Quantum-Chemical Design of Molecular Structures of Tetra-, Penta- and Hexanuclear Metal Clusters Containing Aluminum and 3d-Element Atoms
Abstract
1. Introduction
2. Tetranuclear (AlM) Metal Clusters
3. Pentanuclear (AlM) Metal Clusters
4. Hexanuclear (AlM) Metal Clusters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maroun, F.; Ozanam, F.; Magnussen, O.M.; Behm, R.J. The role of atomic ensembles in the reactivity of bimetallic electrocatalysts. Science 2001, 293, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Derosa, P.A.; Seminario, J.M.; Balbuena, P.B. Properties of Small Bimetallic Ni−Cu Clusters. J. Phys. Chem. A 2001, 105, 7917–7925. [Google Scholar] [CrossRef]
- Eberhardt, W. Clusters as new materials. Surf. Sci. 2002, 500, 242–270. [Google Scholar] [CrossRef]
- Lu, Q.L.; Zhu, L.Z.; Ma, L.; Wang, G.H. Magnetic properties of Co/Cu and Co/Pt bimetallic clusters. Chem. Phys. Lett. 2005, 407, 176–179. [Google Scholar] [CrossRef]
- Yuan, D.W.; Wang, Y.; Zeng, Z. Geometric, electronic, and bonding properties of Au[sub N]M (N=1–7, M=Ni, Pd, Pt) clusters. J. Chem. Phys. 2005, 122, 114310. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Z.; Lu, X.; Lin, M.; Zhang, Q. Structure and stability of binary transition-metal clusters (NbCo)[sub n] (n ≤ 5): A relativistic density-functional study. J. Chem. Phys. 2005, 123, 064315. [Google Scholar] [CrossRef]
- Barcaro, G.; Fortunelli, A.; Rossi, G.; Nita, F.; Ferrando, R. Electronic and Structural Shell Closure in AgCu and AuCu Nanoclusters. J. Phys. Chem. B 2006, 110, 23197–23203. [Google Scholar] [CrossRef]
- Néel, N.; Kröger, J.; Berndt, R.; Wehling, T.O.; Lichtenstein, A.I.; Katsnelson, M.I. Controlling the kondo effectin CoCun clusters atom by atom. Phys. Rev. Lett. 2008, 101, 266803. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Xiao-Yu, K.; Jian-Jun, G.; Ben-Xia, Z. First-principle study of AunFe (n=1–7) clusters. J. Mol. Struct. 2009, 902, 54–58. [Google Scholar]
- Du, J.; Shen, N.; Zhu, L.; Wang, J. Emergence of noncollinear magnetic ordering in bimetallic Co6− n Mnn clusters. J. Phys. D: Appl. Phys. 2010, 43, 015006. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, F.Q.; Jia, J.F.; Xu, X.H.; Wu, H.S. First-principles study of structural, electronic and magnetic properties of Co13−nMn (n=1, 2, M=Mn, V and Al) clusters. J. Mol. Struct. (TheoChem) 2010, 955, 14–22. [Google Scholar] [CrossRef]
- Kilimis, D.A.; Papageorgiou, D.G. Density functional study of small bimetallic Ag–Pd clusters. J. Mol. Struct. 2010, 939, 112–117. [Google Scholar] [CrossRef]
- Lin, L.; Claes, P.; Gruene, P.; Meijer, G.; Fielicke, A.; Nguyen, M.T.; Lievens, P. Far-Infrared Spectra of Yttrium-Doped Gold Clusters AunY (n = 1-9). ChemPhysChem 2010, 11, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Garbounis, D.N.; Tsipis, A.C.; Tsipis, C.A. Structural, electronic, bonding, magnetic, and optical properties of bimetallic [RunAum]0/+ (n+m ≤ 3) clusters. J. Comput. Chem. 2010, 31, 2836–2852. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Fa, W.; Dong, I. Magnetic Properties of Transition-Metal-Doped Tubular Gold Clusters: M@Au24 (M = V, Cr, Mn, Fe, Co, and Ni). J. Phys. Chem. A 2010, 114, 4031–4035. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.S.; Sahara, R.; Mizuseki, H.; Kawazoe, Y. Titanium-Doped Nickel Clusters TiNin (n = 1−12): Geometry, Electronic, Magnetic, and Hydrogen Adsorption Properties. J. Phys. Chem. A 2010, 114, 5049–5057. [Google Scholar] [CrossRef]
- Wang, H.Q.; Kuang, X.Y.; Li, H.F. Density functional study of structural and electronic properties of bimetallic copper-gold clusters: Comparison with pure and doped gold clusters. Phys. Chem. Chem. Phys. 2010, 12, 5156. [Google Scholar] [CrossRef]
- Montejano-Carrizales, J.M.; Aguilera-Granja, F.; Morán-López, J.L. Structural and magnetic properties of FemYn (m+n = 7, Y = Ru, Rh, Pd, and Pt) nanoalloys. Eur. Phys. J. D 2011, 64, 53–62. [Google Scholar] [CrossRef]
- Jin, X.X.; Du, J.G.; Jiang, G.; Luo, X.; Wang, X.W. Geometries and electronic properties of NbnV(0, ±1) (n = 1−6) clusters studied by density-functional theory. Eur. Phys. J. D 2011, 64, 323–329. [Google Scholar] [CrossRef]
- Yang, J.X.; Guo, J.J.; Die, D. Ab initio study of AunIr (n=1–8) clusters. Comput. Theor. Chem. 2011, 963, 435–438. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, Y.; Wang, J.; Yin, W. Density functional study of NO binding on small AgnPdm (n+m≤5) clusters. Comput. Theor. Chem. 2011, 964, 298–303. [Google Scholar] [CrossRef]
- Ma, Q.M.; Xie, Z.; Wang, B.R.; Liu, Y.; You-Cheng Li, Y.C. Structure, stability and magnetic moments of the FenCr clusters: All-electron density functional theory investigations. Solid State Commun. 2011, 151, 806–810. [Google Scholar] [CrossRef]
- Aguilera-Granja, F.; Longo, R.C.; Gallego, L.J.; Vega, A. Magnetic Cooperative Effects in Small Ni–Ru Clusters. J. Phys. Chem. A 2011, 115, 13950–13955. [Google Scholar] [CrossRef] [PubMed]
- Nhatab, P.V.; Nguyen, M.T. Trends in structural, electronic and energetic properties of bimetallic vanadium–gold clusters AunV with n = 1-14. Phys. Chem. Chem. Phys. 2011, 13, 16254–16264. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bai, X.; Jia, J.F.; Xu, X.H.; Wu, H.S. Structural, electronic and magnetic properties of ConRh (n=1–8) clusters from density functional calculations. Phys. B Condens. Matt. 2012, 407, 14–21. [Google Scholar] [CrossRef]
- Hong, L.; Wang, H.; Cheng, J.; Huang, X.; Sai, L.; Zhao, J. Atomic structures and electronic properties of small Au–Ag binary clusters: Effects of size and composition. Comput. Theor. Chem. 2012, 993, 36–44. [Google Scholar] [CrossRef]
- Shewale, V.; Deshpande, M. Structural, electronic, and magnetic properties of NinM clusters (M=Hf, Ta, W) with n=1–12. Comput. Theor. Chem. 2012, 984, 128–136. [Google Scholar] [CrossRef]
- Ding, L.P.; Kuang, X.Y.; Shao, P.; Zhao, Y.R.; Li, Y.F. A comparative study on geometries, stabilities, and electronic properties between bimetallic AgnX (X = Au, Cu; n=1−8) and pure silver clusters. Chin. Phys. B 2012, 21, 043601. [Google Scholar] [CrossRef]
- Yao, J.; Bin Xu, B.; Wang, Y. Ground State Structures, Electronic and Magnetic Properties of ScnFe (n=2-10) Clusters. Chin. J. Chem. 2012, 30, 905–913. [Google Scholar] [CrossRef]
- Florez, E.; Mondragon, F.; Illas, F. Theoretical study of the structure and reactivity descriptors of CunM (M = Ni, Pd, Pt, n = 1–4) bimetallic nanoparticles supported on MgO(001). Surf. Sci. 2012, 606, 1010–1018. [Google Scholar] [CrossRef]
- Ju, W.; Yang, Z. Influence of spin–orbit coupling on electronic structures of TM@Au12 (TM=3d, 4d, and 5d atoms). Phys. Lett. A 2012, 376, 1300–1305. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Hao, Y.; Wang, G. Density functional theory study of FePdn (n=2–14) clusters and interactions with small molecules. Comput. Mater. Sci. 2013, 68, 166–173. [Google Scholar] [CrossRef]
- Liu, X.; Tian, D.; Meng, C. DFT study on stability and H2 adsorption activity of bimetallic Au79−nPdn (n=1–55) clusters. Chem. Phys. 2013, 415, 179–185. [Google Scholar] [CrossRef]
- Tafoughalt, M.A.; Samah, M. Structural properties and relative stability of silver-doped gold clusters AgAun-1 (n = 3–13): Density functional calculations. Comput. Theor. Chem. 2014, 1033, 23–30. [Google Scholar] [CrossRef]
- Wen, J.Q.; Xia, T.; Zhou, H.; Wang, J.F. A density functional theory study of small bimetallic PdnAl (n=1–8) clusters. J. Phys. Chem. Solids 2014, 75, 528–534. [Google Scholar] [CrossRef]
- Singh, N.B.; Sarkar, U. A density functional study of chemical, magnetic and thermodynamic properties of small palladium clusters. Mol. Simul. 2014, 40, 1255–1264. [Google Scholar] [CrossRef]
- Bouderbala, W.; Boudjahem, A.G.; Soltani, A. Geometries, stabilities, electronic and magnetic properties of small PdnIr (n = 1–8) clusters from first principles calculations. Mol. Phys. 2014, 112, 1789–1798. [Google Scholar] [CrossRef]
- Ling, W.; Dong, D.; Shi-Jian, W.; Zheng-Quan, Z. Geometrical, electronic, and magnetic properties of CunFe (n=1–12) clusters: A density functional study. J. Phys. Chem. Solids 2015, 76, 10–16. [Google Scholar] [CrossRef]
- Chaves, A.S.; Rondina, G.G.; Piotrowski, M.J.; Da Silva, J.L.F. Structural formation of binary PtCu clusters: A density functional theory investigation. Comput. Mater. Sci. 2015, 98, 278–286. [Google Scholar] [CrossRef]
- Gong, X.; Ju, W.; Li, T.; Feng, Z.; Wang, Y. Spin–orbit Splitting and Magnetism of Icosahedral M@Ag12 Clusters (M = 3d and 4d atoms). J. Clust. Sci. 2015, 26, 759–773. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Molekularnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterobi)tetrayadernyi klaster Fe2Co2. Herald Technol. Univ. 2016, 19, 20–23. [Google Scholar]
- Die, D.; Zheng, B.X.; Zhao, L.Q.; Zhu, Q.W.; Zhao, Z.Q. Insights into the structural, electronic and magnetic properties of V-doped copper clusters: Comparison with pure copper clusters. Sci. Rep. 2016, 6, 31978. [Google Scholar] [CrossRef] [PubMed]
- Al-Odail, F.; Mazher, J.; Abuelela, A.M. A density functional theory study of structural, electronic and magnetic properties of small PdnAg (n = 1–8) clusters. Comput. Theor. Chem. 2017, 1125, 103–111. [Google Scholar] [CrossRef]
- Deng, M.; Xin, Z.; Yan, X.; Liu, J.; Yu, M. Structural, Electronic, and Magnetic Properties of Bimetallic NimNbn (m+n ≤ 8) Clusters: First Principle Study. J. Supercond. Novel Magn. 2017, 30, 251–260. [Google Scholar] [CrossRef]
- Zhang, G.; Zhai, Z.; Sheng, Y. Structural, electronic and magnetic properties of TinMo (n = 1-7) clusters. Eur. Phys. J. D 2017, 71, 9. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lv, J. Equilibrium geometries, electronic structure and magnetic properties of ConSn (n = 1–12) clusters from density functional calculations. Mod. Phys. Lett. B 2017, 31, 1750171. [Google Scholar] [CrossRef]
- Datta, S.; Raychaudhuri, A.K.; Saha-Dasgupta, T. First principles study of bimetallic Ni13− nAgn nano-clusters (n = 0–13): Structural, mixing, electronic, and magnetic properties. J. Chem. Phys. 2017, 146, 164301. [Google Scholar] [CrossRef]
- Singh, R.K.; Iwasa, T.; Taketsugu, T. Insights into geometries, stabilities, electronic structures, reactivity descriptors, and magnetic properties of bimetallic NimCun-m (m = 1, 2; n = 3-13) clusters: Comparison with pure copper clusters. J. Comput. Chem. 2018, 39, 1878–1889. [Google Scholar] [CrossRef]
- Ma, S.; Fei, S.; Huang, L.; Forrey, R.C.; Cheng, H. Tuning the Catalytic Activity of PdxNiy (x + y = 6) Bimetallic Clusters for Hydrogen Dissociative Chemisorption and Desorption. ACS Omega 2019, 4, 12498–12504. [Google Scholar] [CrossRef]
- Ranjan, P.; Chakraborty, T. Structure and electronic properties of AunPt (n = 1–8) nanoalloy clusters: The density functional theory study. J. Nanopart. Res. 2020, 22, 11. [Google Scholar] [CrossRef]
- Deshpande, M.D.; Roy, S.; Kanhere, D.G. Equilibrium geometries, electronic structure, and magnetic properties of NinSn clusters (n=1–12). Phys. Rev. B 2007, 76, 195423. [Google Scholar] [CrossRef]
- Chen, X.; Deng, K.; Liu, Y.; Tang, C.; Yuan, Y.; Tan, W.; Wang, X. The geometric, optical, and magnetic properties of the endohedral stannaspherenes M@Sn[sub 12] (M=Ti, V, Cr, Mn, Fe, Co, Ni). J. Chem. Phys. 2008, 129, 094301. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, M.; Zhu, W.; Deng, K. Probing the geometric, optical, and magnetic properties of 3d transition-metal endohedral Ge12M (M=Sc–Ni) clusters. Comput. Theor. Chem. 2011, 969, 56–60. [Google Scholar] [CrossRef]
- Rohrmann, U.; Schäfer, R. Stern-Gerlach Experiments on Fe@Sn12: Magnetic Response of a Jahn–Teller Distorted Endohedrally Doped Molecular Cage Cluster. J. Phys. Chem. C 2015, 119, 10958–10961. [Google Scholar] [CrossRef]
- Shewale, V.H.; Deshpande, M.D.; Kanhere, D.G. Structures, energetics and magnetic properties of (NiSn)n clusters with n = 1–6. Pramana 2009, 73, 699–710. [Google Scholar] [CrossRef]
- Jaiswal, S.; Kumar, V. Growth behavior and electronic structure of neutral and anion ZrGen (n = 1–21) clusters. Comput. Theor. Chem. 2016, 1075, 87–97. [Google Scholar] [CrossRef]
- Sosa-Hernández, E.M.; Montejano-Carrizales, J.M.; Alvarado-Leyva, P.G. Global Minimum Structures, Stability and Electronic Properties of Small NiXSnY (X+Y≤ 5) Bimetallic Clusters; a DFT Study. Eur. Phys. J. D 2016, 70, 208. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, J.; Jing, Q.; Luo, Y.; Wang, Y. A density functional study of Y[sub n]Al (n=1–14) clusters. J. Chem. Phys. 2007, 127, 234312. [Google Scholar] [CrossRef]
- Tian, F.Y.; Jing, Q.; Wang, Y.X. Structure, stability, and magnetism of ScnAl (n=1–8,12) clusters: Density-functional theory investigations. Phys. Rev. A 2008, 77, 013202. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, G.; Huang, X.; Du, Z.; Li, Y. Study of the size-dependent properties of ScnAl (n = 1–14) clusters by density-functional theory. J. Phys. Condens. Matter. 2009, 21, 046004. [Google Scholar] [CrossRef]
- Du, J.; Sun, X.; Jiang, G. Structures, chemical bonding, magnetisms of small Al-doped zirconium clusters. Phys. Lett. A 2010, 374, 854–860. [Google Scholar] [CrossRef]
- Hua, Y.; Liu, Y.; Jiang, G.; Du, J.; Chen, J. Geometric Transition and Electronic Properties of Titanium-Doped Aluminum Clusters: AlnTi (n = 2–24). J. Phys. Chem. A 2013, 117, 2590–2597. [Google Scholar] [CrossRef] [PubMed]
- Rusina, G.G.; Borisova, S.D.; Chulkov, E.V. Structure and atomic vibrations in bimetallic Ni13 − nAln clusters. JETP Letters 2015, 101, 474–480. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Hoe, W.M.; Cohen, A.; Handy, N.C. Assessment of a new local exchange functional OPTX. Chem. Phys. Lett. 2001, 341, 319–328. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Paulsen, H.; Duelund, L.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Free Energy of Spin-Crossover Complexes Calculated with Density Functional Methods. Inorg. Chem. 2001, 40, 2201–2203. [Google Scholar] [CrossRef]
- Swart, M.; Groenhof, A.R.; Ehlers, A.W.; Lammertsma, K. Validation of Exchange−Correlation Functionals for Spin States of Iron Complexes. J. Phys. Chem. A 2004, 108, 5479–5483. [Google Scholar] [CrossRef]
- Swart, M.; Ehlers, A.W.; Lammertsma, K. Performance of the OPBE exchange-correlation functional. Mol. Phys. 2004, 102, 2467–2474. [Google Scholar] [CrossRef]
- Swart, M. Metal–ligand bonding in metallocenes: Differentiation between spin state, electrostatic and covalent bonding. Inorg. Chim. Acta 2007, 360, 179–189. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterobi)tetrayadernyi klaster AlFe3. Herald Technol. Univ. 2016, 19, 18–21. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Models of Molecular Structures of Aluminum–Iron Clusters AlFe3, Al2Fe3, and Al2Fe4 according to Quantum-Chemical DFT Calculations. Russ. J. Inorg. Chem. 2017, 62, 336–343. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Modeli molekularnylh struktur alyuminii-zheleznykh klasterov AlFe3, Al2Fe3 i Al2Fe4 po dannym kvantovo-khimicheskogo rascheta metodom DFT. Zh. Neorg. Khim. 2017, 62, 321–329. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Molecular Structures of Tetranuclear (Al, Fe) Metal Clusters. Glass Phys. Chem. 2018, 44, 339–345. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Molekularnye struktury tetrayadernykh (Al, Fe) metalloklasterov. Fizika i Khimiya Stekla 2018, 44, 408–415. [Google Scholar]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterotri)tetrayadernyi klaster Al2FeCo. Herald Technol. Univ. 2016, 19, 30–32. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Quantum Chemical Calculation of Molecular Structures of Al2Fe2 and Al2FeCo Tetranuclear Metalloclusters. Glass Phys. Chem. 2017, 43, 597–604. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Kvantovo-khimicheskii raschyot molekularnykh struktur tetrayadernykh metalloklasterov Al2Fe2 i Al2FeCo. Fizika i Khimiya Stekla 2017, 43, 632–639. [Google Scholar]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterobi)pentayadernyi klaster Al2Ti3. Herald Technol. Univ. 2016, 19, 5–8. [Google Scholar]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterobi)pentayadernyi klaster Al2V3. Herald Technol. Univ. 2016, 19, 12–15. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Molecular structure models of Al2Ti3 and Al2V3 clusters according to DFT quantum-chemical calculation. Eur. Chem. Bull. 2020, 9, 62–68. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Models of Molecular Structures of Al2Cr3 and Al2Mo3 Metal Clusters according to Density Functional Theory Calculations. Russ. J. Inorg. Chem. 2018, 63, 786–799. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Modeli molekularnykh struktur metalloklasterov Al2Cr3 i Al2Mo3 po dannym metoda funktsionala plotnosti. Zh. Neorg. Khim. 2018, 63, 750–763. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Quantum-chemical calculation of molecular structures of Al2Mn3 and Al2Zn3 clusters by using DFT method. Struct. Chem. 2019, 30, 1289–1299. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Models of Molecular Structure of Heteronuclear Clusters Al2Fe3, Al2Co3, and Al2Ni3 According to the Data of Quantum-Chemical Density Functional Simulation. Russ. J. Gen. Chem. 2016, 86, 1991–1998. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Modeli molekularnykh struktur geteroyadernykh klasterov Al2Fe3, Al2Co3 и Al2Ni3 po dannym kvantovo-khimicheskogo raschyota po metody funktsionala plotnosti. Zh. Obshch. Khim. 2016, 86, 1419–1428. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. DFT calculation of molecular structures of Al2Fe3 and Al2Cu3 heterobinuclear clusters. Struct. Chem. 2018, 29, 1543–1549. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. DFT Quantum Chemical Calculation of the Molecular Structures of the Metal Clusters Al2Cu3 and Al2Ag3. Russ. J. Inorg. Chem. 2019, 64, 79–87. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. DFT Kvantovo-khimicheskii raschyot molekularnykh struktur metalloklasterov Al2Cu3 i Al2Ag3 metodom DFT. Zh. Neorg. Khim. 2019, 64, 63–71. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Thermodynamics of Al2M3 Metal Clusters (M = 3d-element) in the framework of quantum-chemical modeling by DFT method. Russ. J. Inorg. Chem. 2020, 65, 646–649. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. Termodinamika metalloklasterov Al2M3 (M = 3d-element) v ramkakh kvantovo-khimicheskogo modelirovaniya metodom DFT. Zh. Neorg. Khim. 2020, 65, 598–602. [Google Scholar]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury poliyadernykh metalloklasterov po dannym raschyota metodom DFT. (Geterobi)hexayadernyi klaster Al3Fe3. Herald Technol. Univ. 2016, 19, 89–93. [Google Scholar]
- Chachkov, D.V.; Mikhailov, O.V. Molecular Structure of Hexatomic Heteronuclear (AlFe) Metal Clusters as Determined by the DFT Quantum-Chemical Calculation. Russ. J. Gen. Chem. 2017, 87, 670–678. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Molekulyarnye struktury shestiatomnykh geteroyadernykh (AlFe) metalloklasterov po dannym po dannym kvantovo-khimicheskogo raschyota metodom DFT. Zh. Obshch. Khim. 2017, 87, 535–543. [Google Scholar]
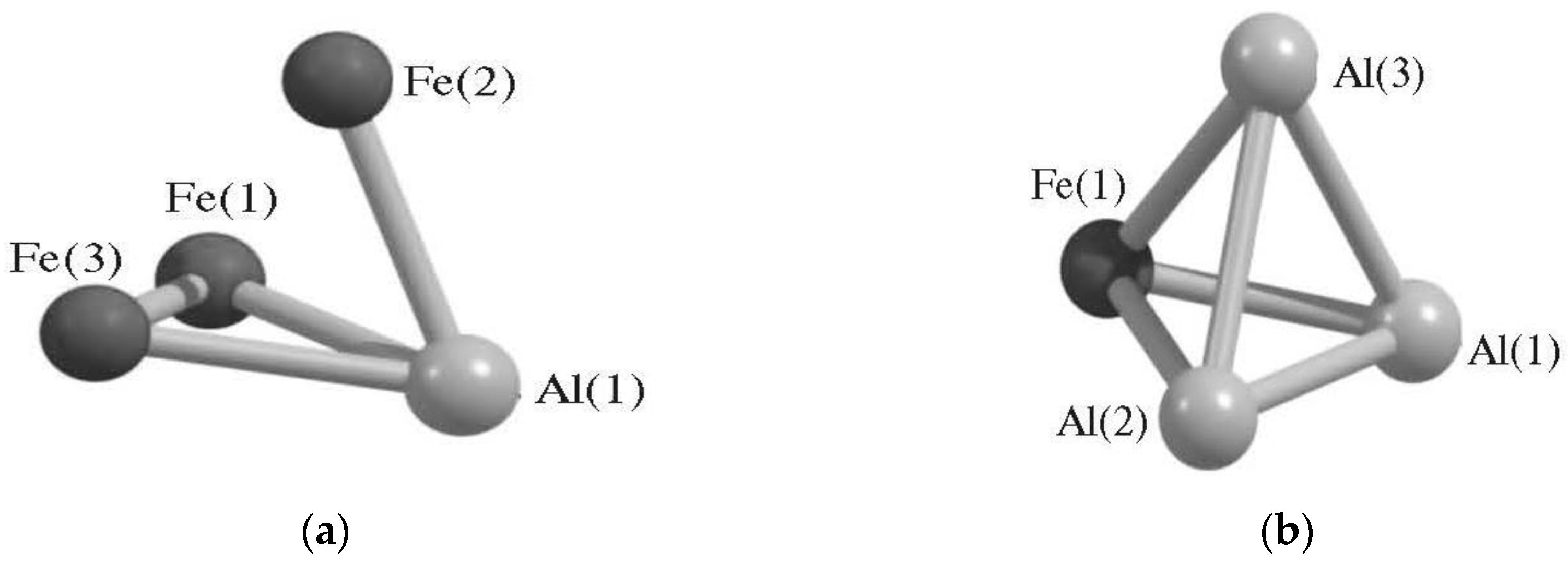
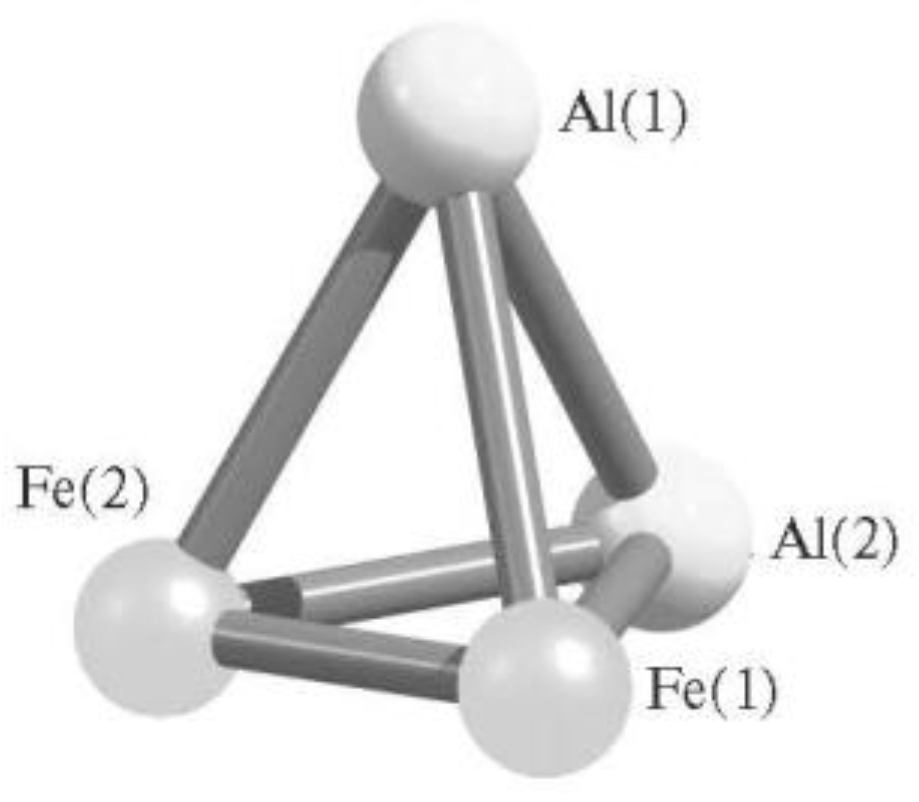
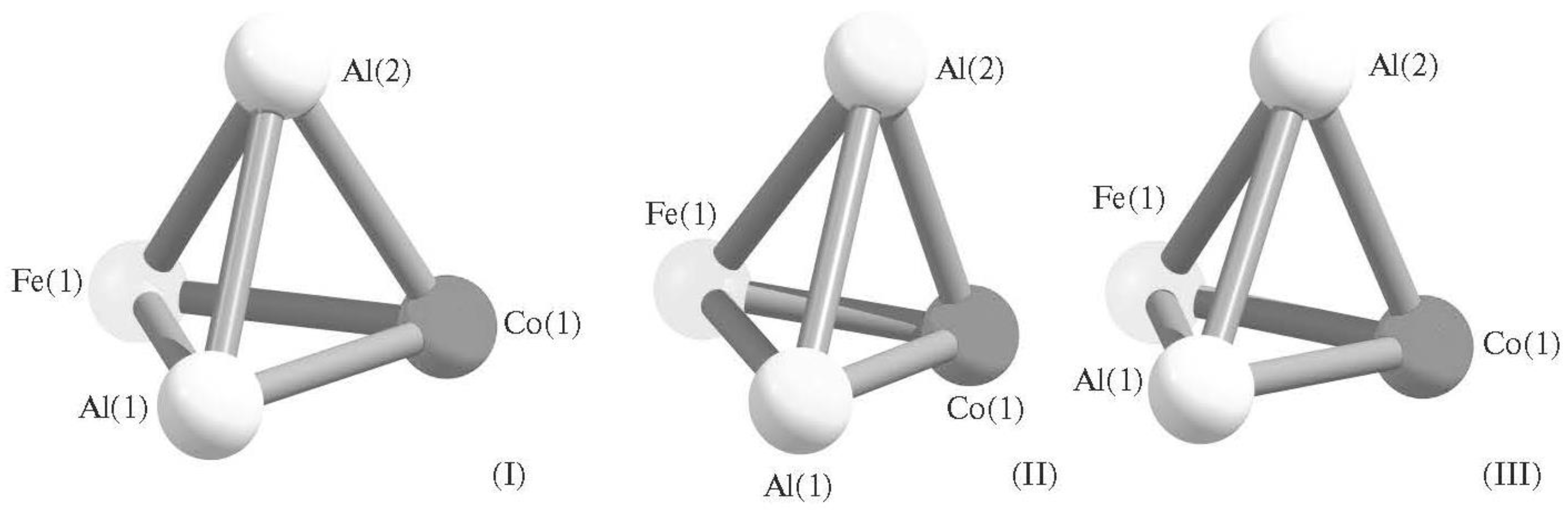
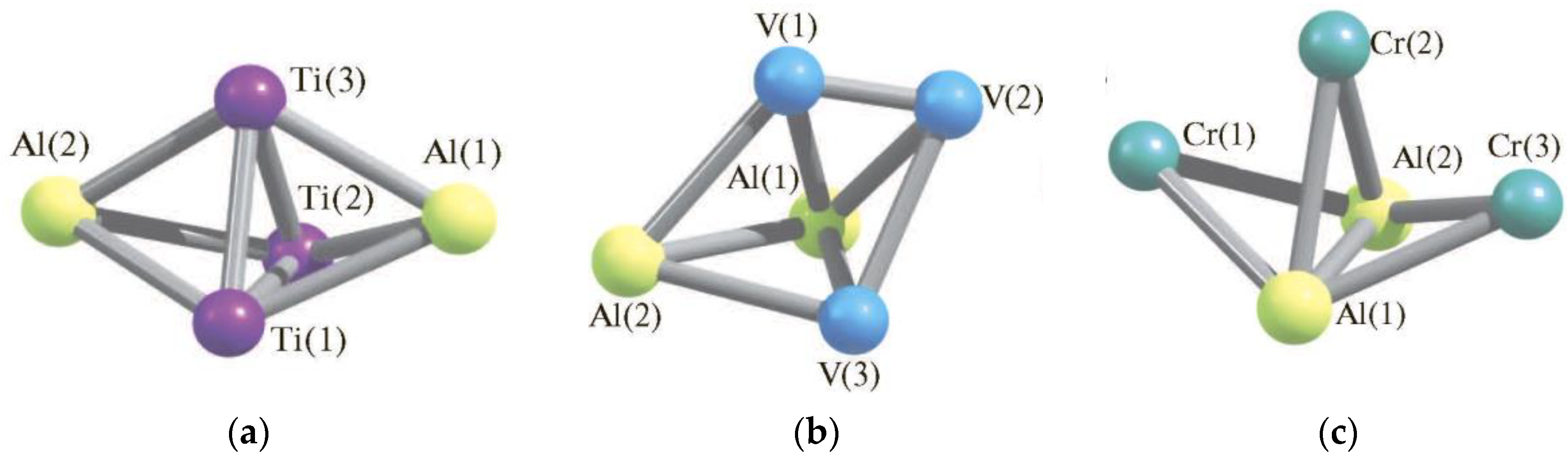
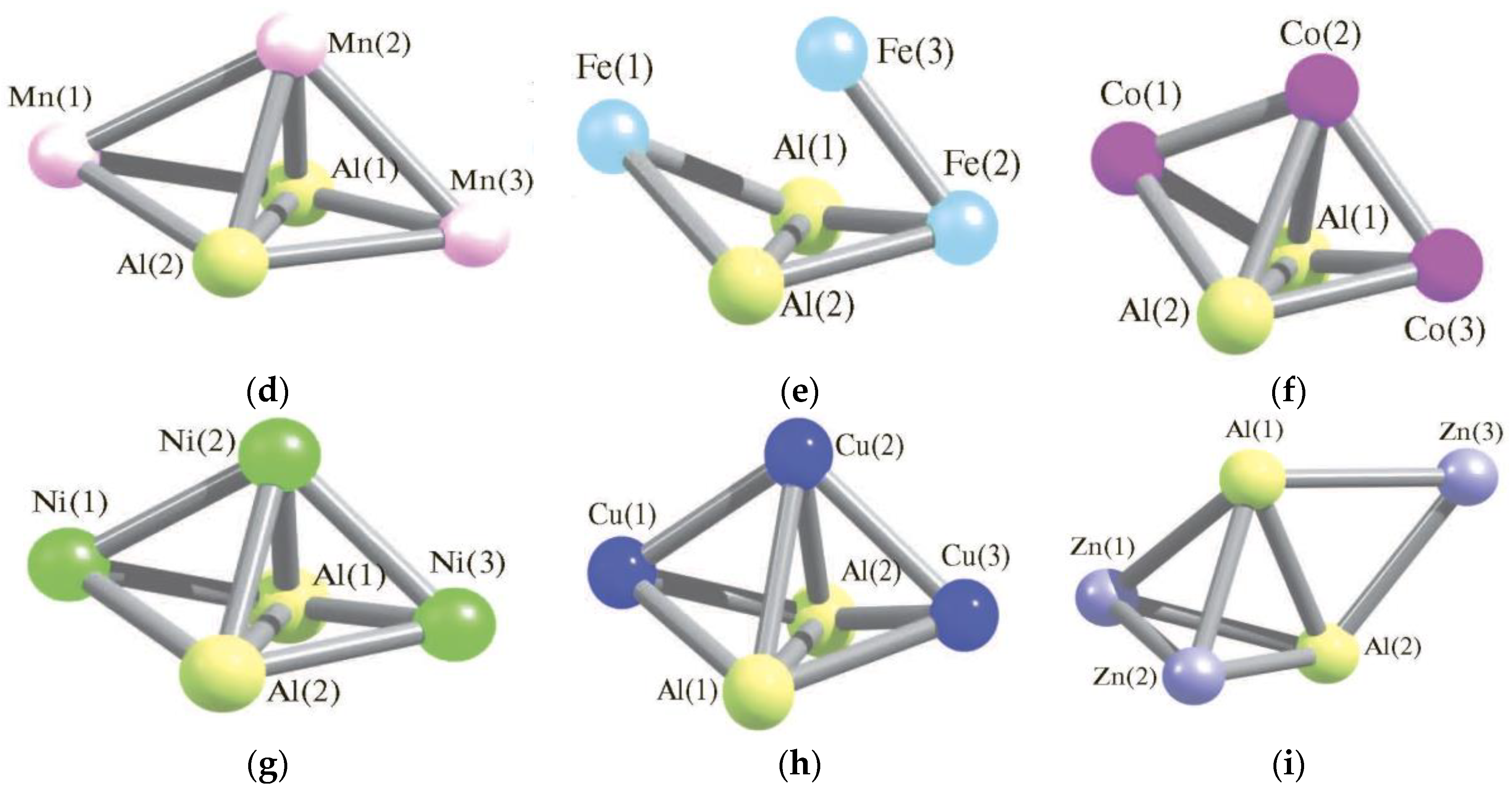
| Structure Designation | Spin Multiplicity of the Ground State | Relative Energy, kJ/mol | Ref. |
|---|---|---|---|
| Al3Fe Metal Cluster | |||
| Al3Fe (I) | 2 | 0.0 | [79,80] |
| Al3Fe (VII) | 2 | 175.1 | |
| Al3Fe (II) | 4 | 32.7 | |
| Al3Fe (IV) | 4 | 153.3 | |
| Al3Fe (III) | 6 | 83.4 | |
| Al3Fe (VI) | 6 | 84.8 | |
| Al3Fe (V) | 6 | 193.1 | |
| AlFe3 Metal Cluster | |||
| AlFe3 (II) | 2 | 11.2 | [79,80] |
| AlFe3 (I) | 2 | 104.0 | |
| AlFe3 (VII) | 2 | 122.8 | |
| AlFe3 (VIII) | 2 | 198.7 | |
| AlFe3 (V) | 4 | 0.0 | |
| AlFe3 (III) | 4 | 11.3 | |
| AlFe3 (IX) | 4 | 150.2 | |
| AlFe3 (VI) | 6 | 17.4 | |
| AlFe3 (IV) | 6 | 41.7 | |
| AlFe3 (X) | 6 | 52.6 | |
| Al2Fe2 Metal Cluster | |||
| Al2Fe2 (XII) | 1 | 45.1 | [78,79,80] |
| Al2Fe2 (VII) | 1 | 46.7 | |
| Al2Fe2 (IX) | 1 | 150.3 | |
| Al2Fe2 (X) | 1 | 209.8 | |
| Al2Fe2 (III) | 1 | 352.5 | |
| Al2Fe2 (VI) | 3 | 66.5 | |
| Al2Fe2 (VIII) | 3 | 68.4 | |
| Al2Fe2 (IV) | 3 | 137.9 | |
| Al2Fe2 (XI) | 3 | 143.6 | |
| Al2Fe2 (II) | 3 | 254.5 | |
| Al2Fe2 (V) | 5 | 0.0 | |
| Al2Fe2 (I) | 5 | 152.7 | |
| Al3Fe Metal Cluster | Al2Fe2 Metal Cluster | AlFe3 Metal Cluster | |||
|---|---|---|---|---|---|
| Metal-Metal Bond Lengths, pm | Metal-Metal Bond Lengths, pm | Metal-Metal Bond Lengths, pm | |||
| Al1Al2 | 263.4 | Al1Al2 | 260.8 | Al1Fe1 | 249.1 |
| Al1Al3 | 263.3 | Al1Fe1 | 249.5 | Al1Fe2 | 249.1 |
| Al2Al3 | 274.3 | Al1Fe2 | 249.4 | Al1Fe3 | 255.5 |
| Al1Fe1 | 245.7 | Al2Fe1 | 249.5 | Fe1Fe2 | 208.6 |
| Al2Fe1 | 235.0 | Al2Fe2 | 249.5 | Fe1Fe3 | 248.7 |
| Al3Fe1 | 235.1 | Fe1Fe2 | 199.2 | Fe2Fe3 | 248.7 |
| Bond Angles, deg | Bond Angles, deg | Bond Angles, deg | |||
| Al1Fe1Al2 | 66.4 | Fe1Al1Fe2 | 47.1 | Fe1Al1Fe2 | 49.5 |
| Fe1Al1Al2 | 54.9 | Fe1Al2Fe2 | 47.1 | Al1Fe1Fe2 | 65.3 |
| Al1Al2Fe1 | 58.7 | Fe1Al1Al2 | 58.5 | Fe1Fe2Al1 | 65.2 |
| Al1Fe1Al3 | 66.4 | Fe1Al2Al1 | 58.5 | Fe1Al1Fe3 | 59.0 |
| Al2Fe1Al3 | 71.4 | Fe2Al1Al2 | 58.5 | Fe2Al1Fe3 | 59.0 |
| Al1Al2Al3 | 58.6 | Fe2Al2Al1 | 58.5 | Fe1Fe2Fe3 | 65.2 |
| Al2Al3Al1 | 58.6 | Al1Fe1Al2 | 63.0 | Fe2Fe3Fe1 | 49.6 |
| Al3Al1Al2 | 62.8 | Al1Fe2Al2 | 63.1 | Fe3Fe1Fe2 | 65.2 |
| M | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| N | 14 | 11 | 20 | 25 | 8 | 9 | 7 | 8 | 14 |
| Ref. | [81,83] | [82,83] | [84,85] | [86] | [87,88,89] | [87,88] | [87,88] | [89,90,91] | [86] |
| Structure Designation | Spin Multiplicity of the Ground State | Relative Energy, kJ/mol | Ref. |
|---|---|---|---|
| Al2Ti3 Metal Cluster | |||
| Al2Ti3 (XIII) | 1 | 21.5 | [81,83] |
| Al2Ti3 (II) | 1 | 24.1 | |
| Al2Ti3 (VII) | 1 | 44.8 | |
| Al2Ti3 (XIV) | 1 | 51.1 | |
| Al2Ti3 (VIII) | 1 | 77.5 | |
| Al2Ti3 (III) | 1 | 93.0 | |
| Al2Ti3 (XII) | 3 | 12.6 | |
| Al2Ti3 (X) | 3 | 37.0 | |
| Al2Ti3 (VI) | 3 | 37.2 | |
| Al2Ti3 (I) | 3 | 80.9 | |
| Al2Ti3 (XI) | 5 | 0.0 | |
| Al2Ti3 (V) | 5 | 19.7 | |
| Al2Ti3 (IV) | 5 | 60.7 | |
| Al2Ti3 (IX) | 5 | 73.0 | |
| Al2V3 Metal Cluster | |||
| Al2V3 (I) | 2 | 25.9 | [82,83] |
| Al2V3 (IV) | 2 | 26.7 | |
| Al2V3 (VII) | 2 | 30.2 | |
| Al2V3 (V) | 4 | 0.0 | |
| Al2V3 (II) | 4 | 2.4 | |
| Al2V3 (X) | 4 | 59.6 | |
| Al2V3 (VIII) | 4 | 71.3 | |
| Al2V3 (III) | 6 | 18.8 | |
| Al2V3 (VI) | 6 | 26.8 | |
| Al2V3 (XI) | 6 | 74.6 | |
| Al2V3 (IX) | 6 | 141.0 | |
| Al2Cr3 Metal Cluster | |||
| Al2Cr3 (XVIII) | 1 | 172.6 | [84,85] |
| Al2Cr3 (XVI) | 1 | 186.5 | |
| Al2Cr3 (V) | 1 | 197.2 | |
| Al2Cr3 (XIII) | 1 | 206.8 | |
| Al2Cr3 (X) | 1 | 219.0 | |
| Al2Cr3 (XIX) | 1 | 266.6 | |
| Al2Cr3 (VI) | 1 | 287.0 | |
| Al2Cr3 (I) | 1 | 396.2 | |
| Al2Cr3 (VII) | 3 | 79.4 | |
| Al2Cr3 (II) | 3 | 92.9 | |
| Al2Cr3 (XIV) | 3 | 109.3 | |
| Al2Cr3 (XI) | 3 | 109.6 | |
| Al2Cr3 (III) | 5 | 0.0 | |
| Al2Cr3 (XVII) | 5 | 13.1 | |
| Al2Cr3 (VIII) | 5 | 19.5 | |
| Al2Cr3 (XII) | 5 | 39.6 | |
| Al2Cr3 (XX) | 5 | 73.4 | |
| Al2Cr3 (XV) | 5 | 86.8 | |
| Al2Cr3 (IV) | 7 | 22.9 | |
| Al2Cr3 (IX) | 7 | 39.6 | |
| Al2Mn3 Metal Cluster | |||
| Al2Mn3 (XIII) | 2 | 29.3 | [86] |
| Al2Mn3 (XVII) | 2 | 34.9 | |
| Al2Mn3 (V) | 2 | 47.5 | |
| Al2Mn3 (XXII) | 2 | 50.5 | |
| Al2Mn3 (XXV) | 2 | 80.8 | |
| Al2Mn3 (VIII) | 2 | 124.0 | |
| Al2Mn3 (XIX) | 2 | 129.4 | |
| Al2Mn3 (XXI) | 4 | 2.2 | |
| Al2Mn3 (XII) | 4 | 5.9 | |
| Al2Mn3 (IV) | 4 | 11.2 | |
| Al2Mn3 (XVI) | 4 | 27.0 | |
| Al2Mn3 (VII) | 4 | 29.6 | |
| Al2Mn3 (XXIV) | 4 | 76.1 | |
| Al2Mn3 (II) | 4 | 82.2 | |
| Al2Mn3 (X) | 4 | 141.7 | |
| Al2Mn3 (VI) | 6 | 0.0 | |
| Al2Mn3 (XX) | 6 | 1.4 | |
| Al2Mn3 (III) | 6 | 19.1 | |
| Al2Mn3 (XV) | 6 | 28.1 | |
| Al2Mn3 (XI) | 6 | 36.6 | |
| Al2Mn3 (XIV) | 6 | 42.3 | |
| Al2Mn3 (XXIII) | 6 | 62.4 | |
| Al2Mn3 (IX) | 6 | 74.6 | |
| Al2Mn3 (I) | 6 | 77.2 | |
| Al2Mn3 (XVIII) | 6 | 149.2 | |
| Al2Fe3 Metal Cluster | |||
| Al2Fe3(I) | 1 | 273.7 | [87,88,89] |
| Al2Fe3(IV) | 1 | 300.6 | |
| Al2Fe3(II) | 3 | 0.0 | |
| Al2Fe3(V) | 3 | 24.1 | |
| Al2Fe3(VII) | 3 | 27.1 | |
| Al2Fe3(III) | 5 | 12.8 | |
| Al2Fe3(VIII) | 5 | 24.7 | |
| Al2Fe3(VI) | 5 | 31.1 | |
| Al2Co3 Metal Cluster | |||
| Al2Co3(I) | 2 | 56.0 | [87,88] |
| Al2Co3(VII) | 2 | 83.6 | |
| Al2Co3(IV) | 2 | 92.0 | |
| Al2Co3(II) | 4 | 86.1 | |
| Al2Co3(VIII) | 4 | 86.8 | |
| Al2Co3(V) | 4 | 96.3 | |
| Al2Co3(III) | 6 | 0.0 | |
| Al2Co3(IX) | 6 | 28.9 | |
| Al2Co3(VI) | 6 | 73.3 | |
| Al2Ni3 Metal Cluster | |||
| Al2Ni3 (I) | 1 | 48.6 | [87,88] |
| Al2Ni3 (IV) | 1 | 70.8 | |
| Al2Ni3 (II) | 3 | 0.0 | |
| Al2Ni3 (V) | 3 | 73.1 | |
| Al2Ni3 (III) | 5 | 102.6 | |
| Al2Ni3 (VI) | 5 | 113.3 | |
| Al2Ni3 (VII) | 5 | 148.4 | |
| Al2Cu3 Metal Cluster | |||
| Al2Cu3 (I) | 2 | 0.0 | [89,90,91] |
| Al2Cu3 (III) | 2 | 20.6 | |
| Al2Cu3 (V) | 2 | 27.0 | |
| Al2Cu3 (VII) | 2 | 40.1 | |
| Al2Cu3 (VIII) | 2 | 67.6 | |
| Al2Cu3 (IV) | 4 | 136.9 | |
| Al2Cu3 (II) | 4 | 144.1 | |
| Al2Cu3 (VI) | 4 | 144.3 | |
| Al2Zn3 Metal Cluster | |||
| Al2Zn3 (III) | 1 | 14.0 | [86] |
| Al2Zn3 (II) | 1 | 17.7 | |
| Al2Zn3 (XII) | 1 | 18.9 | |
| Al2Zn3 (VI) | 1 | 22.1 | |
| Al2Zn3 (XIII) | 1 | 23.8 | |
| Al2Zn3 (XI) | 1 | 25.6 | |
| Al2Zn3 (V) | 1 | 29.6 | |
| Al2Zn3 (IX) | 1 | 30.5 | |
| Al2Zn3 (VIII) | 1 | 79.2 | |
| Al2Zn3 (I) | 3 | 0.0 | |
| Al2Zn3 (XIV) | 3 | 3.2 | |
| Al2Zn3 (IV) | 3 | 11.0 | |
| Al2Zn3 (VII) | 3 | 21.4 | |
| Al2Zn3 (X) | 3 | 29.6 | |
| M | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||||
| Metal–Metal Bond Lengths, pm | ||||||||||
| Al1Al2 | 422.7 | 270.2 | 262.4 | 276.1 | 273.9 | 271.5 | 270.7 | 271.1 | 277.3 | |
| Al1M1 | 254.4 | 263.7 | 262.6 | 260.4 | 244.8 | 233.1 | 229.0 | 244.1 | 267.7 | |
| Al1M2 | 258.2 | 265.7 | 278.1 | 257.6 | 240.9 | 252.8 | 236.1 | 254.2 | 267.8 | |
| Al1M3 | 258.2 | 252.2 | 262.6 | 260.4 | 252.3 | 233.1 | 229.0 | 244.1 | 273.7 | |
| Al2M1 | 254.4 | 261.0 | 262.6 | 260.4 | 244.8 | 233.1 | 229.0 | 244.1 | 267.7 | |
| Al2M2 | 258.2 | 378.8 | 278.1 | 257.7 | 240.9 | 252.8 | 236.1 | 254.2 | 247.9 | |
| Al2M3 | 258.2 | 254.4 | 262.6 | 260.4 | 252.3 | 233.1 | 229.0 | 244.1 | 273.6 | |
| M1M3 | 258.7 | 265.4 | 415.9 | 434.8 | 250.7 | 341.6 | 357.3 | 384.1 | 446.0 | |
| M2M3 | 239.0 | 253.3 | 258.1 | 281.6 | 217.6 | 215.3 | 231.1 | 243.1 | 446.1 | |
| M1M2 | 258.8 | 171.7 | 258.1 | 281.6 | 374.9 | 215.3 | 231.1 | 243.1 | 247.9 | |
| Bond Angles, deg | ||||||||||
| M1Al1M2 | 60.6 | 37.8 | 56.9 | 65.9 | 101.0 | 52.4 | 59.6 | 58.3 | 55.2 | |
| M1Al2M2 | 60.6 | 22.9 | 56.9 | 65.8 | 101.0 | 52.4 | 59.6 | 58.3 | 55.2 | |
| M1Al1Al2 | 33.8 | 58.5 | 60.0 | 58.0 | 56.0 | 54.4 | 53.8 | 56.3 | 58.8 | |
| M1Al2Al1 | 33.8 | 59.5 | 60.0 | 58.0 | 56.0 | 54.4 | 53.8 | 56.3 | 58.8 | |
| M2Al1Al2 | 35.6 | 90.0 | 61.9 | 57.6 | 55.4 | 57.5 | 55.0 | 57.8 | 58.8 | |
| M2Al2Al1 | 35.0 | 44.5 | 61.9 | 57.6 | 55.4 | 57.5 | 55.0 | 57.8 | 58.8 | |
| Al1M1Al2 | 112.3 | 62.0 | 59.9 | 64.0 | 68.0 | 71.2 | 72.5 | 67.4 | 62.4 | |
| Al1M2Al2 | 109.9 | 45.5 | 56.3 | 64.8 | 69.3 | 64.9 | 69.9 | 64.4 | 62.4 | |
| Al1M3Al2 | 109.9 | 64.5 | 59.9 | 64.0 | 65.7 | 68.5 | 72.5 | 67.4 | 60.9 | |
| M1Al1M3 | 60.6 | 61.9 | 104.7 | 113.2 | 60.5 | 94.2 | 102.6 | 103.7 | 110.9 | |
| M1Al2M3 | 60.6 | 62.0 | 104.7 | 113.2 | 60.5 | 94.2 | 102.6 | 103.7 | 111.0 | |
| M1M3M2 | 62.5 | 38.6 | 36.3 | 65.9 | 106.2 | 37.5 | 39.4 | 37.8 | 32.3 | |
| M2Al1M3 | 55.1 | 58.5 | 56.9 | 65.9 | 52.3 | 52.4 | 59.6 | 58.3 | 110.9 | |
| M2Al2M3 | 55.1 | 41.6 | 56.9 | 101.1 | 52.3 | 52.4 | 59.6 | 58.3 | 111.0 | |
| M1M2M3 | 62.5 | 74.5 | 107.3 | 39.5 | 40.0 | 105.0 | 101.3 | 104.4 | 73.9 | |
| Metal Cluster | Standard Thermodynamic Parameters of Formation | ||
|---|---|---|---|
| ΔfH0(298 K) kJ/mol | ΔfS0(298 K) J/mol∙ K | ΔfG0(298 K) kJ/mol | |
| Al2Ti3 | 967.4 | 429.9 | 883.5 |
| Al2V3 | 526.5 | 438.5 | 433.8 |
| Al2Cr3 | 1151.1 | 417.8 | 1067.5 |
| Al2Mn3 | 516.8 | 423.1 | 436.2 |
| Al2Fe3 | 823.4 | 430.0 | 736.5 |
| Al2Co3 | 817.6 | 427.6 | 733.9 |
| Al2Ni3 | 760.9 | 430.0 | 676.3 |
| Al2Cu3 | 812.2 | 406.9 | 737.7 |
| Al2Zn3 | 700.1 | 445.1 | 621.7 |
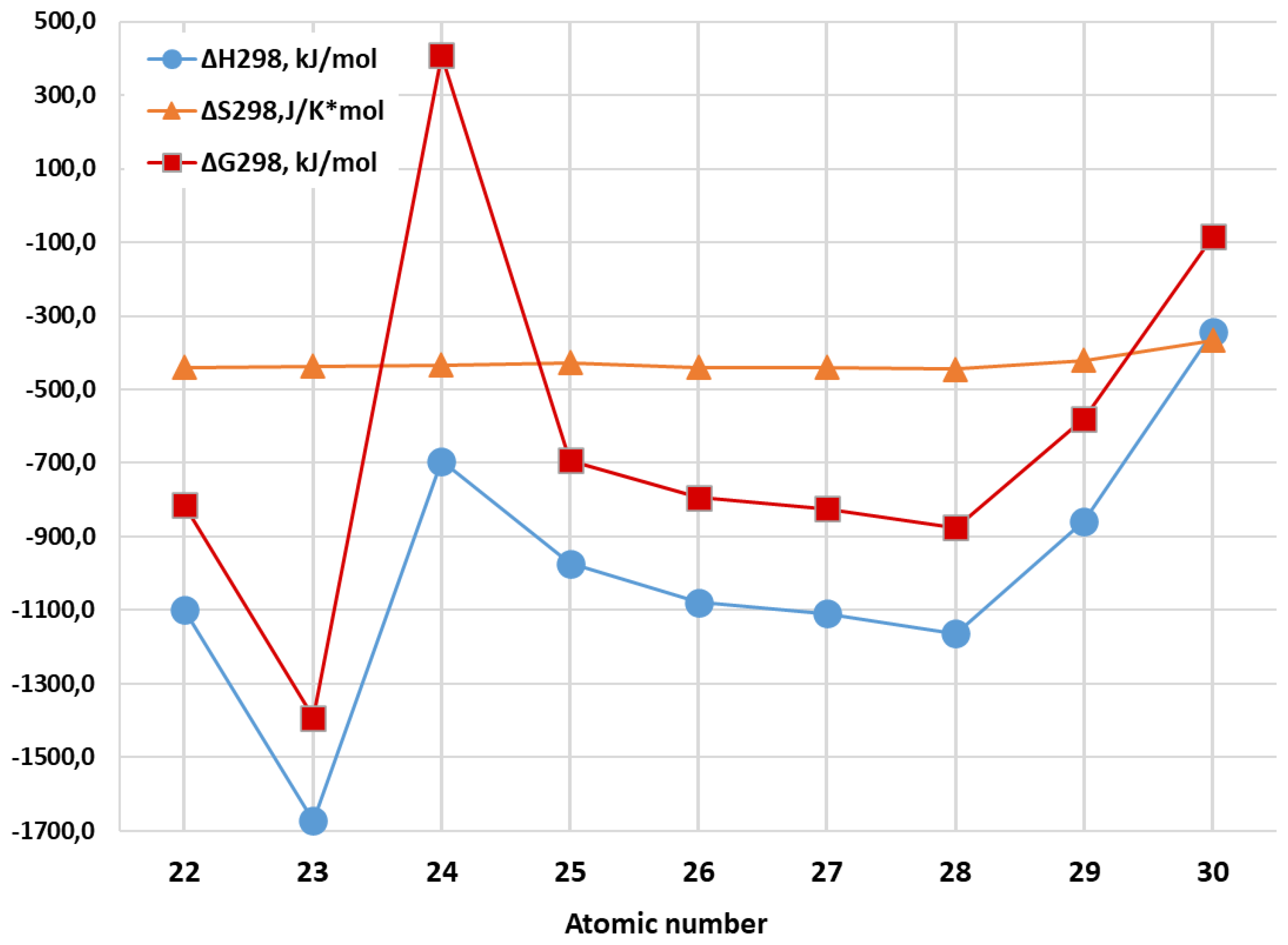
| Metal Cluster | Standard Thermodynamic Parameters of Reactions 2Al(gas) + 3M(gas)→Al2M3 (gas) | ||
| ΔH298, kJ/mol | ΔS298, J/mol∙ K | ΔG298, kJ/mol | |
| Al2Ti3 | –1098.5 | –439.5 | –813.4 |
| Al2V3 | –1672.0 | –436.9 | –1392.1 |
| Al2Cr3 | –694.0 | –433.6 | 407.7 |
| Al2Mn3 | –973.4 | –426.5 | –692.2 |
| Al2Fe3 | –1078.1 | –440.0 | –793.0 |
| Al2Co3 | –1109.1 | –439.4 | –824.1 |
| Al2Ni3 | –1162.8 | –445.1 | –876.0 |
| Al2Cu3 | –858.3 | –420.8 | –578.8 |
| Al2Zn3 | –344.6 | –366.4 | –84.3 |
| Metal Cluster | Atomic Number of M | Ttd, K |
|---|---|---|
| Al2Ti3 | 22 | 2502.2 |
| Al2V3 | 23 | 3826.0 |
| Al2Cr3 | 24 | 1599.0 |
| Al2Mn3 | 25 | 2278.6 |
| Al2Fe3 | 26 | 2450.2 |
| Al2Co3 | 27 | 2526.4 |
| Al2Ni3 | 28 | 2613.0 |
| Al2Cu3 | 29 | 2038.7 |
| Al2Zn3 | 30 | 941.5 |
| Structure Designation | Spin Multiplicity of the Ground State | Relative Energy, kJ/mol | Ref. |
|---|---|---|---|
| Al3Fe3 Metal Cluster | |||
| Al3Fe3 (IV) | 2 | 58.8 | [94,95,96] |
| Al3Fe3 (X) | 2 | 95.2 | |
| Al3Fe3 (XIX) | 2 | 108.3 | |
| Al3Fe3 (VII) | 2 | 134.5 | |
| Al3Fe3 (XIII) | 2 | 137.2 | |
| Al3Fe3 (XVI) | 2 | 153.7 | |
| Al3Fe3 (I) | 2 | 158.3 | |
| Al3Fe3 (V) | 4 | 56.8 | |
| Al3Fe3 (XIV) | 4 | 56.8 | |
| Al3Fe3 (VIII) | 4 | 108.9 | |
| Al3Fe3 (XI) | 4 | 110.1 | |
| Al3Fe3 (II) | 4 | 130.8 | |
| Al3Fe3 (XV) | 6 | 0.0 | |
| Al3Fe3 (XII) | 6 | 40.8 | |
| Al3Fe3 (IX) | 6 | 71.8 | |
| Al3Fe3 (VI) | 6 | 77.3 | |
| Al3Fe3 (III) | 6 | 78.1 | |
| Al3Fe3 (XX) | 6 | 105.9 | |
| Al3Fe3 (XVII) | 6 | 111.4 | |
| Al3Fe3 (XVIII) | 6 | 155.4 | |
| Al2Fe4 Metal Cluster | |||
| Al2Fe4 (V) | 3 | 13.0 | [95,96] |
| Al2Fe4 (VIII) | 3 | 27.5 | |
| Al2Fe4 (III) | 3 | 80.8 | |
| Al2Fe4 (IX) | 3 | 102.8 | |
| Al2Fe4 (VI) | 3 | 115.7 | |
| Al2Fe4 (I) | 3 | 165.5 | |
| Al2Fe4 (II) | 5 | 0.0 | |
| Al2Fe4 (VII) | 5 | 25.5 | |
| Al2Fe4 (IV) | 5 | 79.2 | |
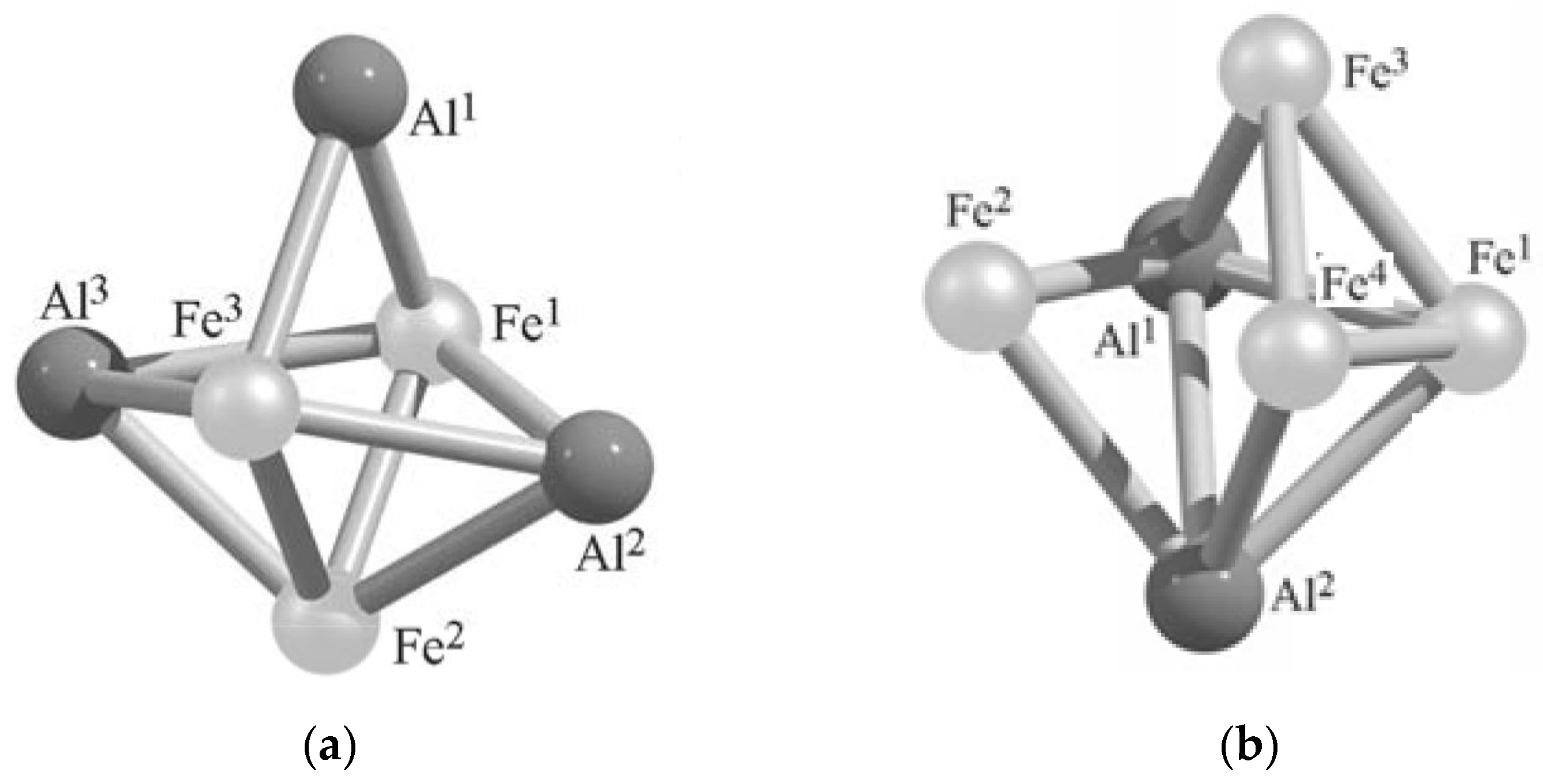
| Al3Fe3 Metal Cluster | Al2Fe4 Metal Cluster | ||
|---|---|---|---|
| Metal–Metal Bond Lengths, pm | Metal–Metal Bond Lengths, pm | ||
| Al1Al2 | 306.2 | Al1Fe1 | 242.7 |
| Al1Al3 | 307.1 | Al1Fe2 | 249.8 |
| Al2Al3 | 398.9 | Al1Fe3 | 243.8 |
| Al1Fe1 | 236.4 | Al1Fe4 | 350.4 |
| Al1Fe2 | 370.1 | Al1Al2 | 274.7 |
| Al1Fe3 | 236.3 | Al2Fe1 | 242.7 |
| Al2Fe1 | 244.4 | Al2Fe2 | 249.7 |
| Al2Fe2 | 242.1 | Al2Fe3 | 350.4 |
| Al2Fe3 | 244.2 | Al2Fe4 | 243.8 |
| Al3Fe1 | 244.3 | Fe2Fe3 | 244.1 |
| Al3Fe2 | 242.1 | Fe1Fe4 | 225.9 |
| Al3Fe3 | 244.2 | Fe1Fe3 | 225.9 |
| Fe1Fe2 | 222.5 | Fe1Fe2 | 329.1 |
| Fe1Fe3 | 270.9 | Fe2Fe4 | 244.1 |
| Fe2Fe3 | 222.4 | Fe3Fe4 | 230.7 |
| Bond Angles, deg | Bond Angles, deg | ||
| Fe2Al1Fe3 | 35.0 | Fe2Al1Fe3 | 59.3 |
| Fe1Al1Fe2 | 35.0 | Fe1Al1Fe2 | 83.9 |
| Fe1Al2Al3 | 35.3 | Fe1Al2Fe4 | 55.4 |
| Fe2Al2Al3 | 34.5 | Fe2Al2Fe4 | 59.3 |
| Al1Al2Fe2 | 84.1 | Al1Al2Fe2 | 56.6 |
| Fe2Al1Al2 | 40.6 | Fe2Al1Al2 | 56.6 |
| Al1Al2Al3 | 49.5 | Al1Al2Fe4 | 84.8 |
| Al2Al3Fe1 | 35.3 | Al2Fe4Fe1 | 62.1 |
| Al3Fe1Al2 | 109.4 | Fe4Fe1Al2 | 62.6 |
| Fe3Al1Al2 | 51.6 | Fe3Al1Al2 | 84.8 |
| Fe1Al1Fe3 | 69.9 | Fe1Al1Fe3 | 55.4 |
| Fe1Al1Al2 | 51.6 | Fe1Al1Al2 | 55.5 |
| Al1Fe1Al2 | 79.1 | Al1Fe1Al2 | 68.9 |
| Al3Fe2Fe3 | 63.3 | Fe4Fe2Fe3 | 56.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailov, O.V.; Chachkov, D.V. Quantum-Chemical Design of Molecular Structures of Tetra-, Penta- and Hexanuclear Metal Clusters Containing Aluminum and 3d-Element Atoms. Materials 2020, 13, 1852. https://doi.org/10.3390/ma13081852
Mikhailov OV, Chachkov DV. Quantum-Chemical Design of Molecular Structures of Tetra-, Penta- and Hexanuclear Metal Clusters Containing Aluminum and 3d-Element Atoms. Materials. 2020; 13(8):1852. https://doi.org/10.3390/ma13081852
Chicago/Turabian StyleMikhailov, Oleg V., and Denis V. Chachkov. 2020. "Quantum-Chemical Design of Molecular Structures of Tetra-, Penta- and Hexanuclear Metal Clusters Containing Aluminum and 3d-Element Atoms" Materials 13, no. 8: 1852. https://doi.org/10.3390/ma13081852
APA StyleMikhailov, O. V., & Chachkov, D. V. (2020). Quantum-Chemical Design of Molecular Structures of Tetra-, Penta- and Hexanuclear Metal Clusters Containing Aluminum and 3d-Element Atoms. Materials, 13(8), 1852. https://doi.org/10.3390/ma13081852






