Glass/Au Composite Membranes with Gold Nanoparticles Synthesized inside Pores for Selective Ion Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. PG Material Synthesis
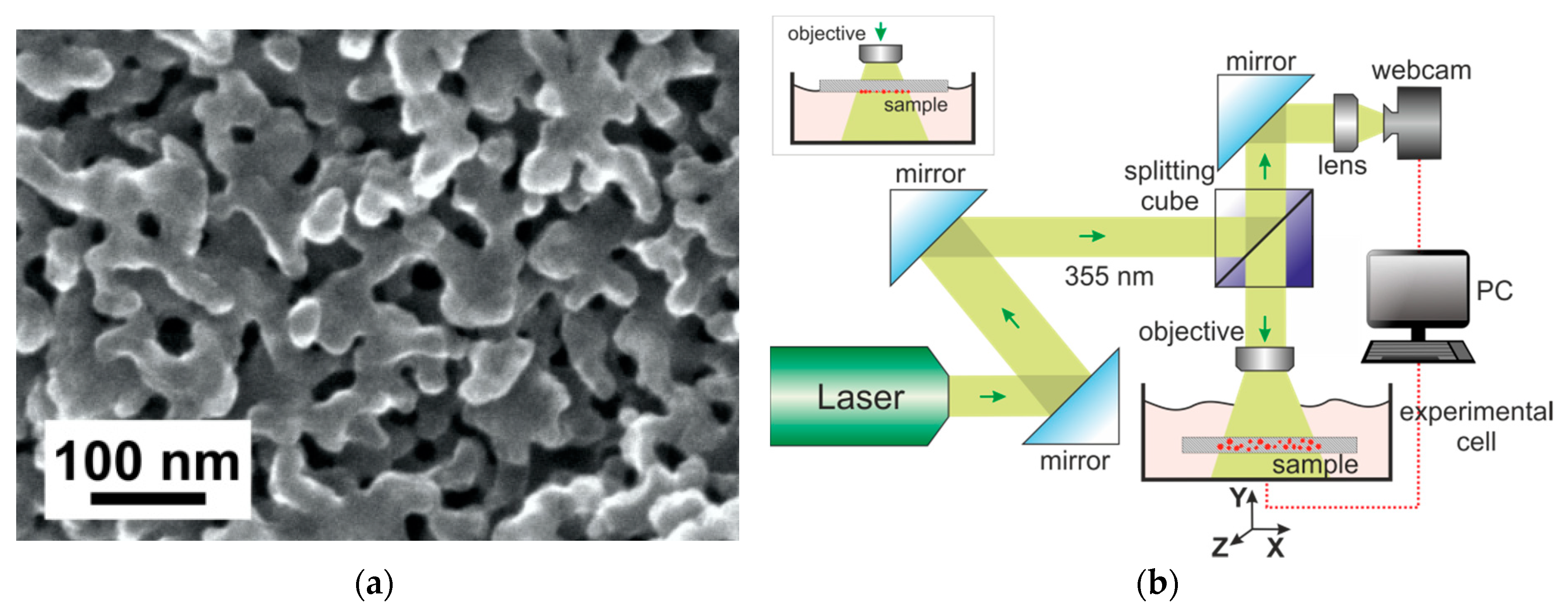
2.2. Laser-Induced Liquid Phase Chemical Deposition Process
2.3. Microscopic and Optical Measurement
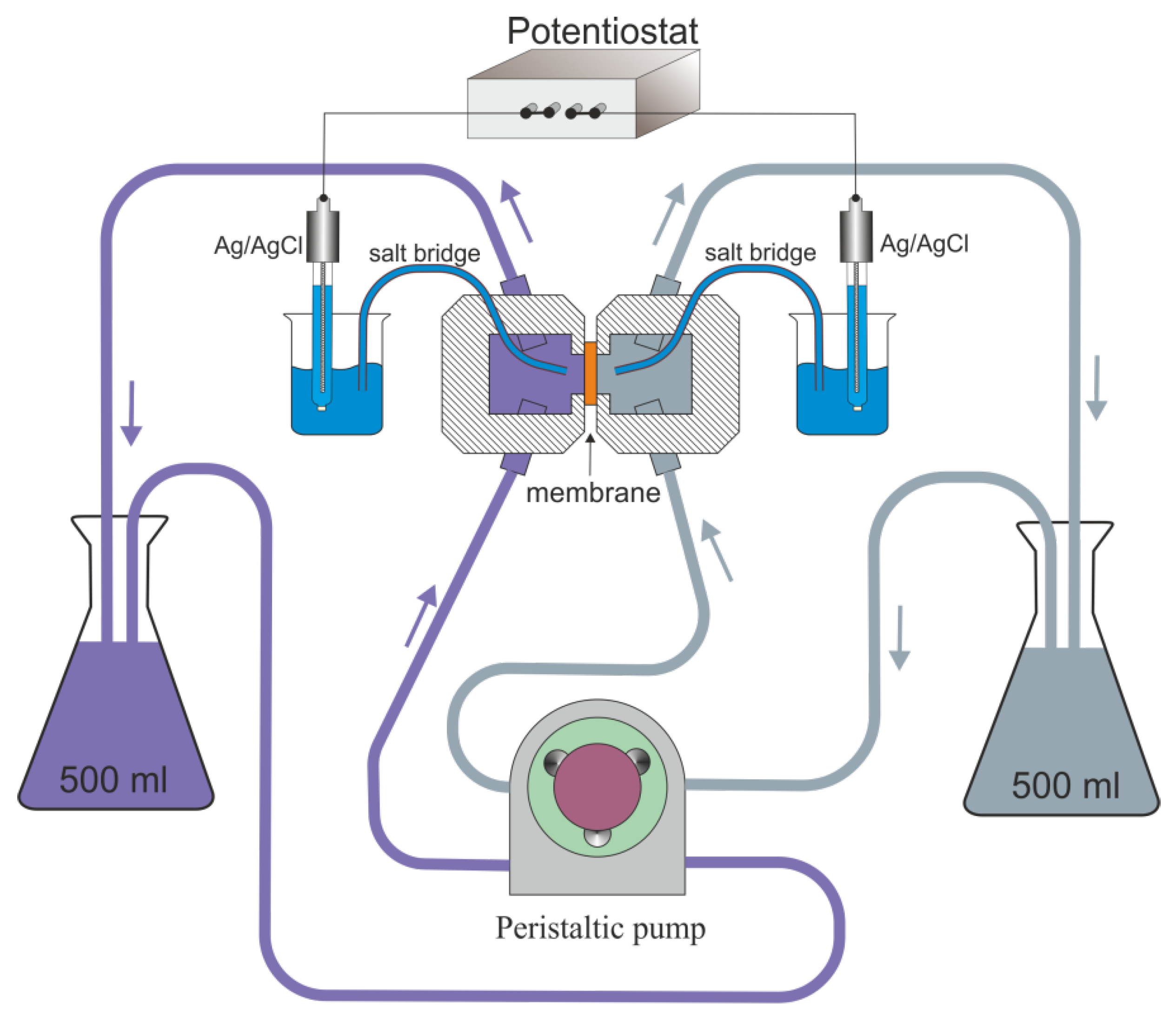
2.4. Ionic Selectivity Measurement
2.5. Mathematical Model
3. Results and Discussion
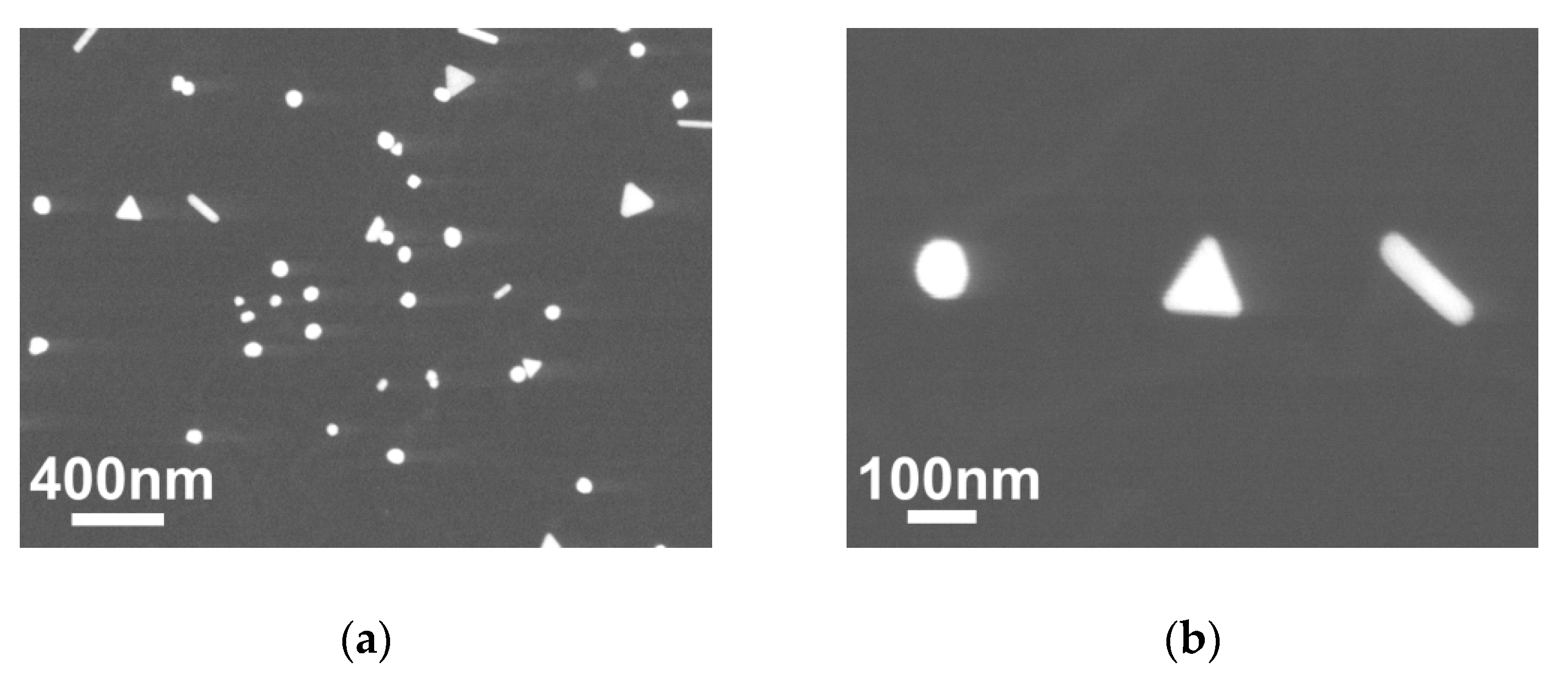
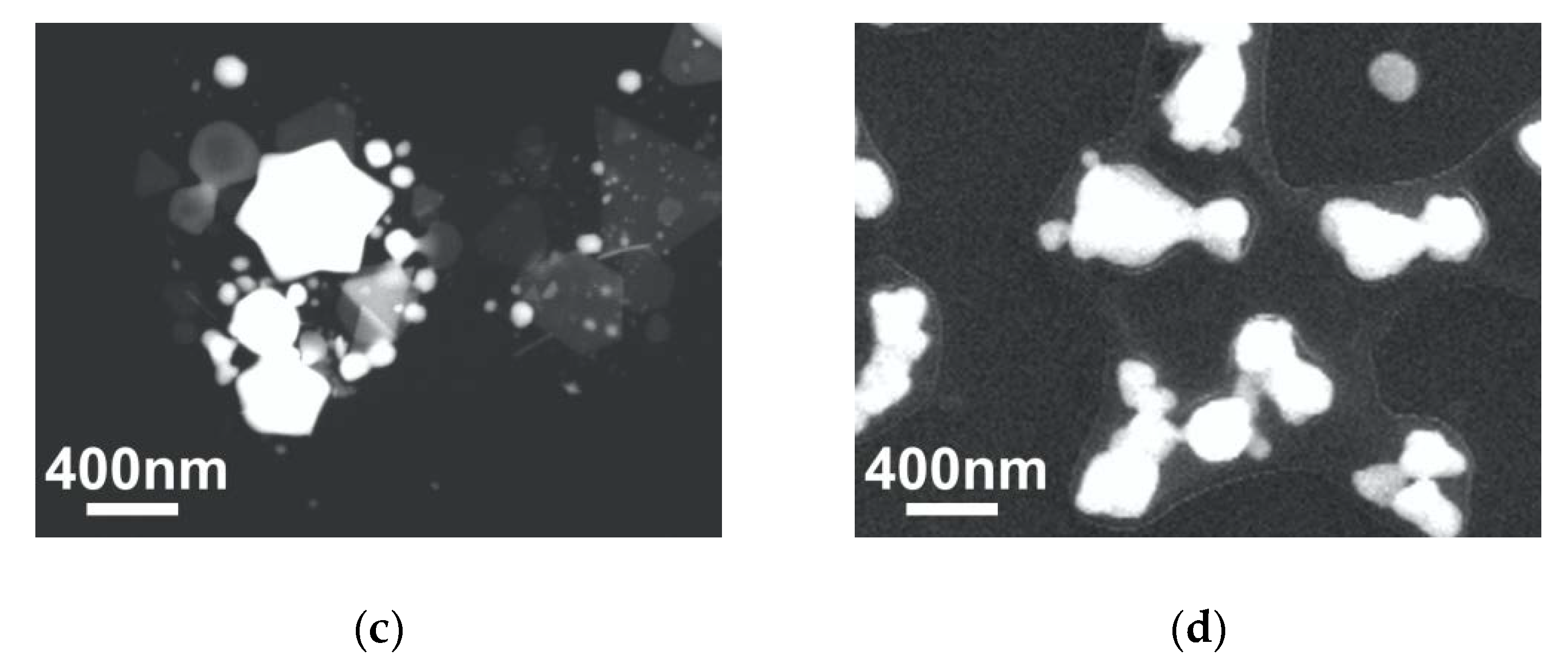
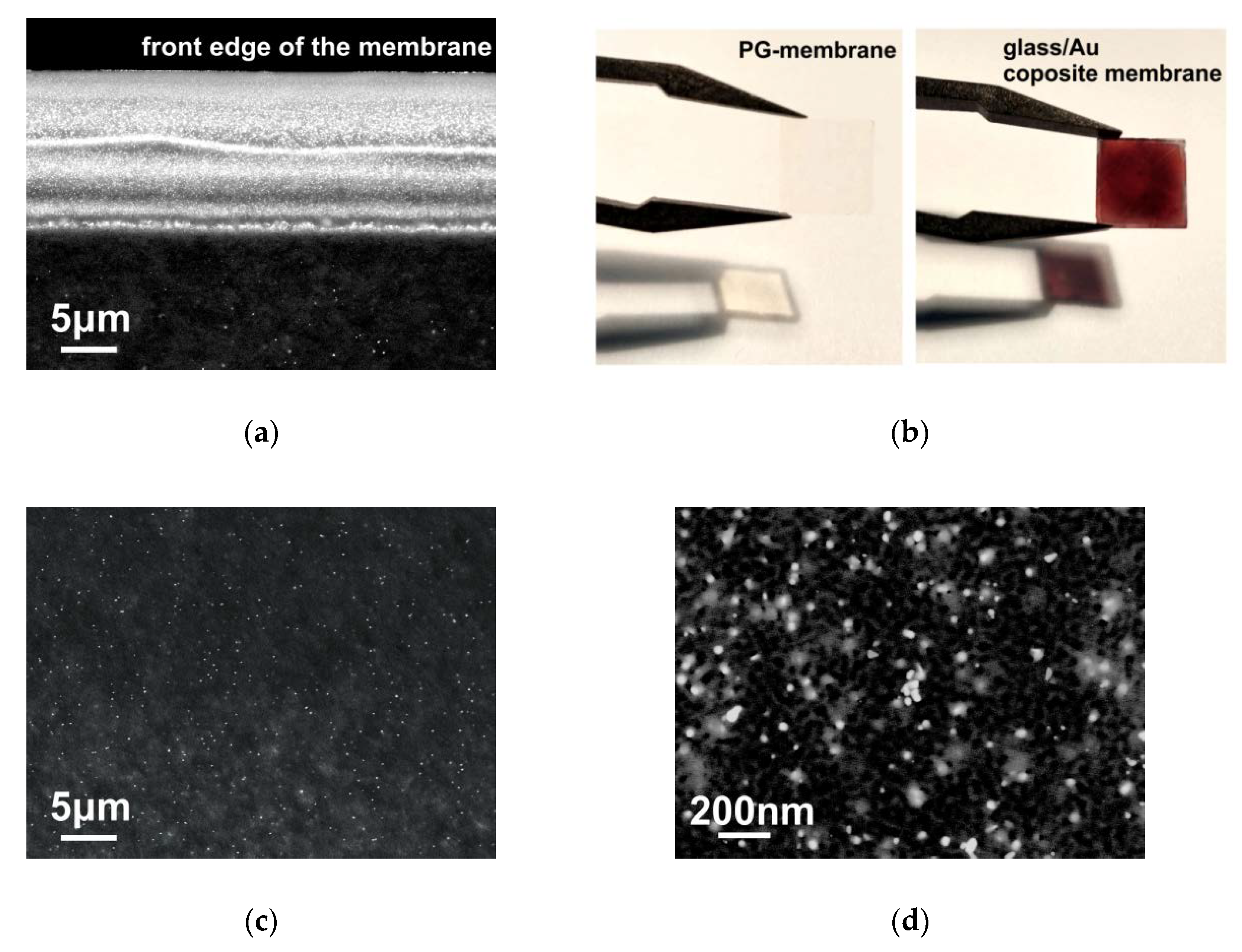
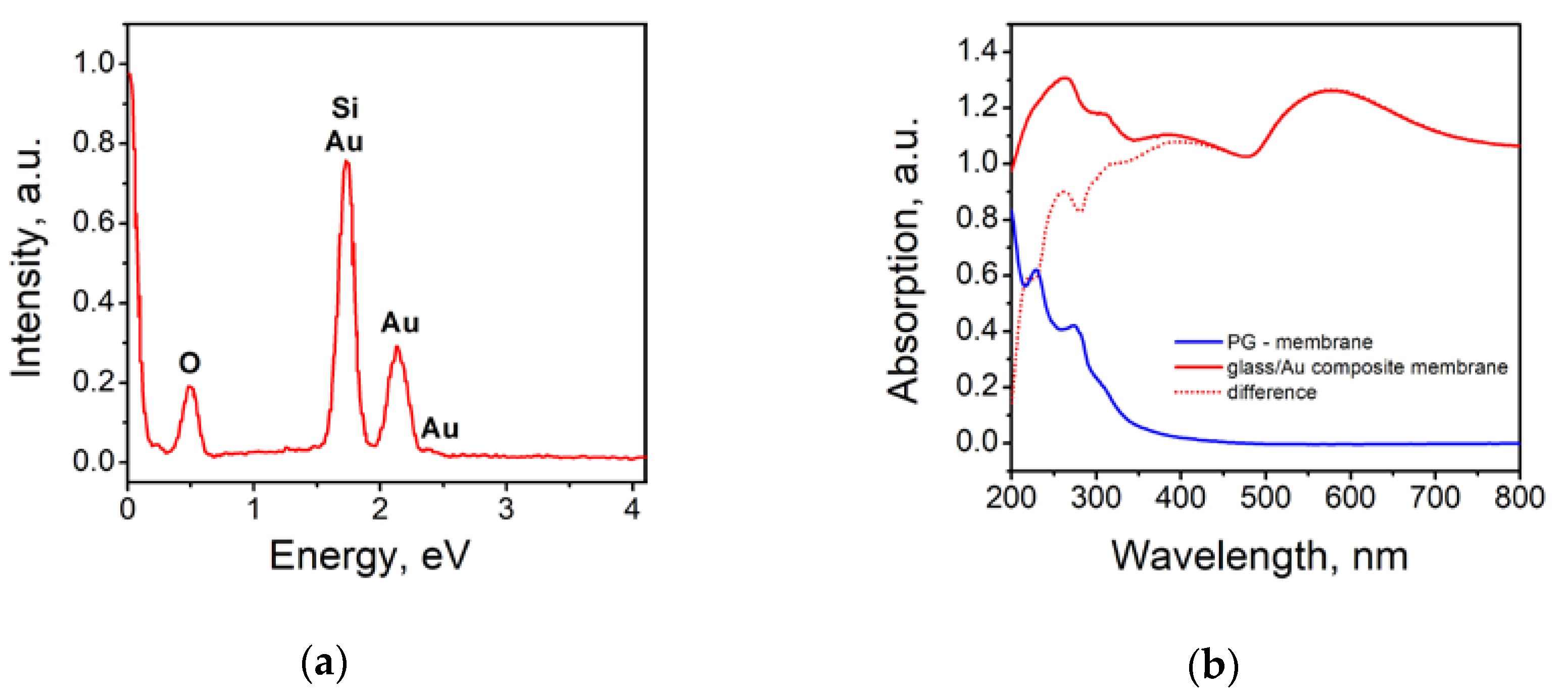
3.1. Glass/Au Composite Membranes Synthesis and Analysis
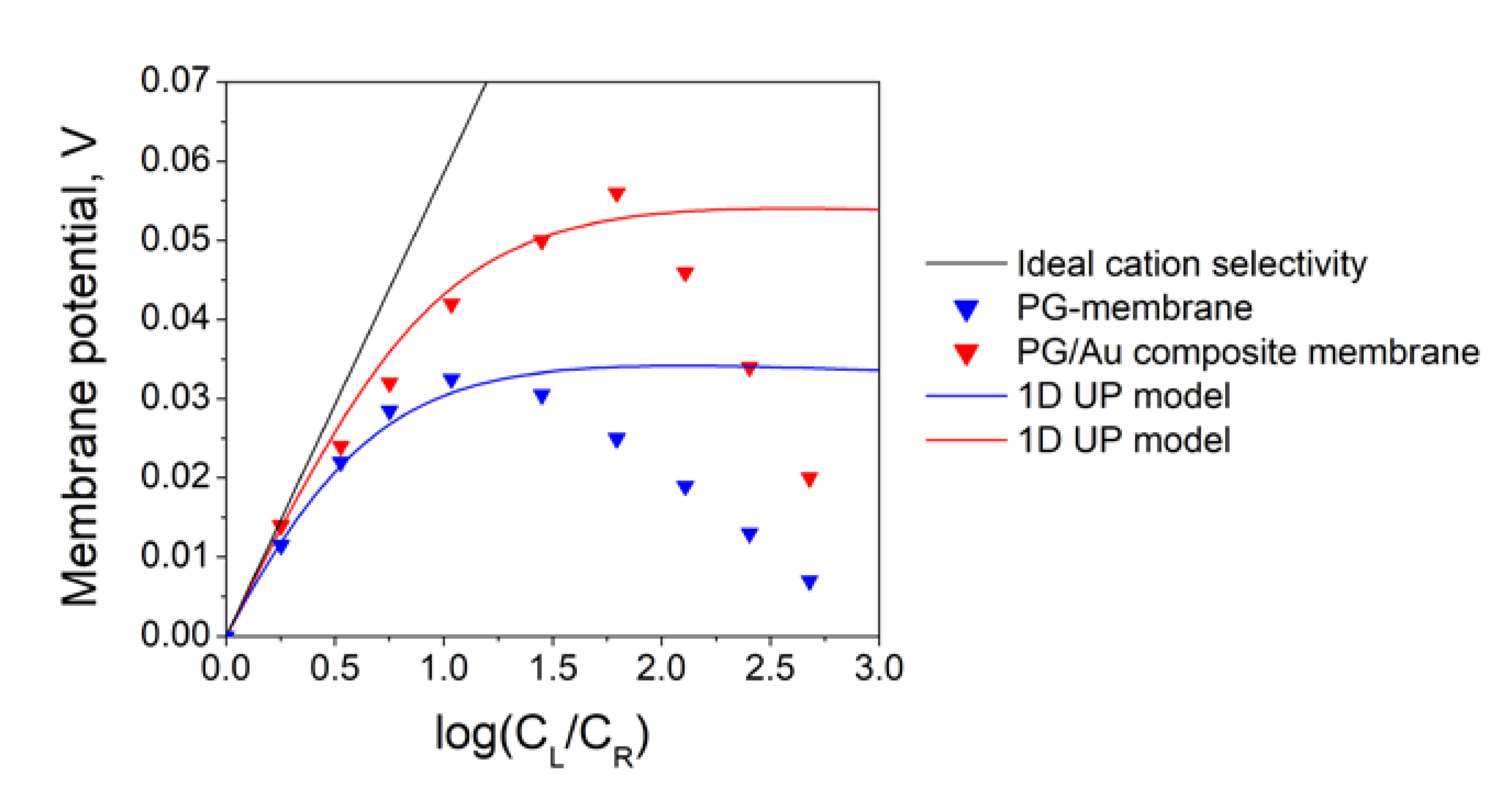
3.2. Study of Ion Transport Properties of the Glass/Au Composite Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Notation List
| electrical potential, V | |
| ion concentration, mol/m3 | |
| pressure, Pa | |
| space coordinate, m | |
| volume flux (velocity), m/s | |
| ion flux, mol/m2 s | |
| charge flux, mol/m2 s | |
| potential difference, V | |
| Faraday constant, C/mol | |
| temperature, K | |
| universal gas constant, J/kg K | |
| relative dielectric permittivity | |
| vacuum permittivity, F/m | |
| Debye length, m | |
| surface charge, C/m2 | |
| volume charge, mol/m3 | |
| ion diffusion coefficients, m2/s | |
| ratio of ion diffusion coefficients | |
| characteristic concentration, 1 mol/m3 | |
| pore radius, m | |
| pore length, m | |
| dimensionless parameter | |
| Subscripts | |
| + | cation |
| – | anion |
| left reservoir | |
| right reservoir | |
| ideal | |
| 0 | pore inlet |
| 1 | pore outlet |
| Superscripts | |
| + | cation selectivity |
| – | anion selectivity |
References
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Nath, K. Membrane Separation Processes; PHI Learning Pvt. Ltd.: New Delhi, India, 2017; ISBN 8120352912. [Google Scholar]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Pourcelly, G.; Nikonenko, V.V.; Pismenskaya, N.D.; Yaroslavtsev, A.B. Applications of charged membranes in separation, fuel cells and emerging processes. Ion. Interact. Nat. Synth. Macromol. 2012, 761–815. [Google Scholar] [CrossRef]
- Pintauro, P.N. Perspectives on membranes and separators for electrochemical energy conversion and storage devices. Polym. Rev. 2015, 55, 201–207. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118354230. [Google Scholar]
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J. Review of membranes in microfluidics. J. Chem. Technol. Biotechnol. 2017, 92, 271–282. [Google Scholar] [CrossRef]
- Hou, X. Smart Gating Multi-Scale Pore/Channel-Based Membranes. Adv. Mater. 2016, 28, 7049–7064. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Lebedev, D.V.; Solodovnichenko, V.S.; Minakov, A.V.; Simunin, M.M. On the origin of membrane potential in membranes with polarizable nanopores. J. Membr. Sci. 2018, 549, 616–630. [Google Scholar] [CrossRef]
- Jeong, B.H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.X.; Reed, M.A. Voltage gated ion and molecule transport in engineered nanochannels: Theory, fabrication and applications. Nanotechnology 2014, 25, 122001. [Google Scholar] [CrossRef]
- Gao, P.; Martin, C.R. Voltage Charging Enhances Ionic Conductivity in Gold Nanotube Membranes. ACS Nano 2014, 8, 8266–8272. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, D.V.; Solodovnichenko, V.S.; Simunin, M.M.; Ryzhkov, I.I. Effect of Electric Field on Ion Transport in Nanoporous Membranes with Conductive Surface. Pet. Chem. 2018, 58, 474–481. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, X.; Hou, J.; Zeng, L.; Tian, Y.; Li, L.; Jiang, L. Synthetic asymmetric-shaped nanodevices with symmetric pH-gating characteristics. Adv. Funct. Mater. 2015, 25, 1102–1110. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Surface-Modified Nanocomposite Membranes. Sep. Purif. Rev. 2018, 47, 288–305. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Ermakova, L.; Sidorova, M.; Antropova, T.; Jura, N.; Lurie, S. Porous glass membranes as model disperse systems. Colloids Surf. A: Physicochem. Eng. Asp. 2006, 282–283, 279–286. [Google Scholar] [CrossRef]
- Inayat, A.; Reinhardt, B.; Herwig, J.; Küster, C.; Uhlig, H.; Krenkel, S.; Raedlein, E.; Enke, D. Recent advances in the synthesis of hierarchically porous silica materials on the basis of porous glasses. New J. Chem. 2016, 40, 4095–4114. [Google Scholar] [CrossRef]
- Enke, D.; Janowski, F.; Schwieger, W. Porous glasses in the 21st century—A short review. Microporous Mesoporous Mater. 2003, 60, 19–30. [Google Scholar] [CrossRef]
- Vasilevskaya, T.N.; Antropova, T.V. Small-angle X-ray scattering study of the structure of glassy nanoporous matrices. Phys. Solid State 2009, 51, 2537–2545. [Google Scholar] [CrossRef]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Bioactive glass-based materials with hierarchical porosity for medical applications: Review of recent advances. Acta Biomater. 2016, 42, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Longley, L.; Krajnc, A.; Smales, G.J.; Qiao, A.; Erucar, I.; Doherty, C.M.; Thornton, A.W.; Hill, A.J.; Ashling, C.W.; et al. Metal-organic framework glasses with permanent accessible porosity. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reisfeld, R.; Levchenko, V.; Saraidarov, T.; Rysiakiewicz-Pasek, E.; Baranowski, M.; Podhorodecki, A.; Misiewicz, J.; Antropova, T. Steady state and femtosecond spectroscopy of Perylimide Red dye in porous and sol–gel glasses. Chem. Phys. Lett. 2012, 546, 171–175. [Google Scholar] [CrossRef]
- Zyryanova, K.S.; Kastro, R.A.; Pshenova, A.S.; Sidorov, A.I.; Antropova, T.V. Dielectric properties of silver-doped nanoporous silicate glasses in the temperature range between −50 and +250 °C. Glass Phys. Chem. 2017, 43, 207–214. [Google Scholar] [CrossRef]
- Sidorov, A.I.; Tung, N.D.; Van Wu, N.; Antropova, T.V.; Nashchekin, A.V. Optical Properties of Nanocomposites Based on Zinc and Tin Sulfides in Nanoporous Silicate Glass. Opt. Spectrosc. 2019, 127, 914–918. [Google Scholar] [CrossRef]
- Veiko, V.P.; Zakoldaev, R.A.; Sergeev, M.M.; Danilov, P.A.; Kudryashov, S.I.; Kostiuk, G.K.; Sivers, A.N.; Ionin, A.A.; Antropova, T.V.; Medvedev, O.S. Direct laser writing of barriers with controllable permeability in porous glass. Opt. Express 2018, 26, 28150–28160. [Google Scholar] [CrossRef]
- Sidorov, A.I.; Tung, N.D.; Van Wu, N.; Antropova, T.V.; Nashchekin, A.V.; Castro, R.; Anfimova, I.N. Optical and Dielectric Properties of Nanocomposites Based on Zinc and Tin Oxides in Nanoporous Glass. Glass Phys. Chem. 2019, 45, 439–446. [Google Scholar] [CrossRef]
- Ciżman, A.; Bednarski, W.; Antropova, T.V.; Pshenko, O.; Rysiakiewicz-Pasek, E.; Waplak, S.; Poprawski, R. Structural, dielectric, thermal and electron magnetic resonance studies of magnetic porous glasses filled with ferroelectrics. Compos. Part. B: Eng. 2014, 64, 16–23. [Google Scholar]
- Ciżman, A.; Rogacki, K.; Rysiakiewicz-Pasek, E.; Antropova, T.; Pshenko, O.; Poprawski, R. Magnetic properties of novel magnetic porous glass-based multiferroic nanocomposites. J. Alloy. Compd. 2015, 649, 447–452. [Google Scholar] [CrossRef]
- Antropova, T.V.; Anfimova, I.N.; Golovina, G.F. Influence of the composition and temperature of heat treatment of porous glasses on their structure and light transmission in the visible spectral range. Glass Phys. Chem. 2009, 35, 572–579. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Skripkin, M.Y.; Tveryanovich, Y.S.; Mereshchenko, A.S.; Gorbunov, A.O.; Panov, M.S.; Tumkin, I.I.; Safonov, S.V. Laser-induced copper deposition from aqueous and aqueous–organic solutions: State of the art and prospects of research. Russ. Chem. Rev. 2015, 84, 1059–1075. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Andrianov, V.S.; Khairullina, E.M.; Lebedev, D.V.; Ryazantsev, M.N.; Panov, M.S.; Tumkin, I.I. In Situ laser-induced synthesis of copper-silver microcomposite for enzyme-free D-glucose and L-alanine sensing. Appl. Surf. Sci. 2019, 488, 531–536. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Novomlinsky, M.O.; Fogel, A.A.; Kochemirovskaia, S.V.; Lebedev, D.V.; Kochemirovsky, V.A. Laser method of microscopic sensor synthesis for liquid and gas analysis using glucose and H2S as an example. J. Solid State Electrochem. 2019, 23, 3173–3185. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Logunov, L.S.; Safonov, S.V.; Tumkin, I.I.; Tver’yanovich, Y.S.; Menchikov, L.G. Sorbitol as an efficient reducing agent for laser-induced copper deposition. Appl. Surf. Sci. 2012, 259, 55–58. [Google Scholar] [CrossRef]
- Smikhovskaia, A.V.; Panov, M.S.; Tumkin, I.I.; Khairullina, E.M.; Ermakov, S.S.; Balova, I.A.; Ryazantsev, M.N.; Kochemirovsky, V.A. In Situ laser-induced codeposition of copper and different metals for fabrication of microcomposite sensor-active materials. Anal. Chim. Acta 2018, 1044, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Panov, M.S.; Vereshchagina, O.A.; Ermakov, S.S.; Tumkin, I.I.; Khairullina, E.M.; Skripkin, M.Y.; Mereshchenko, A.S.; Ryazantsev, M.N.; Kochemirovsky, V.A. Non-enzymatic sensors based on in situ laser-induced synthesis of copper-gold and gold nano-sized microstructures. Talanta 2017, 167, 201–207. [Google Scholar] [CrossRef]
- Eftekhari, F.; Escobedo, C.; Ferreira, J.; Duan, X.; Girotto, E.M.; Brolo, A.G.; Gordon, R.; Sinton, D. Nanoholes As Nanochannels: Flow-through Plasmonic Sensing. Anal. Chem. 2009, 81, 4308–4311. [Google Scholar] [CrossRef]
- Antropova, T.V.; Kalinina, S.V.; Kostyreva, T.G.; Drozdova, I.A.; Anfimova, I.N. Peculiarities of the fabrication process and the structure of porous membranes based on two-phase fluorine- and phosphorus-containing sodium borosilicate glasses. Glass Phys. Chem. 2015, 41, 14–25. [Google Scholar] [CrossRef]
- Gutina, A.; Antropova, T.; Rysiakiewicz-Pasek, E.; Virnik, K.; Feldman, Y. Dielectric relaxation in porous glasses. Microporous Mesoporous Mater. 2003, 58, 237–254. [Google Scholar] [CrossRef]
- Kreisberg, V.A.; Antropova, T.V. Changing the relation between micro- and mesoporosity in porous glasses: The effect of different factors. Microporous Mesoporous Mater. 2014, 190, 128–138. [Google Scholar] [CrossRef]
- Gorshkova, K.O.; Tumkin, I.I.; Myund, L.A.; Tverjanovich, A.S.; Mereshchenko, A.S.; Panov, M.S.; Kochemirovsky, V.A. The investigation of dye aging dynamics in writing inks using Raman spectroscopy. Dye. Pigment. 2016, 131, 239–245. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Vyatkin, A.S.; Mikhlina, E.V. Modelling of Conductive Nanoporous Membranes with Switchable Ionic Selectivity. Membr. Membr. Technol. 2020, 2, 10–19. [Google Scholar] [CrossRef]
- Peters, P.B.; van Roij, R.; Bazant, M.Z.; Biesheuvel, P.M. Analysis of electrolyte transport through charged nanopores. Phys. Rev. E 2016, 93, 053108. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, I.I.; Vyatkin, A.S.; Minakov, A.V. Theoretical study of electrolyte diffusion through polarizable nanopores. J. Sib. Fed. Univ. Math. Phys. 2018, 11, 494–504. [Google Scholar]
- Tanaka, Y. Ion-Exchange Membranes: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444633194. [Google Scholar]
- Zhang, L.; Biesheuvel, P.M.; Ryzhkov, I.I. Theory of Ion and Water Transport in Electron-Conducting Membrane Pores with p H-Dependent Chemical Charge. Phys. Rev. Appl. 2019, 12, 014039. [Google Scholar] [CrossRef]
- Placido, T.; Comparelli, R.; Giannici, F.; Cozzoli, P.D.; Capitani, G.; Striccoli, M.; Agostiano, A.; Curri, M.L. Photochemical Synthesis of Water-Soluble Gold Nanorods: The Role of Silver in Assisting Anisotropic Growth. Chem. Mater. 2009, 21, 4192–4202. [Google Scholar] [CrossRef]
- Kurihara, K.; Kizling, J.; Stenius, P.; Fendler, J.H. Laser and pulse radiolytically induced colloidal gold formation in water and in water-in-oil microemulsions. J. Am. Chem. Soc. 1983, 105, 2574–2579. [Google Scholar] [CrossRef]
- Mohammadnejad, S.; Provis, J.L.; van Deventer, J.S.J. Reduction of gold(III) chloride to gold(0) on silicate surfaces. J. Colloid Interface Sci. 2013, 389, 252–259. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C: Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Ermakova, L.E.; Sidorova, M.P.; Zhura, N.A. Electric transport properties of ultra-and nanoporous glasses in electrolyte solutions. Colloid J. 2007, 69, 571–578. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Niidome, Y.; Hori, A.; Takahashi, H.; Goto, Y.; Yamada, S. Laser-Induced Deposition of Gold Nanoparticles onto Glass Substrates in Cyclohexane. Nano Lett. 2001, 1, 365–369. [Google Scholar] [CrossRef]
- Ngabonziza, P.; Stehno, M.P.; Koster, G.; Brinkman, A. In-Situ Characterization Techniques for Nanomaterials; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-56321-2. [Google Scholar]
- Liu, Y.; Begin-Colin, S.; Pichon, B.P.; Leuvrey, C.; Ihiawakrim, D.; Rastei, M.; Schmerber, G.; Vomir, M.; Bigot, J.Y. Two dimensional dipolar coupling in monolayers of silver and gold nanoparticles on a dielectric substrate. Nanoscale 2014, 6, 12080–12088. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, L.E.; Medvedeva, S.V.; Volkova, A.V.; Sidorova, M.P.; Antropova, T.V. Structure and electrosurface properties of porous glasses with different compositions in KCl and NaCl solutions. Colloid J. 2005, 67, 304–312. [Google Scholar] [CrossRef]
- Nishizawa, M.; Menon, V.P.; Martin, C.R. Metal nanotubule membranes with electrochemically switchable ion-transport selectivity. Science 1995, 268, 700–702. [Google Scholar] [CrossRef]
| Membrane Type | Model | Surface Charge |
|---|---|---|
| PG membrane | 1D UP model | −2.36 mC/m2 |
| PG/Au composite membrane | 1D UP model | −5.42 mC/m2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, D.; Novomlinsky, M.; Kochemirovsky, V.; Ryzhkov, I.; Anfimova, I.; Panov, M.; Antropova, T. Glass/Au Composite Membranes with Gold Nanoparticles Synthesized inside Pores for Selective Ion Transport. Materials 2020, 13, 1767. https://doi.org/10.3390/ma13071767
Lebedev D, Novomlinsky M, Kochemirovsky V, Ryzhkov I, Anfimova I, Panov M, Antropova T. Glass/Au Composite Membranes with Gold Nanoparticles Synthesized inside Pores for Selective Ion Transport. Materials. 2020; 13(7):1767. https://doi.org/10.3390/ma13071767
Chicago/Turabian StyleLebedev, Denis, Maxim Novomlinsky, Vladimir Kochemirovsky, Ilya Ryzhkov, Irina Anfimova, Maxim Panov, and Tatyana Antropova. 2020. "Glass/Au Composite Membranes with Gold Nanoparticles Synthesized inside Pores for Selective Ion Transport" Materials 13, no. 7: 1767. https://doi.org/10.3390/ma13071767
APA StyleLebedev, D., Novomlinsky, M., Kochemirovsky, V., Ryzhkov, I., Anfimova, I., Panov, M., & Antropova, T. (2020). Glass/Au Composite Membranes with Gold Nanoparticles Synthesized inside Pores for Selective Ion Transport. Materials, 13(7), 1767. https://doi.org/10.3390/ma13071767






