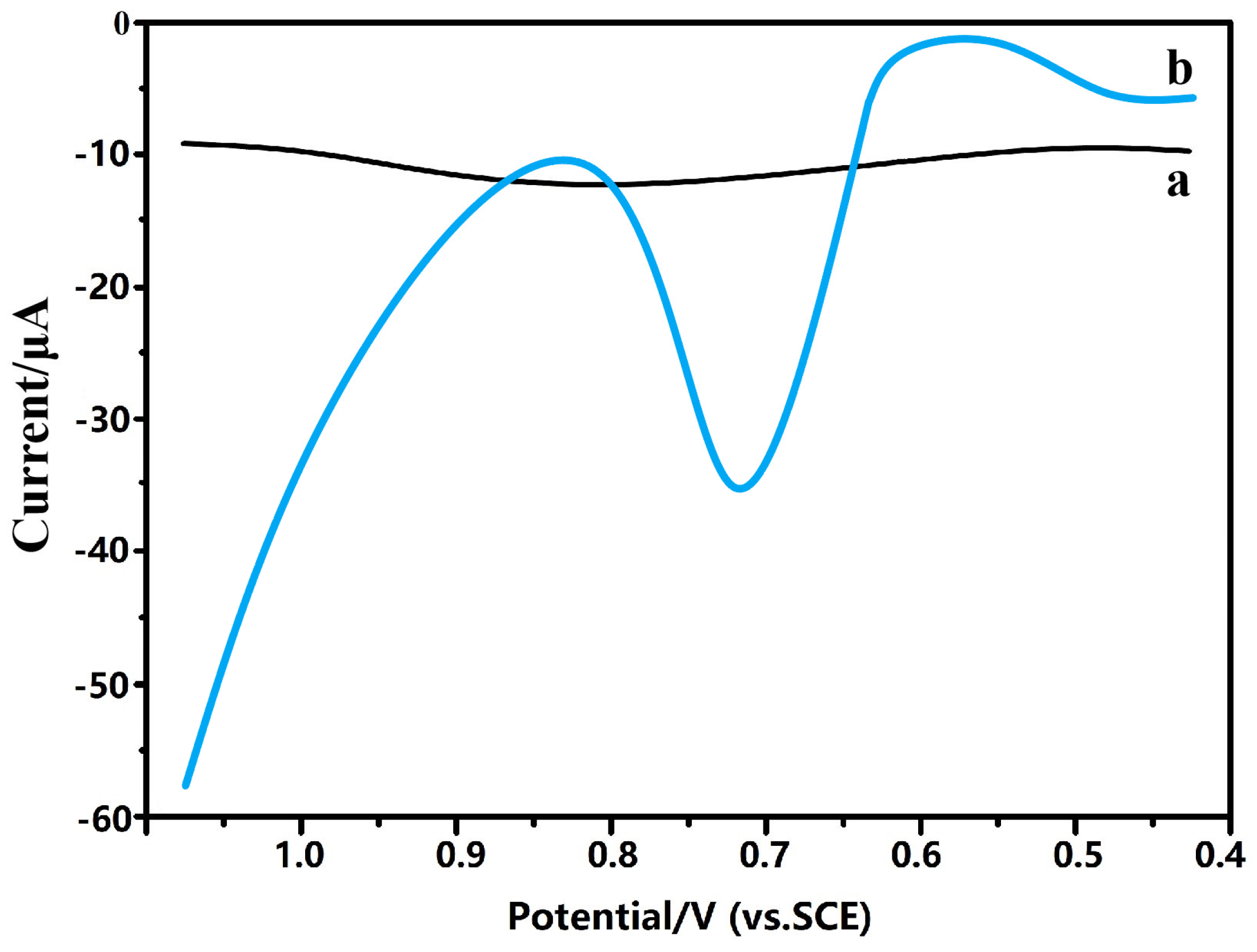
2.3. Response of Amino Acids of Camellia nitidissima Chi Using GR/In2O3 Nanocube-Based Electrochemical Sensors
3. Experimental Details
3.1. Materials and Characterization
3.2. Free Amino Acid Extraction of Camellia nitidissima Chi Leaves
3.3. Typical Procedures for the Preparation of GR/In2O3 Nanocubes
3.4. Construction and Response Measurements of Selective Electrochemical Sensor for L-Lysine Based on GR/In2O3 Nanocubes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mi, B. Scaling up nanoporous graphene membranes. Science 2019, 364, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhong, R.; Wan, W.; Li, H.; Zhu, J. Amino acid detection from the leaves of Camellia nitidissima Chi using novel husk-derived graphene nanoshuttles. Nanosci. Nanotech. Lett. 2017, 9, 1742–1747. [Google Scholar] [CrossRef]
- Cheng, J.; Wan, W.; Zhu, W. One-pot solvothermal synthesis of TiO2 nanobelt/graphene composites for selective renal cancer cells destruction. Chin. J. Chem. 2016, 34, 53–58. [Google Scholar] [CrossRef]
- Cheng, J. Drinking water filtration device and filtration method based on graphene technologies. U.S. Patent US20190070533A1, 7 March 2019. [Google Scholar]
- Zeng, Q.; Cheng, J.; Tang, L.; Liu, X.; Liu, Y.; Li, J.; Jiang, J. Self-sssembled graphene enzyme hierarchical nanostructures for electrochemical biosensing. Adv. Funct. Mater. 2010, 20, 3366–3372. [Google Scholar] [CrossRef]
- Cheng, J.; Du, J. Facile synthesis of germanium-graphene nanocomposites and their application as anode materials for lithium ion batteries. CrystEngComm 2012, 14, 397–400. [Google Scholar] [CrossRef]
- Cao, Y.; Fatemi, V.; Demir, A.; Fang, S.; Tomarken, S.L.; Luo, J.Y.; Sanchez-Yamagishi, J.D.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; et al. Correlated insulator behaviour at half-Filling in magic angle graphene superlattices. Nature 2018, 556, 80–84. [Google Scholar] [CrossRef]
- Lee, M.; Wallbank, J.R.; Gallagher, P.; Watanabe, K.; Taniguchi, T.; Fal’ko, V.I.; Goldhaber-Gordon, D. Ballistic miniband conduction in a graphene superlattice. Science 2016, 353, 1526–1529. [Google Scholar] [CrossRef]
- Solís-Fernández, P.; Bissett, M.; Ago, H. Synthesis, structure and applications of graphene-based 2D heterostructures. Chem. Soc. Rev. 2017, 46, 4572–4613. [Google Scholar] [CrossRef]
- Cheng, J.; Tang, L.; Li, J. Palladium nanoparticles-decorated graphene nanosheets as highly regioselective catalyst for cyclotrimerization reaction. J. Nanosci. Nanotech. 2011, 11, 5159–5168. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven α-Fe2O3 nanorod/graphene/BiV1−xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef]
- Cheng, J.; Zhong, R.; Lin, J.; Zhu, J.; Wan, W.; Chen, X. Linear graphene nanocomposite synthesis and an analytical application for the amino acid detection of Camellia nitidissima Chi seeds. Materials 2017, 10, 443. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Ortega, D.E.; Escobar, N. Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl. Sur. Sci. 2018, 427, 227–236. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z. AuPt/MOF-Graphene: A synergistic catalyst with surprisingly high peroxidase-like activity and its application for H2O2 detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic dirac billiard in graphene quantum dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.; Liu, X.; Bai, H.; Jiang, J. Palladium Nanaparticles/Chitosan-Grafted Graphene Nanocomposites for Construction of a Glucose Biosensor. Biosens. Bioelec. 2011, 26, 3456–3463. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.H.; Wang, J.; Wu, H.; Aksay, A.I.; Liu, J.; Lin, Y.H. Glucose oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Qi, X.X.; Wei, X.; Chen, Z.Y.; Tang, H.; Chai, S.F. Nutrient composition in leaves of cultivated and wild Camellia nitidissima. Pak. J. Bot. 2012, 44, 635–638. [Google Scholar]
- Yin, P.; Zhang, Z.M.; Lv, H.; Li, T.; Haso, F.; Hu, L.; Zhang, B.; Bacsa, J.; Wei, Y.; Gao, Y.; et al. Chiral recognition and selection during the self-assembly process of protein-mimic macroanions. Nat. Commun. 2015, 6, 6475. [Google Scholar] [CrossRef]
- Araki, K.; Inada, K.; Shinkai, S. Chiral Recognition of α-Amino acid derivatives with a homooxacalix[3]arene: Construction of a pseudo-C2-symmetrical compound from a C3-symmetrical macrocycle. Angew. Chem. Int. Ed. 1996, 35, 72–74. [Google Scholar] [CrossRef]
- Krishnan, R.R.; Sanjeev, G.; Prabhu, R.; Pillai, V.P.M. Effect of Electron beam irradiation on structural and optical properties of Cu-doped In2O3 films prepared by RF magnetron sputtering. J. Min Met. Mater. Soc. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, Z.; Ding, Y.; Xuan, Z.; Li, X. Old leaves and tender leaves nutritional composition analysis of Camellia nitidissima. J. Inner Mongolia Agr. Univ. 2016, 37, 52–56. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 208, 1334–1339. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zhong, S.; Wan, W.; Chen, X.; Chen, A.; Cheng, Y. Novel Graphene/In2O3 Nanocubes Preparation and Selective Electrochemical Detection for L-Lysine of Camellia nitidissima Chi. Materials 2020, 13, 1999. https://doi.org/10.3390/ma13081999
Cheng J, Zhong S, Wan W, Chen X, Chen A, Cheng Y. Novel Graphene/In2O3 Nanocubes Preparation and Selective Electrochemical Detection for L-Lysine of Camellia nitidissima Chi. Materials. 2020; 13(8):1999. https://doi.org/10.3390/ma13081999
Chicago/Turabian StyleCheng, Jinsheng, Sheng Zhong, Weihong Wan, Xiaoyuan Chen, Ali Chen, and Ying Cheng. 2020. "Novel Graphene/In2O3 Nanocubes Preparation and Selective Electrochemical Detection for L-Lysine of Camellia nitidissima Chi" Materials 13, no. 8: 1999. https://doi.org/10.3390/ma13081999
APA StyleCheng, J., Zhong, S., Wan, W., Chen, X., Chen, A., & Cheng, Y. (2020). Novel Graphene/In2O3 Nanocubes Preparation and Selective Electrochemical Detection for L-Lysine of Camellia nitidissima Chi. Materials, 13(8), 1999. https://doi.org/10.3390/ma13081999