Effect of the Operational Conditions in the Characteristics of Ceramic Foams Obtained from Quartz and Sodium Silicate
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Materials
2.3. Process of Elaboration of Ceramic Foams
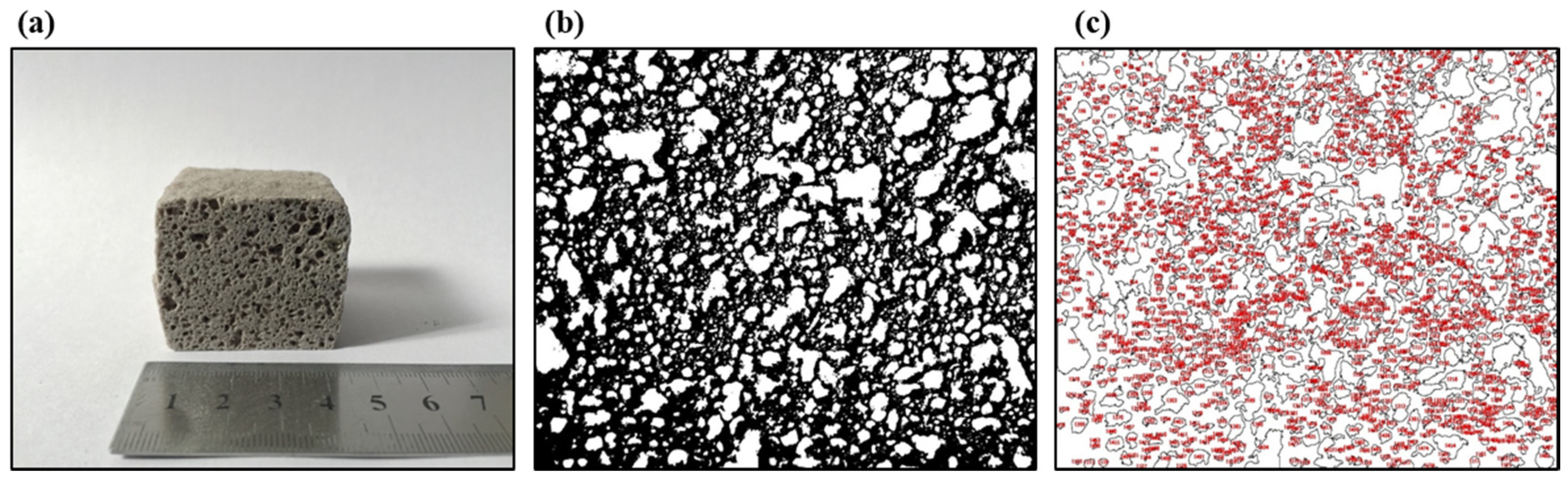
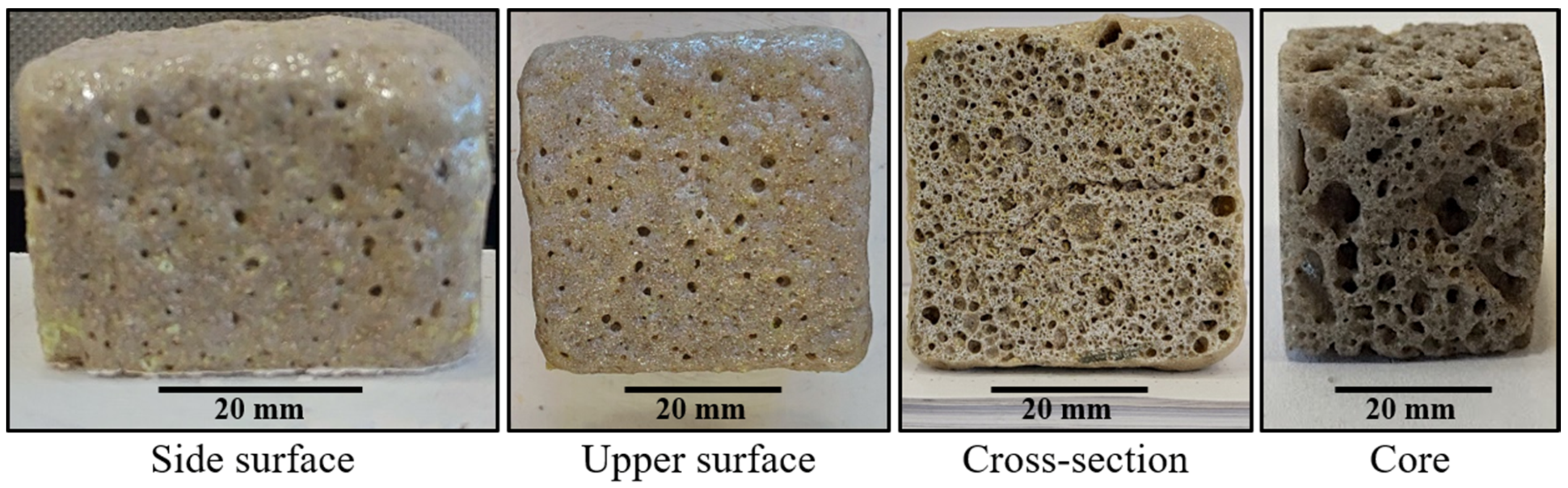
2.4. Ceramic Foams Characterization
2.5. Computational Tools
3. Results and Discussion
3.1. Minimum Temperature and Time
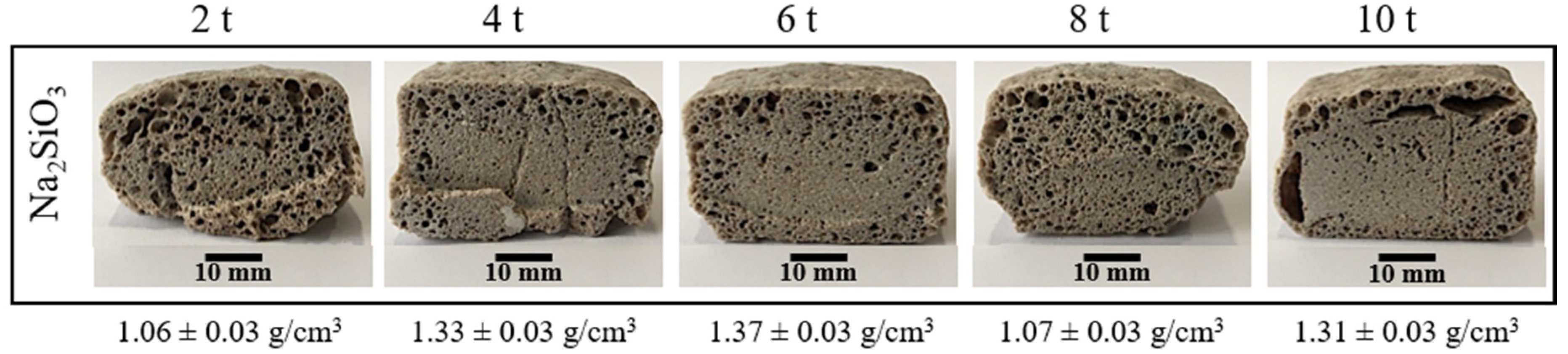
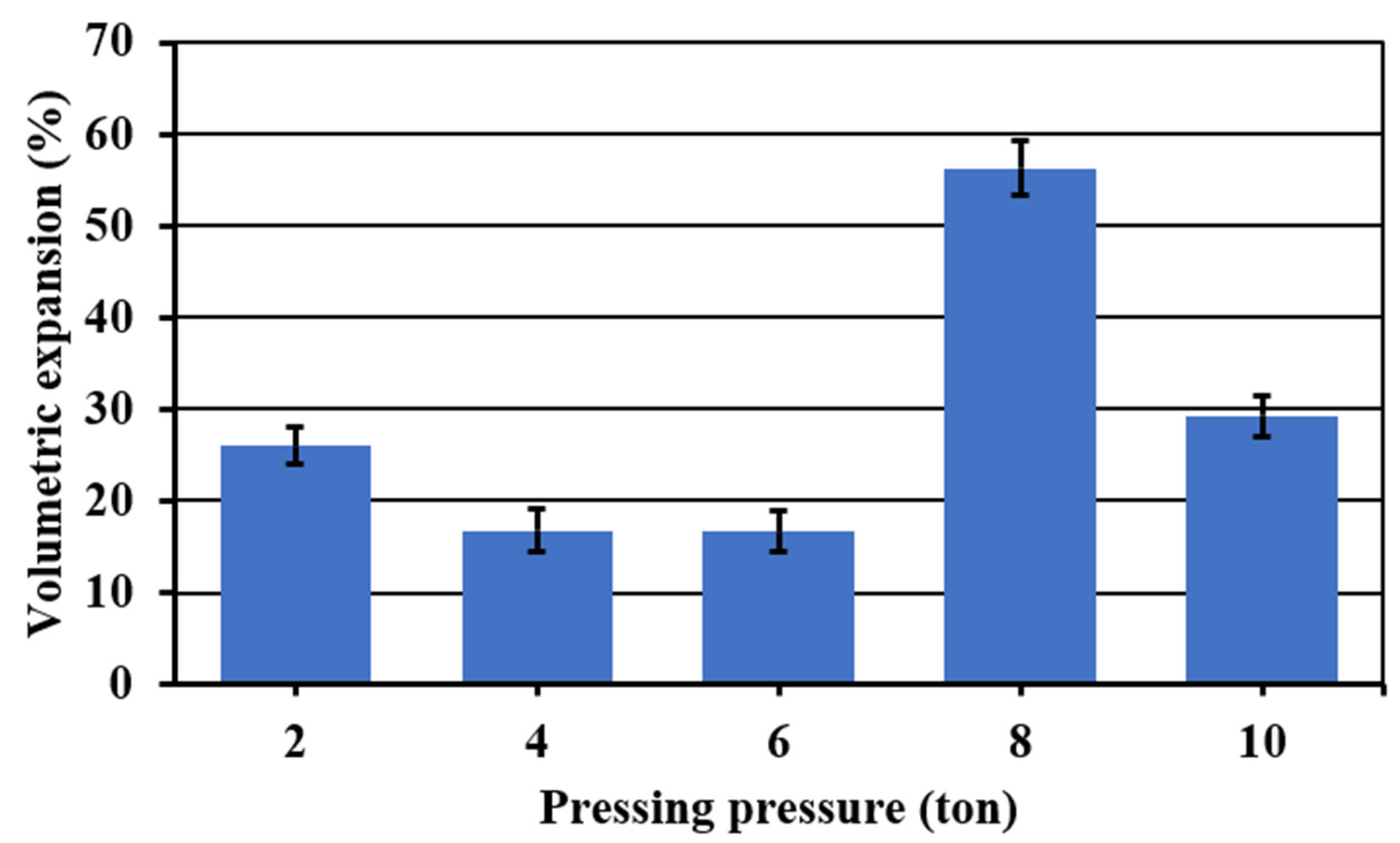
3.2. Effect of the Pressing Pressure
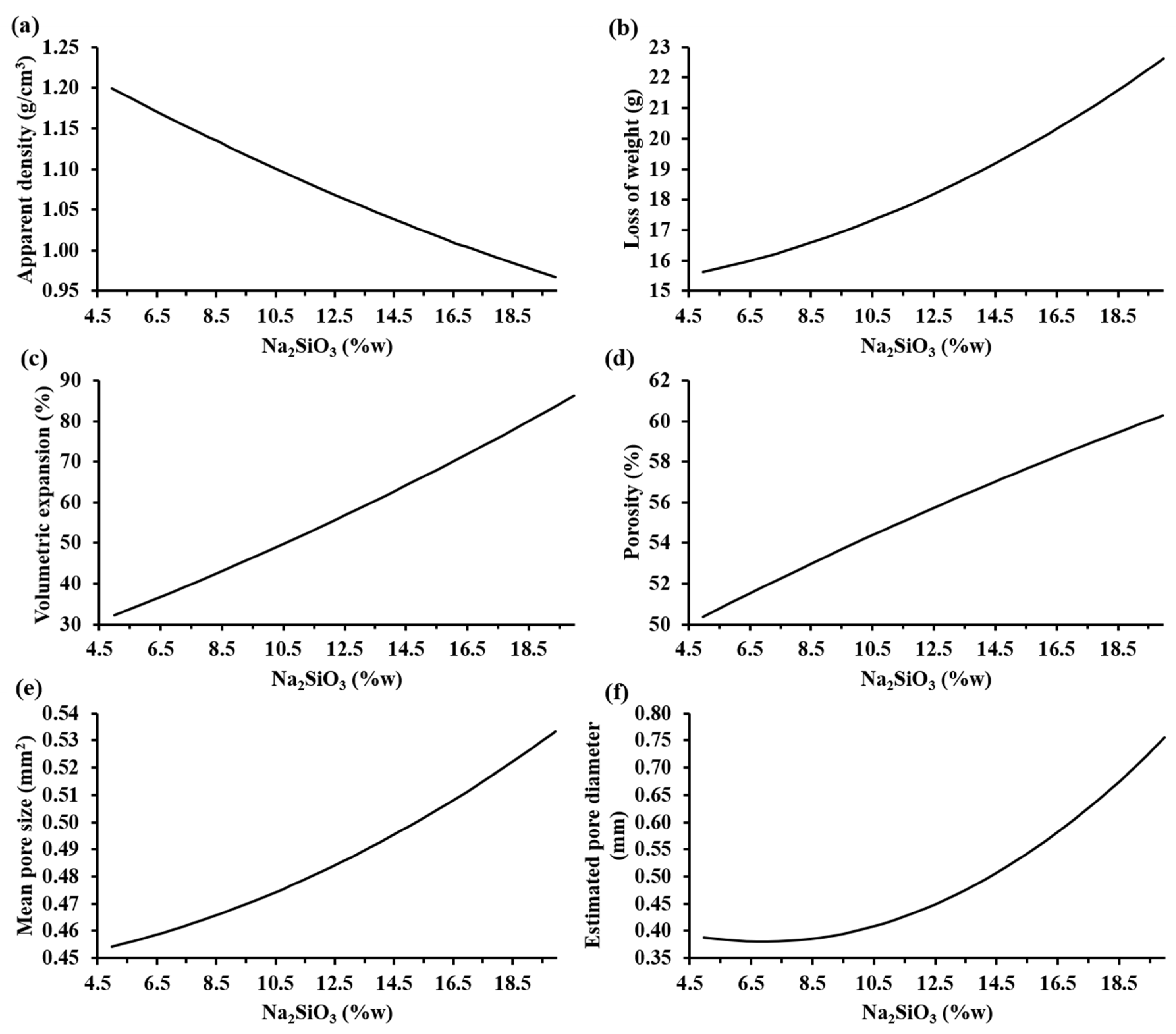
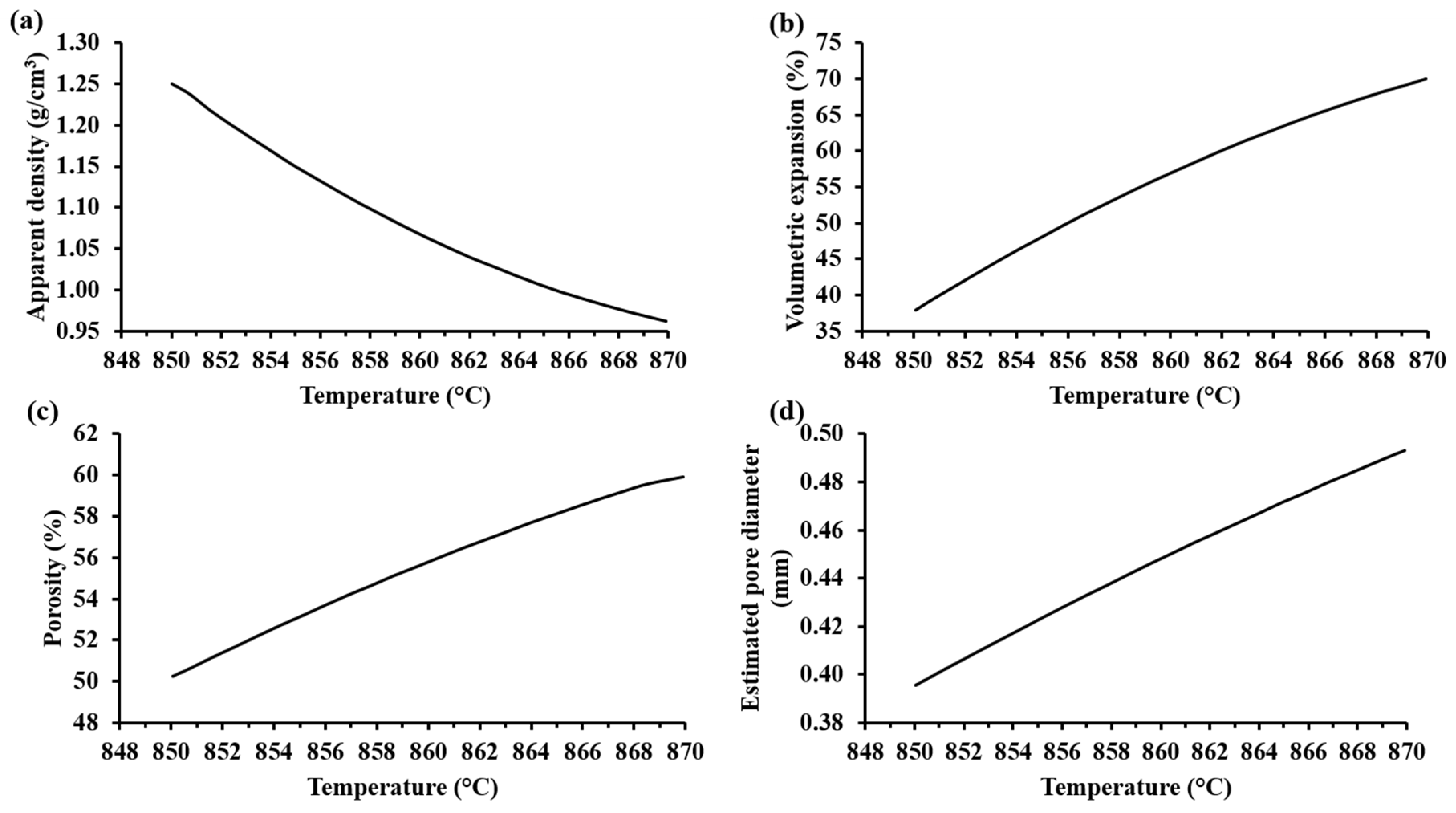
3.3. Effect of the Powder Mix Composition and Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | T (°C) | SiO2 (%w) | Na2SiO3 (%w) | CaO (%w) | Na2CO3 (% w) | (%) | Pore Structures | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SS-01 | 850 | 59.20 | 19.10 | 5.70 | 16.0 | 1.18 | 54.20 | 10.74 | 0.38 |  |
| SS-02 | 59.20 | 17.00 | 4.80 | 19.0 | 0.85 | 66.30 | 137.66 | 1.58 |  | |
| SS-03 | 70.30 | 5.90 | 4.80 | 19.0 | 1.22 | 51.97 | 19.09 | 0.64 |  | |
| SS-04 | 59.20 | 19.00 | 4.80 | 17.0 | 0.95 | 62.38 | 102.50 | 1.13 |  | |
| SS-05 | 59.20 | 16.40 | 5.40 | 19.0 | 0.83 | 68.01 | 124.45 | 0.95 |  | |
| SS-06 | 70.30 | 6.00 | 5.70 | 18.0 | 1.73 | 32.70 | −10.81 | 0.21 |  | |
| SS-07 | 70.30 | 8.60 | 5.10 | 16.0 | 1.54 | 40.00 | −8.57 | 0.18 |  | |
| SS-08 | 70.30 | 8.30 | 5.40 | 16.0 | 1.37 | 46.69 | −5.71 | 0.27 |  | |
| SS-09 | 62.90 | 16.30 | 4.80 | 16.0 | 0.95 | 61.44 | 56.62 | 0.48 |  | |
| SS-10 | 66.60 | 8.70 | 5.70 | 19.0 | 1.43 | 43.04 | 12.88 | 0.29 |  | |
| SS-11 | 870 | 59.20 | 20.00 | 4.80 | 16.0 | 0.80 | 68.38 | 160.42 | 1.39 |  |
| SS-12 | 70.30 | 5.00 | 5.70 | 19.0 | 1.10 | 56.98 | 30.07 | 0.42 |  | |
| SS-13 | 70.30 | 8.90 | 4.80 | 16.0 | 1.19 | 53.01 | 22.77 | 0.37 |  | |
| SS-14 | 59.20 | 17.00 | 4.80 | 19.0 | 0.85 | 66.24 | 105.08 | 1.20 |  | |
| SS-15 | 59.20 | 16.10 | 5.70 | 19.0 | 0.77 | 70.08 | 79.30 | 0.69 |  | |
| SS-16 | 70.30 | 6.90 | 4.80 | 18.0 | 0.92 | 63.87 | 62.85 | 1.13 |  | |
| SS-17 | 70.30 | 7.00 | 5.70 | 17.0 | 1.07 | 57.27 | 25.89 | 0.39 |  | |
| SS-18 | 70.30 | 5.60 | 5.10 | 19.0 | 0.94 | 62.86 | 70.27 | 0.55 |  | |
| SS-19 | 62.90 | 13.30 | 4.80 | 19.0 | 0.66 | 73.41 | 134.38 | 0.56 |  | |
| SS-20 | 62.90 | 15.40 | 5.70 | 16.0 | 0.81 | 67.99 | 100.89 | 0.43 |  | |
| SS-21 | 66.60 | 11.70 | 5.70 | 16.0 | 1.00 | 60.64 | 40.63 | 0.29 |  | |
| SS-22 | 59.20 | 18.05 | 5.25 | 17.5 | 0.79 | 69.22 | 126.85 | 1.12 |  | |
| SS-23 | 860 | 64.75 | 12.50 | 5.25 | 17.5 | 1.20 | 52.66 | 42.05 | 0.40 |  |
| SS-24 | 64.75 | 12.50 | 5.25 | 17.5 | 0.95 | 52.17 | 35.51 | 0.53 |  | |
| SS-25 | 64.75 | 12.50 | 5.25 | 17.5 | 1.19 | 52.94 | 42.05 | 0.34 |  | |
| SS-26 | 64.75 | 12.50 | 5.25 | 17.5 | 1.26 | 50.91 | 39.87 | 0.42 |  |
References
- Scheffer, M.; Colombo, P. Cellular Ceramics: Structure, Manufacturing, Properties and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Smedskjaer, M.M.; Yue, Y. Effect of Na2CO3 as foaming agent on dynamics and structure of foam glass melts. J. Non. Cryst. Solids. 2014, 400, 1–5. [Google Scholar] [CrossRef]
- Zhou, M.; Ge, X.; Wang, H.; Chen, L.; Chen, X. Effect of the CaO content and decomposition of calcium-containing minerals on properties and microstructure of ceramic foams from fly ash. Ceram. Int. 2017, 43, 9451–9457. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda-lime glass matrix. J. Non. Cryst. Solids. 2019, 511, 177–182. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Hesky, D.; Aneziris, C.G.; Groß, U.; Horn, A. Water and waterglass mixtures for foam glass production. Ceram. Int. 2015, 41, 12604–12613. [Google Scholar] [CrossRef]
- Xi, C.; Zheng, F.; Xu, J.; Yang, W.; Peng, Y.; Li, Y.; Li, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Preparation of glass-ceramic foams using extracted titanium tailing and glass waste as raw materials. Constr. Build. Mater. 2018, 190, 896–909. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.; Shu, H.; Qiao, Y.; Mei, S.; Jin, M.; Xie, J. Preparation of high strength glass ceramic foams from waste cathode ray tube and germanium tailings. Constr. Build. Mater. 2016, 111, 105–110. [Google Scholar] [CrossRef]
- Kazmina, O.V.; Tokareva, A.Y.; Vereshchagin, V.I. Using quartzofeldspathic waste to obtain foamed glass material. Resour. Technol. 2016, 2, 23–29. [Google Scholar] [CrossRef]
- Ahmad, S.; Latif, M.A.; Taib, H.; Ismail, A.F. Short review: Ceramic foam fabrication techniques for wastewater treatment application. Adv. Mater. Res. 2013, 795, 5–8. [Google Scholar] [CrossRef]
- Aasly, K. Properties and Behavior of Quartz for the Silicon Process. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2008. [Google Scholar]
- Karandashova, N.S.; Goltsman, B.M.; Yatsenko, E.A. Analysis of influence of foaming mixture components on structure and properties of foam glass. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Z.; Li, X.; Liu, T.; Guan, L.; Wu, T.; Lu, A. Preparation and characterization of glass–ceramic foams with waste quartz sand and coal gangue in different proportions. J. Porous Mater. 2016, 23, 231–238. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.G. Thermal and carbothermic decomposition of Na 2 CO 3 and Li 2 CO 3, Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids Structure and Properties; Cambridge University Press: Cambridge, UK, 1999; Available online: https://books.google.pt/books/about/Cellular_Solids.html?id=IySUr5sn4N8C&source=kp_cover&redir_esc=y (accessed on 7 January 2020).
- Rice, R.W. Porosity of Ceramics; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- D’Amore, G.K.O.; Caniato, M.; Travan, A.; Turco, G.; Marsich, L.; Ferluga, A.; Schmid, C. Innovative thermal and acoustic insulation foam from recycled waste glass powder. J. Clean. Prod. 2017, 165, 1306–1315. [Google Scholar] [CrossRef]
- Lakov, L.; Toncheva, K.; Staneva, A.; Simeonova, T.; Ilcheva, Z. Composition, synthesis and properties of insulation foam glass obtained from packing glass waste. J. Chem. Technol. Metall. 2013, 48, 125–129. [Google Scholar]
- König, J.; Petersen, R.R.; Yue, Y. Influence of the glass particle size on the foaming process and physical characteristics of foam glasses. J. Non. Cryst. Solids. 2016, 447, 190–197. [Google Scholar] [CrossRef]
- Ducman, V.; Kovačević, M. The foaming of waste glass. Key Eng. Mater. 1997, 132–136, 2264–2267. [Google Scholar] [CrossRef]
- Hojaji, H. Development of foam glass structural insulation derived from fly ash. MRS Proc. 1988, 136, 185–206. [Google Scholar] [CrossRef]
- Chen, X.; Lu, A.; Qu, G. Preparation and characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent. Ceram. Int. 2013, 39, 1923–1929. [Google Scholar] [CrossRef]
- Liu, T.; Lin, C.; Liu, J.; Han, L.; Gui, H.; Li, C.; Zhou, X.; Tang, H.; Yang, Q.; Lu, A. Phase evolution, pore morphology and microstructure of glass ceramic foams derived from tailings wastes. Ceram. Int. 2018, 44, 14393–14400. [Google Scholar] [CrossRef]
- Liu, T.; Tang, Y.; Han, L.; Song, J.; Luo, Z.; Lu, A. Recycling of harmful waste lead-zinc mine tailings and fly ash for preparation of inorganic porous ceramics. Ceram. Int. 2017, 43, 4910–4918. [Google Scholar] [CrossRef]
- Liu, T.; Tang, Y.; Li, Z.; Wu, T.; Lu, A. Red mud and fly ash incorporation for lightweight foamed ceramics using lead-zinc mine tailings as foaming agent. Mater. Lett. 2016, 183, 362–364. [Google Scholar] [CrossRef]
- Yin, H.; Ma, M.; Bai, J.; Li, Y.; Zhang, S.; Wang, F. Fabrication of foam glass from iron tailings. Mater. Lett. 2016, 185, 511–513. [Google Scholar] [CrossRef]
- Wang, Y. Performance-Based Fire Engineering of Structures; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]


| Element | (%) |
|---|---|
| Si | 47.410 |
| S | 0.392 |
| Cl | 0.059 |
| K | 0.101 |
| Ca | 0.066 |
| Ti | 0.012 |
| Cr | 0.008 |
| Fe | 1.600 |
| Cu | 0.009 |
| Factors | Evaluated Values |
|---|---|
| Temperature (°C) | 750, 800, 850 |
| Time (min) | 30, 45, 60 |
| Pressing pressure (t) | 2, 4, 6, 8, 10 |
| Mixture composition: | |
| SiO2(s) (%w) | 59.2 |
| Na2CO3(s) (%w) | 19.0 |
| CaO(s) (%w) | 5.4 |
| Na2SiO3(l) (%w) | 16.4 |
| Factors | Ranges and Fixed Values |
|---|---|
| Temperature (°C) | 850–870 |
| Time (min) | 45 |
| Pressing pressure (t) | 4 |
| Mixture composition: | |
| SiO2(s) (%w) | 59.20–70.3 |
| Na2CO3(s) (%w) | 16.0–19.0 |
| CaO(s) (%w) | 4.8–5.7 |
| Na2SiO3(l) (%w) | 5.0–19.1 |
| Essay | T (°C) | SiO2 (%w) | Na2SiO3 (%w) | CaO (%w) | Na2CO3 (%w) |
|---|---|---|---|---|---|
| SS-01 | 850 | 59.20 | 19.10 | 5.70 | 16.0 |
| SS-02 | 59.20 | 17.00 | 4.80 | 19.0 | |
| SS-03 | 70.30 | 5.90 | 4.80 | 19.0 | |
| SS-04 | 59.20 | 19.00 | 4.80 | 17.0 | |
| SS-05 | 59.20 | 16.40 | 5.40 | 19.0 | |
| SS-06 | 70.30 | 6.00 | 5.70 | 18.0 | |
| SS-07 | 70.30 | 8.60 | 5.10 | 16.0 | |
| SS-08 | 70.30 | 8.30 | 5.40 | 16.0 | |
| SS-09 | 62.90 | 16.30 | 4.80 | 16.0 | |
| SS-10 | 66.60 | 8.70 | 5.70 | 19.0 | |
| SS-11 | 870 | 59.20 | 20.00 | 4.80 | 16.0 |
| SS-12 | 70.30 | 5.00 | 5.70 | 19.0 | |
| SS-13 | 70.30 | 8.90 | 4.80 | 16.0 | |
| SS-14 | 59.20 | 17.00 | 4.80 | 19.0 | |
| SS-15 | 59.20 | 16.10 | 5.70 | 19.0 | |
| SS-16 | 70.30 | 6.90 | 4.80 | 18.0 | |
| SS-17 | 70.30 | 7.00 | 5.70 | 17.0 | |
| SS-18 | 70.30 | 5.60 | 5.10 | 19.0 | |
| SS-19 | 62.90 | 13.30 | 4.80 | 19.0 | |
| SS-20 | 62.90 | 15.40 | 5.70 | 16.0 | |
| SS-21 | 66.60 | 11.70 | 5.70 | 16.0 | |
| SS-22 | 59.20 | 18.05 | 5.25 | 17.5 | |
| SS-23 | 860 | 64.75 | 12.50 | 5.25 | 17.5 |
| SS-24 | 64.75 | 12.50 | 5.25 | 17.5 | |
| SS-25 | 64.75 | 12.50 | 5.25 | 17.5 | |
| SS-26 | 64.75 | 12.50 | 5.25 | 17.5 |
| Material | Quartz (Mainly) |
|---|---|
| Apparent density, (g/cm3) | 0.66–1.73 |
| Porosity, (%) | 32.70–73.41 |
| Volumetric expansion, (%) | −10.81–160.42 |
| Mean pore diameter, (mm) | 0.18–1.58 |
| Open or closed cells | Open and closed |
| Symmetry of cell structure | Axisymmetric |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, L.; Giraldo, J.D.; Vargas, A. Effect of the Operational Conditions in the Characteristics of Ceramic Foams Obtained from Quartz and Sodium Silicate. Materials 2020, 13, 1806. https://doi.org/10.3390/ma13081806
Uribe L, Giraldo JD, Vargas A. Effect of the Operational Conditions in the Characteristics of Ceramic Foams Obtained from Quartz and Sodium Silicate. Materials. 2020; 13(8):1806. https://doi.org/10.3390/ma13081806
Chicago/Turabian StyleUribe, Lina, Juan D. Giraldo, and Alejandro Vargas. 2020. "Effect of the Operational Conditions in the Characteristics of Ceramic Foams Obtained from Quartz and Sodium Silicate" Materials 13, no. 8: 1806. https://doi.org/10.3390/ma13081806
APA StyleUribe, L., Giraldo, J. D., & Vargas, A. (2020). Effect of the Operational Conditions in the Characteristics of Ceramic Foams Obtained from Quartz and Sodium Silicate. Materials, 13(8), 1806. https://doi.org/10.3390/ma13081806





