Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Alloy Test Specimens
2.2. Laser Treatment and Titanium Dioxide Coating
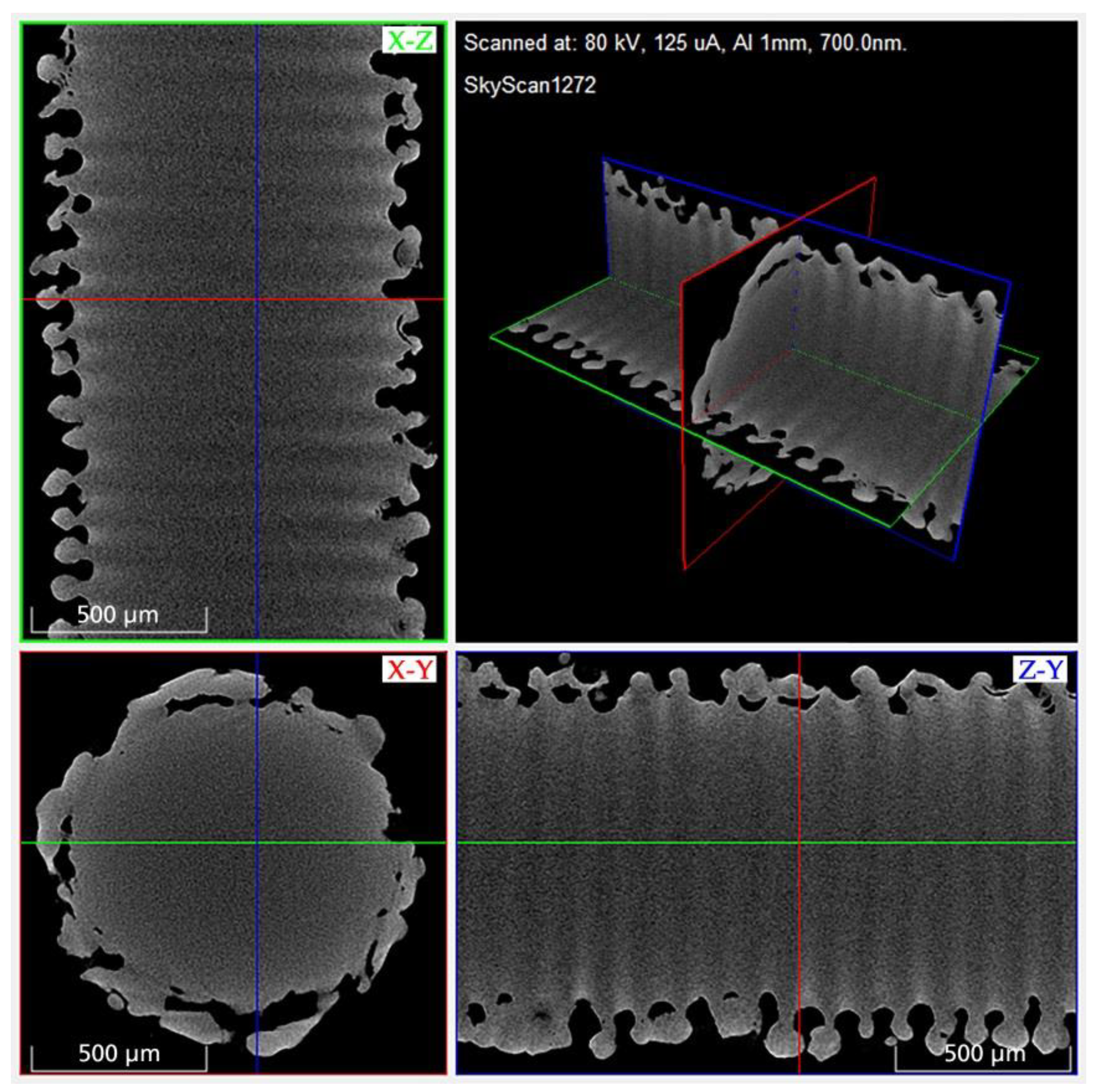
2.3. Micro-Computed Tomography (MicroCT)
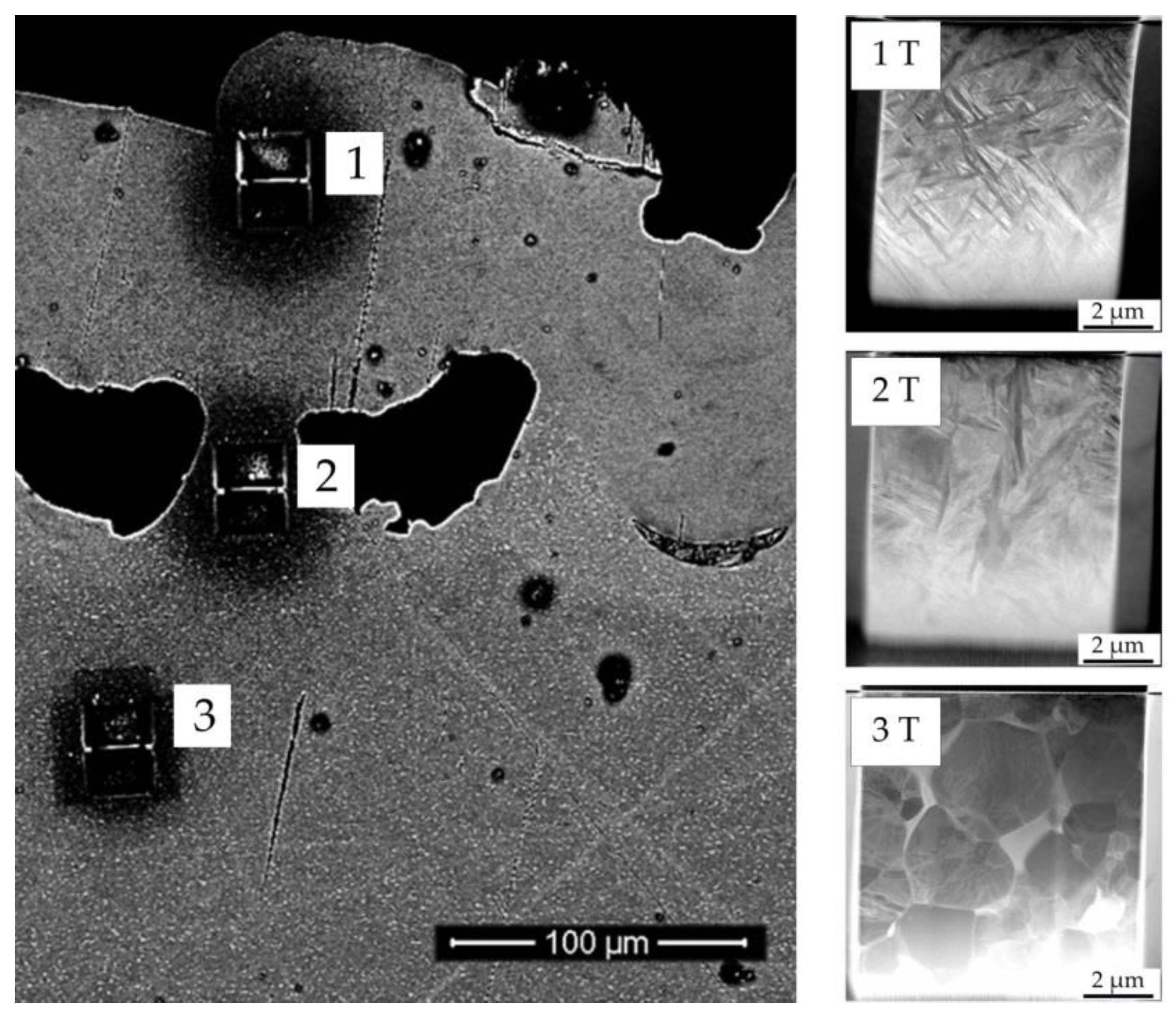
2.4. Electron Microscopy
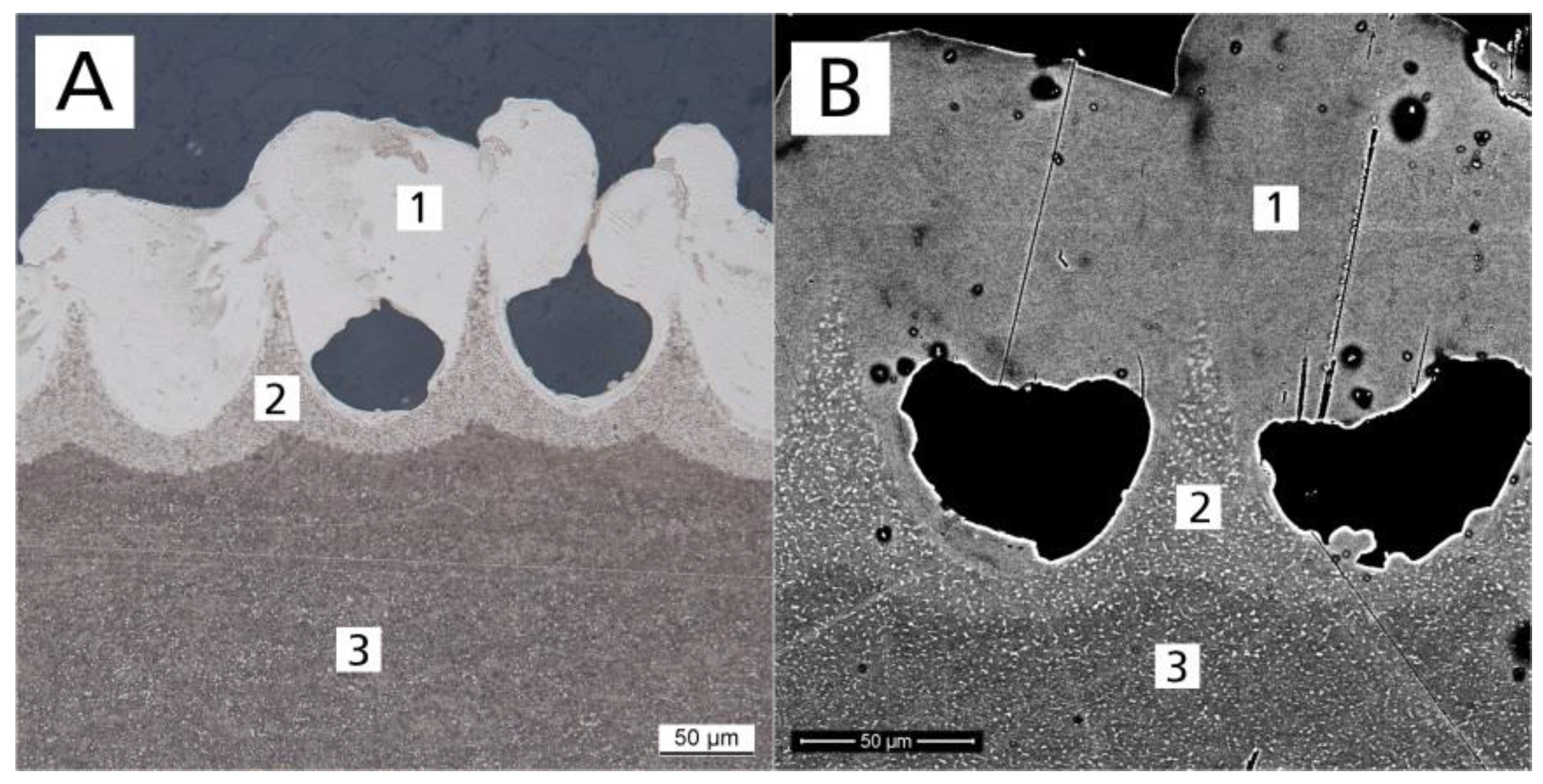
2.5. Metallography
2.6. Osteoblast Growth
2.7. In Vitro Cytotoxicity
2.8. Descriptive Statistics
3. Results
3.1. Microscopic Impact
3.2. Metallographic Impact
3.3. Impact on Osteoblasts (In Vitro)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Southam, J.C.; Selwyn, P. Structural changes around screws used in the treatment of fractured human mandibles. Br. J. Oral Surg. 1970, 8, 211–221. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Bezuidenhout, M.B.; Dimitrov, D.M.; van Staden, A.D.; Oosthuizen, G.A.; Dicks, L.M.T. Titanium-Based Hip Stems with Drug Delivery Functionality through Additive Manufacturing. BioMed. Res. Int. 2015, 2015, 134093. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Hahn, H.; Palich, W. Preliminary evaluation of porous metal surfaced titanium for orthopedic implants. J. Biomed. Mater. Res. 1970, 4, 571–577. [Google Scholar] [CrossRef]
- Branemark, R.; Branemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar] [PubMed]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Borcherding, K.; Marx, D.; Gätjen, L.; Bormann, N.; Wildemann, B.; Specht, U.; Salz, D.; Thiel, K.; Grunwald, I. Burst Release of Antibiotics Combined with Long-Term Release of Silver Targeting Implant-Associated Infections: Design, Characterization and in vitro Evaluation of Novel Implant Hybrid Surface. Materials 2019, 12, 3838. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.; Sefer, B.; Pederson, R.; Antti, M.-L. Study of alpha-case depth in Ti-6Al-2Sn-4Zr-2Mo and Ti-6Al-4V. IOP Conf. Ser.: Mater. Sci. Eng. 2013, 48, 12002. [Google Scholar] [CrossRef]
- Specht, U.; Ihde, J.; Mayer, B. Laser induced nano-porous Ti–O-layers for durable titanium adhesive bonding. Mat. Wiss. U. Werkst. 2014, 45, 1116–1122. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- Long, E.G.; Buluk, M.; Gallagher, M.B.; Schneider, J.M.; Brown, J.L. Human mesenchymal stem cell morphology, migration, and differentiation on micro and nano-textured titanium. Bioact. Mater. 2019, 4, 249–255. [Google Scholar] [CrossRef]
- Iwaszko, J. Microstructural aspects of laser surface treatment of commercially pure titanium. Met. Mater. 2019, 57, 11–18. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Femtosecond laser structuring of titanium implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Zeng, L.; Bieler, T.R. Effects of working, heat treatment, and aging on microstructural evolution and crystallographic texture of α, α′, α″ and β phases in Ti–6Al–4V wire. Mater. Sci. Eng. A 2005, 392, 403–414. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Sundberg, K.L.; Liang, J.; Sisson, R.D. Effect of Annealing Treatments on the Microstructure, Mechanical Properties and Corrosion Behavior of Direct Metal Laser Sintered Ti-6Al-4V. J. Mater. Eng. Perform. 2017, 26, 2572–2582. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Knisley, S.L.; Fagin, P.N.; Barker, D.R.; Zhang, F. Microstructure evolution during alpha-beta heat treatment of Ti-6Al-4V. Metall. Mater. Trans. A 2003, 34, 2377–2386. [Google Scholar] [CrossRef]
- Zimmermann, S.; Specht, U.; Spieß, L.; Romanus, H.; Krischok, S.; Himmerlich, M.; Ihde, J. Improved adhesion at titanium surfaces via laser-induced surface oxidation and roughening. Mater. Sci. Eng. A 2012, 558, 755–760. [Google Scholar] [CrossRef]
- Shanjin, L.; Yang, W. An investigation of pulsed laser cutting of titanium alloy sheet. Opt. Lasers Eng. 2006, 44, 1067–1077. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue failure of metallic biomaterials. In Metals for Biomedical Devices; Elsevier: Duxford, UK, 2019; pp. 153–188. ISBN 9780081026663. [Google Scholar]
- Joshi, K.S.; Rajyalakshmi, G.; Ranjith, G.; Kalainathan, S.; Prabhakaran, S. Optimization of Laser Shock Peening For Titanium. Mater. Today: Proc. 2018, 5, 12174–12186. [Google Scholar] [CrossRef]
- Hartjen, P.; Nada, O.; Silva, T.G.; Precht, C.; Henningsen, A.; Holthaus, M.G.; Gulow, N.; Friedrich, R.E.; Hanken, H.; Heiland, M.; et al. Cytocompatibility of Direct Laser Interference-patterned Titanium Surfaces for Implants. In Vivo 2017, 31, 849–854. [Google Scholar] [CrossRef]
- Sader, M.S.; Balduino, A.; Soares, G.d.A.; Borojevic, R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin. Oral Implant. Res. 2005, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ponader, S.; Vairaktaris, E.; Heinl, P.; Wilmowsky, C.v.; Rottmair, A.; Körner, C.; Singer, R.F.; Holst, S.; Schlegel, K.A.; Neukam, F.W.; et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J. Biomed. Mater. Res. 2008, 84A, 1111–1119. [Google Scholar] [CrossRef]
- Komorowski, P.; Sokołowska, P.; Siatkowska, M.; Elgalal, M.; Rosowski, M.; Makowski, K.; Lipińska, L.; Leszczewicz, M.; Styczyński, A.; Fogel, K.; et al. Designing laser-modified surface structures on titanium alloy custom medical implants using a hybrid manufacturing technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, R.; Erhardt, W.; Kerschbaumer, S.; Schmeller, M.L.; Gradinger, R. Tierexperimentelle Untersuchungen. In Ossäre Integration; Gradinger, R., Gollwitzer, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 30–37. ISBN 978-3-540-35687-5. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcherding, K.; Marx, D.; Gätjen, L.; Specht, U.; Salz, D.; Thiel, K.; Wildemann, B.; Grunwald, I. Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment. Materials 2020, 13, 2000. https://doi.org/10.3390/ma13082000
Borcherding K, Marx D, Gätjen L, Specht U, Salz D, Thiel K, Wildemann B, Grunwald I. Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment. Materials. 2020; 13(8):2000. https://doi.org/10.3390/ma13082000
Chicago/Turabian StyleBorcherding, Kai, Dennis Marx, Linda Gätjen, Uwe Specht, Dirk Salz, Karsten Thiel, Britt Wildemann, and Ingo Grunwald. 2020. "Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment" Materials 13, no. 8: 2000. https://doi.org/10.3390/ma13082000
APA StyleBorcherding, K., Marx, D., Gätjen, L., Specht, U., Salz, D., Thiel, K., Wildemann, B., & Grunwald, I. (2020). Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment. Materials, 13(8), 2000. https://doi.org/10.3390/ma13082000




