Ferromagnetic Half-Metal Cyanamides Cr(NCN)2 Predicted from First Principles Investigation
Abstract
1. Introduction
2. Methods
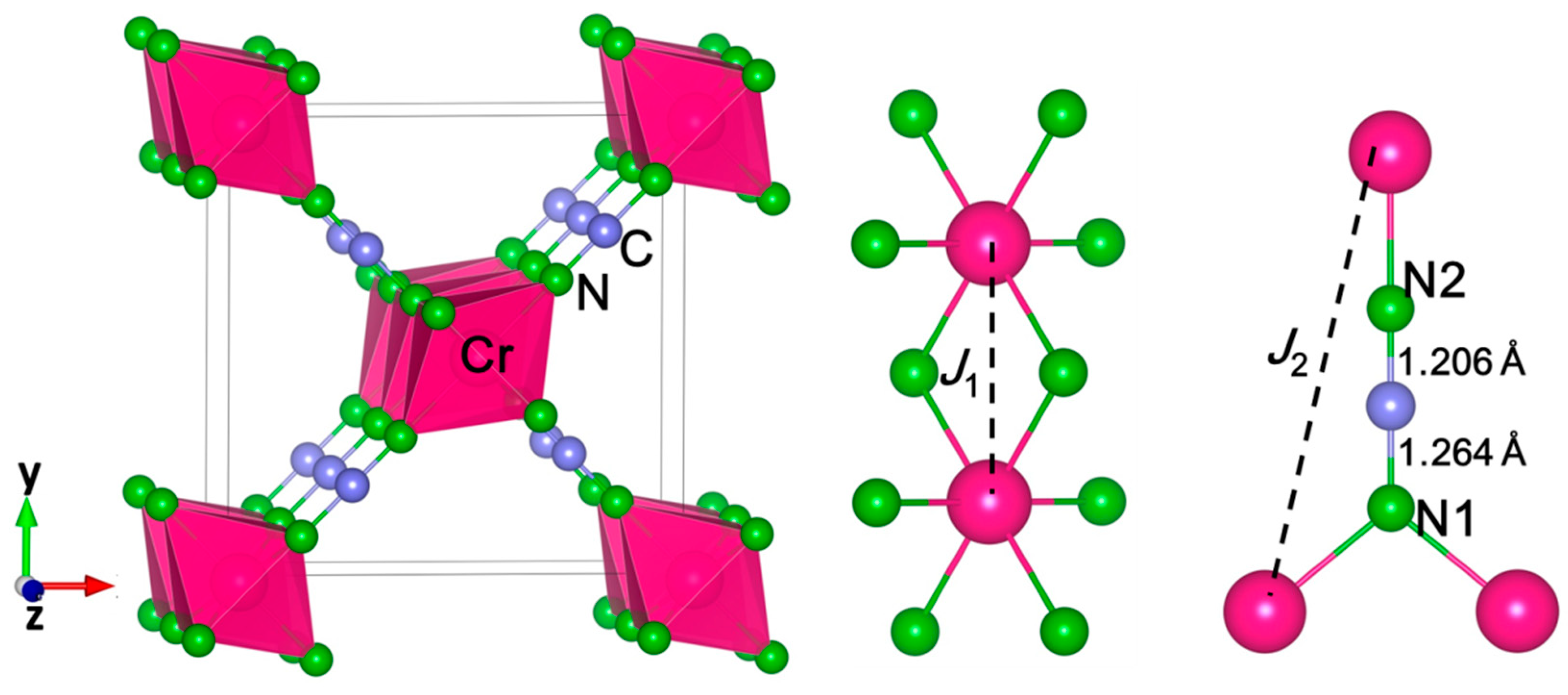
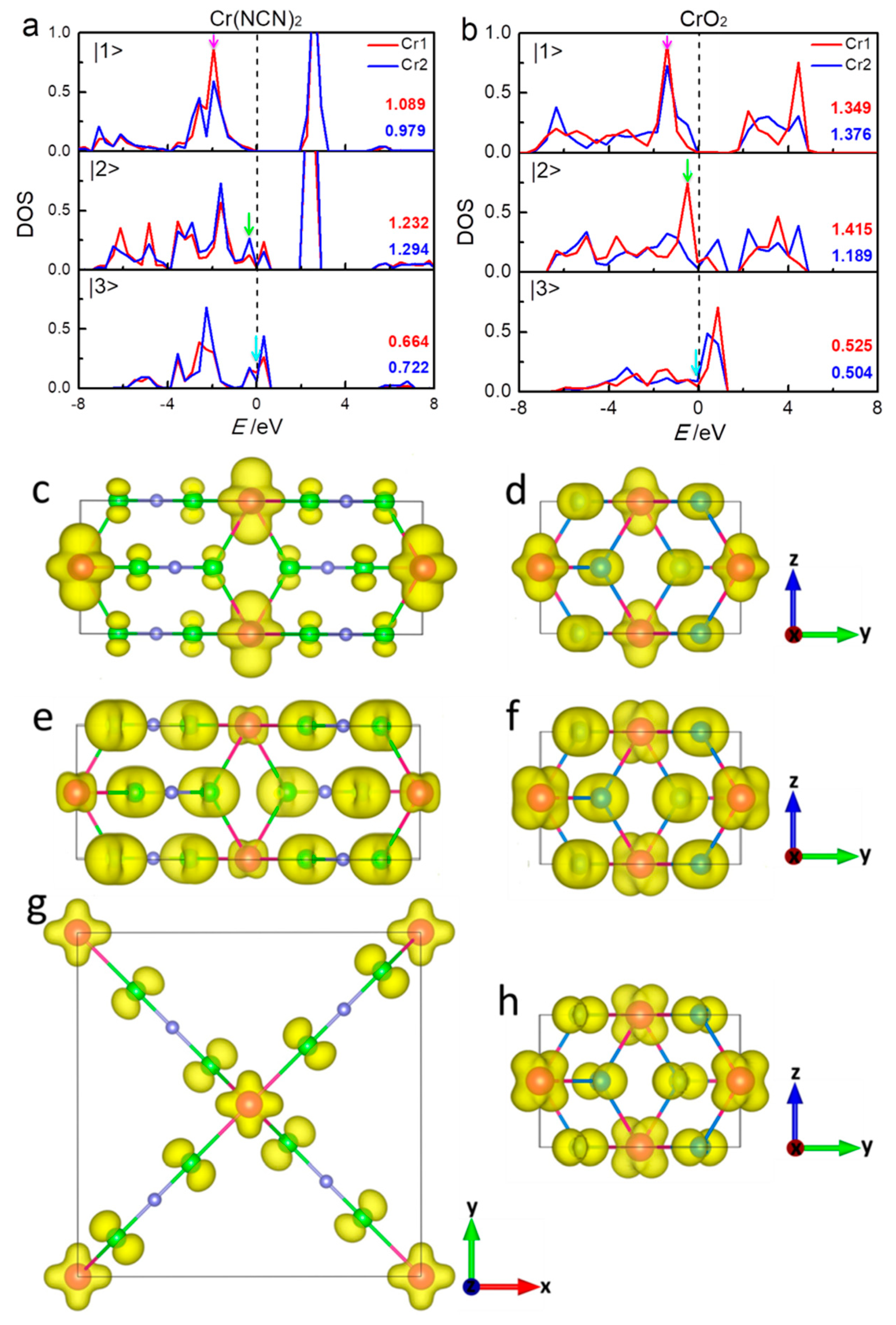
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sakurai, H.; Kolodiazhnyi, T.; Michiue, Y.; Takayama-Muromachi, E.; Tanabe, Y.; Kikuchi, H. Unconventional colossal magnetoresistance in sodium chromium oxide with a mixed-valence state. Angew. Chem. Int. Ed. 2012, 124, 6757–6760. [Google Scholar] [CrossRef]
- Komarek, A.C.; Streltsov, S.V.; Isobe, M.; Möller, T.; Braden, M. CaCrO3: An anomalous antiferromagnetic metallic oxide. Phys. Rev. Lett. 2008, 101, 167204. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, T.; Nakao, A.; Yamaki, Y.; Nakao, H.; Otha, Y. Peierls Mechanism of the metal-insulator transition in ferromagnetic hollandite K2Cr8O16. Phys. Rev. Lett. 2011, 107, 266402. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Ahn, K.H.; Jung, M.C.; Lee, K.W. Strain and spin-orbit coupling induced orbital-ordering in mott insulator BaCrO3. Phys. Rev. B 2014, 9, 205124. [Google Scholar] [CrossRef]
- Komarek, A.C.; Möller, T.; Isobe, M.; Drees, Y.; Braden, M. Magnetic order, transport and infrared optical properties in the ACrO3 system (A = Ca, Sr, and Pb). Phys. Rev. B 2011, 84, 125114. [Google Scholar] [CrossRef]
- Cheng, J.G.; Kweon, K.E.; Larregola, S.A.; Ding, Y.; Shirako, Y.; Marshall, L.G.; Li, Z.-Y.; Li, X.; António, M.; Suchomel, M.R.; et al. Charge disproportionation and the pressure-induced insulator-metal transition in cubic perovskite PbCrO3. Proc. Natl. Acad. Sci. USA 2015, 112, 1670–1674. [Google Scholar] [CrossRef]
- Xiao, W.; Tan, D.; Xiong, X.; Liu, J.; Xu, J. Large volume collapse observed in the phase transition in cubic PbCrO3 perovskite. Proc. Natl. Acad. Sci. USA 2010, 107, 14026–14029. [Google Scholar] [CrossRef]
- Hasegawa, K.; Isobe, M.; Yamauchi, T.; Ueda, H.; Ueda, Y. Discovery of ferromagnetic-half-metal-to-insulator transition in K2Cr8O16. Phys. Rev. Lett. 2009, 103, 146403. [Google Scholar] [CrossRef]
- Korotin, M.A.; Anisimov, V.I.; Khomskii, D.I.; Sawatzky, G.A. CrO2: A self-doped double exchange ferromagnet. Phys. Rev. Lett. 1998, 80, 4305–4308. [Google Scholar] [CrossRef]
- Schlottmann, P. Double-exchange mechanism for CrO2. Phys. Rev. B 2003, 67, 386–393. [Google Scholar] [CrossRef]
- Shim, J.H.; Lee, S.; Dho, J.; Kim, D.H. Coexistence of two different Cr Ions by self-doping in half-metallic CrO2 nanorods. Phys. Rev. Lett. 2007, 99, 057209. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Shimizu, Y.; Kobayashi, Y.; Itoh, M.; Jinno, T.; Isobe, M. Local electronic state in the half-metallic ferromagnet CrO2 investigated by site-selective 53Cr NMR measurements. Phys. Rev. B 2016, 93, 235129. [Google Scholar] [CrossRef]
- Solovyev, I.V.; Kashin, I.V.; Mazurenko, V.V. Mechanisms and origins of half-metallic ferromagnetism in CrO2. Phys. Rev. B 2015, 92, 144407. [Google Scholar] [CrossRef]
- Heffernan, K.H.; Yu, S.; Deckoff-Jones, S.; Zhang, X.; Talbayev, D. The role of spin fluctuations in the conductivity of CrO2. Phys. Rev. B 2016, 93, 165143. [Google Scholar] [CrossRef]
- Toropova, A.; Kotliar, G.; Savrasov, S.Y.; Oudovenko, V.S. Electronic structure and magnetic anisotropy of CrO2. Phys. Rev. B 2005, 71, 172403. [Google Scholar] [CrossRef]
- Boyko, T.D.; Green, R.J.; Dronskowski, R.; Moewes, A. Electronic band gap reduction in manganese carbodiimide: MnNCN. J. Phys. Chem. C 2013, 117, 12754–12761. [Google Scholar] [CrossRef]
- Tang, X.J.; Xiang, H.P.; Liu, X.H.; Speldrich, M.; Dronskowski, R. A ferromagnetic carbodiimide: Cr2(NCN)3. Angew. Chem. Int. Ed. 2010, 49, 4738–4742. [Google Scholar] [CrossRef]
- Liu, X.; Dronskowski, R.; Kremer, R.K.; Ahrens, M.; Lee, C.; Whangbo, M. Characterization of the Magnetic and Structural Properties of Copper Carbodiimide, CuNCN, by Neutron Diffraction and First-Principles Evaluations of Its Spin Exchange Interactions. J. Phys. Chem. C 2008, 112, 11013–11017. [Google Scholar] [CrossRef]
- Xiang, H.P.; Liu, X.H.; Dronskowski, R. Theoretical reinvestigation of the electronic structure of CuNCN: The influence of packing on the magnetic properties. J. Phys. Chem. C 2009, 113, 18891–18896. [Google Scholar] [CrossRef]
- Maddox, B.R. High-pressure structure of half-metallic CrO2. Phys. Rev. B 2006, 73, 144111. [Google Scholar] [CrossRef]
- Tang, X.J.; Houben, A.; Liu, X.H.; Stork, L.; Dronskowski, R. Crystal structure refinement of M(NCNH)2 (M = Fe, Co) based on combined neutron and X-ray diffraction Data. ZAAC 2011, 637, 1089–1091. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Altmeyer, M.; Jeschke, H.O.; Hijano-Cubelos, O.; Martins, C.; Lechermann, F.; Koepernik, K.; Santander-Syro, A.F.; Rozenberg, M.; Valenti, R.; Gabay, M. Magnetism, spin texture, and in-gap states: Atomic specialization at the surface of oxygen-deficient SrTiO3. Phys. Rev. Lett. 2015, 116, 157203. [Google Scholar] [CrossRef]
- Beutier, G.; Collins, S.P.; Dimitrova, O.V.; Dmitrienko, V.E. Band Filling Control of the Dzyaloshinskii-Moriya Interaction in Weakly Ferromagnetic Insulators. Phys. Rev. Lett. 2017, 119, 167201. [Google Scholar] [CrossRef]
- Wen, X.D.; Martin, R.L.; Henderson, T.M.; Scuseria, G.E. Density functional theory studies of the electronic structure of solid state actinide oxides. Chem. Rev. 2013, 113, 1063–1096. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, C.; Yu, T.; Xu, M.; Zhang, S.; Xu, H.; Liu, Y.; Kan, E.; Wang, Y.; Yang, G. High-Temperature Ferromagnetism in Fe3P Monolayer with Large Magnetic Anisotropy. J. Phys. Chem. Lett. 2019, 10, 2733–2738. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Peter, E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for brillouin-zone integrations. Phys Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Poteryaev, A.I.; Korotin, M.A.; Anokhin, A.O.; Kotliar, G. First-principles calculations of the electronic structure and spectra of strongly correlated systems: Dynamical mean-field theory. J. Phys.-Condens. Matter 1997, 9, 7359–7367. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Bhobe, P.A.; Chainani, A.; Taguchi, M.; Eguchi, R.; Shin, S. Electronic structure of an antiferromagnetic metal: CaCrO3. Phys. Rev. B 2010, 83, 165132. [Google Scholar] [CrossRef]
- Liu, X.; Müller, P.; Kroll, P.; Dronskowski, R. Synthesis, structure determination, and quantum-chemical characterization of an alternate HgNCN polymorph. Inorg. Chem. 2002, 41, 4259–4265. [Google Scholar] [CrossRef]
- Baroni, S.; Gironcoli, S.; Corso, A.D.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Becker, M.; Nuss, J.; Jansen, M. ChemInform Abstract: Synthesis and characterization of sodium cyanamide. ChemInform 2010, 32. [Google Scholar] [CrossRef]
- Baldinozzi, G.; Malinowska, B.; Rakib, M.; Durand, G. Crystal structure and characterisation of cadmium cyanamide. J. Mater. Chem. A 2002, 12, 268–272. [Google Scholar] [CrossRef]
- Cooper, M.J. The structures of some inorganic cyanamides. II. The structure of lead cyanamide. Acta Crystallogr. 1964, 17, 1452–1456. [Google Scholar] [CrossRef]
- Yamasaki, A.; Chioncel, L.; Lichtenstein, A.I.; Andersen, O.K. Model hamiltonian parameters for half-metallic ferromagnets NiMnSb and CrO2. Phys. Rev. B 2006, 74, 024419. [Google Scholar] [CrossRef]
- Goodenough, J.B. Magnetism and the Chemical Bond; Interscience Publishers: New York, NY, USA, 1963; p. 180. [Google Scholar]
- Xiang, H.P.; Tang, Y.Y.; Zhang, S.Y.; He, Z. Intra-chain superexchange couplings in quasi-1D 3d transition-metal magnetic compounds. J. Phys.-Condens. Matter 2016, 28, 276003. [Google Scholar] [CrossRef]


| Cr(NCN)2 | CrO2 | ||||
|---|---|---|---|---|---|
| GGA | GGA+U | GGA | GGA+U | ||
| Etotal (meV) | FM | 0 | 0 | 0 | 0 |
| AFM1 | 109.1 | 172.3 | 159.6 | 249.5 | |
| AFM2 | 57.9 | 127.2 | 115.1 | 148.9 | |
| C (meV) | J1 | 1.7 | 20.5 | 17.7 | 12.1 |
| J2 | 13.7 | 21.5 | 19.9 | 31.2 | |
| M (μB) | MCr | 2.18 | 2.63 | 2.11 | 2.38 |
| 2.18 | 2.62 | 2.05 | 2.31 | ||
| MN/O | −0.06 | −0.11 | −0.05 | −0.13 | |
| −0.06 | −0.10 | −0.06 | −0.12 | ||
| −0.06 | −0.12 | ||||
| −0.06 | −0.11 | ||||
| Mtotal | 2.01 | 2.26 | 1.97 | 2.09 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qu, S.; Xiang, H.; He, Z.; Shen, J. Ferromagnetic Half-Metal Cyanamides Cr(NCN)2 Predicted from First Principles Investigation. Materials 2020, 13, 1805. https://doi.org/10.3390/ma13081805
Wang Z, Qu S, Xiang H, He Z, Shen J. Ferromagnetic Half-Metal Cyanamides Cr(NCN)2 Predicted from First Principles Investigation. Materials. 2020; 13(8):1805. https://doi.org/10.3390/ma13081805
Chicago/Turabian StyleWang, Zhilue, Shoujiang Qu, Hongping Xiang, Zhangzhen He, and Jun Shen. 2020. "Ferromagnetic Half-Metal Cyanamides Cr(NCN)2 Predicted from First Principles Investigation" Materials 13, no. 8: 1805. https://doi.org/10.3390/ma13081805
APA StyleWang, Z., Qu, S., Xiang, H., He, Z., & Shen, J. (2020). Ferromagnetic Half-Metal Cyanamides Cr(NCN)2 Predicted from First Principles Investigation. Materials, 13(8), 1805. https://doi.org/10.3390/ma13081805






